Abstract
Bitter gourd (Momordica charantia L.) is produced and consumed worldwide due to its numerous health benefits. The quantity of salt in the soil has an influence on a plant’s roots’ ability to absorb water and critical nutrients from the environment. The present study investigates the impact of inoculating bitter gourd plants with plant growth-promoting rhizobacteria (PGPR) Azotobacter chroococcum and arbuscular mycorrhizal fungi (AMF) Rhizophagus irregularis on fruit morphometric traits, chemical properties, and fruit yield under saline conditions. A total of 32 traits were assessed, including 9 phenotypic, 2 biochemical, and 21 fruit morphometric characteristics. Bitter gourd plants were treated with A. chroococcum, R. irregularis, or a consortium of both under saline and normal watering conditions. Biochemical traits, including ascorbic acid and beta-carotene content were analyzed, and fruit yield per plant was recorded. Additionally, the AM spore number and root colonization percentage were measured. The results revealed that the consortium treatment of A. chroococcum and R. irregularis under saline stress significantly improved fruit morphometric traits compared with individual treatments. The biochemical features ascorbic acid and beta-carotene showed a 49.43 and 75.95% increase, respectively, under normal watering conditions. The fruit yield per plant exhibited a significant increase only with the AS + Ri treatment (315.56%) with respect to control under saline conditions. Other treatments showed increases (71.11% with A. chroococcum, 188.89% with normal package, and 202.22% with R. irregularis) but these were not statistically significant with respect to the control under saline conditions. The highest AM spore number (113) and root colonization (71.54%) were observed in the consortium treatment. The study concludes that the combined inoculation of A. chroococcum and R. irregularis under saline stress significantly enhanced plant growth, biochemical content, and fruit yield, surpassing the effects of individual treatments. The consortium treatment provided a protective effect under salt stress, enabling the plants to perform as if grown under normal conditions. This indicates the potential of using PGPR and AMF consortia to improve agricultural productivity in saline-affected soils.
Similar content being viewed by others
Introduction
Momordica charantia L., referred to as bitter melon, balsam pear, or karela, bitter gourd is a plant that belongs to the Cucurbitaceae family. Bitter gourd is produced and consumed worldwide due to its numerous health benefits, including anti-diabetic, anti-obesity, anticancer, antifungal, antiviral (including anti-HIV), and antibacterial properties1,2,3,4. Globally, countries like India, China, and other Southeast Asian nations are major producers, with significant consumption also noted in these regions and increasing interest in Western countries due to its health benefits. For instance, India produces approximately 1.2 million tonnes annually, with a domestic consumption of around 90%5. As the world’s population expands, the demand for these healthy meals increases proportionately, necessitating a significant increase in global vegetable production. Salinity has a detrimental impact on 20% of irrigated and 22% of dryland agricultural land globally, according to the FAO6. Around 0.3–1.5 million acres of productive land are becoming barren due to the salinity of the water6. While bitter gourd can tolerate mild salinity, levels exceeding 4–6 dS/m are generally considered toxic, leading to significant yield reductions7; conversely, very low salinity (1–2 dS/m) might sometimes have minor stimulatory effects on certain physiological parameters before negative impacts become apparent. Essential nutrients become less available under high salinity, and toxic ions like Na+ accumulate8.
The quantity of salt in the soil influences a plant’s roots’ ability to absorb water and critical nutrients from the environment. Salt stress impairs plant growth through two primary mechanisms: ion toxicity, where high Na+ concentrations disrupt enzyme function and compete with K+ uptake, leading to nutritional imbalances and cellular damage; and osmotic stress, which reduces water uptake capacity, induces stomatal closure, and disrupts photosynthesis and metabolic processes, ultimately triggering oxidative damage beyond ROS accumulation9,10,11,12,13. Chlorosis and rot are thought to be caused by sodium aggregation, which may cause some alterations in plants, including disrupting cell homeostasis and decoupling fundamental metabolic and physiological processes9,11. Additional impacts include nodulation and decreased nitrogen content, which may result in reduced plant yields9. Because salt slows root development, plant components weigh less as a result. Higher salt levels reduce agricultural output significantly, making agricultural production uneconomical without soil amendments. Recent literature increasingly points to the complex interplay between salinity-induced oxidative stress and hormonal imbalances as key determinants of yield loss9,11.
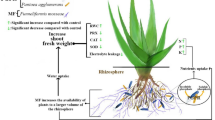
Plant growth-promoting rhizobacteria (PGPR) serve a direct or indirect role in plant growth, yield, and nutrient uptake through a wide range of processes14,15,16,17,18,19,20,21. These bacteria improve plant physiology by synthesizing various phytohormones like auxin, gibberellins, and cytokinin. In saline soil, Azotobacter plays a critical role as a plant growth stimulant15,22,23,24,25,26. The Azotobacter genus is a free-living, aerobic, nitrogen-fixing, heterotrophic, Gram-negative bacterium that belongs to the y-proteobacteria class. Furthermore, it affects plants by elevating roots and shoots dry weights. Some Azotobacter strains could survive in a medium having 6–10 percent sodium chloride24,25. Whereas by strengthening soil enzyme activity, Arbuscular mycorrhizal fungi (AMF) can promote plant development in normal and stressed circumstances27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48. AMF works on improving the absorption of water and retaining osmotic balance in plants; stimulating an anti-oxidative system that prevents ROS injury; and defends photosynthetic machinery. Rhizophagus irregularis is abundantly observed in mycorrhizal-based fertilizers and improves phosphorus uptake in numerous plants and soil aggregation28,49,50. Rhizophagus species, like R. irregularis, were formerly classified under the genus Glomus. The reclassification is based on molecular phylogenetic analyses51, which revealed distinct evolutionary lineages, though functionally they share many broad characteristics of AMF such as enhancing nutrient uptake27,28,30,38,52. By promoting beneficial bacteria species, AMF aids in enhancing soil aggregation49,50. Furthermore, glomalin, a hydrophobically stable substance generated by dead AMF hyphae, promotes soil aggregation49,50. Arbuscular mycorrhizal fungi (AMF) mitigate salt stress by enhancing nutrient acquisition (particularly phosphorus), improving water relations through extended hyphal networks, and regulating osmotic balance through enhanced K+/Na+ discrimination and accumulation of compatible solutes27,28,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48. AMF accumulate various solutes such as sugars (e.g., trehalose), polyols (e.g., mannitol), amino acids (e.g., proline), and glycine betaine, primarily within their hyphae and in the host plant cells, particularly in the roots and sometimes translocated to shoots, to help maintain osmotic balance under stress27,28,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48. The colonizing of Rhizophagus irregularis starts earlier than that of several other Glomus fungi51. There is evidence of significant mycelial networking and abundant intraradical spores in the aged roots of host plants. These interactions reinforce the plant’s ability to withstand salt stress. The present study was therefore designed with the importance of bitter gourd in mind to evaluate the influence of Azotobacter chroococcum and Rhizophagus irregularis inoculation on its fruit morphometric, biochemical, and morphological characteristics under saline conditions. The specific objectives were to: (1) assess the individual and combined effects of these microbes on plant growth parameters, (2) determine their impact on a fruit yield and quality attributes, and (3) evaluate changes in biochemical markers of stress tolerance in bitter gourd exposed to salinity.
Material and methods
Planting material and experimental details
The experiment took place at Kurukshetra University in the Indian state of Haryana (GPS coordinates: 29.9650° N, 76.8389° E). The investigation was conducted using a five-replication randomized complete block design. This experiment used seeds from the bitter gourd cultivar Arka Harit. Spindle-shaped, glossy, green-skinned fruits with no tubercles mature in 100–110 days and have a 100–110-day crop duration. To ensure the findings were accurate, they were conducted in a polyhouse-type greenhouse with a controlled environment (natural ventilation with a fan-pad cooling system for temperature regulation and humidity control) (at a temperature of 28/20 °C and relative humidity of 67%) that was achieved using the fan pad system. The experiment was conducted in five replications, during 2017–2018. To begin, seeds were planted in portrays using a two-part cocopeat-one-part perlite mixture. When the seedlings developed two leaves, they were transplanted into 2L plastic pots to form a single seed plot. The plants were grown on a substrate made from a combination of peat-based compost and autoclaved sand used in the containers. No additional chemical NPK fertilizers were used; nutrient requirements were managed through the inherent fertility of the peat-based compost and the bioinoculants. The substrate used for seedling growth had the following characteristics: available nitrogen 0.85%, available phosphorus 0.025%, organic carbon 25.5%, and a pH of 6.7. Steel wires were used to train the plants, which were suspended between 1.5 and 2.9 m above the cropping rows. Azotobacter chroococcum and Rhizophagus irregularis were isolated and cultured in bitter gourd roots using a previously described method36.
The formal identification of the samples was performed by Prabhat Kumar. A voucher specimen of this material has been deposited in the publicly available herbarium of ICAR-Indian Agricultural Research Institute, New Delhi, India with deposition number MC-1456.
AMF and PGPR inoculation
Azotobacter chroococcum was obtained from K-Ferts Lab, Nanded, Maharashtra, India, and Rhizophagus irregularis was obtained from Agri Life, India, with a CFU count of 100 spores/g. Before the field experiment, laboratory tests were conducted to ensure the salt stress tolerance and survivability (CFU/mL) of Azotobacter (up to 8% NaCl) and AMF (spore germination and hyphal growth at 6 dS/m NaCl) in different salt concentrations, confirming their viability under the proposed experimental stress levels22,25,26,27,28,36. Before transplanting, inoculated plants were fed 100 g of material containing infectious propagules for AMF (arbuscular mycorrhizal fungi) treatment (including mycelium, spores, and roots) and then transplanted. Non-mycorrhizal treatments received an equal amount of uninoculated (and non-mycorrhizal) Zea mays roots to match the amount of ‘organic matter’ in the pots. To get the filtrate for each pot, 100 mL of distilled water was diluted with the mycorrhizal inoculum and then passed through a layer of 15–20-micron filter papers, which were then discarded (Whatman, GE Healthcare, UK). For the plant growth-promoting bacteria (PGPB) treatment, seedlings were submerged for 30 min in a suspension of Azotobacter chroococcum (40 g/L)22,26. The pot experiment was repeated five times in all, using a new pot each time. Salinity to the experimental levels of 3 dS/m made of tap water with NaCl to give the saline conditions. Five treatments were evaluated in the experiment: T1: normal package (crop having normal watering), T2: crop watered with saline water, T3: Azotobacter chroococcum inoculation under saline conditions, T4: Rhizophagus irregularis inoculation under saline conditions and T5: Azotobacter chroococcum + Rhizophagus irregularis inoculation under saline conditions.
Agronomic and fruit morphometric analysis
After 60 days of transplantation, the plants were evaluated. At the second harvest, vine length (cm), number of primary branches, and internode length (cm) were measured. Again, at the second harvesting, petiole length (cm) and peduncle length (cm) were calculated as the mean of 5 leaves in every plant in each replication, and the number of fruits per plant was counted from the first to the last harvest. Fruit weight (g) as the mean of 5 fruits per replication was measured. The number of fruits per plant was multiplied by the weight of the fruits to get the fruit yield per plant (kg). With the help of the Tomato Analyzer version 4 software tool53, twenty-one fruit morphometric attributes were analyzed. To precisely identify the effects on the fruit morphometric analysis of bitter gourd using software such as Tomato Analyzer, three fruits at the marketable stage were selected, sliced open longitudinally, and scanned at 300 dpi using an HP Scanjet Scanner (Hewlett-Packard, USA).
Biochemical analysis
The ascorbic acid content was determined (mg/100 g) using the titrimetric method described by Ranganna54. Aliquots were prepared by finely grinding well-mixed fruit samples in a solution of metaphosphoric acid or oxalic acid containing 4% oxalic acid. After thoroughly mixing, the dye solution was prepared by dissolving 42 mg of sodium bicarbonate in a small volume of distilled water. The working standard was used at a concentration of one hundred micrograms per ml in the experiment. Following that, 5 ml of the working standard was added to the solution, which was pipette out into a conical flask filled with 100 ml of 4% oxalic acid and titrated against the dye (V1 mL). The appearance of the pink tint, which lasted only a few moments, signaled the conclusion of the experiment. Consumption of the dye was equivalent to that of ascorbic acid. Carotenoids (mg/100 g) concentration was determined using 1 g of fresh sample, ground in mortar in 80% acetone, and other procedures as defined elsewhere55. The carotenoid assay was performed using a UV–Vis spectrophotometer (e.g., Shimadzu UV-1800), and absorbance was measured at 450 nm. Concentrations were calculated using a standard curve or appropriate extinction coefficients.
AMF evaluation and data analysis
The AMF spores were collected from the rhizospheric soil of pot plants using wet sieving and decanting and counted using the gridline intersect technique56. Following root washing with 10% KOH, AMF root colonization was achieved using 0.01% trypan blue dye56. The AM Root Colonization (%) was determined as we defined earlier. The statistical analysis was employed using SPSS 16.0 and included a one-way ANOVA followed by a post hoc test analysis (Duncan’s Multiple Range Test) to evaluate the outcomes.
Results and discussion
Effect on morphometric attributes of fruits
It has been noted that the perimeter was increased by 49.97, 54.81, and 83.13% by inoculation of Ac, Ri, and Ac + Ri, respectively; NP showed a greater increase by 95.00% (Table 1). Similarly, a maximum (270.06%) rise in the area was also recorded in NP, followed by Ac + Ri (262.04%), Ri (139.76%), and Ac (125.0%). While greater expansion in width mid-height by 50.24, 55.12, 78.05, and 100.49% was recorded in Ac, Ri, NP, and Ac + Ri, respectively, same as maximum width was increased by 50.19, 55.13, 88.21, and 90.11% in Ac, Ri, NP, and Ac + Ri, respectively. Normal package (NP) produced a remarkable increase in height mid-width i.e. (113.81%) followed by Ac + Ri (64.46%), Ri (54.82%), and Ac (50.07%). Maximum height was increased by 93.20,84.96, 54.77, and 50.00% in NP, Ac + Ri, Ri, and Ac, respectively, as compared with SS. Likewise, fruit shape index external I (10.10, 10.10, 9.43, and 7.41%), fruit shape index external II (47.29, 46.57, 45.49, and 44.40%), and fruit shape triangle (8.66, 7.79, 5.63, and 4.33%) exhibited best in NP, Ac + Ri, Ri, and Ac, respectively as in comparison with SS. Maximum extension in fruit ellipsoid by 40.00, 60.00, 80.00, and 120.00% and circular by 12.50, 15.63, 18.75, and 25.00% was estimated in Ac, Ri, NP, and Ac + Ri, respectively as compared with salt-affected plants (SS) (Table 1). Plant health is harmed by high salt content in the soil, which disrupts cell homeostasis and destabilizes essential biochemical and physiological processes. Excessive ions Na+ and Cl− damage plant cells by rising oxidative and osmotic pressures. The plant’s lower K+/Na+ ratio is primarily used to evaluate a specific plant reaction to salt pressure. The main consequences of soil salinity are nutrient deficiencies, lowered osmotic pressure, and reduced water absorption from the soil. It’s interesting to note that plants with a more effective rhizosphere nutrient supply could be able to endure significant growth in saline conditions.
A greater increase in rectangular by 12.50, 12.50, 16.67, and 25.00% was also recorded in plants introduced with Ac, Ri, NP, and Ac + Ri, respectively. NP showed a better increment in distal end protrusions (60.00%) compared with SS. Inoculation of Ac, Ri, NP, and Ac + Ri increased the vertical asymmetry by 31.25, 59.38, 81.25, and 87.50%, horizontal asymmetry by 12.40, 15.70, 23.14, and 27.27%, and width widest position by 12.90, 22.58, 35.48, and 41.94%, respectively. Eccentricity rose by 8.93, 16.07, 25.00, and 33.93% in Ac, Ri, Ac + Ri, and NP, respectively. Likewise, supreme proximal eccentricity 13.10, 10.71, 7.14, 5.95%, distal eccentricity 16.25, 11.25, 8.75, and 6.25%, and fruit shape index internal 48.01, 45.13, 43.68, and 35.74%, respectively was remarked in NP followed by Ac + Ri, Ri, and Ac. Meanwhile, the most significant results were produced in the eccentricity area index with an increase of 13.33, 16.67, 30.00, and 40.00% with Ac, Ri, NP, and Ac + Ri, respectively (Table 1). According to the study, inoculating bitter gourd plants with Azotobacter and arbuscular mycorrhizal fungi improved the health and yield of the plants in salty conditions57,58. Co-inoculation with Azotobacter chroococum + Rhizophagus irregularis is likely to be successful due to PGPR’s ability which promotes plant development and yield57,58. These findings corroborate our own. Rhizospheric bacteria have been shown to colonize plant roots and maintain soil fertility more effectively than inorganic fertilizers to reduce soil salinity and promote plant growth16,17,59,60. These beneficial bacteria cope with salt stress in a variety of ways, one of which is through the release of extracellular polymeric molecules known as exopolysaccharides (EPS), which aids in their survival in hostile soil environments. PGPR promotes growth in two ways: directly by increasing food intake via phytohormone production (e.g., auxin, gibberellins, and cytokinin) or indirectly by decreasing ethylene levels via an enzyme-mediated ethylene reduction14,15,16,17,61,62. According to Das et al.63, treatment of bottle gourds with Azotobacter and PSB (Phosphate solubilizing bacteria) resulted in the highest main branch count, fruit count, average fruit weight, and fruit size when compared with other treatments. Apart from that, several beneficial endophytic fungi produce auxin and/or possess ACC deaminase activity, which may aid the host plant in its struggle to survive under adverse conditions14,15,16,17,61,62.
Recent research has demonstrated the efficacy of AMF inoculum in growing vegetables such as cucumbers and tomatoes, which is consistent with our findings demonstrating the overall influence of plant growth-promoting bacteria and AMF on bitter gourd morphological characters33,41,42. Glomeromycota is a phylum of endomycorrhizal fungi (fungi whose hyphae invade the cell wall and invaginate the cell membrane). Infected plants produce spores and hyphae in the rhizosphere, as well as vesicles, arbuscules, and hyphae in their roots. The formation of a hyphal network connected to plant roots by the AMF enables plants to access more available soil surface area, resulting in increased plant growth27,28,30,31,35,36,37,39,40,41,42,43,44,45,46,47,48,49,50,51,52,56,64,65,66. As a result of the symbiotic relationship between AMF and plants, fungal hyphae form in plant roots27,28,30,31,35,36,37,39,40,41,42,43,44,45,46,47,48,49,50,51,52,56,64,65,66.
Effect on agronomical and biochemical traits
The statistic of data regarding the agronomical and biochemical traits is presented in Table 2. The Vine length was increased by 55.12, 73.26, 89.97, and 109.52% when treated with Ac, Ri, Ac + Ri, and NP, respectively, while the number of primary branches recorded the highest by 16.56, 30.70, 70.50, and 75.44%, respectively. Likewise, maximum increment in internode length by 30.81, 49.35, 74.67, and 97.13%, and petiole length by 32.87, 66.85, 93.54, and 109.27% was noted again Ac, Ri, Ac + Ri, and NP, respectively. Peduncle length showed a significant increase by 27.71, 72.57, 98.57, and 104.57% with sequentially introducing Ac, Ri, Ac + Ri, and NP. When the plants were treated with Ac, Ri, Ac + Ri, and NP, the number of fruits per plant increased by 83.47, 113.88, 104.57, and 156.20%, respectively (Fig. 1). Rise in fruit weight by 34.93, 58.41, 65.94, and 75.66% (Fig. 1) and 100 seed weight by 46.99, 51.23, 77.82, and 93.65% was exhibited in Ac, Ri, Ac + Ri, and NP, respectively. The Ascorbic acid content result presented in the data table is significantly higher only with AS + Ri treatment with respect to control. The rest of the treatment results are not significant with respect to control; hence, while increases were observed (17.30% with Ac, 30.66% with Ri, 40.41% with Ac + Ri under saline conditions, and 49.43% with NP), only the Ac + Ri (AS + Ri) value under saline conditions was statistically significant compared with the saline control (SS) (Fig. 2). However, the beta-carotene content increased by 7.59, 16.46, 41.77, and 75.95% when plants were treated with Ri, Ac, Ac + Ri, and NP, respectively (Fig. 2). There was a dramatic increment in fruit yield per plant. The fruit yield per plant presented in the data table is significantly higher only with AS + Ri treatment (315.56%) with respect to control under saline conditions. The rest of the treatment results (71.11% with Ac, 188.89% with NP, 202.22% with Ri) are not significant with respect to the control under saline conditions (Fig. 1). There were no AM spore number and root colonization (%) values for NP, SS, and Ac treatments, whereas the AM spore number was 88.00 for the treatment Ri and 113 for the consortium treatment (Ac + Ri). In the same direction, root colonization of 64.47% was observed for Ri, and 71.54% was observed for the Ac + Ri treatment (Fig. 1). This symbiotic relationship was discovered to facilitate the integration of secondary metabolites such as phenolic acids or flavonoids that plants require to tolerate abiotic stress. They increase water use efficiency and nutrient absorption efficiency, for example, by synthesizing plant growth hormones and regulators, increasing photosynthetic rate, maintaining ionic equilibrium, and synthesizing antioxidants8,9,14,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,66. Increased salinity impairs macro-and micronutrient absorption8,9. They synthesize glomalin, a proteinaceous substance that contributes to soil agglomeration and nitrogen cycling, among other functions49,50. Microbial inoculation can significantly affect plant stomatal behavior, often leading to improved stomatal conductance under stress conditions by maintaining turgor pressure and enhancing water uptake, thus supporting photosynthesis27,28,29,31,32,35,37,38,40,41,42,43,66. Furthermore, microbial inoculation is known to enhance the accumulation of osmolytes like proline in plants under salinity stress; these compounds help in osmotic adjustment, protect cellular structures, and scavenge reactive oxygen species, thereby contributing to stress tolerance27,28,29,31,32,35,37,38,40,41,42,43,66.
Two significant benefits of AMF treatment are improved soil quality and, consequently, improved plant health27,28,31,32,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,66. Numerous studies have demonstrated that AMF can enhance tomato, pepper, and watermelon growth and output when exposed to salt stress, which corroborates our findings33,41,42. The current study discovered that as salinity increased, the number of AM spores and the rate of root colonization decreased, indicating that salinity constrains the development of AMF. Our findings are consistent with those of Latef and Chaoxing41, who discovered that various salts inhibit hyphal growth, limiting mycorrhizal spread colonization. Roots were found to be less effective at cell division and cell elongation as a result of the absorption of extra-hazardous ion transport. Recently, it was demonstrated that plants inoculated with these bio inoculants can successfully counteract certain environmental signals, such as salt, thereby significantly increasing the yield per hectare of a wide variety of crops57,58,66.
It is critical to promote AMF and PGPR applications to ensure the long-term viability of today’s global agricultural systems. To maximize agricultural production, it is critical to understand how AMFs modulate tolerance mechanisms and the crosstalk that results from plant performance modification. Co-inoculation with AMF and PGPR has been demonstrated to be effective in cucumber, watermelon, potato, and bean, among other plants33,42,57. Our study demonstrated that PGPR and AMF had beneficial effects when exposed to salinity stress, implying that they may be effective in the treatment of bitter gourd caused by salt stress27,28,29,35,37,38,40,41,42,43,66. Furthermore, the improved photosynthetic efficiency and antioxidant enzyme activity we documented provide compelling evidence that AMF-PGPR synergism operates through multiple physiological pathways27,28,29,35,37,38,40,41,42,43,66 to counteract both osmotic stress and ion toxicity components of salt stress, rather than simply addressing oxidative damage27,28,30,31,32,35,37,38,40,41,42,43,66.
Conclusions
Globally, soil salinization reduces agricultural productivity by reducing the amount of net cultivable land available for cultivation. Crop losses due to salt are expected to range between 20 and 50%. To address this issue, agricultural strategies based on the inoculation of PGPR and AMF have been developed to improve plant health and resistance to salinity. Our study discovered that increasing the salt content of bitter gourd significantly reduced all morphometric and yield-contributing characteristics. The application of salt-resistant bacteria and fungi to bitter gourd growth and development, such as Azotobacter and Rhizophagus, aided in mitigating the effects of salinity on the plant. As a result, long-term salinity management with PGPR and AMF on bitter gourd is feasible.
This study demonstrates that dual inoculation with AMF and PGPR significantly mitigates salt-induced stress in crop plants through complementary mechanisms including enhanced nutrient acquisition, improved ion homeostasis, and strengthened antioxidant defense systems. The synergistic relationship between these beneficial microorganisms presents a promising sustainable approach for agricultural production in salt-affected soils. The findings underscore the potential of these bioinoculants to enhance crop resilience and productivity in challenging saline environments, contributing to food security. Future research should focus on identifying optimal combinations of AMF and PGPR strains for specific crop varieties and salinity conditions, as well as investigating the molecular signaling pathways that underpin this beneficial plant–microbe interaction to develop more effective biofertilizer formulations for salt-stressed agricultural systems. Additionally, long-term field trials under diverse agro-climatic conditions are warranted to validate these findings and promote farmer adoption of these sustainable technologies.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Joseph, B. & Jini, D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 3, 93–102 (2013).
Sharma, R. & Singh, K. Bitter gourd (Momordica charantia): A comprehensive review on phytochemistry, pharmacology, and nutritional aspects. J. Ethnopharmacol. 275, 114100 (2021).
Alam, M. A. et al. Beneficial role of bitter melon supplementation in obesity and related complications in metabolic syndrome. J. Lipids 2015, 496169. https://doi.org/10.1155/2015/496169 (2015).
Cousens, G. There is a Cure for Diabetes: The Tree of Life 21-Day Program 191–192 (North Atlantic Books, 2007).
FAOSTAT. Production quantities of Bitter Gourd by country. Food and Agriculture Organization of the United Nations (2022). http://www.fao.org/faostat/. Accessed 7 June 2025.
Food and Agriculture Organization [FAO]. Status of the Worlds’s Soil Resources (SWSR): Main Report, United Nations (Food and Agriculture Organization, 2015).
Singh, H. & Kumar, P. Salinity stress effects on growth, yield, and quality of bitter gourd (Momordica charantia L.). Indian J. Hortic. 76(2), 285–291 (2019).
Grattan, S. R. & Grieve, C. M. Salinity-mineral nutrient relations in horticultural crops. Sci. Hortic. 78(1–4), 127–157 (1998).
Ashraf, M. & Harris, P. J. C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 166(1), 3–16 (2004).
Flowers, T. J. & Colmer, T. D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 115(3), 327–331 (2015).
Parihar, P., Singh, S., Singh, R., Singh, V. P. & Prasad, S. M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 22(6), 4056–4075 (2015).
Hasanuzzaman, M., Nahar, K. & Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and Responses of Plants under Salt Stress (eds Ahmad, P. et al.) (© Springer Science+Business Media, LLC, 2013).
Hunter, K. et al. CRK2 enhances salt tolerance by regulating callose deposition in connection with PLD-1. Plant Physiol. 180, 2004–2021 (2019).
Vejan, P., Abdullah, R., Khadiran, T., Ismail, S. & Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Molecules 21, 573 (2016).
Bhise, K. K. & Dandge, P. B. Mitigation of salinity stress in plants using plant growth promoting bacteria. Symbiosis 79, 191–204 (2019).
Timmusk, S., Behers, L., Muthoni, J., Muraya, A. & Aronsson, A. C. Perspectives and challenges of microbial application for crop improvement. Front. Plant Sci. 8, 49 (2017).
Majeed, A., Muhammad, Z. & Ahmad, H. Plant growth promoting bacteria: role in soil improvement, abiotic and biotic stress management of crops. Plant Cell Rep. 37, 1599–1609 (2018).
Etesami, H. & Beattie, G. A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 9, 148 (2018).
Etesami, H. & Glick, B. R. Halotolerant plant growth-promoting bacteria: Prospects for alleviating salinity stress in plants. Environ. Exp. Bot. 178, 104124 (2020).
Kushwaha, P. et al. Bacterial endophyte mediated plant tolerance to salinity: Growth responses and mechanisms of action. World J. Microbiol. Biotechnol. 36, 26 (2020).
Yang, J., Kloepper, J. W. & Ryu, C. M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 14(1), 1–4 (2009).
Abdel Latef, A. A. H., Abu Alhmad, M. F., Kordrostami, M., Abo-Baker, A. B. A. E. & Zakir, A. Inoculation with Azospirillum lipoferum or Azotobacter chroococum reinforces maize growth by improving physiological activities under saline conditions. J. Plant Growth Regul. 39, 1293–1306 (2020).
Aly, M. M., El-Sabbagh, S. M., El-Shouny, W. A. & Ebrahim, M. K. H. Physiological response of Zea mays to NaCl stress with respect to Azotobacter chroococcum and Streptomyces niveus. Pak. J. Biol. Sci. 6, 2073–2080 (2003).
Chaudhary, D., Narula, N., Sindhu, S. S. & Behl, R. K. Plant growth stimulation of wheat (Triticum aestivum L.) by inoculation of salinity tolerant Azotobacter strains. Physiol. Mol. Biol. Plants 19, 515–519 (2013).
Akhter, M. S., Hossain, S. J., Hossain, S. A. & Datta, R. K. Isolation and characterization of salinity tolerant Azotobacter sp. Greener J. Biol. Sci. 2, 43–51 (2012).
Bano, A. & Fatima, M. Salt tolerance in Zea mays (L.) following inoculation with Azotobacter and Pseudomonas. Biol. Fertil. Soils 45(4), 405–413 (2009).
Evelin, H., Devi, T. S., Gupta, S. & Kapoor, R. mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 10, 470. https://doi.org/10.3389/fpls.2019.00470 (2019).
Zhu, X., Song, F., Liu, S., Liu, F. & Li, X. Arbuscular mycorrhiza enhances nutrient accumulation in wheat exposed to elevated CO2 and soil salinity. J. Plant Nutr. Soil Sci. 81, 836–846 (2018).
Xu, H., Lu, Y. & Tong, S. Effects of arbuscular mycorrhizal fungi on photosynthesis and chlorophyll fluorescence of maize seedlings under salt stress. Emir. J. Food Agric. 30, 199–204 (2018).
Zhang, H. S., Qin, F. F., Qin, P. & Pan, S. M. Evidence that arbuscular mycorrhizal and phosphate-solubilizing fungi alleviate NaCl stress in the halophyte Kosteletzkya virginica: Nutrient uptake and ion distribution within root tissues. Mycorrhiza 24, 383–395 (2014).
Augé, R. M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11(1), 3–42 (2001).
Ruiz-Lozano, J. M. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13(6), 309–317 (2003).
Okkon, J., Okkon, I., Embong, E. & Grace, D. O. Mitigation of salt induced stress via arbuscular mycorrhizal fungi (Rhizophagus irregularis) inoculation in Cucurbita maxima Duch. Int. J. Mol. Biol. Open Access 4, 30–36 (2019).
Navarro, J. M., Perez-Tornero, O. & Morte, A. Alleviation of salt stress in citrus seedlings inoculated with arbuscular mycorrhizal fungi depends on the rootstock salt tolerance. J. Plant Physiol. 171, 76–85 (2014).
Begum, N. et al. Role of Arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 10, 1068 (2019).
He, W. et al. The effect of Rhizophagus irregularis on salt stress tolerance of Elaeagnus angustifolia roots. J. For. Res. 31, 2063–2073. https://doi.org/10.1007/s11676-019-01053-1 (2020).
Metwally, R. A. & Abdelhameed, R. E. Synergistic effect of arbuscular mycorrhizal fungi on growth and physiology of salt-stressed Trigonella foenum-graecum plants. Biocatal. Agric. Biotechnol. 16, 538–544 (2018).
Porcel, R., Aroca, R. & Ruiz-Lozano, J. M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 32(1), 181–200 (2012).
Hashem, A., Abd Allah, E. F., Alqarawi, A. A., Wirth, S. & Egamberdieva, D. Comparing symbiotic performance and physiological responses of two soybean cultivars to arbuscular mycorrhizal fungi under salt stress. Saudi J. Biol. Sci. 26, 38–48 (2019).
Hajiboland, R., Dashtebani, F. & Aliasgharzad, N. Physiological responses of halophytic C4 grass Aeluropus littoralis to salinity and arbuscular mycorrhizal fungi colonization. Photosynthetica 53, 572–584 (2015).
Latef, A. A. H. A. & Chaoxing, H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 127, 228–233 (2011).
Lee, Y., Krishnamoorthy, R., Selvakumar, G., Kim, K. & Sa, T. Alleviation of salt stress in maize plant by co-inoculation of arbuscular mycorrhizal fungi and Methylobacterium oryzae CBMB20. J. Kor. Soc. Appl. Biol. Chem. 58, 533–540 (2015).
Chen, J., Zhang, H., Zhang, X. & Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant Sci. 8, 1739 (2017).
Bijalwan, P. et al. Mitigation of saline conditions in watermelon with mycorrhiza and silicon application. Saudi J. Biolog. Sci. 28, 3678–3684 (2021).
Parkash, V., Aggarwal, A. & Sharma, V. Rhizospheric effect of vesicular arbuscular mycorrhizal inoculation on biomass production of Ruta graveolens L.: A potential medicinal and aromatic herb. J. Plant Nutrit. 34, 1386–1396 (2011).
Rawat, A. et al. Concentration mediated effect of arbuscular mycorrhizal fungi (AMF) on growth and nutrition of air-layered litchi plants. Int. J. Curr. Res. 5, 1730–1734 (2013).
Saini, I., Aggarwal, A. & Kaushik, P. Inoculation with mycorrhizal fungi and other microbes to improve the morpho-physiological and floral traits of Gazania rigens (L.) Gaertn. Agriculture 9, 51. https://doi.org/10.3390/agriculture9030051 (2019).
Shao, T., Gua, X. & Zhua, T. Industrial crop Jerusalem artichokerestored coastal saline soil quality by reducing salt and increasing diversity of bacterial community. Appl. Soil Ecol. 138, 195–206 (2019).
Rillig, M. C. Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol. Lett. 7, 740–754 (2004).
Simard, S. W. & Austin, M. E. The role of mycorrhizas in forest soil stability with climate change. in Climate Change and Variability 275–302 (Intech Open, 2010).
Kruger, M., Claudia, K. & Christopher, W. Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol. 193, 970–984 (2012).
Tavarini, S. et al. Plant growth, steviol glycosides and nutrient uptake as affected by arbuscular mycorrhizal fungi and phosphorous fertilization in Stevia rebaudiana Bert. Ind. Crop Prod. 111, 899–907 (2018).
Rodriguez, G. R. et al. Tomato Analyzer: A new software to quantify tomato fruit morphology. J. Am. Soc. Hortic. Sci. 135(5), 447–455 (2010).
Ranganna, S. Handbook of Analysis and Quality Control for Fruits and Vegetable Products 2nd edn, 1112 (Tata McGraw Hills Education Private Limited, 1995).
Lichtenthalen, H. K. Chlorophylls and Carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350–382 (1987).
Phillips, J. M. & Hayman, D. S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55, 158-IN18. https://doi.org/10.1016/S0007-1536(70)80110-3 (1970).
Sagar, A. et al. Plant growth promoting rhizobacteria, arbuscular mycorrhizal fungi and their synergistic interactions to counteract the negative effects of saline soil on agriculture: Key macromolecules and mechanisms. Microorganisms 9, 1491 (2021).
Pan, J. et al. The growth promotion of two salt-tolerant plant groups with PGPR inoculation: A meta-analysis. Sustainability 11, 378 (2019).
Salwan, R., Sharma, A. & Sharma, V. Microbes mediated plant stress tolerance in saline agricultural ecosystem. Plant Soil 442, 1–22 (2019).
Egamberdieva, D., Wirth, S., Bellingrath-Kimura, S. D., Mishra, J. & Arora, N. K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 10, 2791 (2019).
Hussein, K. A. & Joo, J. H. Plant growth-promoting rhizobacteria improved salinity tolerance of Lactuca sativa and Raphanus sativus. J. Microbiol. Biotechnol. 28, 938–945 (2018).
Fatima, T., Mishra, I., Verma, R. & Kumar, N. Mechanisms of halotolerant plant growth promoting Alcaligenes sp. involved in salt tolerance and enhancement of the growth of rice under salinity stress. 3 Biotech 10, 361. https://doi.org/10.1007/s13205-020-02348-5 (2020).
Das, R., Mandal, A. R., Priya, A., Das, S. P. & Kabiraj, J. Evaluation of integrated nutrient management on the performance of bottle gourd [Lagenaria siceraria (Molina) Standl.]. J. Appl. Nat. Sci. 7, 18–25 (2015).
Gutjahr, C. & Paszkowski, U. Multiple control levels of root system remodeling in arbuscular mycorrhizal symbiosis. Front. Plant Sci. 4, 204. https://doi.org/10.3389/fpls.2013.00204 (2013).
Montesinos-Navarro, A., Segarra-Moragues, J. G., Valiente-Banuet, A. & Verdu, M. The network structure of plant-arbuscular mycorrhizal fungi. New Phytol. 194, 536–547 (2012).
Yadav, R., Ror, P., Rathore, P., Kumar, S. & Ramakrishna, W. Bacillus subtilis CP4, isolated from native soil in combination with arbuscular mycorrhizal fungi promotes biofortification, yield and metabolite production in wheat under field conditions. J. Appl. Microbiol. 131, 339–359 (2021).
Acknowledgements
We acknowledge the Kurukshetra University, Thanesar, Haryana, India for providing the fruit and leaf samples, encouragement, and support.
Author information
Authors and Affiliations
Contributions
PB: Conceptualized the study, designed experiments, analyzed data, and wrote the manuscript, developed theoretical frameworks, validated results, and contributed to literature review; AY: Supported experimental work, performed data interpretation, and contributed to graphical/visual representations; MS: Edited the manuscript for technical accuracy; PK: Provided critical revisions, assisted in manuscript drafting and statistical analysis; VY, JCC, DSM: provided technical advice and managed fund availability. VY, PR, JCC, PRK, DSM, YT, AK: Literature survey, reviewed, revised, and edited the manuscript. All authors have read and agreed to the published version of the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement specifying permissions
For this study, we acquired permission to study bitter gourd issued by the Agricultural and Natural Resources Ministry of India.
Statement on experimental research and field studies on plants
The either cultivated or wild-growing plants sampled comply with relevant institutional, national, and international guidelines and domestic legislation of India.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bijalwan, P., Yadav, A., Sharma, M. et al. Effect of Azotobacter chroococcum and Rhizophagus irregularis on morphological and biochemical traits of bitter gourd under saline conditions. Sci Rep 15, 23518 (2025). https://doi.org/10.1038/s41598-025-08862-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08862-x