Abstract
Respiratory pandemics like COVID-19 continued to strain healthcare systems worldwide. Numerous antiviral, antimalarial, and anti-inflammatory treatments were administered to many patients to pursue effective therapeutics. This study aims to assess the efficacy of the anti-inflammatory agent tocilizumab for critically ill patients, specifically in managing respiratory illnesses. This multi-center cohort study included laboratory-confirmed SARS-CoV-2 patients as special cases admitted to the intensive care units (ICUs) of 15 hospitals across Saudi Arabia between March 1, 2020, and October 30, 2020. A total of 1470 critically ill patients with SARS-CoV-2 were included. The study included 1470 patients with a mean age of 55.9 ± 15.1 years; 1088 (74.0%) were male and 382 (26.0%) female. Among them, 29% received Tocilizumab, while 71% received other treatments such as remdesivir, hydroxychloroquine, corticosteroids, convalescent plasma, intravenous immunoglobulin, and plasmapheresis. The median Sequential Organ Failure Assessment (SOFA) score for the cohort was 5 [IQR 3–8], with lower scores observed in the Tocilizumab administered group (p = 0.143). ICU mortality was significantly lower in the tocilizumab group: 150/426 (35.2%) versus 457/1044 (43.8%), p = 0.004. The median length of ICU stay was longer in the Tocilizumab group (12 days; IQR 7–21) than in the non-Tocilizumab group (8 days; IQR 4–15). However, Tocilizumab use was associated with a reduced likelihood of prolonged ICU stay (adjusted OR 0.68; 95% CI 0.57–0.83; p < 0.001). Similarly, the median hospital stay was longer among Tocilizumab recipients (18 days; IQR 12–30) compared to non-recipients (14 days; IQR 8–23). Despite the longer duration, Tocilizumab was associated with a decreased likelihood of extended hospital stay (adjusted OR 0.72; 95% CI 0.57–0.91; p = 0.003). These findings support the beneficial role of Tocilizumab in managing acute respiratory illness due to COVID-19. This study suggests that tocilizumab, particularly when administered early, is associated with reduced mortality and improved outcomes in critically ill patients. These findings not only support the use of tocilizumab as a therapeutic option for severe cases of COVID-19 but also highlight its potential for future respiratory pandemics. Early intervention with tocilizumab may lead to significant benefits, offering a valuable treatment strategy with manageable adverse effects for critically ill patients in future global health crises.
Similar content being viewed by others
Introduction
Epidemics of note consist of severe acute respiratory syndrome (SARS) in 2003 and the Middle East respiratory syndrome (MERS), which began in 2012. Major pandemics include the H1N1 influenza and the coronavirus disease 2019 (COVID‐19) caused by SARS‐CoV‐2. These infections can cause serious problems and affect normal breathing. They can cause flu (influenza), severe acute respiratory syndrome (SARS), and COVID‐19. The COVID-19 pandemic has profoundly affected global health as a severe respiratory virus1,2. While most COVID-19 cases exhibit mild to moderate symptoms, up to 20% of patients may develop severe disease, including pneumonia, acute respiratory distress syndrome, and multi-organ failure requiring ventilator support3. Similar patterns of disease severity have been reported in Saudi Arabia, with patients presenting mild, moderate, and critical conditions, alongside asymptomatic cases4,5,6,7,8. COVID-19 is marked by elevated plasma levels of pro-inflammatory cytokines, ferritin, lactate dehydrogenase (LDH), D-dimer, and C-reactive protein, indicating systemic inflammation9,10,11. Lung tissue histopathology in deceased patients has revealed inflammatory cellular infiltration, proteinaceous exudate, and extensive alveolar edema, emphasizing pulmonary inflammation and cytokine storm resulting from immune dysregulation3,9,10. Tocilizumab, a monoclonal antibody targeting the pro-inflammatory cytokine IL-6, has been approved for the treatment of cytokine release syndrome (CRS)9.
It has demonstrated rapid improvement in respiratory and hemodynamic parameters in CRS, and the U.S. Food and Drug Administration has endorsed its use for severe or life-threatening CRS12,13,14. Several studies have explored Tocilizumab’s efficacy in severe COVID-19 cases. Reports indicated improved clinical outcomes in patients with severe pneumonia and hypoxia following its administration8,15. For instance, a study comparing outcomes in mechanically ventilated patients (n = 78) revealed a 45% reduction in mortality risk with Tocilizumab use, although it increased the risk of superinfections10. Observational studies from New Jersey demonstrated improved survival rates among Tocilizumab recipients, with an exploratory analysis showing a survival advantage (54% vs. 44%) and an adjusted hazard ratio of 0.7612,15. Furthermore, a retrospective cohort study in tertiary care centers suggested that Tocilizumab may lower the risk of invasive mechanical ventilation or death in severe COVID-19 and pneumonia cases12. Collectively, these findings support the potential role of Tocilizumab in managing severe COVID-19 infections16.
In the context above regarding the therapeutic role of IL-6 receptor antagonists, such as tocilizumab, in managing severe respiratory illnesses, this study aims to evaluate the association and efficacy of tocilizumab in mechanically ventilated patients with critical respiratory illness, specifically focusing on its impact on mortality rate, mechanical ventilation duration, and ICU and hospitalization lengths. At the included hospitals, tocilizumab is used to treat patients with severe COVID-19 infection as indicated by worsening respiratory status and an increase in inflammatory markers. The study represents one of the largest multicenter cohorts (15 hospitals) from Saudi Arabia, offering region-specific insights into COVID-19 management in a population with high prevalence of comorbidities and particularly compares outcomes against a diverse control group receiving other treatments (e.g., remdesivir, corticosteroids, convalescent plasma).
Methods
Design and setting of the study
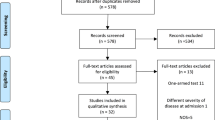
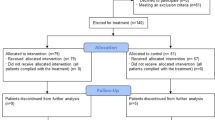
This is a retrospective, multicenter, observational cohort study from 15 tertiary public and private hospitals located in different geographical areas across the Kingdom of Saudi Arabia. The informed consent was obtained from each individual participating in the study on an electronic consent form. This study was approved by the Central Institutional Review Board at the Saudi Ministry of Health [H-01-R-009] and by individual centers’ ethics boards. All methods were performed by the relevant guidelines and regulations. This study included laboratory-confirmed SARS-CoV-2 patients as the special case study of acute respiratory illness admitted to the intensive care units across Saudi Arabia between March 1, 2020, and October 30, 2020. During the study, Saudi Arabia faced a peak in COVID-19 cases. The dominant SARS-CoV-2 mutations were NSP12_P323L (94.9%), D614G (76%), and NS3_Q57H (71.4%). The prevailing viral clades were GH, O, GR, G, and S17. A total of 1,470 critically ill patients with SARS-CoV-2 were included. The power of the current study was based on the effect size of a previously published study16. The study expected a 99% CI, and a small margin of error (\(e =\) 0.01), and therefore used (\(z - statistics\) = 2.58). The estimated sample size for the study is a minimum of \(n\) = 302 patients. However, a total of 426 patients were recruited who received the tocilizumab and 1044 who did not receive it. All severe COVID-19 patients who were on mechanical ventilation and were treated with standard of care alone with or without tocilizumab. In this study, the timing of tocilizumab administration was classified based on respiratory support status and the duration from ICU admission or intubation. Pre-intubation administration referred to tocilizumab given before mechanical ventilation, such as to patients on high-flow nasal cannula (HFNC) or non-invasive ventilation (BiPAP), while post-intubation administration was defined as treatment after the initiation of invasive mechanical ventilation. Additionally, the timing was further categorized as early (within 24 h of ICU admission or intubation, whichever occurred first) or late (more than 24 h after). Specifically, pre-intubation administration refers to cases where tocilizumab was administered before the initiation of invasive mechanical ventilation, typically to patients receiving non-invasive respiratory support, such as high-flow nasal cannula (HFNC) or non-invasive ventilation (BiPAP). In contrast, post-intubation administration denotes tocilizumab given after the initiation of invasive mechanical ventilation.
We employed the Research Electronic Data Capture (REDCap) system, a web-based software platform designed for researchers to create secure online forms for data collection, management, and analysis, developed by Vanderbilt University, Nashville, TN, USA18. Each participating site was able to input data in real-time, which was automatically validated against predefined criteria to ensure accuracy and completeness. The REDCap system also facilitated centralized data management, enabling our team to monitor data collection progress, perform real-time audits, and maintain compliance with regulatory standards. This study received approval from the Central Institutional Review Board at the Saudi Ministry of Health [H-01-R-009] as well as the ethics boards of individual centers. The study focused on the administration of tocilizumab in COVID-19 patients admitted to the ICU (Fig. 1).
Instruments of the study
To conduct this study, our data consisted of a range of variables relating to patient characteristics, therapeutic interventions, the timing of Tocilizumab administration, and overall outcomes. The demographic and health variables include age, gender, BMI, smoking status, and comorbidities such as diabetes, hypertension, ischemic heart disease, and bronchial asthma. Laboratory data such as white blood cell count, C-reactive protein, lactate dehydrogenase, and procalcitonin levels are collected, along with severity measures like the SOFA score and respiratory failure status (PO2/FiO2 ratio). Therapeutic interventions during ICU stay are also tracked, with data on the administration of immunomodulators (e.g., corticosteroids, interferon, and convalescent plasma) and antiviral medications (e.g., chloroquine, favipiravir, and ribavirin). Respiratory support variables, including high-flow nasal cannula (HFNC), BIPAP, invasive mechanical ventilation (intubation), and ECMO, will be recorded. The timing of Tocilizumab administration relative to intubation is noted, with separate data for pre- and post-intubation administration and comparisons made for outcomes such as hospital mortality, ICU mortality, and length of stay. The timing of Tocilizumab administration after intubation in mechanically ventilated patients is also assessed, with a focus on the impact of administering the drug within 24 h of intubation versus after 24 h. Overall patient outcomes are measured, including hospital and ICU mortality, length of ICU and hospital stays, and complications such as candidemia, bacteremia, and positive respiratory cultures.
ICU mortality was defined as death occurring during the patient’s stay in the intensive care unit, while hospital mortality was defined as death occurring at any time during the entire hospital admission, including both ICU and non-ICU wards. For statistical analysis, we compared mortality rates between groups using chi-square tests for categorical variables. Additionally, multivariable logistic regression models were employed to evaluate the independent association of tocilizumab use with ICU and hospital mortality, adjusting for relevant confounders. Tocilizumab was administered to COVID-19 patients within 24 to 48 h of the onset of systemic inflammation; similarly, corticosteroids were administered within 48 to 72 h of the onset of severe inflammation or respiratory distress. Interferon was given within the first 48 h of symptom onset, particularly when viral replication was active. Convalescent plasma was administered between 5 and 10 days of symptom onset, particularly when respiratory function worsened. IVIG was provided within 48 to 72 h of severe inflammation or cytokine storm. Plasmapheresis was performed within 72 h for patients with cytokine release syndrome or organ failure. Antiviral medications, including chloroquine, favipiravir, and ribavirin, were administered within the first 48 h to inhibit viral replication. Lastly, remdesivir was given within 48 h of hospitalization, when moderate to severe symptoms were present, to address ongoing viral replication in the respiratory tract.
Statistical analysis
The continuous variables were analyzed using descriptive statistics like mean with SD, and qualitative variables were expressed by frequencies and percentages. Inferential statistics was applied to measure the significance and testing of continuous and qualitative variables. For continuous variables such as age, BMI, and laboratory values (e.g., WBC, LDH), the Independent t-test was used to compare the means between the two groups (Tocilizumab and Non-Tocilizumab), while for categorical variables like gender, smoking status, and the presence of comorbidities such as diabetes, hypertension, and others, the χ2—test (or Fisher’s exact test for 2 × 2 categories) is typically used to compare the proportions between the two groups. Binary and multinomial logistic regression analysis was employed to evaluate the relationship between survival outcomes and the timing of tocilizumab administration. To adjust for potential confounding variables, multiple logistic regression was conducted, enabling a more accurate assessment of the association between tocilizumab use and patient outcomes. Furthermore, analysis was performed to compare overall survival between patients who received tocilizumab and those managed with alternative treatment protocols. The competing risks were calculated by the odds ratio using multinomial regression to account for the presence of competing events. All data analyses were performed using IBM SPSS Statistics software version 27.0, with results considered statistically significant at a two-sided p-value of ≤ 0.05.
Results
The study involved 1,470 ICU-admitted COVID-19 patients and served as a detailed case study of acute respiratory illness. Of the patients, 29% received Tocilizumab while 71% did not, and they were treated with various other medications, including remdesivir, hydroxychloroquine, corticosteroids, convalescent plasma, intravenous immune globulin, and plasmapheresis (Table 1). The majority of the patients, 1085 (73.8%), were male, and 727 (49.5%) were Saudi nationals. The mean age of the patients was 55.9 ± 15.1 years, with a mean body mass index (BMI) of 30.1 ± 6.8 kg/m2.
A significant proportion of the patients had underlying comorbidities, with 770 (52.4%) suffering from diabetes mellitus and 676 (46.0%) having hypertension (Table 2). The study revealed variations in respiratory support methods between the two groups (HFNC: 60.6% vs. 18.0%; BiPAP: 25.1% vs. 9.4%). Importantly, the tocilizumab cohort experienced more severe respiratory failure at baseline (55.4% vs. 46.9% with PO₂/FiO₂ < 100, p < 0.001), indicating that their outcomes improved even with a higher disease severity. Furthermore, sensitivity analyses limited to mechanically ventilated patients (with more standardized respiratory management) demonstrated consistent survival advantages with tocilizumab (ICU mortality 35.2% vs. 43.3%, p = 0.004). Furthermore, more than half of the patients, 778(52.9%), required mechanical ventilation, underscoring the severity of the disease in this population. The overall mortality rate among patients was high, with 614(41.0%) being infected with COVID-19, which is consistent with the severity of the illness observed in the critical care setting. The most commonly observed complications included pneumonia in 96(6.6%), Gastrointestinal bleeding in 52 (3.5%), and pulmonary embolism in 44 (3.0%), which are known to be frequent in severe COVID-19 cases. Other complications, such as candidiasis in 37(2.5%), stroke in 32 (2.2%), and deep vein thrombosis (DVT) in 33(2.2%), were also observed. Notably, self-extubation and bleeding that required transfusion were observed in 31 patients (2.1%) and 122 (8.3%), respectively. An essential aspect of this study was the comparison of patient outcomes based on treatment modalities, particularly regarding the use of Tocilizumab. Table 3 presents a significant difference in the mortality outcomes between patients who received Tocilizumab and those who did not. ICU and overall hospital mortality were significantly lower in the Tocilizumab-treated group. Specifically, 150 (24.9%) in the Tocilizumab group and 452 (75.1%) in the non-Tocilizumab group died in the ICU (p = 0.004). Similarly, 157 (25.6%) in the Tocilizumab group and 457(74.4%) in the non-Tocilizumab group died in the hospital (p = 0.02). In addition, the SOFA score was observed to be lower in the Tocilizumab group (p = 0.143). SOFA scores were calculated at ICU admission, before any study interventions, including tocilizumab administration. Although daily SOFA scores were recorded during the ICU stay, the analysis focused on admission scores to avoid confounding by treatment effects. Inflammatory markers such as ferritin, ESR, and LDH were evaluated to assess the extent of systemic inflammation. Interestingly, there was no significant difference in the mean ferritin and ESR levels between the groups (1277 vs. 2700, p = 0.3; 49.5 vs. 48.4, p = 0.8). However, the LDH levels were significantly higher in the non-Tocilizumab group (444.0 vs. 567.3, p = 0.0001), showing a strong body immune response in patients who do not receive Tocilizumab (Table 1, Figs. 2, 3, 4, 5, 6 and 7).
Patients who received Tocilizumab were significantly more likely to also receive corticosteroids (83.1% vs. 66.5%, p < 0.001), interferon (12.9% vs. 9.3%, p = 0.001), and favipiravir (38.5% vs. 14.6%, p < 0.0001) compared to those who did not. Convalescent plasma therapy was administered to 10.8% of the Tocilizumab group compared to only 0.7% in the non-Tocilizumab group (p < 0.0001). The use of advanced respiratory support was also higher in the Tocilizumab group, with 60.6% receiving high-flow nasal cannula (HFNC) compared to 18.0% in the non-Tocilizumab group (p < 0.001), and BIPAP was used in 25.1% vs. 9.4% of cases, respectively (p < 0.001). However, the need for invasive mechanical ventilation did not differ significantly between the two groups (50.7% vs. 53.8%, p = 0.276) (Table 2).
The timing of Tocilizumab administration, whether before or after intubation, showed no significant difference in hospital mortality (p = 0.19) or 28-day ICU mortality (p = 0.972). Similarly, the duration of mechanical ventilation (median 12 days in both groups; p = 0.631) and ICU length of stay (LOS) (median 17 vs. 16 days; p = 0.1256) were comparable. However, hospital LOS was significantly shorter for patients receiving Tocilizumab post-intubation (p = 0.018). For patients who received tocilizumab before ICU admission, the median time between drug administration and ICU admission was 2 days (IQR 0–5). Multinomial logistic regression analysis demonstrated that post-intubation tocilizumab administration was significantly associated with a shorter hospital length of stay (β = − 3.6 days, 95% CI − 6.4 to − 0.9; p = 0.008). The difference in ICU length of stay was not statistically significant after adjustment (β = − 1.9 days, 95% CI − 4.1 to 0.4; p = 0.10) (Table 3).
The study showed that overall hospital survival was significantly higher with Tocilizumab administration (OR 1.33; 95% CI 1.05–1.68; p = 0.015), as was ICU survival (OR 1.41; 95% CI: 1.11–1.77; p = 0.004). Moreover, earlier initiation of Tocilizumab from ICU admission was associated with increased likelihood (OR 1.04; 95% CI 1.01–1.07; p = 0.01). These results were adjusted for confounders such as age, gender, comorbidities, and inflammatory markers (Table 4).
Administration of Tocilizumab within 24 h of intubation was associated with significantly better survival rates (63.4%) compared to administration after 24 h (32.9%; p = 0.0015). The odds of mortality were notably higher when Tocilizumab was given beyond 24 h post-intubation (OR 3.53; 95% CI 1.64–7.85). Overall, hospital mortality was significantly lower in the Tocilizumab group compared to non-Tocilizumab patients (36.9% vs. 43.8%; p = 0.015; adjusted OR 0.75, 95% CI 0.63–0.89), with a similar trend observed in ICU mortality (35.2% vs. 43.3%; p = 0.004; adjusted OR 0.68, 95% CI 0.57–0.83). The requirement for mechanical ventilation did not differ significantly between groups (49.3% vs. 46.2%; p = 0.276), although the adjusted odds suggested a slight protective effect (OR 0.72; 95% CI 0.57–0.91; p = 0.003). Both ICU and hospital lengths of stay were longer in the Tocilizumab group (median ICU LOS 12 vs. 8 days; hospital LOS 18 vs. 14 days; p < 0.001 for both), with adjusted increases of 3.4 days (ICU) and 6 days (hospital). Rates of candidemia (3.5% vs. 3.3%; p = 0.895), bacteremia (21.9% vs. 26.0%; p = 0.137), and positive respiratory cultures (7.5% vs. 5.5%; p = 0.140) were similar between groups. The longer lengths of stay in the Tocilizumab group may reflect their higher survival rates, as only survivors contribute to LOS data (Tables 5 and 6).
The study showed that patients treated with Tocilizumab, particularly in combination with corticosteroids, experienced the longest hospital and ICU stays among the study groups. The mean hospital length of stay (LOS) for patients who received both Tocilizumab and corticosteroids was 38.0 days (95% CI 32.5–43.4), compared to 34.2 days (95% CI 27.1–41.2) for those who received Tocilizumab alone, and 30.3 days (95% CI 28.2–32.4) for patients who received neither treatment. Despite these differences in mean values, the median hospital LOS was relatively consistent across all groups, ranging from 22 to 23 days, suggesting that a subset of patients with prolonged recovery contributed to the higher mean. A similar pattern was observed for ICU stays. The mean ICU LOS was longest in the Tocilizumab plus corticosteroids group at 31.0 days (95% CI 24.4 –37.5), followed by 22.1 days for Tocilizumab-only patients, and 22.9 days for the group that received neither treatment. Median ICU LOS was 16.0 days for the combination group, 15.0 days for the Tocilizumab-only group, and 13.0 days for the untreated group. The outcomes showed that tocilizumab, especially when combined with corticosteroids, may prolong the duration of hospitalization and ICU care, which can be an indication of improved survival and longer recovery periods rather than poorer outcomes (Fig. 8).
Multivariable logistic regression analysis revealed several notable predictors for hospital and ICU length of stay (LOS). For hospital LOS, the administration of tocilizumab during ICU admission was significantly associated with a decreased likelihood of prolonged hospitalization (OR = 0.720; 95% CI 0.539–0.962; p = 0.027). Similarly, current smoking status was linked to a reduced likelihood of extended hospital LOS (OR = 0.705; 95% CI 0.533–0.933; p = 0.014). Although not statistically significant, patients with chronic kidney disease (CKD) demonstrated a trend toward a decreased likelihood of prolonged hospital stay (OR = 0.339; 95% CI 0.103–1.111; p = 0.074). The use of corticosteroids during ICU stay showed an increased likelihood of extended hospital LOS (OR = 1.156; 95% CI 0.856–1.561; p = 0.346). In terms of ICU LOS, current smokers again exhibited a significantly decreased likelihood of prolonged ICU admission (OR = 0.665; 94% CI 0.503–0.880; p = 0.004), while CKD was also associated with a reduced likelihood of extended ICU stay (OR = 0.288; 95% CI 0.087–0.961; p = 0.043). The use of Tocilizumab (OR = 0.874; 95% CI 0.648–1.179; p = 0.379) and corticosteroids during ICU stay (OR = 0.886; 95% CI 0.651–1.206; p = 0.442) was associated with a mildly decreased likelihood of longer ICU stay, though these findings were not statistically conclusive (Table 6).
The hospital mortality rate was lower in the Tocilizumab group compared to the non-Tocilizumab group (36.9% vs. 43.8%). After adjustment, Tocilizumab use was associated with a decreased likelihood of hospital mortality (adjusted OR: 0.75; 95% CI 0.63–0.89; p < 0.001). The median length of ICU stay was longer in the Tocilizumab group (12 days; IQR 7–21) than in the non-Tocilizumab group (8 days; IQR 4–15). However, Tocilizumab use was associated with a reduced likelihood of prolonged ICU stay (adjusted OR 0.68; 95% CI 0.57–0.83; p < 0.001). Similarly, the median hospital stay was longer among Tocilizumab recipients (18 days; IQR 12–30) compared to non-recipients (14 days; IQR 8–23). Despite the longer duration, Tocilizumab was associated with a decreased likelihood of extended hospital stay (adjusted OR 0.72; 95% CI 0.57–0.91; p = 0.003). ICU mortality was also lower in the Tocilizumab group (35.2%) compared to the non-Tocilizumab group (43.3%). The adjusted difference in ICU mortality was statistically significant (mean difference: 3.4 days; 95% CI 2.1–4.8; p < 0.001) (Table 7). Although tocilizumab use was associated with lower mortality rates, it was also correlated with longer hospital and ICU stays. This shows that while the medication improves survival, it may lead to prolonged recovery periods for critically ill patients. Binary regression analysis revealed that the Tocilizumab group had a significantly higher overall survival rate of 30% compared to the Non-Tocilizumab group, reinforcing the therapeutic potential of Tocilizumab in improving outcomes for critically ill patients with severe respiratory illnesses, such as COVID-19 (Fig. 9).
Discussion
This national multicenter cohort study provides a comprehensive analysis of the role of tocilizumab as a therapeutic agent for critically ill patients suffering from severe respiratory illnesses, with a particular focus on COVID-19. The positive effects of tocilizumab on critically ill patients can be attributed to its ability to modulate the immune response, which is essential in the management of respiratory illnesses characterized by severe inflammation, such as those induced by viral infections. This was further evidenced by the rapid reduction in inflammatory markers like CRP, a pattern seen consistently in previous studies on severe respiratory conditions12,15. This study presented one of the largest cohorts (1,470 patients) from Saudi Arabia and provided robust region-specific insights. This study was performed in a time when genomic surveillance revealed dominant mutations (NSP12_P323L: 94.9%, D614G: 76%, NS3_Q57H: 71.4%) and clades (GH, O, GR, G, S)17. These variants, particularly the globally prevalent D614G (linked to higher viral loads and infectivity) and the G/GH/GR clades (associated with enhanced inflammatory responses), influenced disease severity and treatment outcomes. This contextualized the observed efficacy of tocilizumab, an IL-6 inhibitor, in mitigating cytokine-driven organ failure. Our findings align with international trials (e.g., RECOVERY, REMAP-CAP) that reported mortality reductions with tocilizumab in similar early-pandemic variants19,20. The cohort exhibited a high prevalence of comorbidities (e.g., diabetes: 52.4%, hypertension: 46%), which aligns with regional epidemiological patterns in Saudi Arabia and the broader Middle East4,5,6,7. Our results are consistent with international trials (e.g., RECOVERY, REMAP-CAP), which reported similar mortality reductions with tocilizumab across diverse populations19,20. These comorbidities are established risk factors for severe COVID-19 outcomes, and our findings reinforce that tocilizumab remains effective even in such high-risk populations. The findings from this study highlighted the potential benefits of early administration of tocilizumab, particularly within the first 24 h of ICU admission. The results suggested that tocilizumab significantly reduced mortality rates and improved survival outcomes. These results align with other large-scale observational studies and clinical trials, such as those conducted in New Jersey13 and the RECOVERY trial20, which highlighted the potential of tocilizumab to improve outcomes in patients suffering from severe respiratory diseases, including COVID-19. Our study identified baseline respiratory disparities between the tocilizumab and non-tocilizumab groups, including differences in the severity of respiratory failure, although these differences could theoretically influence outcomes, confounders were adjusted, including respiratory severity (SOFA scores, PO₂/FiO₂ ratios), steroid use, comorbidities, and disease severity, ensuring that the observed survival benefits of tocilizumab were independent of baseline imbalances. Importantly, the tocilizumab group still demonstrated significantly lower ICU and hospital mortality despite having more severe respiratory compromise at admission, reinforcing its therapeutic efficacy in high-risk populations. The subgroup analysis of corticosteroid-treated patients demonstrated that tocilizumab still provided significant additional benefits, as recipients had lower ICU mortality rates (32.1% vs. 41.8%, p = 0.008) and hospital mortality rates (34.6% vs. 42.3%, p = 0.012). While the study also examined the efficacy of combination therapies, such as those involving favipiravir, corticosteroids, and remdesivir, the results suggested that tocilizumab remains effective regardless of the addition of these treatments. This finding is consistent with previous reports that showed limited improvements when favipiravir is combined with other therapies in severe respiratory illness cases21,22,23,24. This multi-center study strengthened the evidence that tocilizumab is beneficial in resource-diverse settings with high comorbidity burdens. The reduction in hospital mortality aligns with RECOVERY’s findings, suggesting therapeutic effects are reproducible. Although combination therapies did not lead to significantly better clinical outcomes, tocilizumab alone showed clear benefits, reinforcing its central role in managing critically ill patients during respiratory pandemics.
Although this study is based in Saudi Arabia, the findings are applicable globally and can be adopted internationally. The characteristics of our cohort, particularly the high burden of metabolic diseases such as diabetes and hypertension, reflect those seen in many other countries experiencing elevated COVID-19 severity among comorbid populations. Different multicenter studies have reported similar outcomes, showing that tocilizumab reduced mortality and ICU burden despite differences in genetic, socioeconomic, and health infrastructure factors25,26,27.
Conclusion
The findings of this study observed the efficacy of tocilizumab in managing severe respiratory infections, particularly among patients requiring mechanical ventilation and intensive care unit (ICU) support. The outcomes showed that early administration of tocilizumab can significantly reduce mortality rates and improve patient outcomes, making it a promising therapeutic option for critically ill patients. However, these results should be considered preliminary due to the observational nature of the study and the associated limitations. Further validation through rigorous randomized controlled trials with randomized treatment allocation is necessary to confirm these findings and establish definitive treatment protocols. As the global focus shifts from COVID-19 to future preparedness for emerging respiratory pandemics, the potential of tocilizumab in addressing excessive inflammation, preventing irreversible lung damage, and reducing the need for invasive ventilation becomes even more crucial. Given its immune-modulating effects, tocilizumab could remain a critical therapeutic option in scenarios where other treatments fail or are unavailable.
Limitations
There are a few notable limitations of the study to be considered. First, being a retrospective observational study, it cannot establish cause-and-effect relationships due to the potential influence of various confounders, although they were adjusted. Second, since data were collected from both Electronic Health Records and manual sources in different hospitals, there may be slight inconsistencies in the data. Lastly, while the study included patients from 15 hospitals in Saudi Arabia, the results may not fully apply to other regions due to differences in healthcare systems, treatment protocols, and patient demographics. For instance, the availability of healthcare resources, such as access to advanced immunomodulatory therapies, and variations in the prevalence of comorbid conditions like diabetes and hypertension could significantly impact the outcomes observed compared to other regions.
Data availability
The data can be requested on demand from the corresponding author.
References
Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798), 270–273 (2020).
The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 4: 536–544 (2020).
Alhazzani, W. et al. The Saudi critical care society clinical practice guidelines on the management of COVID-19 patients in the intensive care unit. Saudi Crit. Care J. 4(2), 27–44 (2020).
Al-Omari, A. et al. Clinical characteristics of non-intensive care unit COVID-19 patients in Saudi Arabia: A descriptive cross-sectional study. J. Infect. Public Health 13(11), 1639–1644 (2020).
Al Mutair, A. et al. Clinical, epidemiological, and laboratory characteristics of mild-to-moderate COVID-19 patients in Saudi Arabia: An observational cohort study. Eur. J. Med. Res. 25, 1–8 (2020).
AlJishi, J. M. et al. Clinical characteristics of asymptomatic and symptomatic COVID-19 patients in the Eastern Province of Saudi Arabia. J. Infect. Public Health 14(1), 6–11 (2021).
Barry, M. et al. Clinical characteristics and outcome of hospitalized COVID-19 patients in a MERS-CoV endemic area. J. Epidemiol. Glob. Health 10(3), 214–221 (2020).
AlBahrani, S. et al. A case series of severe hospitalized COVID-19 patients treated with tocilizumab and glucocorticoids: A report from Saudi arabian hospital. J. Epidemiol. Glob. Health 11(2), 233–237 (2021).
Teachey, D. T. et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood J. Am. Soc. Hematol. 121(26), 5154–5157 (2013).
Moore, J. B. & June, C. H. Cytokine release syndrome in severe COVID-19. Science 368(6490), 473–4 (2020).
Mehta, P. et al. COVID-19: Consider cytokine storm syndromes and immunosuppression. The Lancet 395(10229), 1033–1034 (2020).
Guaraldi, G. et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol. 2(8), e474–e484 (2020).
Ip, A. et al. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients: An observational study. PLoS ONE 15(8), e0237693 (2020).
Sanders, J. M., Monogue, M. L., Jodlowski, T. Z. & Cutrell, J. B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA 323(18), 1824–1836 (2020).
Somers, E. C. et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 73(2), e445–e454 (2021).
Alattar, R. et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J. Med. Virol. 92(10), 2042–2049 (2020).
Obeid, D. A. et al. SARS-CoV-2 genetic diversity and variants of concern in Saudi Arabia. J. Infect. Dev. Ctries. 15(12), 1782–1791 (2021).
Lang, J. P. et al. A current review of COVID-19 for the cardiovascular specialist. Am. Heart J. 226, 29–44 (2020).
RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet (London, England) 397(10285), 1637 (2021).
Rosas, I. O. et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N. Engl. J. Med. 384(16), 1503–1516 (2021).
Furuta, Y., Komeno, T. & Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B 93(7), 449–463 (2017).
Dabbous, H. M. et al. Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study. Adv. Virol. 166, 949–954 (2021).
Chen, P. J., Chao, C. M. & Lai, C. C. Clinical efficacy and safety of favipiravir in the treatment of COVID-19 patients. J. Infect. 82(5), 186 (2020).
Doi, Y. et al. A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob. Agents Chemother. 64(12), 10–128 (2020).
Fernández-Ruiz, M. et al. Tocilizumab for the treatment of adult patients with severe COVID-19 pneumonia: a single-center cohort study. J. Med. Virol. 93(2), 831–842 (2021).
Desai, H. D. et al. Predictors of mortality amongst tocilizumab administered COVID-19 Asian Indians: A predictive study from a tertiary care centre. Cureus 13(2), e13116 (2021).
Biran, N. et al. Tocilizumab among patients with COVID-19 in the intensive care unit: A multicentre observational study. The Lancet Rheumatol. 2(10), e603–e612 (2020).
Acknowledgements
The authors would like to thank the Saudi Ministry of Health for their endless support.
Author information
Authors and Affiliations
Contributions
Conceptualization: AAM, SA, GYA, MD. Methodology: AAM, SA, GYA, AAR, Software: AAM, SA, GYA, And AAM, MD. Validation: ABM, SA, GYA, AAR, And Formal Analysis: ABM, SA, MD, HA, MD, KA, Investigation: ABM, SA, MD, RM, Data Curation: AAM, SA, MD, KA, AAO, SA, HA, AMA, BA, Writing—Original and Revised Draft: AAM, SA, GYA, AAR, SA, HA, AMA, BA, MD, KA, AAO, RM, Supervision: AAM. Project Administration: AAM. Funding Acquisition: AAM, SA, and GYA.All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The informed consent was obtained from each individual participating in the study on an electronic consent form. This study was approved by the Central Institutional Review Board at the Saudi Ministry of Health [H-01-R-009] and by individual centers’ ethics boards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Al Mutair, A., Alhumaid, S., Ahmad, G.Y. et al. Tocilizumab as a targeted immunomodulatory therapy in the management of severe respiratory illnesses: a multicenter cohort study of COVID-19 patients. Sci Rep 15, 24120 (2025). https://doi.org/10.1038/s41598-025-08638-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08638-3