Abstract
This study aims to explore the impact of NFIC and its regulated signaling factors on epithelial ovarian cancer, providing new insights for the treatment of ovarian epithelial cancer. Bioinformatics methods were applied to predict and analyze NFIC and its downstream signaling factors. In vivo experiments involved dividing 27 purchased female nude mice into three groups: NC group, NFIC-OE group, and NFIC-OE + TBX2-OE group, to observe tumor growth in each group. In vitro experiments involved dividing the human epithelial ovarian cancer cell line SKOV3, OVCAR-3 and A2780 into five groups with different stimuli, using Western blot to observe protein expression, CCK-8 assay to observe cell proliferation, scratch assay to observe cell migration, transwell assay to observe cell invasion, Annexin V/PI assay to study apoptosis and Co-Immunoprecipitation experiment were used to study NFIC action. Bioinformatics revealed that NFIC promotes PTEN and TGFβ1, with TGFβ1 promoting the expression of TBX3 and EGR1, which in turn inhibit TBX2. Additionally, TBX2 inhibits PTEN and promotes MMPs. BRD4 promotes H3K27AC, which leads to TGFβ1 expression, while H3K27me3 inhibits TGFβ1 expression. EZH2 promotes H3K27me3, thereby inhibiting TGFβ1 and TBX3, and SP1 promotes the action of EZH2. In vivo, NFIC alleviated ovarian epithelial cancer, while TBX2 inhibited the effect of NFIC. In vitro, NFIC inhibited the expression of TBX2, nucleus SP1, EZH2, and MMPs, and promoted the expression of PTEN, nucleus BRD4, TGFβ1, and TBX3; TBX2 promoted MMPs expression. NFIC inhibited the migration and proliferation of SKOV3 cells, while TBX2 promoted it; NFIC functioned through TGFβ1 and PTEN, and their inhibition promoted the migration and proliferation of SKOV3 cells. NFIC inhibits the progression of epithelial ovarian cancer by regulating the balance of PTEN/TGFβ1/EGR1/BRD4 and SP1/EZH2 to suppress the TBX2/MMPs signaling pathway.
Similar content being viewed by others
Introduction
Epithelial ovarian cancer (epithelial ovarian cancer, EOC) is one of the most common malignancies in gynecological cancers and is also one of the leading causes of cancer-related deaths in women. Despite significant advancements in diagnostic and therapeutic technologies in recent years, the prognosis for EOC remains pessimistic due to its difficulty in early diagnosis and high recurrence rate. Around 300,000 new cases are identified worldwide each year, and the illness is responsible for roughly 185,000 fatalities, according to the World Health Organisation (WHO)1. As a result, a major focus of current research is finding novel treatment targets and molecular processes.
Epithelial ovarian cancer has a complicated pathophysiology that includes anomalies in many genes and signalling pathways. Two of the most well-researched processes are p53 gene alterations and activation of the PI3K/AKT/mTOR signalling pathway2. Furthermore, the development and metastasis of epithelial ovarian cancer are significantly influenced by the TGFβ signalling system3. Numerous biological processes that contribute to the development of epithelial ovarian cancer, including as cell proliferation, migration, invasion, and fibrosis, are regulated by the TGFβ pathway4. Since epigenetic processes like DNA methylation and histone modification are crucial to the growth and genesis of tumours, they have progressively drawn more attention in recent years.
A transcription factor called nucleus Factor IC (also known as NFIC) is mainly involved in processes including bone differentiation and neurodevelopment5. The function of NFIC in tumours has drawn more interest in recent years. According to studies, NFIC has a variety of regulatory roles in different types of cancer, including increasing tumour metastasis in gastric cancer and preventing tumour development in breast cancer6. NFIC’s precise role and mechanism in epithelial ovarian cancer are yet unknown, however.
PTEN is a key tumour suppressor gene that controls the activity of the phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) signalling pathway7. By controlling the expression of many target genes, TGFβ1, a crucial component of the TGFβ signalling pathway, contributes to a number of cellular biological processes. The immediate early gene known as Early Growth Response 1 (EGR1) has the ability to control gene expression in response to cellular stress signals and a variety of growth factors8. By identifying acetylated histones, the epigenetic regulator bromodomain-containing protein 4 (BRD4) helps control the transcription of genes9. Numerous tumours have been shown to interact with these genes and proteins. For example, TGFβ1 controls the expression of genes linked to fibrosis via EGR1 and BRD4, while PTEN prevents tumour growth by blocking the TGFβ1 signalling pathway10.
A common transcription factor called Specificity Protein 1 (SP1) binds to certain DNA regions to control the expression of many genes11. By catalysing the production of H3K27me3, the significant histone methyltransferase Enhancer of Zeste Homolog 2 (EZH2) contributes to tumour suppression and gene silence12. Research has shown that SP1 stimulates EZH2 expression, which in turn controls H3K27me3 levels, influencing the epigenetic condition of tumours13. In epithelial ovarian cancer, high expression of EZH2 is closely associated with poor prognosis14. T-box transcription factor 2 (TBX2) is a member of the T-box family involved in cell development and differentiation15. TBX2 exhibits tumor-promoting effects in various cancers, such as breast cancer, colorectal cancer, and lung cancer15,16. Matrix metalloproteinases (MMPs) are enzymes capable of degrading the extracellular matrix, participating in tumor invasion and metastasis by regulating the remodeling of the extracellular matrix17.
This study predicted and analyzed the functions of NFIC and its downstream signaling factors through bioinformatics methods. To verify the role of NFIC in epithelial ovarian cancer, in vivo experiments were conducted. Twenty-seven female nude mice were equally divided into three groups: NC group, NFIC-OE group, and NFIC-OE group + TBX2-OE group, observing tumor growth in each group. In vitro experiments used the human epithelial ovarian cancer cell line SKOV3 to perform experiments with different stimuli, observing DNA protein expression and cell behavior. Combining in vivo and in vitro experiments with bioinformatics analysis, this study provides new insights for the treatment of epithelial ovarian cancer.
Materials and methods
Bioinformatics analysis
The ovarian epithelial carcinoma dataset GSE18520, comprising gene expression profiles from 53 tumor samples and 10 normal ovarian epithelial samples, was retrieved from the Gene Expression Omnibus database. Data preprocessing was performed using the Robust Multi-array Average (RMA) algorithm for background correction, quantile normalization, and probe summarization, followed by batch effect correction through the ComBat method. Differential expression analysis was conducted via the limma package with stringent thresholds (absolute log2 fold change > 2 and adjusted p-value < 0.05 using Benjamini-Hochberg false discovery rate correction). We visualized the analytical results using volcano plots and heatmaps generated by the ggplot2 package. We performed functional annotation using ClusterProfiler for Gene Ontology (GO) enrichment analysis, focusing on three categories: cellular components, molecular functions, and biological processes. Additionally, we conducted Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis to identify essential biological pathways. Spearman’s rank correlation analysis was employed to investigate co-expression patterns among differentially expressed genes, with subsequent validation of key findings conducted through cross-platform verification using the TCGA-OV cohort.
Experimental cells and materials
The SKOV3, OVCAR-3 and A2780 human epithelial ovarian cancer cell line and KGN human ovaries cell were purchased from Wuhan Procell Life Science Co., Ltd. The SKOV3, OVCAR-3 and A2780 cells were cultured in RPMI1640 medium supplemented with 100 mL/L fetal bovine serum, 100 IU/mL penicillin, and 100 µg/mL streptomycin. The SKOV3 cell line exhibited abnormal cell growth and differentiation, anti-apoptotic capacity, and the ability to invade surrounding tissues. The OVCAR-3 cell line had natural drug resistance and easily formed tumors in immunodeficient mice. The A2780 cell line was highly sensitive to drugs, proliferated rapidly, had a short experimental cycle, and had a high transfection rate. RPMI1640 medium was obtained from Gibco (USA), fetal bovine serum from Shanghai Solarbio Biotechnology Co., Ltd., and the NFIC overexpression vector and TBX2 overexpression vector were constructed by Shanghai Jikai Gene Technology Co., Ltd. The PTEN inhibitor SF1670 was purchased from SELECK, and the TGFβ1 inhibitor TGFβ1-IN-1 from MCE. Cracking buffer solution:10 mmol/L sodium phosphate, pH 7.2, 100 mmol/L NaCl, 2 mmol/L EDTA, pH 8.0, 10 mL/L NP-40, Supplement protease; washing buffer: same as lysis buffer (without protease inhibitors); elution buffer: 0.1 M glycine (pH 2.5) or SDS loading buffer; Protein A/G magnetic beads or agarose beads; BCA protein quantification kit.
Experimental animals
48 SPF-grade BALB/c-nu female nude mice (6 weeks old, 18 ± 1 g) were purchased from Henan SKBS Biosciences Co., Ltd. (License No.: SCXK (Yu) 2020-0005). The experimental animal facility maintained a constant indoor temperature of 25 ± 2 °C and a relative humidity of 60 ± 4%. The animal facility was strictly disinfected daily, and cages, bedding, and feeding utensils were disinfected and replaced every two days. The nude mice had free access to water (distilled water) and immunodeficiency-specific feed.All experiments were performed in accordance with relevant guidelines and regulations. The study was carried out in compliance with the ARRIVE guidelines (https://arriveguidelines.org).
Cell grouping and transfection
Cells were divided into the NC group (transfected with empty NFIC and TBX2 vectors), the NFIC-OE group (transfected with NFIC overexpression vectors), the TBX2-OE group (transfected with TBX2 overexpression vectors), and the NFIC-OE + TBX2-OE group (transfected with NFIC and TBX2 overexpression vectors), as well as the NFIC-KD group (NFIC gene knockdown to reduce expression levels), NFIC-OE + SF1670 (transfected with NFIC overexpression vectors and treated with the PTEN inhibitor SF1670), and NFIC-OE + SF1670 + TGFβ1-IN-1 (transfected with NFIC overexpression vectors and treated with the PTEN inhibitor SF1670 and the TGFβ1 inhibitor TGFβ1-IN-1). Logarithmic growth phase SKOV3, OVCAR-3 and A2780 cells were seeded at a density of 2 × 10^5 cells per well in 6-well plates and cultured in a humidified incubator at 5% CO2 and 37 °C. After 48 h of culture when the cell confluence reached 50–60%, the cells were transfected with NFIC and TGFβ1 low expression vectors or PTEN, NFIC and TBX2 overexpression vectors according to the Lipo-fectamine2000 protocol. After 6 h of transfection, fresh medium was replaced, and the cells were further cultured for 48 h.
Nude mouse model construction
The SKOV3 epithelial ovarian cancer cells from section “1.4” were processed, counted, and resuspended at a concentration of 5 × 10^6 cells/ml. Forty-eight mice were randomly divided into six groups: NC group, NFIC-OE group, NFIC-OE + TBX2-OE group, TBX2-OE group, NFIC-KD group, NFIC-KD + PTEN-OE group, and NFIC-KD + PTEN-OE + TGFβ1-KD group. The axillary skin of 6-week-old female nude mice was disinfected with iodine tincture. Using a 1 mL syringe, 0.2 mL of the cell suspension was injected subcutaneously at the axillary area. Tumor growth in the transplanted mice was observed daily. The mice had free access to water and feed. After 3 weeks, the xenograft tumors reached a certain size (approximately 1.5 cm in diameter). The tumor volume was calculated using the formula V = π/6×L×W2. The nude mice were euthanized using CO₂. The flow rate of CO₂ was 30% of the container volume per minute. We used 3% isoflurane gas for induction anesthesia and 1.5% isoflurane to maintain anesthesia to reduce anxiety in the nude mice during the euthanasia process. The nude mice were confirmed to have been euthanized successfully 10 min after the cessation of heartbeat.
Immunohistochemical staining
After the experimental nude mice were euthanized with carbon dioxide, tumor tissues were collected, weighed, and fixed in 4% formaldehyde solution. The tissue samples were then deparaffinized, dehydrated with graded alcohol, cleared with xylene, and embedded in paraffin. 4 μm consecutive sections were prepared for immunohistochemistry (IHC). The paraffin-embedded sections were sequentially deparaffinized in xylene, followed by graded alcohol baths, and hydrated. The sections were then subjected to antigen retrieval in a pressure cooker with 0.01 mol/L citrate buffer, washed with PBS, and immersed in freshly prepared 3% H2O2 at room temperature for 15 min to inactivate endogenous peroxidase. The sections were washed three times with PBS, each for 15 min. Normal goat serum was applied to the sections for 30 min to block nonspecific binding. The primary antibody was applied and incubated overnight at 4 °C. The next day, the sections were washed three times with PBS, each for 5 min, and the secondary antibody was applied and incubated at room temperature for 45 min. The sections were then washed with PBS, stained with DAB, and counterstained with hematoxylin. The sections were differentiated, re-blued, dehydrated, and mounted with neutral gum. Sections incubated with PBS instead of the primary antibody served as negative controls. Positive RECK staining was observed in the cytoplasm and appeared yellow or brown.
Cell model construction
The SKOV3, OVCAR-3 and A2780 cells were divided into five groups: NC group (no special treatment), NFIC-OE group (transfected with NFIC overexpression vector), NFIC-OE + TBX2-OE group (transfected with NFIC and TBX2 overexpression vectors), NFIC-OE + SF1670 group (transfected with NFIC overexpression vector and stimulated with PTEN inhibitor SF1670 at 10 µM for 24 h), and NFIC-OE + SF1670 + TGFβ1-IN-1 group (transfected with NFIC overexpression vector and stimulated with PTEN inhibitor SF1670 and TGFβ1 inhibitor TGFβ1-IN-1 at 10 µM for 24 h and 1 µM for 24 h, respectively).
Western blot
SKOV3 and KGN epithelial ovarian cancer cells from section “1.7” were collected, and lysis buffer (10 mmol/L sodium phosphate, pH 7.2, 100 mmol/L NaCl, 2 mmol/L EDTA, pH 8.0, 10 mL/L NP-40, and protease inhibitor cocktail) was added. The cells were incubated on ice for 15 min and then centrifuged at 4 °C and 12,000 r/min for 15 min to prepare the cell lysate. Protein concentration was measured using the BCA method, and the proteins were denatured in a metal bath at 100 °C for 10 min. The proteins were then subjected to SDS-PAGE. The voltage was set to 80 V for about 35 min to pass through the stacking gel, then increased to 120 V for about 60 min to pass through the separating gel. The electrophoresis was stopped when the samples reached the appropriate position in the separating gel. The “sponge-filter paper-PVDF membrane-gel-filter paper-sponge” assembly was transferred, and the PVDF membrane was incubated in skim milk solution on a shaker for 2 h to block non-specific binding. The primary antibody (mouse monoclonal RECK antibody, Santa Cruz, 1:100 dilution) was applied and incubated overnight at 4 °C. The next day, the sections were washed three times with TBST, each for 5 min, and the secondary antibody (HRP-labeled goat anti-mouse IgG, 1:10,000 dilution) was applied and incubated at room temperature for 1 h. The sections were then washed with TBST, stained with ECL, and the images were analyzed using a gel imaging system. The gray values of the target bands were compared with those of the internal control GAPDH to determine the expression levels of each indicator.
Cell scratch wound healing assay
The cell scratch wound healing assay was used to measure the healing rate of the scratch wound. The SKOV3, OVCAR-3 and A2780 cells were seeded in 6-well plates (2 × 10^5 cells per well) and treated with drugs or transfected plasmids. When the cells reached nearly 100% confluence, scratches were made using a 200 µL pipette tip, and the floating cells were washed away with PBS. The initial distance between the scratches (d0 h) was measured. After 48 h of culture in serum-free medium at 37 °C and 5% CO2, the distance between the scratches (d24 h) was measured again. The scratch healing rate was calculated as (d0 h - d48h)/d0 h × 100%.
Transwell invasion assay
The transwell invasion assay was used to count the number of invading cells. Matrigel was diluted to the appropriate concentration (1:10) using pre-cooled serum-free culture medium. The diluted Matrigel was added to the upper chamber of the Transwell chamber, with 100 µL added to each well. The transwell chamber was placed in a 37 °C incubator for 1 h to allow the Matrigel to solidify. After 24 h of culture in complete medium, the SKOV3, OVCAR-3 and A2780 cells were digested with trypsin and resuspended in serum-free DMEM medium. The cell density was adjusted to 1 × 10^5 cells per 200 µL and added to the upper chamber of the transwell plate. The lower chamber was filled with 700 µL of DMEM medium containing 10% fetal bovine serum as a chemoattractant. After 24 h of incubation at 37 °C, the non-migrated cells on the upper surface of the chamber were gently removed with a cotton swab, and the chamber was washed twice with PBS to remove cell debris. The cells that had migrated through the membrane were fixed with 4% paraformaldehyde for 20 min, stained with 0.01% crystal violet for 30 min, and counted under a light microscope.
CCK-8 assay
The SKOV3, OVCAR-3 and A2780 cells treated with different agents were added to 96-well plates, with 2000 cells per well. After 6 h of incubation at 37 °C and 5% CO2, 10 µL of CCK-8 working solution was added to each well, and the absorbance at 450 nm was measured at 0, 12, 24, and 48 h.
Colony formation assay
The SKOV3, OVCAR-3 and A2780 cells in the exponential growth phase were seeded at a density of 600 cells per well in six-well plates and cultured for 3 days. The cells were then incubated in medium with different stimuli for 48 h. The medium containing the drugs was removed, the cells were washed with PBS, and incubated in drug-free medium for 10 days until colonies were visible. The cells were fixed with 4% paraformaldehyde at room temperature for 15 min, washed three times with PBS, stained with 0.01% crystal violet for 30 min, and photographed and counted.
Co-Immunoprecipitation experiment
Three plates were used to cultivate each set of SKOV3 cells. Three PBS washes were performed on the dishes. The cells were then scraped to collect them after 400 µl of Thermo Fisher IP lysis buffer was added to each plate. For a total of thirty minutes, the mixture was vortexed on ice every five minutes. Following a 25-minute centrifugation, the supernatant was kept. After that, 20 µl of the sample was added to a suitable volume of loading buffer and heated for 8 min at 100 °C to act as the input. 400 µl of the protein sample was mixed with a primary antibody, inverted at 4 °C, and then incubated on a shaking incubator for the whole night. Thermo Fisher magnetic bead wash solution was added to the A/G magnetic beads, and they were then gently inverted at room temperature to wash them. To remove the wash solution, the magnetic beads were separated using a magnetic stand. This procedure was carried out three times, each lasting one minute. After adding the produced primary antibody-protein conjugate solution to the cleaned A/G magnetic beads, the mixture was inverted and allowed to sit at room temperature for an hour on a shaking incubator. Following incubation, the magnetic beads were adsorbed using a magnetic stand, and the supernatant was disposed of. The beads were gently inverted and washed three times for five minutes each using 500 µl of washing solution. Double-distilled water was used during the fourth wash. The cleaned magnetic bead-antibody-antigen combination was then heated to 100 °C for 8 min after 50 µl of IP buffer was added. The magnetic beads were adsorbed using a magnetic stand, and the supernatant was taken as a sample and kept at −20 °C. A Western blot analysis was then carried out.
Cell apoptosis measurement using flow cytometry
A 1.5 ml centrifuge tube was filled with 1 × 105–1 × 107 SKOV3 cells, and the tube was spun for 5 minutes at 3000 rpm. To resuspend the cells, 100 µl of FACS buffer was added after the supernatant was disposed of. After adding 0.5 µl of Fc receptor antibody (0.5 mg/ml), the mixture was incubated for three minutes in a water bath. After adding 1 µl of fluorescent antibody (0.5 mg/ml), the mixture was incubated for 30 min in a water bath. 350 µl of FACS buffer was then added, and the mixture was stirred gently. The supernatant was disposed of after it was centrifuged for five minutes at 3000 rpm. Two times, this step was performed. The cells were then resuspended by adding 100 µl of instrument buffer to the cell pellet and gently mixing the combination. The cell solution was prepared for instrument detection and analysis after being moved to a tube designed specifically for FACS.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 software. Each experiment was repeated three times, and the measured data are expressed as the mean ± standard deviation. When comparing two independent samples, if the data conformed to a normal distribution and had equal variances, the t-test was used; if the data did not conform to a normal distribution or had unequal variances, the non-parametric t-test (Wilcoxon test) was used. P-value < 0.05 was considered statistically significant.
Results
Bioinformatics analysis results
The ovarian epithelial cancer dataset GSE18520 identified a total of 731 differentially expressed genes (DEGs) through analysis using the limma package, with 361 upregulated genes and 370 downregulated genes, among which TBX2 was significantly upregulated. GO and KEGG analysis results showed that DEGs were significantly enriched in the phosphoinositide 3-kinase (PI3K), MAPK, and mTOR signaling pathways. These pathways are closely linked to cell growth, proliferation, and survival, indicating their crucial role in the occurrence and development of ovarian epithelial cancer. Additionally, DEGs were significantly enriched in cellular components such as the transferase complex and membrane microdomains, which may be associated with the invasiveness and metastatic ability of tumor cells. Correlation analysis revealed a positive correlation between NFIC and key regulatory factors such as PTEN and TGFβ1, indicating that these factors may interact in the tumor microenvironment, influencing the biological behavior of the tumor. The positive correlation between TGFβ1, TBX3, and Egr1 indicates their significant role in tumor progression. Meanwhile, the negative correlation between EZH2 and TGFβ1 may imply a potential role of EZH2 in tumor suppression. The negative correlation between TGFβ1 and TBX2 suggests that TGFβ1 may play an inhibitory role in the progression of ovarian epithelial cancer. Furthermore, the negative correlation between TBX3 and TBX2 further reveals the interaction between these two transcription factors in ovarian cancer. The above findings were validated in the TCGA-OV cohort, showing high consistency (Fig. 1).
Bioinformatics Analysis Atlas. A: Sample correction box plot; B: Sample difference PCA plot; C: Differential gene volcano plot; D: GO enrichment analysis bar chart; E: KEGG enrichment analysis bubble plot; F: KEGG enrichment analysis distance plot; G: Correlation scatter plot of NFIC and PTEN; H: Correlation scatter plot of NFIC and TGFβ1; I: Correlation scatter plot of TGFβ1 and TBX3; J: Correlation scatter plot of TGFβ1 and Egr1; K: Correlation scatter plot of EZH2 and TGFβ1; L: Correlation scatter plot of TGFβ1 and TBX2; M: Correlation scatter plot of TBX3 and TBX2; N: Validation Heatmap of the TCGA-OV Cohort.
NFIC inhibits the occurrence of epithelial ovarian cancer, while TBX2 promotes its progression
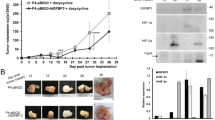
In the subcutaneous tumor transplantation experiment in nude mice, compared with the NC group, the tumor weight and volume of the NFIC-OE group were significantly reduced; compared with the NC group, there was no significant difference in tumor weight and volume between the NFIC-OE + TBX2-OE group; compared with the NFIC-OE group, the tumor weight and volume of the NFIC-OE + TBX2-OE group were significantly increased; compared with the NC group, the NFIC-KD group showed significantly increased tumor weight and volume; compared with the NFIC-KD group, the NFIC-KD + PTEN-OE group showed significantly reduced tumor weight and volume; compared with the NFIC-KD + PTEN-OE group, the NFIC-KD + PTEN-OE + TGFβ1-KD group showed significantly increased tumor weight and volume (Fig. 2A). We then explored the role of TBX2 alone in tumor progression. The results showed that, compared with the NC group, the tumor volume and weight were significantly increased in the TBX2-OE group (Fig. 2B). Immunohistochemical staining results showed that compared with the NC group, NFIC expression levels were significantly increased, and TBX2 expression levels were significantly decreased in the NFIC-OE group; compared with the NC group, NFIC and TBX2 expression levels were significantly increased in the NFIC-OE + TBX2-OE group; compared with the NFIC-OE group, NFIC expression levels did not change significantly in the NFIC-OE + TBX2-OE group, TBX2 expression levels were significantly decreased. Compared with the NC group, NFIC expression levels were significantly decreased in the NFIC-KD group, while TBX2 expression levels were significantly increased; compared with the NFIC-KD group, NFIC expression levels were not significantly different in the NFIC-KD + PTEN-OE group, while TBX2 expression levels were significantly decreased; compared with the NFIC-KD + PTEN-OE group, there was no significant difference in NFIC expression in the NFIC-KD + PTEN-OE + TGFβ1-KD group, while TBX2 expression levels were significantly increased (Fig. 2C). The above results indicated that NFIC inhibits the occurrence of epithelial ovarian cancer, while TBX2 promotes its progression.
NFIC inhibits the development of epithelial ovarian cancer, while TBX2 promotes the progression of epithelial ovarian cancer. A: Subcutaneous tumor transplantation experiment results in nude mice and statistical graphs of tumor volume and weight for the NC group, NFIC-OE group, NFIC-OE + TBX2-OE group, NFIC-KD group, NFIC-KD + PTEN-OE group, and NFIC-KD + PTEN-OE + TGFβ1-KD group; B: Subcutaneous tumor transplantation experiment results in nude mice and statistical graphs of tumor volume and weight for the NC group, and TBX2-OE group; C: Immunohistochemical staining results for the NC group, NFIC-OE group, NFIC-OE + TBX2-OE group, NFIC-KD group, NFIC-KD + PTEN-OE group, and NFIC-KD + PTEN-OE + TGFβ1-KD group, along with statistical graphs showing the expression levels of NFIC and TBX2. Data are expressed as mean ± standard deviation. N = 6; **P<0.01; nsP>0.05.
NFIC acts on epithelial ovarian cancer through PTEN and TGFβ1
Western blot results showed that, compared with the NC group, NFIC and PTEN expression levels were significantly decreased in the NFIC-KD group, while TBX2 and EZH2 expression levels were significantly increased (Fig. 3A). Compared with normal epithelial ovarian cells (KGN), NFIC expression levels were significantly elevated in SKOV3 cells (Fig. 3B). Furthermore, Co-Immunoprecipitation experiments showed that NFIC may interact with PTEN, TGFβ1, and TBX2 separately (Fig. 3C).
NFIC specifically targets PTEN, TBX2, and TGFβ1. A: Western blot analysis of protein bands for NFIC, PTEN, TBX2, and EZH2 in the NC group and NFIC-KD group, along with statistical graphs of relative protein expression levels; B: Western blot analysis of NFIC protein bands in KGN and SKOV3 cells, along with statistical graphs of relative protein expression levels; GAPDH as control protein; C: Co-Immunoprecipitation experiment results diagram. Data are expressed as mean ± standard deviation. N = 3; **P<0.01.
Western blot results showed that, compared with the NC group, the NFIC, PTEN, nucleus BRD4, TGFβ1, and TBX3 relative protein expression levels were significantly increased in the NFIC-OE group, while the nucleus SP1, nucleus EZH2, and TBX2 relative protein expression levels were significantly decreased. Compared with the NFIC-OE group, the NFIC-OE + TBX2-OE group showed no significant differences in the relative protein expression of NFIC, PTEN, nucleus SP1, nucleus EZH2, nucleus BRD4, TGFβ1, and TBX3, but the relative protein expression of TBX2 was significantly increased; compared with the NFIC-OE group, the NFIC-OE + SF1670 group showed no significant difference in NFIC expression, but the relative protein expression of PTEN, nucleus BRD4, TGFβ1, and TBX3 was significantly reduced, while the relative protein expression of nucleus SP1, nucleus EZH2, and TBX2 was significantly increased; compared with the NFIC-OE + SF1670 group, there were no significant differences in NFIC and PTEN expression between the NFIC-OE + SF1670 + TGFβ1-IN-1 group and the NFIC-OE + SF1670 group, but the relative protein expression of nucleus BRD4, TGFβ1, and TBX3 decreased, while the relative protein expression of nucleus SP1, nucleus EZH2, and TBX2 significantly increased (Fig. 4). The above results indicated that NFIC acts on epithelial ovarian cancer through PTEN and TGFβ1.
NFIC acts on epithelial ovarian cancer through PTEN and TGFβ1. A: Western blot protein banding plots of NFIC, TBX2, PTEN, nucleus SP1, nucleus EZH2 and nucleus BRD4 as well as statistical plots of relative protein expression in NC group, NFIC-OE group, NFIC-OE + TBX2-OE group, NFIC-OE + SF1670 group and NFIC-OE + SF1670 + TGFβ1-IN-1 group; B: Western blot protein banding plots of TGFβ1, TBX3, TBX2 as well as statistical plots of relative protein expression in NC group, NFIC-OE group, NFIC-OE + TBX2-OE group, NFIC-OE + SF1670 group and NFIC-OE + SF1670 + TGFβ1-IN-1 group. GAPDH as control protein; Data are expressed as mean ± standard deviation. N = 3; **P<0.01; *P<0.05; nsP>0.05.
NFIC mediates STAT3/TGFβ1 Inhibition of ovarian cancer cell migration and invasion capacity
Western blot results showed that, compared with the NC group, the relative protein expression levels of MMP-2 and MMP-9 were significantly reduced in the NFIC-OE group; compared with the NFIC-OE group, the relative protein expression levels of MMP-2 and MMP-9 were significantly increased in the NFIC-OE + TBX2-OE group; compared with the NFIC-OE group, the NFIC-OE + SF1670 group showed significantly increased relative expression of MMP-2 and MMP-9 compared with the NFIC-OE group; and compared with the NFIC-OE + SF1670 group, the NFIC-OE + SF1670 + TGFβ1-IN-1 group showed significantly increased relative expression of MMP-2 and MMP-9 (Fig. 5). The results of the cell scratch assay showed that, after 48 h, compared with the NC group, the intercellular distance increased in the NFIC-OE group; compared with the NFIC-OE group, the intercellular distance significantly decreased in the NFIC-OE + TBX2-OE group; compared with the NFIC-OE group, the NFIC-OE + SF1670 group showed significantly reduced cell-cell distances; compared with the NFIC-OE + SF1670 group, the NFIC-OE + SF1670 + TGFβ1-IN-1 group showed significantly reduced cell-cell distances (Fig. 6A). Transwell assay results showed that in SKOV3, OVCAR-3, and A2780 cells, the NFIC-OE group had significantly fewer invasive cells compared to the NC group; compared to the NFIC-OE group, the NFIC-OE + TBX2-OE group had more invasive cells; compared to the NFIC-OE group, the NFIC-OE + SF1670 group had more invasive cells; and compared to the NFIC-OE + SF1670 group, the NFIC-OE + SF1670 + TGFβ1-IN-1 group showed an increased number of invading cells (Fig. 6B). The above results demonstrated that NFIC mediates STAT3/TGFβ1 to inhibit the migration and invasion of ovarian cancer cells.
NFIC mediates STAT3/TGFβ1 inhibition of MMP-2 and MMP-9 expression in ovarian cancer cells. A: Western blot protein banding plots of MMP-2, and MMP-9 in NC group, NFIC-OE group, NFIC-OE + TBX2-OE group, NFIC-OE + SF1670 group and NFIC-OE + SF1670 + TGFβ1-IN-1 group; B: Statistical graph of the relative protein expression levels of MMP-2 and MMP-9 in the NC group, NFIC-OE group, NFIC-OE + TBX2-OE group, NFIC-OE + SF1670 group, and NFIC-OE + SF1670 + TGFβ1-IN-1 group. GAPDH as control protein; Data are expressed as mean ± standard deviation. N = 3; **P<0.01; *P<0.05; nsP>0.05.
NFIC mediates STAT3/TGFβ1 inhibition of ovarian cancer cell migration and invasion capacity. A: Diagram showing the results of the cell scratch assay for SKOV3, OVCAR-3, and A2780 cells, as well as statistical graphs of cell scratch spacing at 0 h and 48 h; B: Results of transwell invasion experiments and statistical analysis of invasive cell numbers in SKOV3, OVCAR-3, and A2780 cells. Data are expressed as mean ± standard deviation. N = 3; **P<0.01; *P<0.05; nsP>0.05.
NFIC mediates STAT3/TGFβ1 Inhibition of ovarian cancer cell proliferation and cell viability
Western blot analysis revealed that, compared with the NC group, the relative protein expression levels of P-PI3K, CyclinA1, and CyclinD1 were significantly reduced in the NFIC-OE group; compared with the NFIC-OE group, the relative protein expression levels of P-PI3K, CyclinA1, and CyclinD1 were significantly increased in the NFIC-OE + TBX2-OE group; compared with the NFIC-OE group, the NFIC-OE + SF1670 group showed significantly increased relative protein expression of P-PI3K, CyclinA1, and CyclinD1; compared with the NFIC-OE + SF1670 group, the NFIC-OE + SF1670 + TGFβ1-IN-1 group showed significantly increased relative protein expression of P-PI3K, CyclinA1, and CyclinD1 were significantly increased (Fig. 7A). CCK-8 assay results showed that, in SKOV3, OVCAR-3, and A2780 cells after 72 h, compared with the NC group, the OD values in the NFIC-OE group were significantly reduced; compared with the NFIC-OE group, the OD values in the NFIC-OE + TBX2-OE group were significantly increased; compared with the NFIC-OE group, the OD values in the NFIC-OE + SF1670 group were significantly increased; and compared with the NFIC-OE + SF1670 group, the OD values in the NFIC-OE + SF1670 + TGFβ1-IN-1 group were significantly increased (Fig. 7B). Clonogenic assay results showed that, in SKOV3, OVCAR-3, and A2780 cells, compared with the NC group, the number of clones formed in the NFIC-OE group was significantly reduced; compared with the NFIC-OE group, the number of clones formed in the NFIC-OE + TBX2-OE group was significantly increased; compared with the NFIC-OE group, the number of colonies formed in the NFIC-OE + SF1670 group was significantly increased; compared with the NFIC-OE + SF1670 group, the number of colonies formed in the NFIC-OE + SF1670 + TGFβ1-IN-1 group was increased (Fig. 7C). The above results demonstrate that NFIC mediates STAT3/TGFβ1 inhibition of ovarian cancer cell proliferation and cell viability.
NFIC mediates STAT3/TGFβ1 inhibition of ovarian cancer cell proliferation. A: Western blot protein banding plots of P-PI3K, CyclinA1, CyclinD1 as well as statistical plots of relative protein expression in NC group, NFIC-OE group, NFIC-OE + TBX2-OE group, NFIC-OE + SF1670 group and NFIC-OE + SF1670 + TGFβ1-IN-1 group; B: CCK-8 assay results for SKOV3, OVCAR-3, and A2780 cells; C: Results of cloning formation experiments and statistical graphs of clone numbers for SKOV3, OVCAR-3, and A2780 cells. GAPDH as control protein; Data are expressed as mean ± standard deviation. N = 3; **P<0.01.
NFIC mediates TBX2/PTEN to promote apoptosis in ovarian cancer cells
Flow cytometry results showed that, compared with the NC group, the apoptosis rate was significantly increased in the NFIC-OE group; compared with the NFIC-OE group, the apoptosis rate was significantly decreased in the NFIC-OE + TBX2-OE group; compared with the NFIC-OE group, the NFIC-OE + SF1670 group showed a significantly lower apoptosis rate; compared with the NFIC-OE + SF1670 group, the NFIC-OE + SF1670 + TGFβ1-IN-1 group showed a significantly lower apoptosis rate (Fig. 8). The above results indicated that NFIC mediates TBX2/PTEN to promote apoptosis in ovarian cancer cells.
NFIC mediates TBX2/PTEN to promote apoptosis in ovarian cancer cells. A: Flow cytometry analysis of apoptosis in SKOV3 cells from the NC group, NFIC-OE group, NFIC-OE + TBX2-OE group, NFIC-OE + SF1670 group, and NFIC-OE + SF1670 + TGFβ1-IN-1 group; B: Statistical graph of SKOV3 cell apoptosis rate. Data are expressed as mean ± standard deviation. N = 3; **P<0.01; *P<0.05.
NFIC inhibits the progression of epithelial ovarian cancer by regulating the balance of PTEN/TGFβ1/EGR1/BRD4 and SP1/EZH2, thereby inhibiting the TBX2/MMPs signaling pathway
In summary, NFIC promotes TGFβ1 to induce EGR1 transcription, thereby promoting BRD4 expression and enhancing H3K27AC, further promoting TGFβ1 function; NFIC also promotes PTEN expression, which inhibits HIF1α, and HIF1α promotes SP1 expression, which in turn promotes EZH2 transcription. EZH2, together with HDAC3 and SP1, is recruited to PRC2, and EZH2 promotes H3K27me3, thereby inhibiting the transcriptional expression of TBX3 and TGFβ1. TGFβ1 promotes SMAD2/3 to enhance TBX3 function, and TBX3 inhibits TBX2 expression, which promotes MMPs protein function and activates the PI3K signaling pathway, thereby promoting CyclinA1/D1 function to enhance cancer cell proliferation; PTEN inhibits SP1 function, thereby inhibiting EZH2 and PI3K function (Fig. 9).
NFIC inhibits the progression of epithelial ovarian cancer by regulating the balance of PTEN/TGFβ1/EGR1/BRD4 and SP1/EZH2 to inhibit TBX2/MMPs signaling. A: Transcriptional relationship between TGFβ1 and TBX3, EGR1 expression; B: Interactions between EZH2 and HDAC3, and TBX3/TBX2/TGFβ1; C: SP1 promotes EZH2 transcription; D: NFIC promotes PTEN transcription; E: BRD4 inhibits EZH2 function.
Discussion
Epithelial ovarian cancer (epithelial ovarian cancer, EOC) is a highly malignant gynecological tumor with a complex pathogenesis involving abnormal regulation of multiple signaling pathways and molecules. This study, through bioinformatics analysis, revealed the enrichment of significantly differentially expressed genes (Differentially Expressed Genes, DEGs) in epithelial ovarian cancer, particularly in the PI3K, MAPK, and mTOR signaling pathways. These pathways play key roles in cell growth, proliferation, and survival, and their abnormal activation is considered a significant driver of epithelial ovarian cancer development and progression18,19.
One of the most significant intracellular signalling mechanisms is the PI3K pathway. It controls a number of cellular physiological processes, including as metabolism, cell cycle progression, and cell survival, via downstream effectors like Akt and mTOR20. Tumour development and invasiveness are tightly linked to aberrant PI3K signalling pathway activation in a variety of malignancies21. Similarly, the MAPK signalling pathway contributes to cell proliferation, differentiation, and death by controlling molecules including c-Jun N-terminal kinase (JNK) and extracellular signal-related kinase (ERK)22. The mTOR signalling system controls cell growth, metabolism, and autophagy by combining many intracellular and external inputs. Tumour formation may be aided by abnormal activation of these pathways, which may result in metabolic reprogramming, cell cycle dysregulation, and apoptosis suppression.
Neurofibromin 2-like 1 interacting and corepressor, or NFIC, is a crucial transcription factor whose involvement in a number of malignancies is becoming more clear. According to this research, NFIC uses a variety of molecular pathways to prevent the growth of epithelial ovarian cancer. The specific mechanisms by which NFIC acts during tumor development and progression have been explored by several research teams, primarily involving the following aspects:
PTEN (Phosphatase and Tensin Homolog) is an important tumor suppressor gene that inhibits the PI3K/Akt pathway through its phosphatase activity, thereby reducing cell proliferation and survival. NFIC, by promoting PTEN expression, may inhibit the activity of the PI3K/Akt pathway at multiple levels. For example, PTEN can inhibit the effect of HIF1α (Hypoxia-Inducible Factor 1α), reducing the expression of downstream SP1, and subsequently inhibiting the transcription of EZH2. EZH2 is a core component of PRC2 (Polycomb Repressive Complex 2) and through catalyzing the formation of H3K27me3, inhibits the expression of multiple tumor suppressor genes. Therefore, NFIC, by promoting PTEN expression, not only directly inhibits the PI3K/Akt pathway but also indirectly affects EZH2 expression, further inhibiting tumor development.
TGFβ1 (Transforming Growth Factor β1) is a pleiotropic cytokine that plays important roles in cell proliferation, differentiation, apoptosis, and extracellular matrix remodeling23. In the tumor microenvironment, the expression and activity of TGFβ1 can significantly affect the biological behavior of the tumor. This study found a positive correlation between NFIC and TGFβ1, which may suggest that NFIC promotes the expression of TGFβ1, further activating the TGFβ1 signaling pathway. By encouraging the phosphorylation of SMAD2/3, TGFβ1 increases the transcription and expression of TBX324. An essential transcription factor called TBX3 prevents the production of TBX2, which lowers the activity of matrix metalloproteinases (MMPs) and prevents epithelial ovarian cancer from invading and spreading25. Thus, NFIC may be crucial in preventing cancer cell invasion and metastasis by enhancing the function of TGFβ1.
Early Growth Response 1, or EGR1, is a transcription factor that is momentarily expressed and engaged in a number of gene regulation mechanisms and cellular reactions. Given that NFIC and EGR1 have a positive connection, it is possible that NFIC increases EGR1 expression, which in turn increases BRD4 (Bromodomain Containing 4) transcriptional activity. By attaching itself to histone H3K27ac (Histone H3 lysine 27 acetylation), BRD4 stimulates the transcription of genes like TGFβ126. Thus, via EGR1 and BRD4, NFIC may be crucial in promoting the TGFβ1 signalling pathway.
BRD4 is an important epigenetic regulator that participates in gene transcription activation by recognizing H3K27ac27. The role of BRD4 in various cancers has been widely studied, and it regulates cell proliferation and survival by interacting with multiple key transcription factors and signaling molecules. In epithelial ovarian cancer, BRD4 may enhance the transcriptional activity of TGFβ1, further promoting the phosphorylation of SMAD2/3 and the expression of TBX3, thereby inhibiting the invasion and metastasis of cancer cells. Additionally, BRD4 may also promote tumor development through other pathways, such as enhancing the expression of oncogenes like MYC28.
SP1 (Specificity Protein 1) is a ubiquitously expressed transcription factor that regulates the expression of multiple genes by binding to GC boxes in promoter regions. The overexpression of SP1 in the development and progression of tumors is associated with the activation of multiple oncogenes. NFIC, by inhibiting the expression of SP1, may inhibit the transcription and function of EZH2 at multiple levels. EZH2 promotes the growth of tumours by suppressing the expression of many tumour suppressor genes via the catalysis of H3K27me3. Thus, NFIC may aid in restoring the activity of these tumour suppressor genes and preventing the growth of tumours by suppressing the production of SP1.
As a significant oncogene in a variety of malignancies, EZH2 is extensively researched. By preventing the expression of many tumour suppressor genes and catalysing the production of H3K27me3, EZH2 stimulates the growth of tumours29. A poor prognosis is strongly associated with increased expression of EZH2 in epithelial ovarian cancer12. By suppressing SP1 expression, NFIC may limit EZH2’s transcriptional activity at the epigenetic level, preventing H3K27me3 from forming and resuming the function of tumour suppressor genes.
An essential transcription factor that controls cell division, proliferation, and apoptosis is called TBX2 (T-box Transcription Factor 2)30. High TBX2 expression is strongly correlated with tumour invasiveness and metastasis potential in a variety of malignancies. Matrix metalloproteinases, or MMPs, are a class of proteases that may break down the extracellular matrix and are crucial for tumour invasion and metastasis. By suppressing TBX2 expression, NFIC may lower MMP activity, which would prevent epithelial ovarian cancer from invading and spreading. Furthermore, via activating the PI3K signalling pathway, TBX2 promotes the production of cell cycle proteins including CyclinA1 and CyclinD1, which in turn enhances cell survival and proliferation16. Many types of cancer often exhibit aberrant PI3K signalling pathway activation, which controls cell cycle progression, cell survival, and metabolic reprogramming via a number of downstream effectors, including Akt and mTOR. Thus, by suppressing TBX2 expression, NFIC may suppress the PI3K signalling pathway on many levels, which would stop the growth and survival of ovarian cancer cells that are epithelial6.
Tumour cells, stromal cells, immune cells, extracellular matrix, and different cytokines are all part of the tumour microenvironment, or TME, which plays a crucial role in tumour growth and metastasis31. By controlling TGFβ1 and the TGFβ1 signalling pathway, NFIC may have an impact on how immune cells behave in the tumour microenvironment32. By suppressing T cell and natural killer cell activity and encouraging immunological escape, TGFβ1 plays a significant role in immune control. Thus, NFIC may improve immune suppression in the tumour microenvironment and encourage tumour growth by upregulating TGFβ1 expression. However, further research is needed to determine the precise mechanism behind this impact. By controlling MMP activity, NFIC may have an impact on how tumour cells and stromal cells interact. By breaking down the extracellular matrix during tumour invasion and metastasis, MMPs encourage the movement of tumour cells. NFIC may thus be crucial in lowering the contact between tumour cells and stromal cells, which in turn prevents tumour invasion and metastasis, by blocking the activity of MMPs. The extracellular matrix remodelling process may be impacted by changes in NFIC expression. A crucial stage in tumour invasion and metastasis is extracellular matrix remodelling, which is accomplished by a combination of many cytokines and proteases altering the extracellular matrix’s structure and function. By suppressing MMP activity, NFIC may lessen extracellular matrix remodelling on many levels, which would prevent tumour invasion and metastasis33.
A theoretical foundation for creating novel treatment approaches is provided by the clarification of the NFIC mechanism in epithelial ovarian cancer. Targeted treatment targeting molecules including PI3K, TGFβ1, and EZH2 has shown excellent success in treating a variety of malignancies in recent years. PI3K inhibitors: PI3K inhibitors have been extensively researched, and preliminary evidence has shown their effectiveness in treating a variety of malignancies. For instance, by blocking the PI3K signalling pathway, the potent PTEN inhibitor SF1670 lowers cell survival and proliferation34. According to this research, NFIC suppresses the PI3K signalling pathway’s activity by upregulating PTEN expression, which in turn suppresses the growth and survival of cancer cells. Thus, creating medications that target NFIC may be a novel approach to PI3K inhibition, opening up new therapeutic possibilities for epithelial ovarian cancer. TGFβ1 inhibitors: Tumour invasion and metastasis are significantly influenced by the TGFβ1 signalling system. By preventing the phosphorylation of SMAD2/3, TGFβ1-IN-1, an inhibitor of the TGFβ1 signalling pathway, lowers the expression of TBX3 and further suppresses the expression of TBX2 and MMP activity35. According to this research, NFIC inhibits cancer cell invasion and metastasis by increasing the activity of the TGFβ1 signalling system and enhancing the production of TGFβ1. Thus, creating medications that target NFIC may be crucial to increasing the TGFβ1 signalling pathway’s activity and preventing the invasion and metastasis of epithelial ovarian cancer. Inhibitors of EZH2: In many types of cancer, a poor prognosis is tightly linked to high EZH2 expression. EZH2 inhibitors have been extensively researched, and preliminary evidence has shown their effectiveness in treating a variety of malignancies. Tazemetostat, an EZH2 inhibitor, has shown excellent success in treating certain solid tumour types. By suppressing SP1 expression, NFIC lowers EZH2 transcriptional activity, which prevents the production of H3K27me3 and restores the function of many tumour suppressor genes, according to this research. Consequently, the development of medications that target NFIC may reduce EZH2 function on many levels, opening up new therapeutic possibilities for the treatment of epithelial ovarian cancer.
Even though the mechanism of NFIC in epithelial ovarian cancer has been uncovered by this work, other concerns remain that need more research: NFIC’s upstream regulatory mechanisms: Numerous variables, including as transcription factors, microRNAs, and epigenetic changes, control the expression and function of NFIC. Our knowledge of NFIC’s involvement in tumour growth and progression will be strengthened by more research into its upstream regulatory mechanisms, which will serve as a foundation for the creation of novel treatment approaches. NFIC’s relationship with other signalling pathways: Multiple signalling pathways are often abnormally activated during the formation and progression of tumours. A more thorough understanding of NFIC’s function in the tumour microenvironment will be possible with more research on its interactions with other signalling pathways, including Wnt/β-catenin, Notch, and Hippo. This will serve as a theoretical foundation for the development of combination treatment approaches.
Although the mechanism of NFIC in the laboratory has been preliminarily revealed, its application in the clinic still needs further verification. For example, by analyzing the expression level of NFIC in clinical samples, exploring its relationship with the prognosis of epithelial ovarian cancer patients may provide new biomarkers for clinical diagnosis and treatment. The development of drugs targeting NFIC requires a clear understanding of its specific action sites within cells. Therefore, further study of the structure and function of NFIC, determining its interaction sites with key signaling molecules, will provide target information for drug development.
In summary, this study, through bioinformatics analysis and in vitro and in vivo experimental validation, revealed the mechanism of NFIC in epithelial ovarian cancer. NFIC inhibits cancer cell proliferation, invasion, and metastasis by promoting the expression of PTEN, TGFβ1, and EGR1, inhibiting the transcriptional activity of SP1/EZH2, and further regulating multiple key signaling pathways such as PI3K/Akt, MAPK, and mTOR. Additionally, NFIC may further inhibit tumor development by regulating immune cell function, stromal cell interaction, and extracellular matrix remodeling in the tumor microenvironment. These findings not only provide new perspectives on understanding the development and progression of epithelial ovarian cancer but also provide a theoretical basis for developing new therapeutic strategies. Future research should further explore the upstream regulatory mechanisms of NFIC, its interaction with other signaling pathways, and its potential clinical applications.
This study is the first to systematically reveal the tumor-suppressing role of NFIC in epithelial ovarian cancer (EOC) and its molecular mechanisms. Although previous studies have reported the dual regulatory functions of NFIC in breast cancer and gastric cancer (such as inhibiting breast cancer progression or promoting gastric cancer metastasis), its role in ovarian cancer remains unclear. Through integrated bioinformatics analysis and in vitro/in vivo experimental validation, this study has for the first time elucidated that NFIC dynamically regulates the balance between PTEN/TGFβ1/EGR1/BRD4 and SP1/EZH2, thereby inhibiting the TBX2/MMPs signaling axis. This regulatory network has not been reported in ovarian cancer, particularly the mechanism by which NFIC enhances the TGFβ1-EGR1-BRD4 positive feedback loop to promote H3K27ac modification, while simultaneously inhibiting SP1/EZH2-mediated H3K27me3 silencing through PTEN, thereby bidirectionally inhibiting the oncogenic function of TBX2/MMPs; it also revealed for the first time that NFIC regulates the PI3K/Akt/mTOR signaling pathway by targeting TBX2, and confirmed that TBX2 promotes ovarian cancer invasion and proliferation by activating MMPs and cell cycle proteins (Cyclin A1/D1). This finding provides direct experimental evidence for the functional role of TBX2 in ovarian cancer. Combined with inhibitor intervention experiments, it confirms that the anticancer effects of NFIC depend on the dual synergistic action of PTEN and TGFβ1, and proposes for the first time that targeting the NFIC-TBX2 regulatory axis may serve as a potential strategy to overcome EOC drug resistance, offering new directions for the development of multi-targeted combination therapies. In summary, this study not only expands the functional understanding of NFIC in the tumor microenvironment but also provides novel molecular targets and theoretical basis for precision treatment of ovarian cancer, distinguishing itself from previous studies that were limited to single pathways or specific cancer types. This study reveals the tumor-suppressive role of the NFIC-TGFβ1 axis in a specific model, which is not contradictory to the tumor-promoting function of TGFβ1 in advanced cancers but rather reflects its stage-dependent nature. Future studies should systematically evaluate the dynamic regulation of the NFIC-TGFβ1-TBX2 network in different disease progression models, clinical samples, and immune microenvironments, and explore the optimal timing and combination strategies for targeting this pathway (e.g., combining TGFβ inhibitors for advanced patients). Additionally, further investigation is needed to elucidate the molecular switches regulating TGFβ1 signaling differentiation (e.g., SMAD4 deficiency or activation of non-canonical pathways) to refine the theoretical framework of its biphasic action.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
WEBB P M, JORDAN, S. J. Global epidemiology of epithelial ovarian cancer [J]. Nat. Reviews Clin. Oncol. 21 (5), 389–400 (2024).
RINNE, N. et al. Targeting the PI3K/AKT/mTOR pathway in epithelial ovarian cancer, therapeutic treatment options for platinum-resistant ovarian cancer [J]. Cancer Drug Resist. (Alhambra Calif). 4 (3), 573–595 (2021).
YANG H W, LAN, Y. et al. Myricetin suppresses TGF-β-induced epithelial-to-mesenchymal transition in ovarian cancer [J]. Front. Pharmacol. 14, 1288883 (2023).
RAFEHI, S. et al. TGFβ signaling regulates epithelial-mesenchymal plasticity in ovarian cancer ascites-derived spheroids [J]. Endocr. Relat. Cancer. 23 (3), 147–159 (2016).
LIANG, X. et al. ECRG4 represses cell proliferation and invasiveness via NFIC/OGN/NF-κB signaling pathway in bladder Cancer [J]. Front. Genet. 11, 846 (2020).
LV, S. et al. Association of miR-9-5p and NFIC in the Progression of Gastric cancer [J]419603271221084671 (Human & experimental toxicology, 2022).
PAPA, A. & PANDOLFI, P. P. The PTEN⁻PI3K Axis in Cancer [J]. Biomolecules, 9(4):153 (2019).
WANG, B. et al. The role of the transcription factor EGR1 in Cancer [J]. Front. Oncol. 11, 642547 (2021).
DONATI, B. BRD4 and cancer: going beyond transcriptional regulation [J]. Mol. Cancer. 17 (1), 164 (2018).
TIAN, H. et al. Early-immediate gene Egr1 is associated with TGFβ1 regulation of epigenetic reader Bromodomain-containing protein 4 via the canonical Smad3 signaling in hepatic stellate cells in vitro and in vivo [J]. FASEB J. Off. Publ. Fed. Amer. Soc. Exp. Biol. 36(11):e22605 (2022)
JIANG, J. F. et al. Role of Sp1 in atherosclerosis [J]. Mol. Biol. Rep. 49 (10), 9893–9902 (2022).
VERMA, A. et al. EZH2-H3K27me3 mediated KRT14 upregulation promotes TNBC peritoneal metastasis [J]. Nat. Commun. 13 (1), 7344 (2022).
PU F F, SHI D Y, CHEN, T. et al. SP1-induced long non-coding RNA SNHG6 Facilitates the Carcinogenesis of Chondrosarcoma Through Inhibiting KLF6 by Recruiting EZH2 [J]1259 (Cell death & disease, 2021). 1.
PAN, Y. M. et al. STAT3 signaling drives EZH2 transcriptional activation and mediates poor prognosis in gastric cancer [J]. Mol. Cancer. 15 (1), 79 (2016).
GARCíA-AñOVEROS, J. et al. Tbx2 is a master regulator of inner versus outer hair cell differentiation [J]. Nature 605 (7909), 298–303 (2022).
MI, R. et al. Expression and prognosis analysis of TBX2 subfamily in human lung carcinoma [J]. Discover Oncol. 15 (1), 51 (2024).
ROBICHAUD, S. et al. Autophagy is differentially regulated in leukocyte and nonleukocyte foam cells during atherosclerosis [J]. Circul. Res. 130 (6), 831–847 (2022).
KUROKI, L. & GUNTUPALLI, S. R. Treatment of epithelial ovarian cancer [J]. BMJ (Clinical Res. ed). 371, m3773 (2020).
LHEUREUX, S. et al. Epithelial ovarian cancer [J]. Lancet (London England). 393 (10177), 1240–1253 (2019).
DENG, J. et al. Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression [J]. BMC cancer. 19 (1), 618 (2019).
YU, L. & WEI, J. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer [J]. Sem. Cancer Biol. 85, 69–94 (2022).
GUO, Y. J. et al. ERK/MAPK signalling pathway and tumorigenesis [J]. Experimental Therapeutic Med. 19 (3), 1997–2007 (2020).
PENG, D. et al. Targeting TGF-β signal transduction for fibrosis and cancer therapy [J]. Mol. Cancer. 21 (1), 104 (2022).
HU H H et al. New insights into TGF-β/Smad signaling in tissue fibrosis [J]. Chemico-Biol. Interact. 292, 76–83 (2018).
LOPATKA, A. & MOON, A. M. Complex functional redundancy of Tbx2 and Tbx3 in mouse limb development [J]. Dev. Dynamics: Official Publication Am. Association Anatomists. 251 (9), 1613–1627 (2022).
TAN, Z. & WANG, Z. Natural intestinal metabolite xylitol reduces BRD4 levels to mitigate renal fibrosis [J]. Clin. Transl. Sci. 17 (3), e13770 (2024).
EFE, G. et al. p53 Gain-of-Function mutation induces metastasis via BRD4-Dependent CSF-1 expression [J]. Cancer Discov. 13 (12), 2632–2651 (2023).
YU, H. & SHI, T. Elevated nucleus PIGL disrupts the cMyc/BRD4 axis and improves PD-1 Blockade therapy by dampening tumor immune evasion [J]. Cell Mol. Immunol. 20 (8), 867–880 (2023).
PUZYRENKO, A. et al. Inverse co-expression of EZH2 and acetylated H3K27 in prostatic tissue [J]. Annals Diagn. Pathol. 59, 151956 (2022).
PATEL G K, DUTTA, S. et al. TBX2 Drives Neuroendocrine Prostate Cancer through Exosome-Mediated Repression of miR-200c-3p [J]. Cancers, 13(19). (2021).
XIAO, Y. YU D. Tumor Microenvironment as a Therapeutic Target in cancer [J]221107753 (Pharmacology & therapeutics, 2021).
HE, W. et al. Regulatory interplay between NFIC and TGF-β1 in apical papilla-derived stem cells [J]. J. Dent. Res. 93 (5), 496–501 (2014).
FINK, K. BORATYŃSKI J. [The role of metalloproteinases in modification of extracellular matrix in invasive tumor growth, metastasis and angiogenesis] [J]. Postepy higieny i medycyny doswiadczalnej (Online), 66: 609 – 28. (2012).
YUE, C. et al. DOK7 Inhibits Cell Proliferation, Migration, and Invasion of Breast Cancer via the PI3K/PTEN/AKT Pathway [J]. Journal of oncology, 2021: 4035257. (2021).
LEE Y J, PARK J H, OH, S. M. TOPK promotes epithelial-mesenchymal transition and invasion of breast cancer cells through upregulation of TBX3 in TGF-β1/Smad signaling [J]. Biochem. Biophys. Res. Commun. 522 (1), 270–277 (2020).
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Yanan Ren participated in the conception and design of the study, organized the database, and drafted the initial version of the manuscript. Ren Xu、Di Zhang and Luyang Su conducted the experimental treatments. Ying Jin、Na Li and Yazhuo Wang performed the statistical analysis of the data. All authors were involved in revising the manuscript, reviewed, and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was supervised and approved by the Ethics Committee of Hebei General Hospital, Hebei Province. [Ethics Number: 2022002.]
Patient consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanan Ren: First author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ren, Y., Xu, R., Zhang, D. et al. NFIC suppressed the epithelial ovarian cancer via modulating the balance of PTEN/TGFβ1/EGR1/BRD4 and SP1/EZH2 induced Inhibition of TBX2/MMPs signaling. Sci Rep 15, 26593 (2025). https://doi.org/10.1038/s41598-025-09653-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09653-0