Abstract
One of the crucial risk factors for tuberculosis (TB) is diabetes mellitus (DM), and physical activity could afford protective effects for the former disease entity. We aimed to evaluate the association between physical activity (intensity and amount) and TB development in individuals with type 2 DM (T2DM) among the South Korean nationwide cohort. Using the Korean National Health Information Database, we screened individuals who underwent the national health examination between 2009 and 2012 and identified 2,437,443 eligible individuals with T2DM. They were followed up to the date of TB notification, death, censor, or until December 2018. We identified 21,275 individuals with newly developed TB (active TB, either pulmonary or extrapulmonary). Physical activity was evaluated according to the health examination questionnaire, categorized them by activity intensity (walking, moderate, and vigorous) and amount measured by metabolic equivalent task minutes per week (METs-min/week). To estimate the adjusted hazard ratio (aHR) of risk factors for TB, we used the multivariate Cox proportional hazard models. The risk of developing TB declined with increasing activity intensity. Individuals with vigorous activity had the lowest risk for TB (aHR 0.85, 95% confidence interval [CI] 0.82–0.89) compared with individuals without vigorous activity. The risk of TB development decreased with increasing amount of activity. Individuals ≥ 1,500 METs-min/week had the lowest risk for TB (incidence rate 1.22/1000 person-years, aHR 0.84, 95% CI 0.80–0.88) compared with individuals < 500 METs-min/week. Physical activity intensity and amount were inversely correlated with TB risk in individuals with T2DM.
Similar content being viewed by others
Introduction
Globally, one of the leading causes of death from infectious agents is tuberculosis (TB), making it an important public health concern1. Diabetes mellitus (DM) is an important risk factor of TB, which is associated with a two to fourfold increased risk for TB2. Globally, it is estimated that approximately 1 million people both have TB and DM3. By 2050, at least one-third of TB incidence and approximately half of TB mortality would be attributed to DM in Asia–Pacific countries4,5. Thus, modifiable risk factor management for TB is pivotal for TB control in this high-risk population.
Several epidemiological studies have demonstrated that DM increases the risk of developing active TB2,3,6 primarily due to impaired cellular immunity associated with poorly controlled hyperglycemia7,8,9. Physical activity plays a key role in improving glycemic control in individuals with DM10,11. Moreover, physical activity maintenance has been suggested to decrease TB risk through systemic anti-inflammatory effects and enhanced immune surveillance and lung function12,13,14,15. In this context, physical activity may confer protective benefits against TB among patients with DM.
Unfortunately, few reports elucidate the association between physical activity and TB in patients with DM. Park et al. reported that those who failed to exercise persistently or quit regular exercise had a higher risk of TB than those who newly started exercise. However, they only dealt with exercise behavior changes between two serial health examinations and lacks details of physical activity, such as intensity and amount16. Qiu et al. investigated activity intensity (sedentary, mild, and moderate to vigorous) and duration (< 1, 1–2, and > 2 h) separately. Nevertheless, they found that physical activity was associated with decreased TB risk in women but not in men17.
Recent studies have explored the associations between physical activity and several health outcomes (e.g., colorectal cancer, infective endocarditis, hip fracture, and atrial fibrillation) in individuals with DM18,19,20,21. To our knowledge, there has been no study that explored the association between physical activity and TB incidence among individuals with DM, considering activity intensity and amount in a dose–response manner. Thus, we aimed to investigate the association between physical activity and development of TB in individuals with DM using the Korean National Health Information Database (NHID).
Methods
Data sources
The Korean National Health Insurance Service (NHIS) provides a public database, the NHID, which collates data on national health examination, socio-demographic characteristics, medical treatment and claims information, health care use, and mortality of the entire South Korean population since 200122,23. The NHIS, a mandatory universal public health insurance system, covers approximately 97% of the Korean population. The “Medical Aid” is a public assistance that covers 3% of the population in the lowest income bracket; however, the NHIS also covers all the administrative processes for Medical Aid beneficiaries.
For the prevention and early detection of diseases, the NHIS has provided a national health examination24. Until 2018, all adult employees or adults aged ≥ 40 years with a national health insurance underwent a national health examination biannually (annually for manual workers), including laboratory tests, simple chest radiographs, and questionnaires regarding lifestyle behaviors and medical history22,25.
Study population
In this study, 23,452,862 individuals who underwent a national health examination between 2009 and 2012 (the index year) were screened for inclusion. Among them, we identified 2,746,079 individuals with type 2 DM (T2DM) according to the operating definition as follows: (1) insurance claim with International Classification of Diseases 10th Revision (ICD-10) codes for T2DM (E11–E14) with at least one prescription of oral hypoglycemic agents (OHAs) or insulin within a year prior to the health examination or (2) fasting blood glucose (FBG) ≥ 126 mg/dL in the health examination data16,26,27.
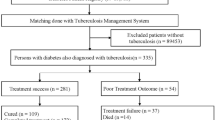
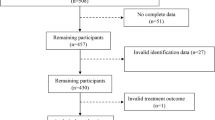
Thereafter, we excluded 441 individuals aged < 20 years, 121,265 individuals with any insurance claim with ICD-10 codes for TB (A15–19) before their health examination (TB wash-out), 162,666 individuals with insufficient medical records, and 24,264 individuals with any TB registration within 1 year after the index date (1-year lag period). We employed a 1-year lag period and started follow-up 1 year after the index date (time zero) to exclude the over-detection of TB after the health examination (surveillance bias). The remaining 2,437,443 eligible individuals with T2DM were included (Fig. 1).
Main exposure: physical activity
Within 2 years prior to the index date, physical activity information was collected from self-reported questionnaires during the national health examination. Physical activity was measured via the Korean version of the International Physical Activity Questionnaire (IPAQ) short form28,29. The IPAQ short form included seven questions intended to identify the frequencies and durations of walking, moderate, and vigorous physical activity during the previous 7 days30,31.
Activity intensity was categorized as follows: (1) walking: walking (e.g., walking at a slow pace) for at least 30 min per day and 5 days per week; (2) moderate physical activity: activity (e.g., brisk walking, slow cycling, or tennis doubles) for at least 30 min per day and 5 days per week; and (3) vigorous physical activity: activity (e.g., running, aerobics, or fast cycling) for at least 20 min per day and 3 days per week.
Amount of activity was calculated through metabolic equivalent task minutes per week (METs-min/week) according to the IPAQ scoring protocol30,31. The following values were utilized for calculating the METs score: walking = 3.3 METs, moderate physical activity = 4.0 METs, and vigorous physical activity = 8.0 METs. The total METs-min/week were calculated as duration × frequency per week × METs intensity value, which were summed across activity domains (walking + moderate + vigorous activity)29. The activity level was categorized into 0–499, 500–999, 1000–1,499, and ≥ 1,500 METs-min/week18,19,20,21.
Study outcome: TB diagnosis
The study outcome was new TB diagnosis (active TB, either pulmonary or extrapulmonary), according to the rare intractable disease (RID) registration codes for TB (V206, V246, and V000). Since 2005, for all patients with TB, the NHIS has provided a special copayment reduction program (90–100%) for the national TB control policy. In South Korea, it is mandatory for attending physicians to notify all new TB cases to nearby public health centers. Thereafter, a TB diagnosis is reviewed by the NHIS to provide copayment reduction. Under the current mandatory reporting system, the RID registration codes for TB provides a valid tool in identifying individuals with TB in the Korean population12,16,27.
Our study cohort were followed up until December 31, 2018, and 21,275 individuals were identified with newly developed TB according to the RID registry data. The follow-up ended at TB development (outcome), death, or censor (e.g., out-migration). The follow-up duration had a mean of 6.87 ± 1.60 years (Fig. 1).
Covariates
Information on anthropometric measurements (body weight, height, and blood pressure) and lifestyle behaviors (cigarette smoking and alcohol consumption) from self-reported questionnaires was collected on the national health examination. Body mass index (BMI) was calculated by dividing the body weight by the height squared (kg/m2) and categorized into five groups (< 18.5, 18.5–22.9, 23–24.9, 25–29.9, and ≥ 30 kg/m2) according to the Asian BMI criteria32. Cigarette smoking was categorized into never-smoker, former-smoker, and current-smoker groups33. Alcohol consumption was categorized into none, mild (< 30 g/day), or heavy (≥ 30 g/day) drinker34. The household income level was categorized into quartiles (Q1 = the lowest and Q4 = the highest) according to the subscribers’ annual national health insurance premium. Individuals receiving Medical Aid benefits were included into the Q1 category35.
Comorbidities were identified according to the NHIS and national health examination data within 1 year before the index date. Definitions were as follows: (1) hypertension, either an insurance claim for ICD-10 codes I10–13 and I15 with a prescription of antihypertensive medications or high blood pressure (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg) measured during a health examination; (2) dyslipidemia, either an insurance claim for ICD-10 code E78 with a prescription of lipid-lowering medications or serum total cholesterol ≥ 240 mg/dL during a health examination; (3) chronic kidney disease (CKD), either an insurance claim for ICD-10 codes N18–19 or an estimated glomerular filtration rate < 60 mL/min/1.73 m2 by the Modification of Diet in Renal Disease equation during a health examination.
Statistical analysis
Continuous variables are presented as mean ± standard deviation, and categorical variables are expressed as numbers (percentage). To compare continuous and categorical variables, Student’s t-test and χ2 test were used, respectively. The TB incidence rate was calculated as the ratio between the number of patients with newly diagnosed TB and the number of person-years at risk of TB (per 1,000). To evaluate the impact of risk factors on the time-to-event of TB development, a multivariate Cox proportional hazards model was utilized. The proportional hazards assumption was evaluated using the Schoenfeld residuals test. Model 1 was non-adjusted. In Model 2, the covariates included age and sex. Model 3 included the covariates in Model 2 and BMI, income, cigarette smoking (non, former, and current), alcohol consumption (non, mild, and heavy), hypertension, and dyslipidemia. Hypertension and dyslipidemia were considered proxies for broader metabolic health status and healthcare utilization36. Model 4 (the main analysis model) contained the covariates in Model 3 and FBG concentration, CKD (DM-related renal complication), T2DM duration (< 5 vs. ≥ 5 years), number of OHAs (< 3 vs. ≥ 3), and insulin37,38,39. The last covariates were selected as surrogate markers to adjust DM severity, which was also associated with TB risk27. Stratified analysis by age (< 40, 40–64, and > 65 year) and sex was performed for model 4. In the multivariate analyses, age, BMI, and FBG concentration were included as continuous variables. All P-values were two-tailed, and statistical significance was set at P < 0.05. All statistical analyses were conducted using SAS version 9.4 (SAS institute, Cary, NC, United States), and the PHREG procedure was utilized for the Cox proportional hazards model.
Ethics statement
This study protocol was approved by the Institutional Review Board of Samsung Medical Center, Seoul, Republic of Korea (approval No. SMC 2022-07-072). The requirement for informed consent was waived by Institutional Review Board of Samsung Medical Center as it was a retrospective study and the data used were anonymized. This study was conducted in accordance with the Declaration of Helsinki guidelines, and all methods were performed in accordance with the relevant guidelines.
Results
Baseline characteristics
Table 1 illustrates the baseline characteristics of the study population according to the physical activity (METs-min/week) categories. The mean age of all participants was 57.3 ± 12.3 years with men accounting for 59.7%. The participants ≥ 1,500 METs-min/week were predominantly male (66.8%) compared with participants < 500 METs-min/week (55.9%). In terms of DM severity, 30.3% had DM duration ≥ 5 years, 8.3% used insulin, and 11.3% had CKD.
Risk of TB development according to physical activity
Table 2 demonstrates the associations between physical activity intensity and TB development. The risk of developing TB declined with increasing physical activity intensity (walking, aHR 0.94; moderate, aHR 0.88; vigorous, aHR 0.85). Individuals with vigorous physical activity showed the lowest risk for TB development (aHR 0.85, 95% CI 0.82–0.89). Table 3; Fig. 2 reveal the association between physical activity amount and TB development. The risk reduced with increasing physical activity level, resulting in the lowest risk among individuals ≥ 1,500 METs-min/week (aHR 0.84, 95% CI 0.80–0.89) and 10th decile category (aHR 0.81, 95% CI 0.77–0.85).
Incidence and relative hazard ratio of tuberculosis according to the decile category of the physical activity. The left Y axis and bar graph reflect the incidence of tuberculosis per 1,000 person-year. The right Y axis and line graph represent the relative hazard ratio of tuberculosis. Both 1st and 2nd decile categories are corresponded to 0 METs-min/week; thus, these 2 categories are plotted as 2nd decile.
Risk of TB development stratified by age and sex
The relationship between physical activity and TB was observed significantly in individuals aged 40–64 and ≥ 65 years group in the stratified analysis by age. The relationship was more prominent in females compared with males (Fig. 3).
Discussion
In this nationwide population-based retrospective cohort study, which included more than 2 million individuals with T2DM, we explored the relationship between physical activity and the new development of TB. Physical activity was significantly associated with a decreased risk for TB development and the risk declined with increasing physical activity intensity and amount. To our knowledge, this is the first report that showed the significant relationship between physical activity and TB among individuals with DM in a dose response manner.
Several epidemiological studies elucidated that T2DM increased the risk of active TB, and it could be attributed to the fact that an impaired host-defense mechanism promoted latent TB to active TB infection2,3,40,41. Previous studies have exhibited that poor DM control status, reflected in FBG concentration and hemoglobin A1c, were associated with an increased risk of TB6,27,42. T2DM, particularly with chronic hyperglycemia, is associated with altered immune responses to Mycobacterium tuberculosis, which are higher innate and type 1 cytokine responses compared with those of non-DM control8. T2DM is also associated with altered CD8 + T and natural killer cell function in TB7. Moreover, macrophage function, such as phagocytic and bactericidal activity, is decreased in patients with DM9. Thus, DM, particularly uncontrolled hyperglycemia, impairs cellular immunity, resulting in more susceptible host condition to M. tuberculosis43.
Regular physical exercise, including both aerobic and resistance training, is considered an essential component of lifestyle behavior; furthermore, physical exercise enhances glucose regulation in patients with DM10,11. Previous studies showed that DM severity, including DM duration, number of OHAs, insulin use, FBG concentration, and organ complication, is associated with TB risk2,6,27. Hence, physical activity could decrease TB risk in patients with DM through improving DM control status. However, our results reveal that physical activity was inversely correlated with the risk of TB even after adjusting for DM severity. This indicates that physical activity may have other beneficial effects in patients with DM beyond glycemic control.
Regular physical activity has systemic anti-inflammatory effects mediated by the reduction in visceral adipose tissue and/or an anti-inflammatory environment13. Regular moderate to vigorous-intensity exercise is beneficial for the normal immunity by enhancing immune surveillance and likely to lower the risk of respiratory infection and illness14. Physical activity is also linked with increased lung function even in individuals with a smoking history15. Physical activity is inversely associated with the risk of acute respiratory infection, including the common cold, pneumonia, influenza, and coronavirus disease 201944. Furthermore, the risk of TB increases with worsening physical inactivity and sarcopenia among older adults12,45. In this context, physical activity itself may be associated with a decreased risk of TB, which also requires further investigation.
In the stratified analysis by age, the risk of TB considerably reduces with increasing physical activity among individuals aged ≥ 65 years. Particularly in these older adults, some of them could become physically inactive because of their medical problems or poor health status, which were not fully adjusted in our analyses, and they may have an increased risk of developing TB. In this context, a reverse causality would also partially explain the lowest risk of TB among the most physically active individuals. However, we deem that the dose response relationship between physical activity and TB risk and the lag period between health examination and follow-up in our study design reflect the true association between physical activity and TB development. To elucidate these points, further prospective studies are required. In individuals aged < 40 years, the association between physical activity and TB risk tended to be non-significant, as this age group generally has fewer other risk factors for TB—such as undernutrition and low BMI—compared to older adults, making the relative impact of physical inactivity more pronounced in this younger population.
This study had some notable strengths. First, the large sample size and long-term follow-up of the nationwide population-based cohort enhanced the statistical power to show the association between physical activity and TB development individuals with T2DM in a dose response relationship. Second, under the national reporting system, the RID registry-based TB diagnosis provided high accuracy among the Korean population. Additionally, T2DM diagnosis which was based on the combination of insurance claim with ICD-10 diagnostics codes, medication records, and laboratory data on the health examination, also provided a high accuracy under the current NHIS system26. Third, using the NHID database, we could adjust DM severity as covariates in the multivariate analysis, which might be confounders for the TB risk27.
This study also had some limitations. First, we determined the physical activity (e.g., METs-min/week) of the study population in accordance with the baseline health examination data, IPAQ short form, which showed limited correlations to objective standard measurements46. Thus, a considerable temporal interval between the time of health examination and the time of TB diagnosis may exist. Unfortunately, because the national health examination is under voluntary participation, several study participants lack the data at the time of TB diagnosis. Second, as FBG was recorded as a single baseline value in the health examination data, it may not adequately reflect sustained glycemic control. Additionally, the absence of serial FBG measurements limited the feasibility of a time-weighted analysis. Third, during the health examination, the self-reported questionnaire might have a recall bias. Fourth, voluntary participation to health examinations might lead to a selection bias. Fifth, recent studies indicate that sarcopenia is associated with TB12,45,47; however, sarcopenia could not be evaluated owing to the limitations of the source data. Sixth, as this was an observational study, the association between physical activity and TB might not be causal. Finally, this study was conducted among the Korean population (foreign nationals accounting for 5.3% of the total TB cases in 2022)48; thus, the results should be cautiously interpreted to other ethnicities or countries, particularly those with a low TB prevalence. Nevertheless, our findings emphasize that physically inactive individuals with T2DM are more susceptible to TB. Further prospective large-scale cohort studies are required to ascertain our findings.
In conclusion, physical activity was significantly associated with a decreased risk for TB development, and the risk declined with increasing physical activity intensity and amount in a dose response manner. These findings also reveal that attending physicians and the national healthcare policy should underscore physical activity among individuals with DM.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- aHR:
-
Adjusted hazard ratio
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- DM:
-
Diabetes mellitus
- FBG:
-
Fasting blood glucose
- ICD-10:
-
International classification of diseases 10th revision
- IPAQ:
-
International physical activity questionnaire
- METs-min/week:
-
Metabolic equivalent task minutes per week
- NHID:
-
Korean National Health Information Database
- NHIS:
-
Korean National Health Insurance Service
- OHS:
-
Oral hypoglycemic agents
- RID:
-
Rare intractable disease
- T2DM:
-
Type 2 diabetes mellitus
References
World Health Organization. Global tuberculosis report 2022. World Health Organization (2022). https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022
Al-Rifai, R. H., Pearson, F., Critchley, J. A. & Abu-Raddad, L. J. Association between diabetes mellitus and active tuberculosis: A systematic review and meta-analysis. PLoS One. 12, e0187967. https://doi.org/10.1371/journal.pone.0187967 (2017).
Lonnroth, K., Roglic, G. & Harries, A. D. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol. 2, 730–739. https://doi.org/10.1016/S2213-8587(14)70109-3 (2014).
Awad, S. F. et al. Forecasting the impact of diabetes mellitus on tuberculosis disease incidence and mortality in India. J. Glob Health. 9, 020415. https://doi.org/10.7189/jogh.09.020415 (2019).
Oh, K. H., Kim, H. J. & Kim, M. H. Non-communicable diseases and risk of tuberculosis in Korea. Int. J. Tuberc Lung Dis. 20, 973–977. https://doi.org/10.5588/ijtld.15.0684 (2016).
Yoo, J. E. et al. Diabetes status and association with risk of tuberculosis among Korean adults. JAMA Netw. Open. 4, e2126099. https://doi.org/10.1001/jamanetworkopen.2021.26099 (2021).
Kumar, N. P. et al. Type 2 diabetes mellitus is associated with altered CD8(+) T and natural killer cell function in pulmonary tuberculosis. Immunology 144, 677–686. https://doi.org/10.1111/imm.12421 (2015).
Restrepo, B. I. et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin. Infect. Dis. 47, 634–641. https://doi.org/10.1086/590565 (2008).
Pavlou, S., Lindsay, J., Ingram, R., Xu, H. & Chen, M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 19, 24. https://doi.org/10.1186/s12865-018-0261-0 (2018).
Kirwan, J. P., Sacks, J. & Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin. J. Med. 84, S15–S21. https://doi.org/10.3949/ccjm.84.s1.03 (2017).
Syeda, U. S. A., Battillo, D., Visaria, A. & Malin, S. K. The importance of exercise for glycemic control in type 2 diabetes. Am. J. Med. Open. 9, 100031. https://doi.org/10.1016/j.ajmo.2023.100031 (2023).
Yoo, J. E. et al. Anemia, sarcopenia, physical activity, and the risk of tuberculosis in the older population: a nationwide cohort study. Ther. Adv. Chronic Dis. 12, 20406223211015959. https://doi.org/10.1177/20406223211015959 (2021).
Gleeson, M. et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11, 607–615. https://doi.org/10.1038/nri3041 (2011).
Simpson, R. J. et al. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 26, 8–22 (2020). https://www.ncbi.nlm.nih.gov/pubmed/32139352
Collaud, S., Touilloux, B., von Garnier, C., Marques-Vidal, P. & Kraege, V. Physical activity and lung function association in a healthy community-dwelling European population. BMC Pulm Med. 24, 169. https://doi.org/10.1186/s12890-024-02979-x (2024).
Park, J., Yoon, J. H., Ki, H. K., Han, K. & Kim, H. Lifestyle changes and risk of tuberculosis in patients with type 2 diabetes mellitus: A nationwide cohort study. Front. Endocrinol. (Lausanne). 13, 1009493. https://doi.org/10.3389/fendo.2022.1009493 (2022).
Qiu, H. et al. Incident rate and risk factors for tuberculosis among patients with type 2 diabetes: retrospective cohort study in shanghai, China. Trop. Med. Int. Health. 22, 830–838. https://doi.org/10.1111/tmi.12884 (2017).
Choi, J. et al. Effect of physical activity on incident atrial fibrillation in individuals with varying duration of diabetes: a nationwide population study. Cardiovasc. Diabetol. 23, 115. https://doi.org/10.1186/s12933-024-02194-2 (2024).
Kim, K. M., Kim, K. J., Han, K. & Rhee, Y. Associations between physical activity and the risk of hip fracture depending on glycemic status: A nationwide cohort study. J. Clin. Endocrinol. Metab. 109, e1194–e1203. https://doi.org/10.1210/clinem/dgad601 (2024).
Lee, H. H. et al. Association between regular physical activity and lower incidence of colorectal cancer in patients with diabetes mellitus: a nationwide cohort study. Colorectal Dis. 25, 1588–1597. https://doi.org/10.1111/codi.16631 (2023).
Lee, S. J. et al. Effect of physical activity on risk reduction of infective endocarditis among patients with diabetes: a nationwide cohort study. Sci. Rep. 14, 22254. https://doi.org/10.1038/s41598-024-73993-6 (2024).
Seong, S. C. et al. Cohort profile: the National health insurance Service-National health screening cohort (NHIS-HEALS) in Korea. BMJ Open. 7, e016640. https://doi.org/10.1136/bmjopen-2017-016640 (2017).
Seong, S. C. et al. Data resource profile: the National health information database of the National health insurance service in South Korea. Int. J. Epidemiol. 46, 799–800. https://doi.org/10.1093/ije/dyw253 (2017).
Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol 46, e15. (2017). https://doi.org/10.1093/ije/dyv319
Shin, D. W., Cho, J., Park, J. H. & Cho, B. National general health screening program in korea: history, current status, and future direction. Precis Future Med. 6, 9–31. https://doi.org/10.23838/pfm.2021.00135 (2022).
Baek, J. H. et al. Comparison of operational definition of type 2 diabetes mellitus based on data from Korean National health insurance service and Korea National health and nutrition examination survey. Diabetes Metab. J. 47, 201–210. https://doi.org/10.4093/dmj.2022.0375 (2023).
Kang, J. Y., Han, K., Lee, S. H. & Kim, M. K. Diabetes severity is strongly associated with the risk of active tuberculosis in people with type 2 diabetes: a nationwide cohort study with a 6-year follow-up. Respir Res. 24, 110. https://doi.org/10.1186/s12931-023-02414-5 (2023).
Oh, J. Y., Yang, Y. J., Kim, B. S. & Kang, J. H. Validity and reliability of Korean version of international physical activity questionnaire (IPAQ) short form. J. Korean Acad. Fam Med. 28, 532–541 (2007). http://www.kjfm.or.kr/journal/view.php?number=318(
Chun, M. Y. Validity and reliability of Korean version of international physical activity questionnaire short form in the elderly. Korean J. Fam Med. 33, 144–151. https://doi.org/10.4082/kjfm.2012.33.3.144 (2012).
Craig, C. L. et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 35, 1381–1395. https://doi.org/10.1249/01.MSS.0000078924.61453.FB (2003).
Ainsworth, B. E. et al. Compendium of physical activities: an update of activity codes and MET intensities. Med. Sci. Sports Exerc. 32, 498–504. https://doi.org/10.1097/00005768-200009001-00009 (2000).
Anuurad, E. et al. The new BMI criteria for Asians by the regional office for the Western Pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J. Occup. Health. 45, 335–343. https://doi.org/10.1539/joh.45.335 (2003).
Chung, C., Lee, K. N., Han, K., Shin, D. W. & Lee, S. W. The effect of smoking on nontuberculous mycobacterial pulmonary disease and tuberculosis: a nationwide retrospective cohort study. Sci. Rep. 14, 22653. https://doi.org/10.1038/s41598-024-72438-4 (2024).
Chung, C. et al. Association between alcohol consumption and risk of developing tuberculosis in patients with diabetes: a nationwide retrospective cohort study. Respir Res. 25, 420. https://doi.org/10.1186/s12931-024-03047-y (2024).
Chung, C., Lee, K. N., Shin, D. W., Lee, S. W. & Han, K. Low household income increases risks for chronic obstructive pulmonary disease in young population: a nationwide retrospective cohort study in South Korea. BMJ Open. Respir Res. 11. https://doi.org/10.1136/bmjresp-2024-002444 (2024).
Adegbite, B. R. et al. Non-communicable disease co-morbidity and associated factors in tuberculosis patients: A cross-sectional study in Gabon. EClinicalMedicine 45, 101316. (2022). https://doi.org/10.1016/j.eclinm.2022.101316
Kim, K. S., Kim, B. & Han, K. Big data research for Diabetes-Related diseases using the Korean National health information database. Diabetes Metab. J. 49, 13–21. https://doi.org/10.4093/dmj.2024.0780 (2025).
Kim, N. H. et al. Diabetic Kidney Disease Fact Sheet in Korea. Diabetes Metab J 48, 463–472. (2023). https://doi.org/10.4093/dmj.2023.0310 (2024).
Basu, M. et al. Prevalence of non-diabetic kidney disease and inability of clinical predictors to differentiate it from diabetic kidney disease: results from a prospectively performed renal biopsy study. BMJ Open. Diabetes Res. Care. 10. https://doi.org/10.1136/bmjdrc-2022-003058 (2022).
Liu, Q. et al. The association between diabetes mellitus and the risk of latent tuberculosis infection: A systematic review and Meta-Analysis. Front. Med. (Lausanne). 9, 899821. https://doi.org/10.3389/fmed.2022.899821 (2022).
Boadu, A. A., Yeboah-Manu, M., Osei-Wusu, S. & Yeboah-Manu, D. Tuberculosis and diabetes mellitus: the complexity of the comorbid interactions. Int. J. Infect. Dis. 146, 107140. https://doi.org/10.1016/j.ijid.2024.107140 (2024).
Antonio-Arques, V. et al. Glycemic control and the risk of tuberculosis in patients with diabetes: A cohort study in a mediterranean City. Front. Public. Health. 10, 1017024. https://doi.org/10.3389/fpubh.2022.1017024 (2022).
Thong, P. M., Wong, Y. H., Kornfeld, H., Goletti, D. & Ong, C. W. M. Immune dysregulation of diabetes in tuberculosis. Semin Immunol. 78, 101959. https://doi.org/10.1016/j.smim.2025.101959 (2025).
Nieman, D. C. & Sakaguchi, C. A. Physical activity lowers the risk for acute respiratory infections: time for recognition. J. Sport Health Sci. 11, 648–655. https://doi.org/10.1016/j.jshs.2022.08.002 (2022).
Mohamad Zani, R. A. et al. Sarcopenia and it’s influencing factors among adults with asthma, chronic obstructive pulmonary disease, and tuberculosis in penang, Malaysia. BMC Public. Health. 25, 1572. https://doi.org/10.1186/s12889-025-22819-9 (2025).
Lee, P. H., Macfarlane, D. J., Lam, T. H. & Stewart, S. M. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int. J. Behav. Nutr. Phys. Act. 8, 115. https://doi.org/10.1186/1479-5868-8-115 (2011).
Shin, M. K. et al. Association of protein consumption and energy intake on sarcopenia in tuberculosis survivors. Ther. Adv. Chronic Dis. 12, 20406223211056712. https://doi.org/10.1177/20406223211056712 (2021).
Min, J., Jeong, Y., Kim, H. W. & Kim, J. S. Tuberculosis notification and incidence: Republic of korea, 2022. Tuberc Respir Dis. (Seoul). 87, 411–413. https://doi.org/10.4046/trd.2024.0018 (2024).
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2023-NR077159), the Bio&Medical Technology Development Program of the NRF funded by the MSIT (RS-2022-NR067421 and RS-2023-00222687), and the National Institute of Health research project (2024ER080601).
Author information
Authors and Affiliations
Contributions
Chiwook Chung, Kyu Na Lee, Kyungdo Han, Junhee Park, Dong Wook Shin, and Sei Won Lee conceived and designed the study. Kyu Na Lee, Kyungdo Han, and Junhee Park contributed to the data collection and data analysis. Chiwook Chung, Dong Wook Shin, and Sei Won Lee contributed to data interpretation and drafted the manuscript. The final manuscript was revised and approved by all authors. All authors accept responsibility for the accuracy of the content in the final manuscript. Generative artificial intelligence was not utilized in any portion of the manuscript writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study protocol was approved by the Institutional Review Board of Samsung Medical Center, Seoul, Republic of Korea (approval No. SMC 2022-07-072). The requirement for informed consent was waived by Institutional Review Board of Samsung Medical Center as it was a retrospective study and the data used were anonymized. This study was conducted in accordance with the Declaration of Helsinki guidelines, and all methods were performed in accordance with the relevant guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chung, C., Lee, K.N., Han, K. et al. Physical activity intensity and amount are inversely correlated with the risk of tuberculosis in patients with diabetes. Sci Rep 15, 24701 (2025). https://doi.org/10.1038/s41598-025-09593-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09593-9