Abstract
Rice straw (RS), a major agricultural byproduct in Thailand, holds significant potential for biochar production. This study investigated the physicochemical properties, pore structure, and yield of rice straw-derived biochar (RSBC) produced using a traditional drum kiln, compared to raw RS. Synchrotron-based X-ray tomographic microscopy (XTM) was employed to characterize changes in pore type, size, and volume. The pyrolysis of RS resulted in a 70% mass reduction; however, RSBC exhibited significantly higher fixed carbon content, cation exchange capacity (CEC), and water-holding capacity (WHC), attributed to increased aromatic functional groups following lignin enrichment and the decomposition of cellulose and hemicellulose. Synchrotron-based XTM analysis revealed an 18% increase in total porosity, driven by a 19% rise in open-pore porosity and a 91% reduction in closed-pore porosity. Additionally, macropore volume (> 100 μm) expanded by 271%, leading to a 267% increase in total pore volume. These structural modifications, particularly the enhanced open-pore porosity and macropore expansion, along with the formation of aromatic and phenolic functional groups, are key contributors to the improved WHC and CEC observed in RSBC. Overall, these findings highlight the potential of rice straw-derived biochar produced via traditional drum kiln pyrolysis as an effective soil amendment, offering a viable alternative to agricultural waste management in rice cultivation systems.
Similar content being viewed by others
Introduction
Rice straw (RS) is the predominant agricultural residue in Thailand, with an annual production of approximately 26 million tons. Conventional post-harvest RS management practices include off-field utilization, open burning, and left in the field after harvest1, each of which carries significant environmental and agronomic implications2. In particular, open burning is widely practiced due to the immediate benefits, such as pests and weeds elimination3,4 and increased soil pH and nutrient enrichment from the remaining ash3. However, the combustion of RS leads to substantial carbon (C) losses, contributing to global CO2 and black C emissions, exacerbating air pollution2,5 and accelerating long-term soil organic carbon (SOC) depletion6,7. As an alternative, RS incorporation into paddy soils is often recommended to improve SOC levels, soil fertility, and crop productivity8,9. While this practice introduces organic carbon inputs into the soil, it also presents agronomic and environmental trade-offs. High RS inputs can increase pathogen pressure, elevating the risk of disease and pest outbreaks in rice cultivation10. Furthermore, RS is considered a low-quality organic amendment due to its high cellulose and low lignin content, which accelerates decomposition and limits long-term SOC accumulation9,11,12. More critically, RS incorporation in flooded rice systems stimulates microbial-mediated anaerobic decomposition, resulting in substantial methane (CH₄) emissions13,14. Given that CH₄ has a 100-year global warming potential 28 times greater than CO₂15, the role of RS incorporation in greenhouse gas emissions necessitates careful evaluation.
To address the limitations of conventional RS management while enhancing soil C sequestration, thermochemical conversion of RS into biochar via pyrolysis has gained increasing attention16. Biochar is a highly recalcitrant, C-rich material that resists microbial degradation17,18, thereby providing long-term SOC stabilization and reducing greenhouse gas emissions from paddy soils19,20,21,22. Additionally, biochar application has shown to improve soil physicochemical properties, increase nutrient retention, and enhance crop productivity, particularly in sandy and low-organic-matter soils20,22,23. These benefits are particularly relevant in northeastern Thailand, where poor soil conditions limit rice yield potential24,25. In our previous study, the rice straw-derived biochar (RSBC) was applied under field conditions and demonstrated significant environmental and agronomic benefits, including reduced methane emissions, improved soil organic carbon, and increased rice yield21. However, the underlying chemical and structural mechanisms that contribute to these beneficial effects remain insufficiently understood. Additionally, biochar production is more labor-intensive than in-field burning or direct RS incorporation, and its properties are influenced by several technological parameters, primarily pyrolysis conditions and feedstock composition26.
Pyrolysis, an anaerobic thermochemical conversion process which transforms biomass into biochar, liquid fuel, and gases27. Among several pyrolysis methods, slow pyrolysis is widely used for biochar production27, typically operating at temperatures ranging from 300 to 600 °C over several hours28. The physicochemical properties and yield of biochar are significantly influenced by feedstock characteristics and pyrolysis conditions29,30,31, particularly temperature29,32. Higher pyrolysis temperatures typically increase biochar pH, surface area, porosity, ash content, and hydrophobicity, while reducing its overall yield and hydrophilicity29. Based on pyrolysis temperature, biochar is classified into three categories: low-temperature (< 450 °C), moderate-temperature (450–550 °C), and high-temperature (> 550 °C) biochar33. In northeastern Thailand, biochar is typically produced using traditional drum kilns, which operate at low pyrolysis temperatures14,34,35,36.
Despite the increasing interest in RS-derived biochar, there remains a lack of comprehensive analysis regarding the transformation of its physicochemical properties and microstructural characteristics during pyrolysis. To address this gap, this study employed synchrotron-based X-ray tomographic microscopy (XTM), an advanced imaging technique capable of providing high-resolution, three-dimensional microstructural insights into biochar porosity and pore connectivity37,38. Thus, the objectives of this study were to (i) characterize the physicochemical properties and yield of biochar derived from leftover RS compared with its raw material, and (ii) investigate the transformation of biochar’s pore type, size, and volume using synchrotron-based XTM analysis. We hypothesized that (i) pyrolysis-induced changes in chemical composition, functional groups, and pore structure of RS account for differences in key soil-relevant properties such as CEC and WHC. Further, (ii) synchrotron-based XTM analysis would provide precise insights into the microstructural changes governing biochar’s physicochemical properties. (iii) Pore distribution and interconnectivity, along with variations in functional group composition, could explain the observed differences in WHC and CEC between RS and RSBC. Understanding of these transformations is essential for optimizing RS-derived biochar as a soil amendment, thereby improving soil management strategies and enhancing agronomic sustainability in rice-based systems.
Results
Pyrolysis product yield and surface morphology
The pyrolysis of RS to RSBC yielded on average 30.1% of the original feedstock biomass, including the ash content (Table 1). Field emission scanning electron microscopy (FE-SEM) images of the milled RS and RSBC samples revealed differences in surface morphology. Variations were observed in RS (Fig. 1a–c) and RSBC (Fig. 1d–f) at low (100×), intermediate (500×), and high (2500×) magnifications. The RS particles exhibited a flat shape, with surfaces showing slight roughness. Conversely, the RSBC particles showcased a porous structure with tubes and a distribution of numerous small particles.
Physicochemical properties
The physicochemical properties of RS and RSBC are shown in Table 1 and are consistent with our previous findings21. RSBC had a pH value of 8.9, total C of 46.9%, carbon to nitrogen (C: N) ratio of 93.8, CEC of 35.7 cmol(+) kg− 1, and WHC of 7.2 ml g− 1, which were significantly higher than those of RS (p < 0.05; Table 1). In contrast, total N content in RSBC (0.50%) was significantly lower than in RS (0.64%). The contents of cellulose (48.9%), hemicellulose (29.2%), volatile matter (65.3%), and moisture (4.1%) of RS were significantly higher than RSBC (p < 0.05). In contrast, RSBC had significantly higher contents of lignin (56.3%), fixed C (40.7%), and ash (25.1%) than RS (p < 0.05; Table 1).
The total elemental contents of RS and RSBC are presented in Table 2. Both RS and RSBC exhibited the highest total potassium (K) contents and the lowest total copper (Cu) contents. RSBC had significantly higher contents of total phosphorus (P) (1281.6 mg kg− 1), total K (15,775.1 mg kg− 1), total calcium (Ca) (7,981.2 mg kg− 1), total magnesium (Mg) (3273.1 mg kg− 1), total sodium (Na) (560.5 mg kg− 1), total iron (Fe) (445.8 mg kg− 1), total manganese (Mn) (999.9 mg kg− 1), and total zinc (Zn) (112.5 mg kg− 1) compared to RS (p < 0.05). Conversely, RSBC had significantly lower contents of total sulfur (S), averaging 94.5 mg kg− 1and total Cu, averaging 6.6 mg kg− 1 compared to RS (p < 0.05).
Functional group
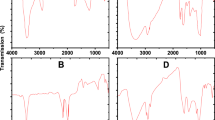
Fourier transform infrared (FTIR) spectra of both RS and RSBC, as illustrated in Fig. 2, exhibited similar functional groups, including (i) C–H stretching of cellulose, (ii) acetyl (C= O) group in hemicellulose or lignin, C=Cstretching of aromatic components, (iii) C–H deformation of lignin, C= C stretching, indicative of lignin and aromatic C, (ix) guaiacyl lignin, (x) C–O–C symmetric stretching in ester groups of cellulose and hemicellulose, and (xi) aromatic C–H out of plane bending (Table 3). Furthermore, the FTIR spectra of RS showed additional functional groups, including (i) O–H stretching of cellulose or hemicelluloses and (ii) C–H deformation in various polysaccharides (Table 3). The result indicated that O–H stretching of cellulose or hemicelluloses and C–H deformation in various polysaccharides in RS had a positive correlation with cellulose, and hemicellulose, but a negative correlation with lignin (Table 4), WHC, and CEC (Table 6). In contrast, the FTIR spectra of RSBC showed additional functional groups, including (i) free O–H stretching of phenolic and alcoholic –OH and (ii) aromatic skeleton stretching of lignin (Table 3). Functional groups of RSBC, including free O–H stretching of phenolic and alcoholic –OH and aromatic skeleton stretching of lignin, had a positive correlation with lignin (Table 4), WHC, and CEC (Table 6), and a negative correlation with cellulose and hemicellulose (Table 4).
RSBC had a higher proportion of aromatic functional groups than RS, such as guaiacyl lignin, aromatic C–H out of plane bending, aromatic skeleton stretching of lignin, and free O–H stretching of phenolic and alcoholic –OH (Fig. 3). Moreover, RS had the main functional groups related to cellulose, including O–H stretching of cellulose or hemicelluloses, C-H deformation in various polysaccharides, C–H stretching of cellulose, and C–O–C symmetric stretching in ester groups of cellulose and hemicellulose (Fig. 3).
Porous characterization
The 3D visualizations of RS and RSBC (Fig. 4a, b) revealed differences in pore structure and morphology. RSBC exhibited a significantly higher total porosity (88.61%) compared to RS (75.36%). Additionally, RSBC had a substantially lower closed-pore porosity (0.07%) than RS (0.81%), indicating a 91% reduction in closed pores (Fig. 5). Consequently, RSBC displayed a 19% increase in open-pore porosity relative to RS. Regarding pore size distribution, both RS and RSBC were dominated by ultramicropores and micropores (1–30 μm), with average counts of 5,361 and 5,768, respectively, showing no significant difference (p > 0.05; Table 5). However, RSBC exhibited a significantly lower total number of mesopores (30–70 μm) and macropores (70–100 μm and > 100 μm) than RS. Despite these differences, the total pore number between RS (6,456) and RSBC (6,043) was not significantly different (p > 0.05). Although total pore counts remained similar, RSBC exhibited a significantly higher total pore volume (1.71 × 1010 µm³) than RS (4.67 × 10⁹ µm³) (p < 0.05). Among the overall pore volume, macropores contributed the most, followed by mesopores and ultramicropores/micropores. Compared to RS, RSBC demonstrated a significantly greater total macropore volume, primarily driven by an increase in pores > 100 μm (1.71 × 1010 m2; p < 0.05). Conversely, RSBC exhibited significantly lower total mesopore volume (7.85 × 10⁶ µm³) and ultramicropore/micropore volume (5.44 × 10⁶ µm³) than RS (p < 0.05; Table 5).
A three-dimensional (3-D) renderings of the synchrotron-based-XTM images of rice straw (a) and rice straw-derived biochar (b) representing the pore types including the open-pore and closed-pore. Volume rendering of the pore architecture in a representative straw/biochar fragment (scale bar = 500 μm). Higher-magnification orthoslice from the same dataset illustrating pore types, including open pores and closed pores (scale bar = 100 μm).
These findings highlight the substantial structural transformations induced by pyrolysis, particularly the described increase in total porosity and pore volume, which plays a crucial role in enhancing the functional properties of RSBC. Pearson correlation analysis (Table 6) further revealed strong positive correlations between total porosity, total pore volume, open-pore porosity, and macropore volume (> 100 μm) with WHC and CEC (p < 0.05, 0.01). In contrast, closed-pore porosity exhibited a significant negative correlation with WHC and CEC (p < 0.01), emphasizing the importance of pore accessibility in influencing RSBC’s functional properties.
Discussion
Biochar is a C-rich solid product generated through the thermal decomposition of biomass, a process governed by key factors such as pyrolysis temperature, residence time, and feedstock composition29,30,31. In this study, RS was converted into RSBC using a traditional drum kiln at approximately 350 °C for 2 h. This low-cost and easily reproducible method offers a practical solution for small-scale, low-mechanized agricultural systems, which are in Thailand14,34,35,36. To enable a comprehensive characterization of biochar functionality, unmilled RS and RSBC samples were used for analyses requiring preservation of the original structure, such as pore structure (via synchrotron-based XTM), WHC, and CEC. In addition, milled samples were used for detailed assessments of surface morphology (via FE-SEM), chemical and elemental composition, and functional groups (via synchrotron-based FTIR), ensuring sample homogeneity and analytical precision. The pyrolysis process resulted in a biochar yield of approximately 30.1%, consistent with previous studies reporting biochar yields ranging from 21 to 80% under slow pyrolysis conditions (300–700 °C)45,46. Under slow-heating conditions below 600 °C, yields cluster around 25–50% because prolonged vapour–char contact promotes secondary carbon deposition46. Residence time also plays a critical role in determining biochar yield. At temperatures below 400 °C, short holding times of less than 30 min can preserve more than 60% of the initial biomass mass. However, extending the holding time to 2 h or longer significantly reduces the yield to 30–40%, primarily due to continued devolatilization and mild oxidation remove additional mass47. Given that residual RS in northeastern Thailand typically is available at an average rate of 10 Mg ha⁻¹11,48, pyrolyzing this biomass via drum-kiln pyrolysis would therefore produce approximately 3 Mg ha⁻¹ of biochar, providing an opportunity for more efficient field-based RS management and soil amendment strategies.
The physicochemical properties of biochar are largely influenced by pyrolysis conditions and feedstock composition, resulting in variations in volatile matter, ash content, C composition, pH, surface area, pore volume, and CEC49. During thermal decomposition, organic biomass undergoes significant mass loss due to the expulsion of volatile compounds, while thermally stable C structures remain partially intact50,51,52. Besides the overall mass loss, RSBC exhibited a substantial reduction in volatile matter content (approximately 50%) compared to RS, consistent with previous findings on wheat straw biochar, where nearly half of the volatile fraction was lost during pyrolysis50. This mass loss was primarily attributed to the degradation of cellulose (37%) and hemicellulose (97%), along with reductions in moisture (53%) and N (22%). Further, RSBC showed a notable increase in total C content (approximately 35%), driven by an enrichment in stable C fractions. Lignin and fixed C content increased nearly tenfold and fourfold, respectively, compared to RS, highlighting structural transformations that enhance RSBC’s stability and persistence in the environment.
The C content of organic polymers such as cellulose, hemicellulose, and lignin is converted into recalcitrant aromatic C compounds during pyrolysis49,53. Compared to lignin, a complex hydrophobic polymer which consists of diverse aromatic substructures and functional groups, cellulose and hemicellulose, which are composed of simple sugar monomers, decompose at lower temperatures (200–400 °C)54,55. Our findings indicate that the increase in lignin content and the reduction of cellulose and hemicellulose in RSBC were associated with the formation of additional aromatic functional groups. These functional groups, including free O–H stretching of phenolic and alcoholic –OH and aromatic skeleton stretching of lignin, exhibited a negative correlation with cellulose and hemicellulose and a positive correlation with lignin, reinforcing RSBC’s enhanced structural recalcitrance. Furthermore, the correlation analysis reveals that these aromatic and phenolic groups are strongly and positively associated with WHC, suggesting that these functional groups considerably improve RSBC’s ability to retain water. Oxygen-containing functional groups such as hydroxyl (–OH) groups, contribute to increased enhancing hydrophilicity of the biochar surface, thereby improving water retention56. These findings indicate that the same chemical transformations that stabilize the carbon matrix by enriching lignin-derived aromatic structures also augment its capacity to retain water. In contrast, the cellulose’s main functional groups such as O–H and C –H groups prevalent in RS exhibit a strong negative correlation with its WHC and CEC, explaining the poorer nutrient‑ and water‑retention properties of the raw material. Overall, these findings indicate that pyrolysis drives a coordinated chemical shift from a cellulose‑rich to a lignin‑enriched matrix, thereby simultaneously enhancing structural stability and functional performance. This dual enhancement of structural recalcitrance and water retention underscores the effectiveness of RS-derived biochar as a soil amendment, capable of improving soil quality while contributing to long‑term soil‑carbon sequestration and climate‑change mitigation in agricultural systems.
The transformation of RS to RSBC also resulted in changes in ash content and pH value. The ash content of biochar is primarily determined by the composition of the raw material, as the thermal degradation of organic matter during pyrolysis concentrates mineral components on the biochar surface while total ash content remains largely unchanged57,58. Zhang et al.58 reported that the ash content of biochars derived from wheat, corn, rape, and rice straw increased after pyrolysis, directly correlating with the mineral composition of the feedstock. Similarly, in this study, the measured ash content of raw RS (19.5%) increased to 25.1% in RSBC. This accumulation of ash plays a crucial role in biochar alkalinity, as mineral-rich biochars typically exhibit elevated pH levels59. The rise in ash content also contributed to higher concentrations of key minerals such as K, Ca, Mg, and Na, which are known to accumulate in biochar at higher pyrolysis temperatures as overall mass decreases60. Our results showed a 5.6% increase in ash content in RSBC, leading to a significant enrichment of P, K, Ca, Mg, Na, Fe, Mn, and Zn. These findings suggest that RSBC has a potential for ameliorating soil acidity and enhancing nutrient availability, particularly for macronutrients. However, the application rate must be carefully considered, as the 70% mass reduction from RS to RSBC alters the overall nutrient contribution compared to raw RS incorporation.
The homogeneity, structure, and quality of raw materials, along with pyrolysis temperature and duration, are critical factors influencing the pore structure of biochar61,62. Biochar exhibits a diverse range of pore sizes, which makes direct measurement difficult63. Common methods for analyzing pore size distribution primarily rely on indirect techniques, such as mercury porosimetry and gas adsorption analysis63,64,65. High resolution structural analysis, such as synchrotron-based X-ray tomographic microscopy (XTM), can more precisely measure porosity of materials on a micro-scale and distinguish between open and closed pores66. In this study, RSBC exhibited significantly higher total porosity (88.6%) compared to RS (75.4%) (p < 0.05). This increase in total porosity was primarily driven by a 271% expansion in macropore volume (> 100 μm), despite a 70% reduction in the total number of macropores (> 75 μm Mesopore number and volume decreased by 75% and 80%, respectively. Similarly, for larger micropores (20–30 μm), RSBC had 45% fewer pores and 52% lower pore volume than RS. However, for smaller micropores (1–20 μm), RSBC exhibited a 20% increase in pore number and an 11% increase in pore volume, though these differences were not statistically significant (p > 0.05). Despite the overall increase in porosity, the total pore number between RS and RSBC remained statistically comparable (p > 0.05), suggesting that the original pore structure of RS underwent shrinkage but largely remained intact. During pyrolysis, the dehydration process and release of volatile constituents from the C matrix facilitate pore formation, increasing porosity and structural modifications67. Higher pyrolysis temperatures generally enhance biochar porosity by removing volatiles and promoting the condensation of amorphous C into crystalline forms. This transition leads to the formation of cracks and void spaces, significantly modifying the biomass structure and increasing the overall porosity of biochar68,69.
Therefore, we conclude that the expansion of pore volume during pyrolysis primarily results from mass reduction, which leads to a substantial increase in a few large macropores that form around the largest pores of the leading tissue in RS. Meanwhile, the smaller pores in RS shrink in volume and diameter but not decrease in number, indicating that the skeletal structure of RS remains partially intact. Consequently, ultramicropores and micropores (0.1–30 μm) were more prevalent in RS than in RSBC, accounting for approximately 83% of the total pore count in RS and 95% in RSBC. These findings align with previous studies. For instance, Edeh et al.69 reported an increase in the frequency of smaller pores and a concurrent expansion in macropore volume in rice husk biochar produced below 500 °C. However, when the biomass had a high lignin content or was pelletized before pyrolysis, this effect was less pronounced69,70.
Furthermore, we observed a 91% reduction in closed-pore porosity and a 19% increase in open-pore porosity, suggesting that pyrolysis effectively modifies the biomass structure through perforation, thereby expanding the intraparticle porous network. The conversion of closed pores into open pores enhances the surface area and accessibility of biochar, which is crucial for its effectiveness in soil applications71. Strong positive correlations were observed between total porosity, open-pore porosity, and macropore volume (> 100 μm) with WHC and CEC (p < 0.05, 0.01), while closed-pore porosity exhibited a significant negative correlation with WHC and CEC (p < 0.01). These results highlight RSBC’s potential as an effective soil amendment by improving water retention and nutrient adsorption. Enhanced porosity and surface area provide more active sites for water and nutrient retention, which are crucial for soil amendments72,73.
In conclusion, this study demonstrated that pyrolyzing rice straw in a traditional drum kiln at approximately 350 °C enhances its physicochemical properties and pore structure, improving its functionality as a soil amendment. The process yielded 30.1% biochar, with the primary loss of cellulose, hemicellulose, and volatile matter, while enriching stable C fractions such as lignin, fixed C, and aromatic functional groups. The increased ash content elevated pH and macronutrient concentrations. Structural transformations, including greater total porosity, open-pore formation, macropore expansion, and the formation of aromatic and phenolic functional groups, were pivotal in enhancing WHC and CEC of RSBC. These findings suggest that biochar derived from rice straw can be a viable alternative to traditional straw burning and direct incorporation, offering both agronomic and environmental benefits for rice cultivation systems.
Methods
Rice straw and biochar preparation
Rice straw (RS) residues, consisting of the stems, leaves, and panicles of the rice plant (excluding rice grains and roots), were air-dried in the field for two weeks before collection for analysis and biochar production. The RS used in this study was of the RD6 variety, cultivated in Ban Non Muang, Tambon Sila, Mueang, Khon Kaen, Thailand (48Q 265026 N, 1825361E). Biochar was produced from 25 kg of air-dried rice straw via pyrolysis at approximately 350 °C for 2 h under oxygen-limited conditions in a 200 L traditional drum kiln, with three replications. After pyrolysis, the rice straw-derived biochar (RSBC) was removed, weighed, and prepared for analysis. Both RS and RSBC samples were oven-dried at 60 °C for 72 h and then divided into three portions: (i) milled using a hammer mill for chemical property and surface morphology analyses, (ii) finely milled using a ball mill for synchrotron-based FTIR analysis, and (iii) unmilled for water holding capacity (WHC) and cation exchange capacity (CEC) measurements, and Synchrotron-based XTM analysis.
Physicochemical properties analysis
pH was determined using a pH meter (HANNA Instruments, HI8424) with a material-to-distilled water ratio of 1:5. A 5.00 g milled sample of RS or RSBC was mixed with 25 mL of distilled water in a 50 mL Erlenmeyer flask and shaken at 250 rpm for 1 h and 30 min. The mixture was then allowed to rest for 1 h before measurement74. All measurements were performed in triplicate. The C: N ratio was calculated from the total C and total N contents, which were analyzed using a TOC/TN analyzer (Multi N/C 2100s, Analytik Jena, Jena, Germany). The CEC was measured using the ammonium acetate (NH₄OAc) method at pH 7.0, as described by Berek and Hue75. RS and RSBC were oven-dried at 60 °C for 72 h and cut into pieces of around 0.5–1 cm length. Subsequently, 2.00 g of unmilled RS or RSBC sample was added to 100 mL of 1 M NH₄OAc (pH 7.0) and shaken intermittently for 24 h. The mixture was vacuum-filtered through a Buchner funnel lined with filter paper no. 6 S and rinsed with an additional 20 mL of NH₄OAc. The residue in the funnel was washed four times with 10 mL of methyl alcohol and transferred into an Erlenmeyer flask. To extract ammonium ions, 50 mL of 4% KCl was added to the residue, shaken for 30 min, filtered through another Buchner funnel, and washed three times with 4% KCl. A 40 mL aliquot of the filtrate was transferred to a micro-Kjeldahl flask, to which 1 mL of 1 M NaOH was added. The solution was distilled, and the distillate was titrated with standardized 0.04 M HCl to determine the CEC.
Cellulose, hemicellulose, and lignin contents in RS and RSBC were determined following the procedure described by Aravantinos-Zafiris et al.76. This method involves the initial treatment of samples with dilute nitric acid to extract pectin, as outlined by Aravantinos-Zafiris et al.77. Subsequently, samples undergo aqueous ethanol treatment for total sugar determination, protease treatment to remove proteins, NaOH extraction for hemicellulose content, and H₂SO₄ treatment for the determination of cellulose, lignin, and ash contents.
Volatile matter, fixed C, ash, and moisture contents in RS and RSBC were analyzed according to the American Standard Test Method (ASTM D1762)78. Moisture content (M) was determined by weighing approximately 1.00 g of biochar, placing it in a porcelain crucible, and drying it in an oven at 105 ± 5 °C for 24 h. The dried material was then transferred to a muffle furnace preheated to 950 ± 10 °C for 6 min to determine volatile matter (V). The residual material was subsequently heated in the muffle furnace at 750 °C for 6 h to measure ash content (A). Fixed C content was calculated using the formula:
WHC was determined using a previously developed in-house method43. RS and RSBC samples were cut into pieces of around 0.5–1 cm length. Before analysis, glass beakers and filter papers were oven-dried, and unmilled RS and RSBC samples were heated in an oven at 105 °C for 10 h (or until a constant mass was achieved). The dried samples were then submerged in deionized water for 24 h to achieve full saturation. Excess water was drained over 30 min, during which dry filter papers were used to hold the biochar samples. The wet samples were then weighed using a digital balance. Any RS or RSBC particles remaining on the filter papers were dried and weighed separately to account for their contribution. WHC was calculated using the following equation:
Total concentrations of elements (P, K, Ca, Mg, S, Na, Fe, Mn, Cu, and Zn) in RS and RSBC samples were determined after digestion in concentrated perchloric acid (HClO4)79. In brief, 0.20 g of milled RS or RSBC sample was digested with 5 mL of HClO4 heated at 180–200 °C until obtaining a clear solution. The final extract was then diluted with Milli-Q water to a final volume of 20 mL. Elemental concentrations in the diluted solution were analyzed using inductively coupled plasma-optical emission spectrometry (ICP-OES, Analytik Jena, PQ 9000).
Surface morphology analysis
The surface morphology of RS and RSBC materials was analyzed using field emission scanning electron microscopy (FE-SEM; FEI Helios NanoLab G3 CX). Prior to imaging, milled samples were prepared by placing them onto carbon adhesive tape mounted on aluminum stubs to ensure stability during the analysis. The analysis was conducted at multiple magnification levels (100×, 500×, and 2500×) to capture detailed variations in surface features.
Synchrotron-based FTIR analysis
The RS and RSBC were ball-milled (Retsch Mixer MM301, Leeds, UK) into a fine powder to ensure sample homogeneity and dried overnight at 32 °C to remove the absorbed moisture80,81. Sample characterization was done by synchrotron-based Fourier transform infrared (FTIR) microspectroscopy. The dried sample was prepared using a diamond window, which is available to assist with sample preparation for transmission studies. Diamond windows have many advantages that exhibit a broadband transparency ranging from the UV to the far IR. Numerical simulations indicate that diamond windows can offer an attractive, and at times, the only alternative to beryllium windows for use on the third-generation x-ray synchrotron radiation beamlines82. There is only one minor absorption band around 5 μm resulting from two-phonon absorption (maximum absorption coefficient: 12 cm− 1). Hence, it is an ideal material for soil sample preparation with multi-wavelength spectroscopy. Spectral data were collected at an infrared microspectroscopy beamline (BL4.1 Infrared Spectroscopy and Imaging) at the Synchrotron Light Research Institute. Spectra were acquired with a Synchrotron radiation-based FTIR (Hyperion 2000, Bruker Optics, Ettlingen, Germany) coupled with an infrared microscope (Hyperion 2000, Bruker) using the 36x objective with an MCT detector cooled with liquid nitrogen over the measurement range from 4000 to 800 cm− 1. The measurements were performed in transmission mode, using an aperture size of 20 × 20 µm2 with a spectral resolution of 4 cm− 1, with 64 scans co-added. Spectral acquisition and instrument control were performed using OPUS 7.2 (Bruker Optics Ltd., Ettlingen, Germany) software.
Synchrotron-based XTM analysis
RS and RSBC were oven-dried at 60 °C for 72 h and cut into pieces of around 1 cm length. Sample characterization was done by synchrotron-based X-ray tomographic microscopy (XTM) at beamline 1.2 W, which was operated at 1.2 GeV, 150 mA in the Synchrotron Light Research Institute. Samples were fixed into the stub by glue before mounting on the stage. 350-micron thickness of the alumina foil were attenuated to minimize an artifact with a mean energy of 11.5 keV. 100 microns thickness of the YAG: Ce scintillator (Crytur, Czech Republic), lens-coupled X-ray microscope (Optique Peter, France), and the sCMOs camera (Andor NEO 5.5, 2560 × 2160 pixels, 16 bits) were employed to collect the X-ray radiographies from 0 to 180 with an angular increment of 0.2 degree. Tomographic scans were acquired at an isotropic voxel size of 0.72 μm. The acquired projections were preprocessed using flat-field correction and then reconstructed into cross-sectional slices using a filtered back-projection algorithm with Octopus Reconstruction software (TESCAN, Gent, Belgium).
Following image reconstruction, segmentation and 3D analysis were performed using Octopus Analysis and Visualization software. Pores were identified through grayscale thresholding and connected component labeling. Individual pores were segmented and color-coded for visualization, distinguishing open pores (connected to the sample surface) from closed pores (isolated internal pores). The analysis included calculations of porosity, total pore volume, and equivalent pore diameter (EPD). Porosity was defined as the ratio of pore voxels to the total number of voxels within the representative elementary volume. Pore sizes were quantified using EPD, defined as the diameter of a circle with the same area-to-perimeter ratio. Pore size distribution was calculated and expressed as a frequency distribution of equivalent pore diameter (EPD), divided into 10 μm intervals within the 1–100 μm range, with an additional class for pores larger than 100 μm. For interpretive context, we referenced the classification by Cameron and Buchan83, which categorizes pores into four functional classes: ultramicropores (0.1–5 μm), micropores (5–30 μm), mesopores (30–75 μm), and macropores (> 75 μm). The total pore volume within each class was also calculated to evaluate the structure and distribution of pores within the sample matrix. To visualize the 3D microstructure and pore segmentation in the samples, the reconstructed volumes were rendered using Drishti software.
Statistical analysis
The data are expressed as mean ± standard deviation of three replicates. Statistical analyses were performed using IBM SPSS Statistics software (version 28). The means of the data were subjected to a t-test at a significance level of 0.05. Pearson’s correlation was used to determine the relationships between physicochemical and pore properties.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request (P.L., phrula@kku.ac.th).
References
Cheewaphongphan, P., Junpen, A., Kamnoet, O. & Garivait, S. Study on the potential of rice straws as a supplementary fuel in very small power plants in Thailand. Energies 11(2), 270. https://doi.org/10.3390/en11020270 (2018).
Singh, Y. et al. Strategies for economic utilization of rice straw residues into value-added by-products and prevention of environmental pollution. Sci. Total Environ. 906, 167714. https://doi.org/10.1016/j.scitotenv.2023.167714 (2024).
Arunrat, N., Sereenonchai, S., Sansupa, C., Kongsurakan, P. & Hatano, R. Effect of rice straw and stubble burning on soil physicochemical properties and bacterial communities in central Thailand. Biology 12(4), 501. https://doi.org/10.3390/biology12040501 (2023).
Parihar, D. S., Narang, M. K., Dogra, B., Prakash, A. & Mahadik, A. Rice residue burning in Northern India: An assessment of environmental concerns and potential solutions–a review. Environ. Res. Commun. 5(6), 062001. https://doi.org/10.1088/2515-7620/acb6d (2023).
Nguyen, M. N. et al. Thermal induced changes of rice straw phytolith in relation to arsenic release: A perspective of rice straw arsenic under open burning. J. Environ. Manag. 304, 114294. https://doi.org/10.1016/j.jenvman.2021.114294 (2022).
Ku, H. H., Ryu, J. H., Bae, H. S., Jeong, C. & Lee, S. E. Modeling a long-term effect of rice straw incorporation on SOC content and grain yield in rice field. Arch. Agron. Soil. Sci. 65 (14), 1941–1954. https://doi.org/10.1080/03650340.2019.1583330 (2019).
Halder, M. et al. Effects of straw incorporation and straw-burning on aggregate stability and soil organic carbon in a clay soil of Bangladesh. Geoderma Reg. 32, e00620. https://doi.org/10.1016/j.geodrs.2023.e00620 (2023).
Zhang, J. et al. Long-term straw incorporation increases rice yield stability under high fertilization level conditions in the rice–wheat system. Crop J. 9 (5), 1191–1197. https://doi.org/10.1016/j.cj.2020.11.007 (2021).
Li, N., Lei, W., Sheng, M., Long, J. & Han, Z. Straw amendment and soil tillage alter soil organic carbon chemical composition and are associated with microbial community structure. Eur. J. Soil. Biol. 110, 103406. https://doi.org/10.1016/j.ejsobi.2022.103406 (2022).
Shan, A. et al. Effects of straw return with N fertilizer reduction on crop yield, plant diseases and pests and potential heavy metal risk in a Chinese rice paddy: A field study of 2 consecutive wheat-rice cycles. Environ. Pollut. 288, 117741. https://doi.org/10.1016/j.envpol.2021.117741 (2021).
Pingthaisong, W. & Vityakon, P. Nonadditive effects on decomposition of a mixture of rice straw and groundnut Stover applied to a sandy soil. Agronomy 11(6), 1030. https://doi.org/10.3390/agronomy11061030 (2021).
Chivenge, P. et al. Rice straw incorporation influences nutrient cycling and soil organic matter. in Sustainable Rice Straw Management (eds Gummert, M., Hung, N., Chivenge, P. & Douthwaite, B.) 131–144 (Springer, Cham, 2020).
Cao, Y. et al. Mitigating the global warming potential of rice paddy fields by straw and straw-derived biochar amendments. Geoderma 396, 115081. https://doi.org/10.1016/j.geoderma.2021.115081 (2021).
Kumputa, S., Vityakon, P., Saenjan, P. & Lawongsa, P. Carbonaceous greenhouse gases and microbial abundance in paddy soil under combined biochar and rice straw amendment. Agronomy 9(5), 228. https://doi.org/10.3390/agronomy9050228 (2019).
IPCC & Climate change : Synthesis report in Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change (eds. Pachauri, R. K. & Meyer, L. A.) 151 (IPCC, 2014).
Seow, Y. X. et al. A review on biochar production from different biomass wastes by recent carbonization technologies and its sustainable applications. J. Environ. Chem. Eng. 10(1), 107017. https://doi.org/10.1016/j.jece.2021.107017 (2022).
Ahsaan, M., Tripathi, P. & Khare, P. A relationship paradigm between biochar amendment and greenhouse gas emissions in Advances in Chemical Pollution, Environmental Management and Protection 203–220 (Elsevier, 2021).
Budai, A., Rasse, D. P., Lagomarsino, A., Lerch, T. Z. & Paruch, L. Biochar persistence, priming and microbial responses to pyrolysis temperature series. Biol. Fertil. Soils. 52, 749–761. https://doi.org/10.1007/s00374-016-1116-6 (2016).
Gross, A., Bromm, T. & Glaser, B. Soil organic carbon sequestration after biochar application: A global meta-analysis. Agronomy 11(12), 2474. https://doi.org/10.3390/agronomy11122474 (2021).
Gupta, D. K. et al. Role of Biochar in carbon sequestration and greenhouse gas mitigation in Biochar Applications. in Agriculture and Environment Management (eds Singh, J. & Singh, C.) 141–165 (Springer, Cham, 2020).
Somboon, S. et al. Mitigating methane emissions and global warming potential while increasing rice yield using biochar derived from leftover rice straw in a tropical paddy soil. Sci. Rep. 14 (1), 8706. https://doi.org/10.1038/s41598-024-59352-5 (2024).
Joseph, S. et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. Glob Change Biol. Bioenergy. 13(11), 1731–1764. https://doi.org/10.1111/gcbb.12885 (2021).
Bolan, N. et al. Multifunctional applications of biochar beyond carbon storage. Int. Mat. Rev. 67(2), 150–200. https://doi.org/10.1080/09506608.2021.1922047 (2022).
Arunrat, N., Kongsurakan, P., Sereenonchai, S. & Hatano, R. Soil organic carbon in sandy paddy fields of Northeast Thailand: A review. Agronomy 10(8), 1061. https://doi.org/10.3390/agronomy10081061 (2020).
Vityakon, P. Degradation and restoration of sandy soils under different agricultural land uses in Northeast thailand: a review. Land. Degrad. Dev. 18(5), 567–577. https://doi.org/10.1002/ldr.798 (2007).
Tomczyk, A., Sokołowska, Z. & Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio 19(1), 191–215. https://doi.org/10.1007/s11157-020-09523-3 (2020).
Suliman, W. et al. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 84, 37–48. https://doi.org/10.1016/j.biombioe.2015.11.010 (2016).
Gabhane, J. W., Bhange, V. P., Patil, P. D., Bankar, S. T. & Kumar, S. Recent trends in biochar production methods and its application as a soil health conditioner: A review. SN Appl. Sci. 2(7), 1307. https://doi.org/10.1007/s42452-020-3121-5 (2020).
Hassan, M. et al. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis. Sci. Total Environ. 744, 140714. https://doi.org/10.1016/j.scitotenv.2020.140714 (2020).
Mukherjee, A., Patra, B. R., Podder, J. & Dalai, A. K. Synthesis of biochar from lignocellulosic biomass for diverse industrial applications and energy harvesting: Effects of pyrolysis conditions on the physicochemical properties of biochar. Front. Mater. 9, 870184. https://doi.org/10.3389/fmats.2022.870184 (2022).
Wystalska, K. & Kwarciak-Kozłowska, A. The effect of biodegradable waste pyrolysis temperatures on selected biochar properties. Materials 14, 1644. https://doi.org/10.3390/ma14071644 (2021).
Tag, A. T., Duman, G., Ucar, S. & Yanik, J. Effects of feedstock type and pyrolysis temperature on potential applications of Biochar. J. Anal. Appl. Pyrol. 120, 200–206. https://doi.org/10.1016/j.jaap.2016.05.006 (2016).
Roshan, A., Ghosh, D. & Maiti, S. K. How temperature affects biochar properties for application in coal mine spoils? A meta-analysis. Carbon Res. 2(1), 3. https://doi.org/10.1007/s44246-022-00033-1 (2023).
Thammasom, N., Vityakon, P., Lawongsa, P. & Saenjan, P. Biochar and rice straw have different effects on soil productivity, greenhouse gas emission and carbon sequestration in Northeast Thailand paddy soil. Agr Nat. Resour. 50 (3), 192–198. https://doi.org/10.1016/j.anres.2016.01.003 (2016).
Haruthaithanasan, M. et al. Cambridge University Press,. The role of biochar production in sustainable development in Thailand, Lao PDR and Cambodia in Biochar: A Regional Supply Chain Approach in View of Climate Change Mitigation (eds. Bruckman, V. J., Apaydın Varol, E., Uzun, B. B. & Liu, J.) 266–288 (2016).
Sangsuk, S., Buathong, C. & Suebsiri, S. High-energy conversion efficiency of drum kiln with heat distribution pipe for charcoal and biochar production. Energy Sustain. Dev. 59, 1–7. https://doi.org/10.1016/j.esd.2020.08.008 (2020).
DeGostin, M. B., Cocco, A. P. & Chiu, W. K. Synchrotron-based transmission x-ray microscopy: A tool for three-dimensional spectroscopic imaging and numerical simulations. Annu. Rev. Heat Transf. 19. https://doi.org/10.1615/AnnualRevHeatTransfer.2017013698 (2016)
Fife, J. L. et al. Development of a laser-based heating system for in situ synchrotron-based X-ray tomographic microscopy. J. Synchrotron Radiat. 19(3), 352–358. https://doi.org/10.1107/S0909049512003287 (2012).
Bhattacharyya, P. et al. Characterization of rice straw from major cultivars for best alternative industrial uses to cutoff the menace of straw burning. Ind. Crops Prod. 143, 111919. https://doi.org/10.1016/j.indcrop.2019.111919 (2020).
Kalina, M. et al. The effect of pyrolysis temperature and the source biomass on the properties of biochar produced for the agronomical applications as the soil conditioner. Materials 15 (24), 8855. https://doi.org/10.3390/ma15248855 (2022).
Janu, R. et al. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 4, 36–46. https://doi.org/10.1016/j.crcon.2021.01.003 (2021).
Zhou, G. et al. Influence of enhanced ultraviolet-B radiation during rice plant growth on rice straw decomposition with nitrogen deposition. Sci. Rep. 8(1), 14512. https://doi.org/10.1038/s41598-018-32863-8 (2018).
Chen, C. et al. Profiling of chemical and structural composition of lignocellulosic biomasses in tetraploid rice straw. Polymers 12(2), 340. https://doi.org/10.3390/polym12020340 (2020).
Ghaffar, S. H. & Fan, M. Structural analysis for lignin characteristics in biomass straw. Biomass Bioenergy 57, 264–279. https://doi.org/10.1016/j.biombioe.2013.07.015 (2013).
Chi, N. T. L. et al. A review on biochar production techniques and biochar based catalyst for biofuel production from algae. Fuel 287, 119411. https://doi.org/10.1016/j.fuel.2020.119411 (2021).
Safarian, S. To what extent could biochar replace coal and coke in steel industries? Fuel 339, 127401. https://doi.org/10.1016/j.fuel.2023.127401 (2023).
Sakhiya, A. K., Baghel, P., Pathak, S., Vijay, V. K. & Kaushal, P. Effect of process parameters on slow pyrolysis of rice straw: Product yield and energy analysis, in International Conference and Utility Exhibition on Energy, Environment and Climate Change (ICUE). (IEEE, 2020). https://doi.org/10.1109/ICUE49301.2020.9306945 (2020).
Vityakon, P., Meepech, S., Cadisch, G. & Toomsan, B. Soil organic matter and nitrogen transformation mediated by plant residues of different qualities in sandy acid upland and paddy soils. Neth. J. Agric. Sci. 48(1), 75–90. https://doi.org/10.1016/S1573-5214(00)80006-8 (2000).
Aboelela, D. et al. Recent advances in biomass pyrolysis processes for bioenergy production: Optimization of operating conditions. Sustainability 15(14), 11238. https://doi.org/10.3390/su151411238 (2023).
Mierzwa-Hersztek, M., Gondek, K., Jewiarz, M. & Dziedzic, K. Assessment of energy parameters of biomass and biochars, leachability of heavy metals and phytotoxicity of their ashes. J. Mater. Cycles Waste Manag. 21, 786–800. https://doi.org/10.1007/s10163-019-00832-6 (2019).
Oginni, O. & Singh, K. Influence of high carbonization temperatures on microstructural and physicochemical characteristics of herbaceous biomass derived biochars. J. Environ. Chem. Eng. 8(5), 104169. https://doi.org/10.1016/j.jece.2020.104169 (2020).
Zheng, Q. et al. Unraveling the synergistic development of carbon skeleton and pore networks involved in lignin pyrolysis. J. Anal. Appl. Pyrol. 170, 105912. https://doi.org/10.1016/j.jaap.2023.105912 (2023).
Tripathi, M., Sahu, J. N. & Ganesan, P. Effect of process parameters on production of Biochar from biomass waste through pyrolysis: A review. Renew. Sust Energ. Rev. 55, 467–481. https://doi.org/10.1016/j.rser.2015.10.122 (2016).
Lee, Y. et al. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 °C. Bioresour. Technol. 148, 196–201. https://doi.org/10.1016/j.biortech.2013.08.135 (2013).
Maziarka, P. et al. Do you BET on routine? The reliability of N2 physisorption for the quantitative assessment of biochar’s surface area. J. Chem. Eng. 418, 129234. https://doi.org/10.1016/j.cej.2021.129234 (2021).
Zhang, K. et al. Easily pyrolyzable biomass components significantly affect the physicochemical properties and water-holding capacity of the pyrolyzed biochar. Agriculture 13(11), 2053. https://doi.org/10.3390/agriculture13112053 (2023).
Allen, J. A. & Downie, A. E. Predicting slow pyrolysis process outcomes with simplified empirical correlations for a consistent higher heating temperature: Biochar yield and ash content. Energy Fuels 34(11), 14223–14231. https://doi.org/10.1021/acs.energyfuels.0c02597 (2020).
Zhang, X., Zhang, P., Yuan, X., Li, Y. & Han, L. Effect of pyrolysis temperature and correlation analysis on the yield and physicochemical properties of crop residue biochar. Bioresour. Technol. 296, 122318. https://doi.org/10.1016/j.biortech.2019.122318 (2020).
Zhou, D. et al. Role of Ash content in Biochar for copper immobilization. Environ. Eng. Sci. 33(12), 962–969. https://doi.org/10.1089/ees.2016.0042 (2016).
Chatterjee, R. et al. Effect of pyrolysis temperature on physicochemical properties and acoustic-based amination of biochar for efficient CO2 adsorption. Front 8, 85. https://doi.org/10.3389/fenrg.2020.00085 (2020).
Hyväluoma, J. et al. Effects of pyrolysis temperature on the hydrologically relevant porosity of Willow biochar. J. Anal. Appl. Pyrol. 134, 446–453. https://doi.org/10.1016/j.jaap.2018.07.011 (2018).
Yang, C., Liu, J. & Lu, S. Pyrolysis temperature affects pore characteristics of rice straw and canola stalk biochars and biochar-amended soils. Geoderma 397, 115097. https://doi.org/10.1016/j.geoderma.2021.115097 (2021).
Brewer, C. E. et al. New approaches to measuring biochar density and porosity. Biomass Bioenergy. 66, 176–185. https://doi.org/10.1016/j.biombioe.2014.03.059 (2014).
Tian, Y. Z. & Chen, K. F. Determination of pore size distribution and surface area of several materials using mercury porosimetry and gas adsorption. China Pulp Paper. 23 (4), 21–23 (2004).
Maziarka, P. et al. Do you BET on routine? The reliability of N2 physisorption for the quantitative assessment of biochar’s surface area. Chem. Eng. J. 418. https://doi.org/10.1016/j.cej.2021.129234 (2021).
Wildenschild, D. & Sheppard, A. P. X-ray imaging and analysis techniques for quantifying pore-scale structure and processes in subsurface porous medium systems. Adv. Water Resour. 51, 217–246. https://doi.org/10.1016/j.advwatres.2012.07.018 (2013).
Bagreev, A., Bandosz, T. J. & Locke, D. C. Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage sludge-derived fertilizer. Carbon 39(13), 1971–1979. https://doi.org/10.1016/S0008-6223(01)00026-4 (2001).
Fu, P. et al. Evaluation of the porous structure development of Chars from pyrolysis of rice straw: Effects of pyrolysis temperature and heating rate. J. Anal. Appl. Pyrol. 98, 177–183. https://doi.org/10.1016/j.jaap.2012.08.005 (2012).
Edeh, I. G., Masek, O. & Fusseis, F. 4D structural changes and pore network model of biomass during pyrolysis. Sci. Rep. 13(1), 22863. https://doi.org/10.1038/s41598-023-49919-z (2023).
Srocke, F. et al. Synchrotron X-ray microtomography and multifractal analysis for the characterization of pore structure and distribution in softwood pellet biochar. Biochar 3(4), 671–686. https://doi.org/10.1007/s42773-021-00104-3 (2021).
Ngambia, A., Mašek, O. & Erastova, V. Development of Biochar molecular models with controlled porosity. Biomass Bioenergy 184, 107199. https://doi.org/10.1016/j.biombioe.2024.107199 (2024).
Leng, L. et al. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 763, 144204. https://doi.org/10.1016/j.scitotenv.2020.144204 (2021).
Weber, K. & Quicker, P. Properties of biochar. Fuel 217, 240–261. https://doi.org/10.1016/j.fuel.2017.12.054 (2018).
Santos, J. A. et al. Characterization, water retention and availability of different types of biochar from animal and plant origin. Res. Soc. Dev. 11(5). https://doi.org/10.33448/rsd-v11i5.28360 (2022).
Berek, A. K. & Hue, N. V. Characterization of biochars and their use as an amendment to acid soils. Soil. Sci. 181(9/10), 412–426. https://doi.org/10.1097/SS.0000000000000177 (2016).
Aravantinos-Zafiris, G., Oreopoulou, V., Tzia, C. & Thomopoulos, C. D. Fibre fraction from orange peel residues after pectin extraction. LWT-Food Sci. Technol. 27 (5), 468–471. https://doi.org/10.1006/fstl.1994.1094 (1994).
Aravantinos-Zafiris, G., Oreopoulou, V., Tzia, C. & Thomopoulos, C. D. Utilisation of orange by‐products—orange peel carotenoids. J. Sci. Food Agric. 59(1), 77–79. https://doi.org/10.1002/jsfa.2740590111 (1992).
American Society Testing and Materials (ASTM). D1762-84: Standard Test Method for Chemical Analysis of Wood Charcoal 1–2 (ASTM International, 2013).
Ksawery, K., John, G. R., Gorm, P. T. & Emielda, Y. B. The composition and dissolution in citric extractants of ash from the thermal gasification of pig manure. Chem. Eng. J. 163(1–2), 1–9. https://doi.org/10.1016/j.cej.2010.06.024 (2010).
Somboon, S., Kamolmanit, B., Namanusart, W., Thammanu, K. & Lawongsa, P. Changes in soil organic carbon composition resulting from long-term application of biochemical contrasting organic residues monitoring by synchrotron-based FTIR microspectroscopy. Biosci. Res. 15(3), 2542–2550 (2018).
Puttaso, P. et al. Assessing the effect of rubber (Hevea Brasiliensis (Willd. Ex A. Juss.) muell. Arg.) leaf chemical composition on some soil properties of differently aged rubber tree plantations. Agronomy 10 (12), 1871. https://doi.org/10.3390/agronomy10121871 (2020).
Khounsary, A. M. & Kuzay, T. M. On diamond windows for high power synchrotron x-ray beams. ucl. Instrum. Methods Phys. Res. 319(1–3), 233–239. https://doi.org/10.1016/0168-9002(92)90559-M (1992).
Cameron, K. C. & Buchan, G. D. Porosity and pore-size distribution. in Encyclopedia of Soil Science (ed Lal, R.) 1350–1353 (CRC, 2006).
Acknowledgements
This research was funded by the Basic Research Fund of Khon Kaen University and the National Science, Research and Innovation Fund (NSRF) (No. 49019, 49020, 49021). Saowalak Somboon was recipient of a Thesis Support Scholarship from the Graduate School, Khon Kaen University (Project no. 641T217).
Author information
Authors and Affiliations
Contributions
S.S. (Somboon): data curation, formal analysis, investigation, conceptualization, methodology, project administration, writing—original draft, writing—review and editing; S.S. (Schlichenmaier): writing—review and editing; K.T.: methodology, writing—review and editing; P.P.: methodology, writing—review and editing; S.Y.: writing—review and editing; T-S.S.: funding acquisition, conceptualization, methodology, resources, writing—review and editing; P.L.: conceptualization, data curation, methodology, project administration, supervision, resources, Validation, funding acquisition, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study does not include human or animal subjects. The plant collection and use were in accordance with all the relevant guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Somboon, S., Schlichenmaier, S., Thumanu, K. et al. Transformations in physicochemical properties and pore structure of biochar derived from rice straw revealed by synchrotron techniques. Sci Rep 15, 23641 (2025). https://doi.org/10.1038/s41598-025-08772-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08772-y