Abstract
Dementia is a degenerative and chronic disorder, increasingly prevalent among older adults, posing significant challenges in providing appropriate care. As the number of dementia cases continues to rise, delivering optimal care becomes more complex. Machine learning (ML) plays a crucial role in addressing this challenge by utilizing medical data to enhance care planning and management for individuals at risk of various types of dementia. Magnetic resonance imaging (MRI) is a commonly used method for analyzing neurological disorders. Recent evidence highlights the benefits of integrating artificial intelligence (AI) techniques with MRI, significantly enhancing the diagnostic accuracy for different forms of dementia. This paper explores the use of AI in the automated detection and classification of dementia, aiming to streamline early diagnosis and improve patient outcomes. Integrating ML models into clinical practice can transform dementia care by enabling early detection, personalized treatment plans, and more effectual monitoring of disease progression. In this study, an Enhancing Automated Detection and Classification of Dementia in Thinking Inability Persons using Artificial Intelligence Techniques (EADCD-TIPAIT) technique is presented. The goal of the EADCD-TIPAIT technique is for the detection and classification of dementia in individuals with cognitive impairment using MRI imaging. The EADCD-TIPAIT method performs preprocessing to scale the input data using z-score normalization to obtain this. Next, the EADCD-TIPAIT technique performs a binary greylag goose optimization (BGGO)-based feature selection approach to efficiently identify relevant features that distinguish between normal and dementia-affected brain regions. In addition, the wavelet neural network (WNN) classifier is employed to detect and classify dementia. Finally, the improved salp swarm algorithm (ISSA) is implemented to choose the WNN technique’s hyperparameters optimally. The stimulation of the EADCD-TIPAIT technique is examined under a Dementia prediction dataset. The performance validation of the EADCD-TIPAIT approach portrayed a superior accuracy value of 95.00% under diverse measures.
Similar content being viewed by others
Introduction
Modifications in brain structure are a severe concomitant of ageing and dementia. From a medical view, conventional MRI became a normal element of dementia valuation, assisting in preventing infarcts and other non-neurodegenerative causes of dementia1. Dementia is an irreversible and progressive cognitive deterioration. About 40 million people are suffering from dementia worldwide, and so it is identified as the primary medical issue of the present century2. The dual main reasons for dementia are dementia and Alzheimer’s disease (AD) produced by vascular components. AD is the most common reason for dementia. Nowadays, it is responsible for 70–80% of cases influencing 50 million individuals3. Dementia symptoms are related to ageing, but some indications are generated at an initial age. AD produces changes in the brain, changing its functional and structural features. Due to the initial and serious participation of the hippocampus by neurofibrillary pathologic alteration in AD, it is not peculiar that hippocampal atrophy viewed on MRI is often related to the gathering of neurofibrillary tangles at post-mortem check-ups. There are various kinds of dementia, each with unique treatment options, reasons, and symptoms4. The initial-stage symptoms could be separated into dual types: (1) cognitive symptoms, like language problems and memory loss, and (2) non-cognitive symptoms, including mood instability, behavioural changes, and other psychotic symptoms. Dementia disproportionately affects women in both indirect and direct ways. Women experience higher disabilities-adjusted life years and death rates related to dementia5.
In addition, they present 70% of the care hours for persons with dementia. Amongst the elderly, dementia is an essential contributor to dependencies and disabilities, and it can significantly affect their quality of life. Analyzing dementia includes neuropsychological testing, imaging studies, and clinical assessment6. However, it is a difficult task, particularly during the initial stages of the disease, when symptoms are inconspicuous and might be incorrect for normal ageing. Additionally, diverse kinds of dementia have unique neuropathological and clinical features that are tough to differentiate utilizing traditional diagnostic methods7. Deep Learning (DL) and ML were considered an essential function of AI as data investigation method that computerizes the explanatory method structure8. In some common cases, depending on the learning model, dual kinds of ML Models, unsupervised and supervised, were utilized. Currently, these models appeal to all the primary industries, such as transport, social media, banking, healthcare, and more. Recently, there has been an increasing interest in the possibility of ML approaches for categorizing and analyzing several kinds of dementia9. ML models can handle intricate and vast imaging, genetic datasets, and clinically revealing biomarkers and patterns that may contain classical diagnostic methods. DL and ML models are part of a broader diagnostic process, like neuroimaging and clinical evaluation10.
In this study, an Enhancing Automated Detection and Classification of Dementia in Thinking Inability Persons using Artificial Intelligence Techniques (EADCD-TIPAIT) technique is presented. The goal of the EADCD-TIPAIT technique is for the detection and classification of dementia in individuals with cognitive impairment using MRI imaging. The EADCD-TIPAIT method performs preprocessing to scale the input data using z-score normalization to obtain this. Next, the EADCD-TIPAIT technique performs a binary greylag goose optimization (BGGO)-based feature selection approach to efficiently identify relevant features that distinguish between normal and dementia-affected brain regions. In addition, the wavelet neural network (WNN) classifier is employed to detect and classify dementia. Finally, the improved salp swarm algorithm (ISSA) is implemented to choose the WNN technique’s hyperparameters optimally. The stimulation of the EADCD-TIPAIT technique is examined under a Dementia prediction dataset. The key contribution of the EADCD-TIPAIT technique is listed below.
-
The EADCD-TIPAIT model incorporates z-score normalization to standardize the data, ensuring that all features are on a consistent scale. This approach assists in mitigating bias from varying feature scales, resulting in an improved model performance. The normalization process enhances the model’s ability to detect and classify dementia-related patterns in the data accurately.
-
The EADCD-TIPAIT approach employs BGGO for feature selection, efficiently identifying the most relevant features contributing to the model’s performance. By choosing key features, BGGO assists in enhancing the classification accuracy of dementia detection. This optimization process confirms that only the most informative features are used, improving overall model efficiency.
-
The EADCD-TIPAIT model implements a WNN classifier known for its capability to capture intricate patterns in data, ensuring accurate dementia detection and classification. The model effectively handles complex datasets with varying characteristics using the wavelet transform. This approach improves the model’s robustness, leading to reliable performance in diagnosing dementia.
-
The ISSA model fine-tunes the WNN’s hyperparameters, improving its performance in classifying dementia. By optimizing the search process, ISSA confirms that the model’s parameters are set for optimal efficiency. This results in improved accuracy and more reliable dementia detection.
-
The novelty lies in the innovative integration of BGGO for feature selection and ISSA for hyperparameter optimization, which is applied within the WNN framework. This unique integration enhances the efficiency and accuracy of the dementia detection model. The model attains superior performance in identifying dementia-related patterns by optimizing the feature selection and hyperparameters.
Literature survey
Tufail et al.11 developed various transfer learning (TL) models. Initially, pre-processing is performed. The model also utilized diverse convolutional neural network (CNN) models, namely VGG-16, VGG-19, DenseNet-121, and InceptionResNet-V2, to improve the classifying process. Rectified Linear Unit (RLU) and leaky activation are also integrated to counter issues related to vanishing gradient. Mariappan and Valarmathi12 introduced a novel ensemble-based classification methodology for classifying dementia stages. The pre-processing is accomplished by resizing, CLAHE, augmenting data models, and several datasets. The pre-trained techniques are trained with all four dataset forms. Vishwanadham et al.13 proposed a model by implementing ML methods, particularly incorporating the Dense Net121 feature extractor and a support vector machine (SVM) classifier. The MRI dataset obtained from Kaggle is utilized for investigation. The Flask web application has been developed to ease user-friendly interaction for healthcare professionals, presenting a practical tool for effectual dementia diagnosis. Ilias et al.14 implemented diverse pretrained approaches with vision transformer (ViT) methodology. Additionally, the method presents multimodal models. Moreover, the presented approaches comprise a Gated Multimodal Unit for regulating modality influence and effectual cross-modal attention. Cheung et al.15 incorporated radiomics, volumetry, and demographics as inputs to construct an AI technique. Freesurfer is used for segmentation. The 3D Slicer software is implemented for 107 radiomics extractions. The feedforward neural network (FFNN) and general AI approaches encompassing SVM, decision tree (DT), and ensemble classifier (EC) are utilized to construct the techniques using several features. Mathkunti et al.16 presented a methodology employing the power of image processing and ML methods. The Gaussian filtering (GL) model is used for preprocessing. Furthermore, Otsu’s segmentation technique is utilized for the pre-processed images. Grey-level co-occurrence matrix (GLCM)-based removal captures complex textural details indicative of dementia-related changes. The Probabilistic Neural Network (PNN) approach is employed for classification.
Huang and Qiu17 proposed a novel approach, Monte Carlo Ensemble ViT (MC-ViT), which develops an ensemble model with ViT. The presented approach utilizes a single-ViT learner. This methodology produces a broad spectrum of classification decisions by employing Monte Carlo sampling, improving the MC-ViT performance. Veluchamy et al.18 developed a hybrid Deepnet (HY-Deepnet) framework by utilizing AlexNet, GoogLenet, SqueezeNet, VGGNet, and ResNet for accurate detection and diagnosis of AD, optimized with COATI techniques to improve performance. Khosravi, Parsaei, and Rezaee19 proposed a cascade attention model-CNN (CAM-CNN) methodology that integrates CNNs with an attention mechanism. Haq et al.20 proposed a multimodal DL technique by integrating CNN and LSTM models for accurate AD detection using the ADNI-1 dataset. Chui et al.21 presented a model to improve AD detection from MRI scans using a CNN technique improved by TL and generative adversarial networks (GANs), attaining higher accuracy across OASIS-series benchmark datasets. Medani et al.22 proposed the leveraging explainability AI and optimization algorithm for accurate dementia prediction and classification model (LXAIOA-ADPCM), which integrates explainable AI (EAI) with optimization techniques and ensemble DL models such as bidirectional LSTM (BiLSTM), sparse autoencoder (SAE), and temporal convolutional network (TCN) methods to predict and classify dementia accurately. Mohana et al.23 presented a technique to improve the CBAMWDNet DL model for accurately classifying multiple dementia subtypes using a diverse MRI dataset, assisting in personalized diagnosis and treatment. Chowdary et al.24 developed and evaluated a collaborative entertainment robot that utilizes advanced speech recognition, natural language processing (NLP), and ML methods to assist cognitive health monitoring and early detection of mental decline in ageing populations. Castellano et al.25 proposed uni-modal and multimodal models by utilizing 2D/3D MRI and PET scans for AD classification, attaining advanced performance and interpretability through Grad-CAM.
Gandhi, Thakkar, and Milanova26 developed a DL-based model using MRI to accurately classify stages of cognitive impairment in AD, improving early detection and clinical decision-making. Vimbi, Shaffi, and Mahmud27 explored local interpretable model-agnostic explanations (LIME) and SHapley Additive exPlanations (SHAP) explainable AI (XAI) frameworks to improve the interpretability and trust of AD detection models. Cohen et al.28 developed and validated the emergency department dementia algorithm (EDDA) to detect dementia in adults and assist clinical decisions in the emergency department. Jahan, Khan, and Saraee29 analyzed natural language processing (NLP) techniques applied to text data from the DementiaBank corpus for effective dementia detection using ML methods. Mekulu, Aqlan, and Yang30 proposed a model to compare encoder-based pretrained language models (PLMs) and autoregressive large language models (LLMs) techniques for detecting cognitive impairment from narrative speech using the DementiaBank Pitt Corpus. Anuradha, Davu, and Karri31 developed and optimized a genetic algorithm (GA)-based DT for early and accurate dementia detection using MRI data. Vivekanand, Nayagam, and Rajeev32 developed and evaluated ML models using acoustic features from speech data for early detection of dementia to enable timely intervention and cost-effective care. Poonam, Guha, and Chakrabarti33 classified subtypes of frontotemporal dementia (FTD) using MRI-based cortical and subcortical features with hierarchical ML models for improved diagnostic accuracy. Al-Qazzaz, Ali, and Ahmad34 analyzed electroencephalograms differences among vascular dementia (VD), mild cognitive impairment (MCI), and control subjects using hybrid preprocessing and classify dementia severity with DL and ML models. Thanarajan and Rajendran35 analyzed the impact of anticholinergic medications on dementia risk through meta-analysis and uses AI-based random forest (RF) and XGBoost techniques with sensor data to predict sleep disorders and dementia.
While the existing studies exhibit considerable progress in AD and dementia classification, various limitations still exist. Most works depend on single-modality data, restricting generalization across diverse clinical scenarios. Some models achieve high accuracy but lack interpretability, making clinical deployment challenging. A significant research gap exists in incorporating XAI techniques like LIME or SHAP within DL frameworks. Few studies utilize ensemble methods optimized through advanced models while maintaining low computational complexity. Data imbalance and the absence of cross-dataset validation also affect robustness. Moreover, the multi-class classification of dementia subtypes remains underexplored despite its clinical relevance.
The proposed method
This study presents an EADCD-TIPAIT technique. The technique aims to detect and classify dementia in individuals with cognitive impairment using MRI imaging. It contains step-by-step processes: data normalization, BGGO-based feature selection, WNN-based classification, and parameter tuning. Figure 1 represents the complete workflow of the EADCD-TIPAIT model.
Stage I: Z-score normalization
At the initial phase, the EADCD-TIPAIT method performs pre-processing to scale the input data utilizing z-score normalization36. Z-Score Normalization is chosen because it can standardize the data into a distribution with a mean of 0 and a standard deviation of 1. This technique confirms that all features contribute equally to the model’s performance, regardless of their original scales. By eliminating unit bias, Z-score normalization improves the convergence of ML models, making them more efficient and faster in training. It also handles outliers better than other normalization methods, ensuring that extreme values do not disproportionately affect the model. This is significant in dementia detection, where some features might vary significantly. Furthermore, Z-Score normalization is simple to implement, computationally efficient, and widely used in various fields, making it a reliable choice for standardizing complex medical datasets.
Z-score normalization is a statistical method that normalizes data by converting values to have a standard deviation of \(\:1\) and a mean of \(\:0\). In automatic dementia classification and detection, using z-score normalization to MRI images aids in extracting intensity variations and improves data consistencies. This pre-processing stage guarantees that the data connected to the classification method is uniformly scaled, enhancing model precision in differentiating dementia-affected areas from normal brain tissue. By utilizing z-score normalization, the system gains more interpretable and reliable outcomes in identifying cognitive damages in MRI data.
Stage II: BGGO-based feature selection
Next, the EADCD-TIPAIT technique performs a BGGO-based feature selection approach to efficiently identify relevant features that distinguish between normal and dementia-affected brain regions37. This technique is chosen for its ability to efficiently identify and select the most relevant features from a large set, which is crucial for improving the accuracy and efficiency of dementia detection models. BGGO is inspired by the migration behaviour of Greylag geese, where the algorithm mimics their cooperative search and dynamic adaptation for optimal solutions. This technique outperforms handling binary feature selection problems, making it well-suited for selecting features in high-dimensional medical data. Compared to other methods like GAs or particle swarm optimization (PSO), BGGO is more effective in finding optimal feature subsets while avoiding overfitting. It also presents faster convergence and better scalability in complex datasets, ensuring that only the most significant features are used, thus enhancing classification performance. BGGO’s robustness in exploring the search space and minimizing feature redundancy further improves the model’s predictive power.
Recently, feature selection has been the most crucial step in data analysis. This is an outcome of feature selection, which extracts unimportant or redundant features to lower the higher data dimensionality. This feature selection optimizer method intends to recognize related features that reduce classifier errors, an aim that has resulted in its application in different areas. Mathematically, feature selection is a problem of minimum optimization. Owing to the rise of feature selection problems, the results of the GGO model should be truly binary, containing both \(\:0\) and 1. The series of values of the recommended GGO model should be changed to binary values \(\:\left[\text{0,1}\right]\) as exposed in the steps of Algorithm 1 to enable selected features from the dataset. The sigmoid function is the basis for the subsequent equation.
whereas \(\:{x}_{d}^{t+1}E\) signifies the dual solution at\(\:\:t\)he iteration and \(\:d\) dimension. The Sigmoid function is applied to generate the resulting binary solutions. The parameter \(\:m\) shows the features designated by Algorithm 1. Algorithm 1 explains the binary GGO model in detail. Examining the GGO model exposes that the computational cost is \(\:0\left({t}_{\text{m}\text{a}\text{x}}\times\:n\right)\) \(\:and\) should be \(\:0\left({t}_{\text{m}\text{a}\text{x}\:}\times\:n\times\:d\right)\:\)or the dimension\(\:\:d\).
The binary GGO approach utilizes the objective equation. \(\:{F}_{n}\) to assess the quality of the solution. \(\:{F}_{n}\) symbolizes the error percentage in classification Algorithm 1 in the succeeding equation: Error means feature selection. \(\:s\) and a set of absent features \(\:S.\)
K-nearest neighbour (k-NN) is a popular, fundamental classification method for designating the population position of traits. It is successfully measured when the model gives a subset of features using a lower classifier rate of error. To guarantee the validity of the chosen features, this model employs the k‐NN as a classifier. This study doesn’t use a k‐NN method; the only condition for classifier choice is the minimum distance between the training and query instances.
The fitness function (FF) employed in the BGGO technique is intended to have a balance between the preferred feature sums in each solution (minimal) and the classification precision (maximal) gained by applying these chosen features, Eq. (4) signifies the FF to assess solutions.
Whereas \(\:{\gamma\:}_{R}\left(D\right)\) signifies the classifier error rate in a specified classifier. \(\:\left|R\right|\:\)means cardinalities of the preferred subset, and \(\:\left|C\right|\) means entire feature sums in the dataset, \(\:\alpha\:\) and \(\:\beta\:\) are dual parameters equal to the impact of classifier qualities and subset lengths. ∈ [1,0] and \(\:\beta\:=1-\alpha\:.\)
Stage III: dementia detection using WNN
In addition, the WNN classifier is employed for detecting and classifying dementia38. This technique is chosen for dementia detection due to its exceptional ability to capture frequency and temporal data characteristics. It is significant for analyzing complex, high-dimensional medical datasets like brain imaging or cognitive data. Unlike conventional neural networks, WNN integrates the power of wavelet transform and neural networks, enabling it to extract multi-resolution features from the data efficiently. This allows the model to handle non-linearities and irregularities often present in medical data, improving detection accuracy. Compared to other techniques, such as CNNs or SVMs, WNN presents improved generalization capabilities, especially for small or imbalanced datasets, which are common in medical research. Additionally, the model’s capability to reduce noise and enhance signal quality in the data makes it more robust for early detection of dementia. Overall, WNN’s ability to effectively process and interpret complex medical features leads to improved classification performance for dementia detection. Figure 2 depicts the infrastructure of WNN.
The WNN is a function network based on RBF or wavelet functional networks. Compared with traditional feedforward networks, WNN shows strong adaptability, a direct network architecture, and outstanding fault tolerance. Therefore, it is essential in NN studies. The WNN is built upon the BPNN architecture, using the wavelet foundational function as the transfer function. Concurrently, the first parameters of the wavelet functions in the NN are enhanced with error backpropagation.
This method represents the WNN’s inputs as \(\:{x}_{1},{x}_{2},\dots\:{x}_{k}\) and the equivalent predicted outputs as \(\:{y}_{1},{y}_{2},\dots\:,{y}_{m}\). \(\:{\omega\:}_{ij}\) denotes the connectional weights connecting the input to the hidden layer (HL), and \(\:{\omega\:}_{jk}\) symbolizes the weights linking the HL to the output layer. The output made through the HL regarding the sequence of input signal \(\:{x}_{i}(i=\text{1,2},\:\dots\:,\:k)\) is verified with Eq. (5).
Whereas \(\:h\left(j\right)\) means the \(\:jth\) node output within the HL, h specifies the wavelet foundational functions. The parameter \(\:{a}_{j}\) signifies the scaling feature, and \(\:{b}_{j}\) refers to the translation factor related to \(\:h\). The Mexican-hat wavelet foundational functions are selected as the transferring functions, as exposed in Eq. (6).
The Mexican-hat wavelet foundational functions are chosen depending on their better performance in taking limited features. Being an additional derivation of the Gaussian, has proved efficient in taking transient designs and abnormalities in time-series data, which supports the quick transformations. Furthermore, it properly balances frequencies and time localizations, making it perfect for the prediction method. Hence, the WNN output is stated by the succeeding Eq. (7):
Meanwhile, \(\:y\left(k\right)\) denotes the \(\:kth\) node within the output layer, \(\:f\) signifies the total node counts in the HL, and m represents the total node counts in the output layer.
Stage IV: ISSA-based parameter tuning
Eventually, the ISSA is utilized to optimally select the hyperparameters of the WNN technique39. This approach is chosen for parameter tuning due to its improved search capability, inspired by the social behaviour of salps. Unlike conventional optimization techniques like grid or random search, ISSA effectively explores the solution space to find optimal hyperparameters with reduced computational costs. The improvement in ISSA comes from its enhanced exploration and exploitation mechanisms, which allow for better convergence to optimal solutions. Compared to other swarm-based models like PSO, ISSA avoids local optima more effectively, resulting in greater accuracy in hyperparameter tuning. Its adaptability and robustness in complex optimization problems make it particularly suitable for tuning the hyperparameters of complex models like WNN. Additionally, ISSA’s ability to balance exploration and exploitation enhances the performance of the dementia detection model, ensuring better classification results.
Optimization models are associated with several limitations in either science or engineering domains. A few detailed necessities must be observed for structural analysis problems in the engineering field, like the welded portions of the box sections. These plate widths are typically integers; however, the plate thickness is generally normal, and most are balanced. Moreover, the wing plate widths are greater than the web plates, and so on. Executing these working strategy limitations precisely is a problem for conventional swarm optimizer methods. To address this challenge, this work presented the ISSA as an appropriate optimizer model to determine the welded cross-sectional dimensions. The ISSA improves iterative capability, enhances the solution qualities, and preserves strong optimizer performance in organized issues. The basic logic of the model is to upgrade the locations of the autonomous variables. \(\:{x}_{1}\) and \(\:{x}_{2}\), whereas \(\:{x}_{3}\) and \(\:{x}_{4}\) are applied to select plate thickness T from the regular data to gain a solution set that fulfils the necessities for obtaining. The plate thickness data \(\:T\) value is exposed in Eq. (8).
The multi-dimensional setting approach of the model is to generate permutations and combinations from the known dataset of plate thickness. For instance, if \(\:T=[4-\text{6,8},10]\), the algorithm dimensionalities is \(\:N=size\left(T\right)\times\:size\left(T\right)=25\). Every size is associated with a type of \(\:{x}_{3}\) and \(\:{x}_{4}\) solution set. Otherwise specified separately, the succeeding policy upgrades are processes of the optimizer variables \(\:{x}_{1}\) and \(\:{x}_{2}.\)
Population initialization
The conventional SSA uses an initial population generated randomly, which may result in unequal coverage of the first solutions inside the search area. To deal with these problems, chaos-based methods are extensively used to improve the functioning of meta‐inspired models. Between the commonly applied chaotic maps, Tent mapping presents a more even distribution of first solutions compared to Logistic maps. In this work, Tent chaotic mapping is applied to the generation of pseudo‐random first solutions, which can make the initial population’s distribution in the description field more standardized and enhance the system’s capability for traversing worldwide among the lower and upper limits of the optimizer variable.
The optimizer variables are spread chaotically for every size, as presented in Eq. (10).
Here, \(\:x[n{]}_{j}^{i}\) represents the \(\:nth\) size, the \(\:ith\) Salp, and the \(\:jth\) variable; \(\:ub\) and \(\:lb\) denote the lower and upper limits of the optimizer variables.
Then, the fitness of the first solution is calculated and chosen for every size with the food resource \(\:F\left[n\right].\)
Leader renewal
During this SSA, the location of the food source is the targeted location of each salp individual, for example, the globally optimum solution throughout the searching procedure, which impacts the leader location upgrade, and the leader counts are engaged to be partial of the population counts. The leader of every size carries out an upgrade operation for the source of food of every size, as in Eq. (11).
Here, \(\:F[n{]}_{j}\) denotes the position of the \(\:jth\) variable of the \(\:n\)-dimensional food resource. \(\:{c}_{1}\) means convergence feature in the model, which plays as the balancing portion of local exploitation and global exploration and is the most significant parameter. \(\:{c}_{2}\) and \(\:{c}_{3}\) are [0,1] randomly generated numbers applied to improve diversity and randomness. \(\:l\) refers to present iteration counts. \(\:L\) means maximal iteration counts.
Follower updates
The location of the \(\:ith\) follower Salp in the conventional Salp model is only associated with itself and the \(\:i‐1th\) Salp. When the leader Salp meets a local optimal, the Salp follower will also be surrounded by a similar local optimum solution, preventing the globally optimum solution from being achieved. This work is enhanced by utilizing Levy’s fights and greedy choices to generate the model that can leap out at the local optimal. The primary is the former leader or the follower itself, as in Eq. (13); the next is that the previous executes a Levy fight perturbation, as in Eq. (14), and the last is that the follower follows the former individual and their movements follow to Newton’s Laws of Motion as in Eq. (15).
Here, \(\:{c}_{4}\) and \(\:{c}_{5}\) are randomly generated numbers of [0,1], and \(\:s\) refers to the Levy fight stage as exposed in Eq. (16).
Now \(\:{\sigma\:}_{\mu\:}\) and \(\:{\sigma\:}_{\nu\:}\) are gained from Eq. (17), as exposed below:
The parameter value ranges between \(\:(0\)-2) and is selected as \(\:=1.5\) in this work.
Here, the greedy choice model, a tactic that continuously chooses the choice with the finest instant fitness or prize, is applied to select the location of the updated follower. For example, the one with the best fitness among the other choices is nominated, as exposed in Eq. (18).
In this approach, the solutions should be different, and the model is more likely to leap out of the local optimal. The ISSA method obtains an FF to enhance classifier performance. It describes a positive integer to imply the better performance of the candidate solutions. In this paper, the decrease of the classifier error rate is established as the FF, as specified in Eq. (19).
Result analysis and discussion
In this section, the stimulated validation of the EADCD-TIPAIT method is performed under the Dementia prediction dataset40 (https://www.kaggle.com/datasets/shashwatwork/dementia-prediction-dataset)/ doi: https://doi.org/10.1109/ACCESS.2021.3127394. The technique is simulated using Python 3.6.5 on a PC with an i5-8600k, 250GB SSD, GeForce 1050Ti 4GB, 16GB RAM, and 1 TB HDD. Parameters include a learning rate of 0.01, ReLU activation, 50 epochs, 0.5 dropouts, and a batch size of 5. The dataset contains 200 instances under dual-class labels, as illustrated in Table 1. The total no. of features is twelve, namely MR Delay, Visit, Hand, M/F, Age, SES, EDUC, CDR, MMSE, nWBV, eTIV, and ASF. However, only seven features have been chosen: MR Delay, Visit, Age, M/F, CDR, MMSE, and ASF.
Figure 3 provides the classifier results of the EADCD-TIPAIT model. Figure 3a and b shows the confusion matrix with precise classification and identification of each class under 70%TRPH and 30%TSPH. Figure 3c presents the PR investigation, demonstrating reduced performance through all two classes. Eventually, Fig. 3d portrays the ROC examination, showing successful outcomes with significant ROC values for several classes.
Table 2; Fig. 4 determined the Dementia prediction result of the EADCD-TIPAIT approach under 70%TRPH and 30%TSPH. The outcomes indicate that the EADCD-TIPAIT approach accurately recognized all the samples. With 70%TRPH, the EADCD-TIPAIT model presents average \(\:acc{u}_{y}\), \(\:pre{c}_{n}\), \(\:rec{a}_{l},\) \(\:{F}_{score}\) and \(\:MCC\) of 88.57%, 88.57%, 88.57%, 88.57%, and 77.14%, individually. Moreover, With 30%TRPH, the EADCD-TIPAIT methodology gives average \(\:acc{u}_{y}\), \(\:pre{c}_{n}\), \(\:rec{a}_{l},\) \(\:{F}_{score}\) and \(\:MCC\) of 95.00%, 95.09%, 94.94%, 94.99%, and 90.03%, respectively.
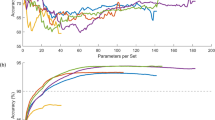
In Fig. 5, the training (TRA) \(\:acc{u}_{y}\) and validation (VAL) \(\:acc{u}_{y}\) outcomes of the EADCD-TIPAIT method are portrayed. The \(\:acc{u}_{racy}\:\)values are computed for 0–25 epoch counts. The figure noted that the TRA and VAL \(\:acc{u}_{y}\) values present increasing tendencies that indicate the capabilities of the EADCD-TIPAIT method with superior performance through several iterations. In addition, the TRA and VAL \(\:acc{u}_{y}\:\)stay nearer across the epoch counts, which specifies the least overfitting and demonstrates superior performance of the EADCD-TIPAIT approach, encouraging steady prediction on undetected instances.
Figure 6 describes the TRA loss (TRALOS) and VAL loss (VALLOS) graphs of the EADCD-TIPAIT technique. The loss values are calculated for 0–25 epoch counts. The TRALOS and VALLOS values exemplify declining tendencies, indicating the EADCD-TIPAIT technique’s ability to balance a trade-off between generality and data fitting. The repeated fall in loss values also pledges the advanced functioning of the EADCD-TIPAIT method and, eventually, tuning of the prediction results.
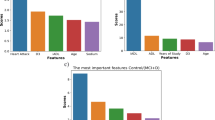
The comparison investigation of the EADCD-TIPAIT method with recent techniques is established in Table 3; Fig. 717,18,41,42. The experimental result identified that the EADCD-TIPAIT method outshined superior performances. According to\(\:\:acc{u}_{y}\), the EADCD-TIPAIT method has greater \(\:acc{u}_{y}\) of 95.00% however the SVM, AdaBoost, DT, Extra Tree (ET), Gradient Boost (GB), K-NN, Logistic Regression (LR), NB, MC-ViT, ResNet, and VGGNet models have least \(\:acc{u}_{y}\) of 89.72%, 94.40%, 91.99%, 91.99%, 93.60%, 94.41%, 94.42%, 94.41%, 92.06%, 92.04%, and 93.67%, individually. Besides, depending on \(\:{F1}_{score}\), the EADCD-TIPAIT techniques have a superior \(\:{F1}_{score}\) of 94.99%. In contrast, the SVM, AdaBoost, DT, ET, GB, K-NN, LR, NB, MC-ViT, ResNet, and VGGNet methodologies have lower \(\:{F1}_{score}\) of 91.91%, 91.92%, 89.48%, 90.51%, 93.38%, 90.93%, 94.94%, 90.91%, 89.55%, 90.56%, and 93.46%, correspondingly. Furthermore, depending on the \(\:rec{a}_{l}\), the EADCD-TIPAIT technique has an advanced \(\:rec{a}_{l}\) of 94.94%. At the same time, the SVM, AdaBoost, DT, ET, GB, K-NN, LR, NB, MC-ViT, ResNet, and VGGNet approaches have minimum \(\:rec{a}_{l}\) of 89.52%, 92.59%, 90.12%, 92.46%, 92.37%, 92.18%, 90.41%, 90.83%, 90.20%, 92.54%, and 92.44%, respectively.
In Table 4; Fig. 8, the comparison results of the EADCD-TIPAIT approach are stated under execution time (ET). The results imply that the EADCD-TIPAIT approach attains superior performance. According to ET, the EADCD-TIPAIT approach presents least ET of 8.56s while the SVM, AdaBoost, DT, ET, GB, K-NN, LR, NB, MC-ViT, ResNet, and VGGNet methodologies obtain better ET values of 14.49s, 12.04s, 10.89s, 17.36s, 18.25s, 17.24s, 18.38s, 17.55s, 13.04s, 11.90s, and 14.11s, consistently.
Conclusion
In this article, an EADCD-TIPAIT model was presented. The EADCD-TIPAIT technique was used to preprocess and scale the input data using z-score normalization. Next, the EADCD-TIPAIT technique utilized a BGGO-based feature selection approach to efficiently detect relevant features that distinguish between normal and dementia-affected brain regions. In addition, the WNN classifier was employed to detect and classify dementia. Eventually, the improved salp swarm algorithm (ISSA) was used to choose the WNN technique’s hyperparameters optimally. The stimulation of the EADCD-TIPAIT technique is examined under a Dementia prediction dataset. The performance validation of the EADCD-TIPAIT approach portrayed a superior accuracy value of 95.00% under diverse measures. The limitations of the EADCD-TIPAIT approach include reliance on a relatively small dataset, which may limit the model’s generalizability to diverse populations and conditions. Furthermore, noisy or incomplete data could impact the model’s performance, which was not fully addressed in the current work. Another limitation is the computational complexity of the model, which could be prohibitive in resource-constrained environments. Moreover, the study concentrates on a specific type of dementia, and its applicability to other neurodegenerative diseases has not been explored. In future work, expanding the dataset to comprise a broader range of dementia types and improving data preprocessing methods could enhance the model’s robustness. Incorporating real-time data processing and optimizing the model for edge devices could also facilitate practical implementation. Furthermore, investigating multimodal data integration, such as genetic or behavioural data, could result in more comprehensive detection systems.
Data availability
The data supporting this study’s findings are openly available in the Kaggle repository at https://www.kaggle.com/datasets/shashwatwork/dementia-prediction-dataset, reference number [40].
References
Tanveer, M. et al. Machine learning techniques for the diagnosis of alzheimer’s disease: A review. ACM Trans. Multimed. Comput. Commun. Appl. 16 (1s), 1–35 (2020).
Fadhel, Z., Attia, H. & Ali, Y. H. Optimized and comprehensive fake review detection based on Harris Hawks optimization integrated with machine learning techniques. J. Cybersecur. Inform. Manag., 15(1). (2025).
Lopez, O. L. & Kuller, L. H. Epidemiology of aging and associated cognitive disorders: Prevalence and incidence of alzheimer’s disease and other dementias. Handb. Clin. Neurol. 167, 139–148 (2019).
Maqsood, M. et al. Transfer learning assisted classification and detection of Alzheimer’s disease stages using 3D MRI scans. Sensors, 19(11), 2645 (2019).
Sappok, T., Diefenbacher, A. & Winterholler, M. The medical care of people with intellectual disability. Deutsches Ärzteblatt Int., 116(48), 809 (2019).
Spooner, A. et al. A comparison of machine learning methods for survival analysis of high-dimensional clinical data for dementia prediction. Sci. Rep. 10 (1), 20410–20410 (2020).
Gupta, Y. et al. Early diagnosis of alzheimer’s disease using combined features from voxel-based morphometry and cortical, subcortical, and hippocampus regions of MRI T1 brain images. PLoS One. 14 (10), e0222446 (2019).
Lombardi, G. et al. Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer’s disease in people with mild cognitive impairment. Cochrane Dataset Syst. Rev. 3 (2020).
Fymat, A. L. On dementia and other cognitive disorders. Clin. Res. Neurol. 2 (1), 1–4 (2019).
Atassi, R., Alhosban, F. & Dordevic, M. A new data fusion model for medical image encryption in IoT environment. Fusion Pract. Appl. (1), 16 – 6 (2022).
Tufail, H., Ahad, A., Naqvi, M. H., Maqsood, R. & Pires, I. M. Classification of Vascular Dementia on magnetic resonance imaging using deep learning architectures. Intell. Syst. Appl. 22, 200388. (2024).
Mariappan, P. & Valarmathi, R. S. Deep learning techniques in classification of stages in dementia: An ensemble approach. J. Adv. Res. Appl. Sci. Eng. Technol. 36 (1), 131–146 (2023).
Vishwanadham, Y. K. et al. May. Revolutionizing dementia diagnosis: Integrating DenseNet121 and SVM for early detection through MRI analysis. In 2024 International Conference on Advances in Computing, Communication and Applied Informatics (ACCAI) (pp. 1–7). IEEE. (2024).
Ilias, L., Askounis, D. & Psarras, J. Detecting dementia from speech and transcripts using transformers. Comput. Speech Lang. 79, 101485. (2023).
Cheung, E. Y., Wu, R. W., Chu, E. S. & Mak, H. K. Integrating lification: Feed forward neural network multi-class approach. Biomedicines 12(4), 896. (2024).
Mathkunti, N. M., Ananathnagu, U. & Ebin, P. M. Dementia diagnosis through artificial intelligence in medical images. In 2023 Global Conference on Information Technologies and Communications (GCITC) (pp. 1–6). IEEE. (2023).
Huang, F., Qiu, A. & Alzheimer’s Disease Neuroimaging Initiative. and, Ensemble vision transformer for dementia diagnosis. IEEE J. Biomedical Health Informatics (2024).
Veluchamy, S., Bhuvaneswari, R., Ashwini, K., Alshathri, S. & El-Shafai, W. HY-Deepnet: A new Optimal Deep transfer learning empowered framework for an autonomous Alzheimer’s disease detection and diagnosis system. Eng. Sci. Technol. Int. J., 68, 102058. (2025).
Khosravi, M., Parsaei, H. & Rezaee, K. Novel Classification Scheme for Early Alzheimer’s Disease (AD) Severity Diagnosis Using Deep Features of the Hybrid Cascade Attention Architecture: Early Detection of AD on MRI Scans (Tsinghua Science and Technology, 2024).
Haq, E. U., Yong, Q., Yuan, Z., Huarong, X. & Haq, R. U. Multimodal fusion diagnosis of the Alzheimer’s disease via lightweight CNN-LSTM model using magnetic resonance imaging (MRI). Biomed. Signal Process. Control 104, 107545. (2025).
Chui, K. T., Gupta, B. B., Alhalabi, W. & Alzahrani, F. S. An MRI scans-based Alzheimer’s disease detection via convolutional neural network and transfer learning. Diagnostics, 12(7), 1531. (2022).
Medani, M. et al. Leveraging explainable artificial intelligence with ensemble of deep learning model for dementia prediction to enhance clinical decision support systems. Sci. Rep. 15 (1), 1–17 (2025).
Mohana, R. M., Zuhaibuddin, M. A., Hussain, M. F. & Reddy, K. S. Enhanced CBAMWDNet: A deep learning approach for accurate dementia multiclassification using MRI scans. In MATEC Web of Conferences (Vol. 392, p. 01132). EDP Sciences. (2024).
Chowdary, M. K. et al. Entertainment robots for automatic detection and mitigation of cognitive impairment in elderly populations. Entertainment Comput., 52, 100803. (2025).
Castellano, G., Esposito, A., Lella, E., Montanaro, G. & Vessio, G. Automated detection of Alzheimer’s disease: A multimodal approach with 3D MRI and amyloid PET. Sci. Rep. 14(1), 5210. (2024).
Gandhi, V. C., Thakkar, D. & Milanova, M. Unveiling Alzheimer’s progression: AI-Driven models for classifying stages of cognitive impairment through medical imaging. In International Conference on Pattern Recognition pp. 55–87 (Springer, 2025).
Vimbi, V., Shaffi, N. & Mahmud, M. Interpreting artificial intelligence models: A systematic review on the application of LIME and SHAP in Alzheimer’s disease detection. Brain Inform. 11(1), 10. (2024).
Cohen, I. et al. Detection of emergency department patients at risk of dementia through artificial intelligence. Alzheimer’s Dement. 21 (6), e70334 (2025).
Jahan, Z., Khan, S. B. & Saraee, M. Early dementia detection with speech analysis and machine learning techniques. Discov. Sustain. 5(1), 65. (2024).
Mekulu, K., Aqlan, F. & Yang, H. Automated detection of early-stage dementia using large language models: A comparative study on narrative speech. medRxiv, 2025-06. (2025).
Anuradha, G., Davu, H. & Karri, M. Genetic algorithm-based decision tree optimization for detection of dementia through MRI analysis. Informatyka, Automatyka, Pomiary w Gospodarce i Ochronie Środowiska, 14. (2024).
Vivekanand, C. V., Nayagam, S. & Rajeev, S. Speech-based dementia prediction using artificial intelligence. In 2025 International Conference on Visual Analytics and Data Visualization (ICVADV) (pp. 1293–1298). IEEE. (2025).
Poonam, K., Guha, R. & Chakrabarti, P. P. Artificial intelligence based hierarchical classification of frontotemporal dementia. In 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 1–4). IEEE. (2024).
Al-Qazzaz, N. K., Ali, S. H. B. M. & Ahmad, S. A. Early dementia detection and severity classification with deep squeezenet convolutional neural network using EEG images. In Artificial Intelligence and Multimodal Signal Processing in Human-Machine Interaction (171–183). Academic. (2025).
Thanarajan, T. & Rajendran, S. Sensor based EEG signal based dementia disease detection using artificial intelligence. J. New. Mater. Electrochem. Syst., 27(4). (2024).
Prihanditya, H. A. The implementation of z-score normalization and boosting techniques to increase accuracy of c4. 5 algorithm in diagnosing chronic kidney disease. J. Soft Comput. Explor. 1 (1), 63–69 (2020).
Ahmad, I. & Alqurashi, F. Early Cancer detection using deep learning and medical imaging: A survey. Crit. Rev. Oncol./Hematol., 104528. (2024).
Liang, Y. et al. Fouling prediction of a heat exchanger based on wavelet neural network optimized by improved particle swarm optimization algorithm. Processes, 12(11), 2412. (2024).
Yang, H., Ren, Y. & Xu, G. Optimization of rotary drilling rig mast structure based on multi-dimensional improved salp swarm algorithm. Appl. Sci. 14(21), 10040. (2024).
https://www.kaggle.com/datasets/shashwatwork/dementia-prediction-dataset
Battineni, G., Chintalapudi, N. & Amenta, F. Machine learning in medicine: Performance calculation of dementia prediction by support vector machines (SVM). Inform. Med .Unlocked, 16, 100200. (2019).
Dhakal, S. et al. Dementia prediction using machine learning. Procedia Comput. Sci. 219, 1297–1308 (2023).
Acknowledgments
The authors extend their appreciation to the King Salman center For Disability Research for funding this work through Research Group no KSRG-2024- 091.
Author information
Authors and Affiliations
Contributions
Shoayee Dlaim Alotaibi: Conceptualization, methodology, validation, investigation, writing—original draft preparation, fundingAbeer A. K. Alharbi: Conceptualization, methodology, writing—original draft preparation, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alotaibi, S.D., Alharbi, A.A.K. Enhancing automated detection and classification of dementia in individuals with cognitive impairment using artificial intelligence techniques. Sci Rep 15, 24659 (2025). https://doi.org/10.1038/s41598-025-09124-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09124-6