Abstract
The use of beneficial microorganisms to enhance phosphate fertilizer use efficiency and solubilize residual phosphorus (P) is a promising strategy to improve soil P availability for plants. This study tested the hypothesis that inoculation with arbuscular mycorrhizal fungi, either alone or in combination with Bacillus subtilis, enhances maize growth and optimizes nutrient availability, particularly phosphorus. The experiment was conducted under greenhouse conditions with four replications, following an 8 × 3 factorial design. Treatments included individual inoculations with Bacillus subtilis (IPACC26), Rhizophagus clarus (RJN102A), Claroideoglomus etunicatum (SCT101A), and a commercial inoculant of Rhizophagus intraradices (Rootella BR ULTRA), as well as three co-inoculations (IPACC26 combined with each fungus) and a non-inoculated control. These treatments were combined with three levels of phosphate fertilization (0, 50, and 100% of the recommended P level). Mycorrhizal colonization improved root architecture and increased photosynthetic pigments and uptake of P and other nutrients, resulting in greater plant growth and biomass production. The most pronounced effects were observed in plants inoculated with R. clarus and C. etunicatum, either alone or in combination with B. subtilis, at the 0 and 50% P levels. At 0% P, inoculated plants accumulated significantly more biomass, with root and shoot dry mass up to 3,000% and 680% higher, respectively, than those of uninoculated plants; this effect was associated with a 1,700% increase in shoot P accumulation compared to the control. These findings highlight the potential of these inoculants as biofertilizers for more sustainable and efficient phosphorus management in maize cultivation.
Similar content being viewed by others
Introduction
The continuous growth of the global population has intensified the demand for higher agricultural productivity to meet global food requirements, leading to the widespread use of external inputs, particularly chemical fertilizers. Fertilizers containing nitrogen (N), phosphorus (P), and potassium (K) are extensively applied, posing significant risks to soil health and water quality1. In tropical soils, phosphorus availability is typically low due to strong fixation by soil minerals, and phosphate fertilizers are often applied in excessive amounts to support crop production. The low P availability results from adsorption onto iron and aluminum oxides or precipitation with aluminum and iron ions, forming mineralogically stable compounds (fixed P)2,3,4.
Agricultural systems are increasingly encouraged to adopt sustainable practices that promote soil health and environmental conservation. Consequently, strategies to optimize phosphate use are critical for minimizing the environmental impacts associated with excessive P application. Soil microorganisms offer a promising alternative for improving nutrient availability while reducing dependence on chemical fertilizers. Well-known beneficial microorganisms, such as arbuscular mycorrhizal fungi (AMF) and plant growth-promoting bacteria (PGPB) play key roles in enhancing plant growth, nutrient uptake, and tolerance to biotic and abiotic stresses5. AMF are obligate biotrophs that colonize the root cortex and develop an extensive mycelial network in the soil surrounding the roots, providing an additional absorptive surface area for the plant. The symbiotic association enhances the plant’s capacity to absorb nutrients with low soil mobility, such as phosphorus6, as well as potassium, manganese, and zinc7.
PGPB can also enhance plant development through various mechanisms, such as the production of indole-3-acetic acid (IAA), which stimulates root growth and consequently nutrient uptake8. These bacteria can colonize the plant rhizosphere or act as endophytes without causing harm. Among the well-known PGPB, Bacillus subtilis exhibits important biochemical capabilities, including IAA production and phosphorus solubilization. In terms of phosphorus solubilization, B. subtilis produces siderophores which, together with other phosphate-solubilizing mechanisms, increase the availability of phosphorus and associated cations in the soil9.
Several studies have shown that co-inoculation with AMF and B. subtilis can benefit plants while reducing dependence on P fertilizers, an approach particularly relevant for economically important crops such as maize (Zea mays L.)10,11,12. We hypothesize that the co-inoculation of AMF and B. subtilis improves maize growth and nutrient uptake by increasing P availability, thereby reducing reliance on phosphate fertilizers and mitigating its associated environmental impacts. This hypothesis was tested by growing maize plants in a greenhouse, inoculated with AMF either individually or in combination with B. subtilis, and evaluating plant growth and nutrient uptake under varying P levels.
Materials and methods
Location, experimental design and treatments
The experiment was conducted in a greenhouse at the Professora Cinobelina Elvas Campus of the Federal University of Piauí (CPCE/UFPI), located in Bom Jesus, Piauí, a semi-arid region of Brazil (9º05’02.0’’ S, 44º19’32.0’’ W; 277 m above sea level). The study was carried out from November 2023 to January 2024, using a randomized block design arranged in an 8 × 3 factorial scheme, with four replicates per treatment.
The factors evaluated were: (1) eight microorganism treatments, including individual inoculations with Bacillus subtilis (IPACC26), Rhizophagus clarus (RJN102A), Claroideoglomus etunicatum (SCT101A), a commercial inoculant (Rootella BR ULTRA, based on Rhizophagus intraradices), and three co-inoculations (B. subtilis + Rootella BR ULTRA, B. subtilis + R. clarus, and B. subtilis + C. etunicatum), and a non-inoculated control; and (2) three phosphate fertilization levels, with P at 0, 50, and 100% of recommended level for maize14.
Soil preparation and experimental conditions
Plants were cultivated in polyethylene pots filled with 8 kg (dry weight) of soil, which had been sterilized in an autoclave at 121 °C and 1.0 atm for 1 h. The soil employed for experiment, classified as dystrophic Yellow Latosol13, was collected at 0–20 cm depth in an area with of native vegetation in the semi-arid region of Piauí, Brazil (9°04’45.2” S and 44°19’36.8” W). Simple soil samples were homogenized to form a composite sample, which exhibited the following characteristics: pH = 4.8; available P (Mehlich-1) = 4.1 mg dm⁻³; K = 62 mg dm⁻³; Ca = 0.55 cmolc dm⁻³; Mg = 0.05 cmolc dm⁻³; Al = 2.5 cmolc dm⁻³; H + Al = 3.67 cmolc dm⁻³; sum of bases = 0.76 cmolc dm⁻³; potential cation exchange capacity = 4.43 cmolc dm⁻³; Cu = 0.32 mg dm⁻³; Mn = 16.9 mg dm⁻³; Fe = 63.5 mg dm⁻³; Zn = 1.39 mg dm⁻³; base saturation = 17.1%; aluminum saturation = 76.7%; organic matter = 1.83%; sand = 760 g kg⁻¹; silt = 27 g kg⁻¹; clay = 213 g kg⁻¹.
Soil correction was carried out by increasing base saturation to 60%, following the recommended guidelines for maize cultivation14. Thirty days after liming, phosphate fertilization was applied at three levels: 0, 50, and 100% of the recommended level, corresponding to 0, 50, and 100 kg ha⁻¹ of P₂O₅ (as single superphosphate, 18%), respectively. Additionally, 120 kg ha⁻¹ of K₂O (as potassium chloride, 60%), 2.0 kg ha⁻¹ of Cu (as copper sulfate, 26.23%), 1.5 kg ha⁻¹ of Zn (as zinc sulfate, 22.73%), and 160 kg ha⁻¹ of N (as urea, 45%) were applied14. Fertilizers were applied to each pot and incorporated into the soil prior to sowing. The nitrogen fertilization was done in three applications: the first one incorporated at planting, and two subsequent topdressings applied as urea solution in circular bands around the plants.
The strains Rhizophagus clarus (RJN102A) and Claroideoglomus etunicatum (SCT101A) were obtained from the International Culture Collection of Glomeromycota (CICG) at FURB, in Santa Catarina, Brazil. The inoculum consisted of a soil-based mixture containing root fragments, hyphae, and spores. The C. etunicatum and R. clarus inoculants contained approximately 8 and 10 spores per gram of soil, respectively. The commercial inoculant Rootella BR ULTRA, based on vermiculite and clay, contained approximately 167,000 propagules of Rhizophagus intraradices per gram of product. The Bacillus subtilis strain (IPACC26), selected by Aquino et al.15 for its potential in IAA production and maize growth promotion, was cultured in TSB medium at 28 °C and 120 rpm for three days.
At sowing, three maize seeds (hybrid BM970VIP3) were sown per pot after surface disinfection with 70% ethanol for 30 s and 2% sodium hypochlorite for two minutes, followed by rinsing with distilled water. The inoculants of R. clarus and C. etunicatum were applied at 5.0 g per pot, directly into the sowing furrow beneath the seeds, in accordance with CICG guidelines. The Rootella BR ULTRA inoculant was applied according to the manufacturer’s recommended rate (16 g ha⁻¹), adjusted to 0.052 mg per pot, and placed in the sowing furrow beneath the seeds to ensure effective root contact during germination. The B. subtilis inoculant was applied at a rate of 1.0 mL of bacterial suspension (~ 1 × 10⁸ cells mL⁻¹) per seed, directly onto the seed surface at sowing.
Thinning was performed 10 days after sowing (DAS), leaving one plant per pot. Soil moisture was maintained at 80% of field capacity throughout the experiment. During the experimental period, air temperature inside the greenhouse ranged from 25 to 34 °C (minimum) and 27 to 38 °C (maximum), while relative humidity varied between 39% and 80%.
Morphological assessments of shoots and roots and biomass production
Plants were harvested at 55 DAS at the onset of flowering stage. Firstly, photographic records of the plants were taken and later processed using CorelDraw software. Stem length, basal diameter, and number of leaves were measured. Root morphology images were captured using an EPSON LA2400 scanner and processed using RhizoVision Explorer software, in order to obtain total root length, average root diameter, number of branches, and root surface area. Subsequently, 10 g of roots per treatment were separated for mycorrhizal colonization assessment. Shoots and the remaining roots were oven-dried at 60 °C in a forced-air chamber until reaching constant weight.
Mycorrhizal colonization
To determine the mycorrhizal colonization rate (% MC), 10 g of fresh roots were cleared with 10% KOH, acidified with 1% HCl, and stained with Trypan Blue, following the method of Phillips and Hayman16. Colonization was quantified by counting fungal structures on microscope slides using the gridline intersection method17. Observations were made using a plan achromatic optical microscope equipped with LED illumination and 40× magnification. Images were captured using a 12-megapixel mobile camera mounted on the microscope lens. For each sample, 50 intersections were analyzed, and the mycorrhizal structures were quantified separately (hyphae, arbuscules, and vesicles). The total colonization percentage was determined according to the following equation:
The colonization percentages for hyphae, arbuscules, and vesicles were also calculated separately using the same equation, replacing the numerator with the number of intersections containing each specific structure, according to the methodology adapted from Giovannetti and Mosse18.
Quantification of soil protein related to easily extractable glomalin (SPRG-EE)
To obtain SPRG-EE, 1.0 g of dry rhizospheric soil from each treatment was sieved through a 2.0 mm mesh and mixed with 8.0 mL of sodium citrate solution (0.02 mol L⁻¹, pH 7.0). The mixture was autoclaved at 121 °C for 30 min. Subsequently, samples were centrifuged at 3,500 rpm for 10 min, and the supernatant was collected. Protein quantification was performed using the Bradford method19, employing Coomassie Brilliant Blue G-250 dye and spectrophotometric readings at 590 nm. The readings, based on the method of Wright and Upadhyaya, were calibrated against a standard protein concentration curve using bovine serum albumin (BSA)20. Finally, SPRG-EE concentration was expressed as mg of glomalin per gram of soil, accounting for the total supernatant volume and dry soil weight.
Nutrient accumulation and phosphorus use efficiency (PUE)
Phosphorus (P), potassium (K), zinc (Zn), manganese (Mn), and iron (Fe) were selected for quantification due to their low mobility in the soil, while calcium (Ca) and magnesium (Mg) were included because their uptake is strongly influenced by root interception21. Other nutrients were not measured due to the limited availability of plant dry mass, particularly in the non-inoculated control. Samples of 0.5 g of dried and ground shoot or root material were subjected to nitro-perchloric acid digestion22. P content was determined by spectrophotometry using the yellow vanadate method, while K content was assessed by flame photometry22. The concentrations of Ca and Mg, as well as those of the micronutrients (Fe, Zn, and Mn), were quantified using atomic absorption spectrophotometry. Nutrient accumulation was calculated by multiplying the dry mass (g) by the nutrient concentration (%) in the shoot and root, and then dividing the result by 100. Phosphorus use efficiency (PUE) was evaluated using the balance method described by Syers, Johnston, and Curtin23, in which the percentage recovery of applied P is calculated as:
Photosynthetic pigments and leaf temperature
To determine photosynthetic pigments, 1.0 cm diameter leaf discs were collected from the middle third of the leaf at 55 DAS. The discs were incubated in calcium carbonate (CaCO₃) saturated with DMSO and kept in the dark for five days. Subsequently, spectrophotometric readings were taken using a microplate reader (MRXA200) at wavelengths of 665, 649, and 480 nm. The concentrations of chlorophyll a, chlorophyll b, and carotenoids were calculated using the equations proposed by Wellburn24. Leaf temperature was measured using a thermographic camera between 10:00 and 11:00 a.m., during a period of stable ambient temperature and transpiration rates. Measurements were taken from the middle third of the leaf to minimize variability between younger and older leaves.
Statistical analysis
The data were subjected to normality testing (Shapiro-Wilk) and homogeneity of variance testing (Bartlett). Upon meeting the assumptions, analysis of variance (ANOVA) and the Scott-Knott mean comparison test (α = 0.05) were conducted using Sisvar 5.6. Means were graphically represented using SigmaPlot (version 14.0). Additionally, Principal Component Analysis (PCA) and hierarchical cluster analysis (dendrogram) were performed to evaluate the relationships among variables, inoculation treatments, and phosphorus levels.
Results
Mycorrhizal colonization
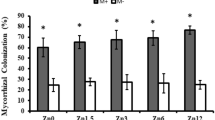
Microscopic images of maize roots confirmed the presence of fungal structures, such as arbuscules, hyphae, vesicles, and spores, in all AMF-treated plants (Fig. S1B-G). Hyphae were the most abundant fungal structures. In contrast, no AMF colonization was detected in the individual B. subtilis inoculation or in the non-inoculated control (Fig. S1A, H). Colonization rates in treatments without P or with 50% P were higher than those in 100% P treatments (Fig. 1A). Colonization rates ranged from 33 to 90% in plants without P fertilization and from 20 to 65% in plants with 50% P, when inoculated with Rootella BR ULTRA or B. subtilis + R. clarus, respectively.
Plants grown without P exhibited greater hyphal colonization when inoculated with R. clarus, C. etunicatum, or their respective co-inoculations by B. subtilis, with colonization rates ranging from 37 to 45% (Fig. 1B). In contrast, inoculation with Rootella BR ULTRA resulted in low hyphal colonization rates. The C. etunicatum was the AMF that promoted the highest arbuscular (35%) and vesicular (32%) colonization rates in plants without P fertilization (Fig. 1C, D).
Mycorrhizal colonization (A), colonization by hyphae (B), colonization by arbuscules (C), and colonization by vesicles (D) observed in roots of maize plants with different inoculations (Rootella BR ULTRA, Rhizophagus clarus, Claroideoglomus etunicatum, Bacillus subtilis + Rootella BR ULTRA, B. subtilis + R. clarus, B. subtilis + C. etunicatum) and phosphorus levels (0, 50, and 100%). Uppercase letters compare inoculations within each phosphorus level, and lowercase letters compare levels within each inoculation. Means followed by the same letters do not differ significantly by the Scott-Knott test at 5%. ANOVA F values: ** = significant at 5%; ns = not significant.
Soil protein related to easily extractable glomalin (SPRG-EE)
SPRG-EE levels significantly decreased at the 100% P level, except in treatments with R. clarus and B. subtilis + Rootella BR ULTRA (Fig. 2). Without P fertilization, all inoculated treatments increased SPRG-EE, particularly B. subtilis + R. clarus with a ~ 200% increase compared to the control. At 50% P, most inoculations enhanced SPRG-EE, with increases ranging from 30% for C. etunicatum to 45% for B. subtilis + C. etunicatum compared to the control. Under 100% P, only R. clarus and B. subtilis + Rootella BR ULTRA promoted significantly increases at approximately 36% and 45%, respectively, compared to the control.
Soil protein related to easily extractable glomalin (SPRG-EE) from maize plants with different inoculations (Bacillus subtilis, Rootella BR ULTRA, Rhizophagus clarus, Claroideoglomus etunicatum, B. subtilis + Rootella BR ULTRA, B. subtilis + R. clarus, B. subtilis + C. etunicatum, and non-inoculated control) and phosphorus levels (0, 50, and 100%). Uppercase letters compare inoculations within each phosphorus level, and lowercase letters compare levels within each inoculation. Means followed by the same letters do not differ significantly by the Scott-Knott test at 5%. ANOVA F values: ** = significant at 5%; ns = not significant.
Morphological characteristics of roots and shoots and biomass production
Root development was significantly reduced in the absence of P compared to the 50% and 100% P levels (Figs. 3, S2). Almost all inoculations improved root architecture compared to the non-inoculated control (Fig. 3), particularly at the 0% and 50% P levels. Without P fertilization, inoculations with R. clarus, C. etunicatum, and their co-inoculations enhanced root length (RL), mean root diameter (MRD), average number of branches (ANB), and root surface area (RSA) (Fig. 3A-D). Additionally, B. subtilis and Rootella BR ULTRA, when applied individually, also increased ANB and SRA. For root dry mass (RDM), only R. clarus, C. etunicatum, and B. subtilis + R. clarus significantly increased biomass, with gains of up to 3,000% compared to the non-inoculated control (Fig. 3E).
Root length (A), Mean root diameter (B), average number of branches (C), root surface area (D), and root dry mass (E) of maize plants with different inoculations (Bacillus subtilis, Rootella BR ULTRA, Rhizophagus clarus, Claroideoglomus etunicatum, B. subtilis + Rootella BR ULTRA, B. subtilis + R. clarus, B. subtilis + C. etunicatum, and non-inoculated control) and phosphorus levels (0, 50, and 100%). Uppercase letters compare inoculations within each phosphorus level, and lowercase letters compare levels within each inoculation. Means followed by the same letters do not differ significantly by the Scott-Knott test at 5%. ANOVA F values: ** = significant at 5%; ns = not significant.
At 50% P, the highest RL values were observed in plants inoculated with B. subtilis, Rootella BR ULTRA, R. clarus, or B. subtilis + C. etunicatum (Fig. 3A), whereas the highest RDM was recorded in plants inoculated with B. subtilis + R. clarus (Fig. 3E). At 100% P, inoculations had no effect on RL, MRD, or SRA, but enhanced ANB and RDM (Fig. 3C, E). The most pronounced effects were observed in plants treated with Rootella BR ULTRA and B. subtilis + R. clarus, which increased RDM by 87% and 89%, respectively, relative to the control (Fig. 3E).
All treatments without P application resulted in reduced plant length (PL), number of leaves (NL), basal stem diameter (BSD), and shoot dry mass (SDM) compared to those with 50% and 100% P (Figs. 4A-D, S3). Inoculations with R. clarus, B. subtilis + R. clarus, C. etunicatum, and B. subtilis + C. etunicatum significantly increased PL, NL, and BSD compared to the control at the 0% P level (Fig. 4A-C). Furthermore, these treatments markedly enhanced shoot dry mass (SDM), with increases ranging from 557% for B. subtilis + C. etunicatum to 680% for B. subtilis + R. clarus relative to the control (Fig. 4D).
Plant length (A), number of leaves (B) and basal stem diameter (C), and shoot dry mass (SDM) (D) of maize plants with different inoculations (Bacillus subtilis, Rootella BR ULTRA, Rhizophagus clarus, Claroideoglomus etunicatum, B. subtilis + Rootella BR ULTRA, B. subtilis + R. clarus, B. subtilis, C. etunicatum, and non-inoculated control) and phosphorus levels (0, 50, and 100%). Uppercase letters compare inoculations within each phosphorus level, and lowercase letters compare levels within each inoculation. Means followed by the same letters do not differ significantly by the Scott-Knott test at 5%. ANOVA F values: ** = significant at 5%; ns = not significant.
At the 50% P level, all inoculations increased PL, NL, and BSD compared to the control, except for C. etunicatum, which had no effect on PL (Fig. 4A-C). Significant increases in SDM were also observed with B. subtilis, R. clarus, B. subtilis + R. clarus, and Rootella BR ULTRA, with gains of up to 18%. At the100% P, inoculation had no significant effect on the shoot morphological parameters (Fig. 4D).
Photosynthetic pigments and leaf temperature
Co-inoculation with B. subtilis + C. etunicatum and B. subtilis + R. clarus significantly increased chlorophyll a, b, and total chlorophyll concentrations at the 0% P level compared to higher P levels (Fig. 5A-C). In contrast, carotenoid content was generally higher at the 100% P level across most treatments compared to the 0% P level (Fig. 5D). At the 0% P level, B. subtilis + R. clarus and B. subtilis + C. etunicatum significantly increased chlorophyll a, b, and total chlorophyll content. Almost all inoculated treatments increased carotenoid content compared to the control, with B. subtilis + C. etunicatum promoting an increase of approximately 65%. At the 50% P, Rootella BR ULTRA, C. etunicatum, B. subtilis + R. clarus, and B. subtilis + C. etunicatum increased chlorophyll b levels. At the 100% level, most inoculations increased photosynthetic pigment levels.
Leaf temperature was higher in non-inoculated plants or those treated solely with B. subtilis and Rootella BR ULTRA in the absence of P fertilization (Fig. 5E). Conversely, plants inoculated with R. clarus, B. subtilis + R. clarus, C. etunicatum, and B. subtilis + C. etunicatum exhibited lower leaf temperatures. At 50% and 100% P levels, no significant differences were observed among treatments.
P accumulation and use efficiency (PUE)
Phosphorus application at the 50% and 100% levels significantly increased P accumulation in the shoot and roots, as well as total P accumulation, compared to the 0% P level (Fig. 6A-C). Phosphorus use efficiency (PUE) at the 100% P level was higher than at 50%, except in the B. subtilis + R. clarus treatment, which resulted in similar PUE values at both levels (Fig. 6D).
Chlorophyll a (A), chlorophyll b (B), total chlorophyll (C), and carotenoid (D) contents, and leaf temperature (E) of maize plants with different inoculations (Bacillus subtilis, Rootella BR ULTRA, Rhizophagus clarus, Claroideoglomus etunicatum, B. subtilis + Rootella BR ULTRA, B. subtilis + R. clarus, B. subtilis + C. etunicatum, and non-inoculated control) and phosphorus levels (0, 50, and 100%). Uppercase letters compare inoculations within each phosphorus level, and lowercase letters compare levels within each inoculation. Means followed by the same letters do not differ significantly by the Scott-Knott test at 5%. ANOVA F values: ** = significant at 5%; ns = not significant.
Phosphorus accumulation in the shoots (A), in the root (B), and total (C), and phosphorus use efficiency (D) of maize plants with different inoculations (Bacillus subtilis, Rootella BR ULTRA, Rhizophagus clarus, Claroideoglomus etunicatum, B. subtilis + Rootella BR ULTRA, B. subtilis + R. clarus, B. subtilis, C. etunicatum, and non-inoculated control) and phosphorus levels (0, 50, and 100%). Uppercase letters compare inoculations within each phosphorus level, and lowercase letters compare levels within each inoculation. Means followed by the same letters do not differ significantly by the Scott-Knott test at 5%. ANOVA F values: ** = significant at 5%; ns = not significant.
In the absence of P fertilization, only R. clarus increased P accumulation in the roots (Fig. 6B), while R. clarus, C. etunicatum, B. subtilis + R. clarus, and B. subtilis + C. etunicatum enhanced both shoot and total P accumulation (Fig. 6A, C). At this P level, inoculation with R. clarus resulted in an increase of up to 1,700% in shoot P accumulation compared to the control. At the 50% P level, B. subtilis, R. clarus, and B. subtilis + R. clarus increased shoot P accumulation and PUE, with gains of up to 47% and 38%, respectively, compared to the control. At the 100% P level, inoculations increased root P accumulation, except for R. clarus. Additionally, PUE was improved by Rootella BR ULTRA, R. clarus, and C. etunicatum, with the latter promoting a 19% increase compared to the control.
The highest accumulations of K, Ca, Mg, Fe, Zn, and Mn were observed at the 100% and 50% P levels, in both shoot and roots, with variations depending on the inoculation treatment (Figs. S4, S5). Overall, treatments involving R. clarus, C. etunicatum, B. subtilis + R. clarus, and B. subtilis + C. etunicatum were key contributors to the accumulation of macro- and micronutrients in plant tissues, regardless of the P level.
Principal component analysis (PCA) and a cluster dendrogram
The first and second principal components explained 57.06 and 21.19% of the data variability, respectively, accounting for a total of 78.25% of the variance (Fig. 7A).
Principal component analysis (PCA) (A) and cluster dendrogram (B) for variables total phosphorus (P total), phosphorus in shoots (P in shoots), phosphorus in roots (P in roots), shoot dry mass (SDM), plant length (PL), root dry mass (RDM), root length (RL), mean root diameter (MRD), average number of root branches (ANB), specific root surface area (SRA), basal stem diameter (BSD), chlorophyll a, chlorophyll b, total chlorophyll, carotenoids, leaf temperature (LT), and soil protein related to easily extractable glomalin (SPRG-EE) of maize plants with different inoculations (Bacillus subtilis, Rootella BR ULTRA, Rhizophagus clarus, Claroideoglomus etunicatum, B. subtilis + Rootella BR ULTRA, B. subtilis + R. clarus, B. subtilis + C. etunicatum, and non-inoculated treatment) and phosphorus levels (0, 50, and 100%).
Overall, the PCA revealed that both P levels and inoculation types significantly influenced the analyzed variables. Four distinct groups were identified based on the evaluated parameters across treatments (Fig. 7A, B). Groups 1 and 2 comprised treatments without P fertilization. Group 1 included R. clarus and C. etunicatum, either alone or in combination with B. subtilis, and exhibited the strongest association with photosynthetic pigments and SPRG-EE. Group 2 consisted of inoculations with B. subtilis, Rootella BR ULTRA, B. subtilis + Rootella BR ULTRA, and the uninoculated control, which was closely associated with leaf temperature. Group 3 included all treatments at the 50% P level and three treatments at the 100% P level (C. etunicatum, Rootella BR ULTRA, and the uninoculated treatment). This group was associated with variables related to growth, biomass production, and P accumulation in plants. Group 4 comprised exclusively treatments at the 100% P level and was primarily associated with root length and the average number of root branches.
Discussion
Our study provides evidence that inoculation with AMF, either alone or in combination with B. subtilis, benefits maize plants depending on phosphorus availability. The positive effect of AMF on root colonization rates at the 0% and 50% P levels (Fig. 1) suggests effective symbiosis even under P-limited conditions. Notably, B. subtilis enhanced AMF colonization, highlighting the potential of co-inoculation to strengthen the AMF–maize symbiosis under reduced P fertilization. In contrast, colonization decreased at the 100% P level, likely due to plant-mediated suppression of symbiosis-related genes essential for mycorrhizal colonization, including those involved in carotenoid and strigolactone synthesis25,26. This feedback mechanism helps conserve plant carbon under P-sufficient conditions, thereby reducing the incentive for mycorrhizal associations27.
The reduced AMF colonization at the 100% P level may have also limited glomalin production (Fig. 2), a glycoprotein synthesized by AMF that is crucial for soil structure and plant growth28,29,30. Variability in glomalin synthesis among AMF species, potentially influenced by high P availability, could have further contributed to this decline. Interestingly, the detection of glomalin (SPRG-EE) in uninoculated control suggests the presence of residual glomalin from native AMF populations, consistent with studies reporting glomalin persistence in areas of native vegetation within stable ecosystems29.
Phosphorus availability is key determinant for cell division, nutrient uptake and photosynthesis due to its role as a structural component and cofactor in biological reactions31. Phosphorus deficiency commonly results in sharp declines in biomass and morphological traits (Figs. 3, 4). Our findings indicate that AMF inoculation partially mitigates P deficiency in maize, particularly in treatments with R. clarus, C. etunicatum, and their co-inoculation with B. subtilis (Figs. S2, S3). This effect was associated with the highest mycorrhizal colonization rates (Fig. 1), which facilitated improved root exploration and nutrient acquisition. The enhanced root architecture and biomass production are also attributed to the B. subtilis-mediated synthesis of indole-3-acetic acid (IAA), a phytohormone that stimulates root development and subsequently enhances nutrient uptake15.
Under P deficiency, maize plants typically exhibit reduced photosynthetic pigments32,33. However, AMF-inoculated plants at 0% and 50% P showed increased chlorophyll and carotenoid contents compared to non-inoculated plants (Fig. 5), suggesting that AMF contribute to maintaining photosynthetic efficiency under nutrient stress33,34. Carotenoids, which also function as antioxidants, were especially elevated in B. subtilis + C. etunicatum co-inoculated plants (Fig. 5), indicating an enhanced antioxidant defense against free radicals and lipid peroxidation35,36. Additionally, AMF inoculation reduced leaf temperature under P deficiency, an indicator of stress alleviation, likely through improved photosystem II regulation37.
At 50% P fertilization, plants inoculated with AMF, either alone or in combination with B. subtilis, accumulated P and other nutrients at levels nearly equivalent to those observed at the 100% P level (Fig. 6, S4, S5), confirming the potential of microorganisms to enhance maize nutrition. Increases in P accumulation promoted by AMF were notably greater in treatments without P than at higher P levels, indicating enhanced mycorrhizal uptake efficiency when phosphorus is limited. The most pronounced benefits were observed with R. clarus, C. etunicatum, B. subtilis + R. clarus, and B. subtilis + C. etunicatum. Mycorrhizal pathways provide a more energy-efficient alternative to root-mediated nutrient uptake by extending hyphal networks into the soil without the need for extensive root growth38,39.
The greatest increases in P accumulation and phosphorus use efficiency (PUE) observed with B. subtilis + R. clarus at the 50% P suggest that co-inoculation is particularly effective in improving phosphorus nutrition in maize under moderate P availability. Phosphate-solubilizing Bacillus species, such as the one used in this study (B. subtilis strain IPACC26), can further enhance P availability when associated with AMF by stimulating root exudation, promoting the activity of enzymes such as phosphatases, and increasing the release of organic acids40,41. Additionally, the IPACC26 strain produces IAA15, which contributes to improved root architecture and enhanced nutrient uptake.
Our results are consistent with the findings of Stoffel et al.42, who observed increases in biomass and P uptake in maize following inoculation with Rhizophagus intraradices, particularly in soils with low to moderate phosphorus levels. Similarly, Song et al.10 demonstrated that co-inoculation with R. intraradices and PGPB at 50% of the recommended P level led to significant improvements in maize growth and P acquisition. Collectively, these findings highlight the synergistic potential of AMF and PGPB to enhance phosphorus use efficiency and plant performance under suboptimal phosphorus conditions.
Finally, principal component analysis showed that both single AMF inoculation and co-inoculation with B. subtilis, combined with 50% of the recommended P level, improved biomass, morphological traits, and P accumulation in maize plants, achieving efficiency comparable to that of the uninoculated control under full P fertilization (100%) (Fig. 7). These results highlight the potential of microbial inoculants for maize cultivation, representing a viable alternative to reduce dependence on chemical phosphate fertilizers and promote more sustainable agricultural practices.
Conclusion
This research demonstrates that mycorrhizal colonization, either alone or in combination with Bacillus subtilis, increases soil glomalin content and enhances root architecture, plant growth, and phosphorus nutrition in maize plants. Rhizophagus clarus and Claroideoglomus etunicatum showed the greatest potential as biofertilizers, particularly in soils with low to moderate phosphorus availability. Future field studies are needed to validate the effectiveness observed in greenhouse trials. The use of AMF inoculation represents a promising strategy to reduce reliance on chemical phosphate fertilizers, thereby promoting more sustainable and cost-effective agricultural practices.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and supplementary material of this article.
References
Wang, N et al. Impacts of nitrogen (N), phosphorus (P), and potassium (K) fertilizers on maize yields, nutrient use efficiency, and soil nutrient balance: insights from a long-term diverse NPK omission experiment in the North China plain. Field Crops Res. 318, 109616; https://doi.org/10.1016/j.fcr.2024.109616 (2024).
Paiva, M et al. Phosphorus in alkaline soils of the semiarid region, brazil: inorganic fractions, capacity factor, and availability. Int. J. Phytoremediation 25, 965–980; https://doi.org/10.1080/15226514.2022.2124232 (2022).
Szerlag, KD et al. Systematic study of legacy phosphorus (P) desorption mechanisms in high-P agricultural soils. Minerals 12(4), 458; https://doi.org/10.3390/min12040458 (2022).
Bedassa, M. Fractionation and distribution of phosphorus in acid soils: review. Int. J. Hortic. Food Sci. 5(1), 64–70; https://doi.org/10.33545/26631067.2023.v5.i1b.159 (2023).
Obianuju, CE & Olubukola, OB. Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria. Microbiol. Res. 239, 126569; https://doi.org/10.1016/j.micres.2020.126569 (2020).
Ezawa, T & Saito, K. How do arbuscular mycorrhizal fungi handle phosphate? New insight into fine-tuning of phosphate metabolism. New Phytol. 220(4), 1116–1121; https://doi.org/10.1111/nph.15187 (2018).
Rocha, I et al. Seed coating with inocula of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria for nutritional enhancement of maize under different fertilization regimes. Arch. Agron. Soil Sci. 65(1), 31–43; https://doi.org/10.1080/03650340.2018.1479061 (2018).
Silvestrini, GR et al. Potential use of phosphate-solubilizing bacteria in soybean culture. AgriEngineering 5(3), 1544–1554; https://doi.org/10.3390/agriengineering5030095 (2023).
Cui, K et al. Siderophores, a potential phosphate solubilizer from the endophyte Streptomyces sp. Cot10, improved phosphorus mobilization for host plant growth and rhizosphere modulation. J. Clean. Prod. 367, 133110367; https://doi.org/10.1016/j.jclepro.2022.133110 (2022).
Song, C et al. Mycorrhizosphere bacteria and plant-plant interactions facilitate maize P acquisition in an intercropping system. J. Clean. Prod. 314, 127993; https://doi.org/10.1016/j.jclepro.2021.127993 (2021).
Liu, W et al. Arbuscular mycorrhizal fungi in soil and roots respond differently to phosphorus inputs in an intensively managed calcareous agricultural soil. Sci. Rep. 6(24902), 1–11; https://doi.org/10.1038/srep24902 (2016).
Battini, F, Gronlund, M, Agnolucci, M, Giovannetti, M & Jakobsen, I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 7(4686); https://doi.org/10.1038/s41598-017-04959-0 (2017).
Santos, HG. Sistema Brasileiro de Classificação de Solos (5ª ed.) 180–181 (Embrapa, 2018).
Sousa, DMG & Lobato, E. Cerrado: Correção Do Solo E Adubação 416 (Embrapa Cerrados, 2004).
Aquino, JPA et al. Plant growth-promoting bacteria improve growth and nitrogen metabolism in maize and sorghum. Theor. Exp. Plant Physiol. 33, 249–260; https://doi.org/10.1007/s40626-021-00209-x (2021).
Phillips, JM & Hayman, DS. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Brit. Mycol. Soc. 55(18), 158–161; https://doi.org/10.1016/S0007-1536(70)80110-3 (1970).
Mcgonigle, TP, Miller, MH, Evans, DG, Fairchild, GL & Swan, JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 115(3), 495–501; 10.1111/j.1469-8137.1990.tb00476.x (1990).
Giovannetti, M & Mosse, B. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol. 84(3), 489–500; https://doi.org/10.1111/j.1469-8137.1980.tb04556.x (1980).
Bradford, MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72(1–2), 248–254; https://doi.org/10.1016/0003-2697(76)90527-3 (1976).
Wright, SF & Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant and Soil 198, 97–107; https://doi.org/10.1023/A:1004347701584 (1998).
Prado, RM. Nutrição De Plantas (2ª ed.) (Editora Unesp, 2020).
Silva, FC. Manual De Análises Químicas De Solos, Plantas E Fertilizantes (2ª ed.) 627 (Embrapa Informação Tecnológica & Embrapa Solos, 2009).
Syers, JK, Johnston, A & Curtin, D. Efficiency of soil and fertilizer phosphorus use: reconciling changing concepts of soil phosphorus behaviour with agronomic information. Preprint at https://www.fao.org/4/a1595e/a1595e00.htm
Wellburn, AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol.144(3), 307–313; https://doi.org/10.1016/S0176-1617(11)81192-2 (1994).
Nguyen, T, Cavagnaro, T & Watts-Williams, S. The effects of soil phosphorus and zinc availability on plant responses to mycorrhizal fungi: a physiological and molecular assessment. Sci. Rep. 9(14880); https://doi.org/10.1038/s41598-019-51369-5 (2019).
Chen, P et al. Strigolactones shape the assembly of root-associated microbiota in response to phosphorus availability. mSystems 9(6), e0112423; https://doi.org/10.1128/msystems.01124-23 (2024).
Lucic-Mercy, E, Mercy, L, Jeschke, A, Schneider, C & Franken, P. Short-term artificial adaptation of Rhizoglomus irregulare to high phosphate levels and its implications for fungal-plant interactions: phenotypic and transcriptomic insights. Front. Plant Sci. 15; https://doi.org/10.3389/fpls.2024.1385245 (2024).
Lovelock, C, Wright, S, Clark, D & Ruess, R. Soil stocks of glomalin produced by arbuscular mycorrhizal fungi across a tropical rain forest landscape. J. Ecol. 92(2), 278–287; https://doi.org/10.1111/j.0022-0477.2004.00855.x (2004).
Rillig, MC, Ramsey, PW, Morris, S & Paul, EA. Glomalin, an arbuscular-mycorrhizal fungal soil protein, responds to land-use change. Plant Soil 253, 293–299; https://doi.org/10.1023/A:1024807820579 (2003).
Driver, JD, Holben, WE, Rilling, MC. Characterization of glomalin as a hyphal wall component of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 37(1), 101–106; https://doi.org/10.1016/j.soilbio.2004.06.011 (2005).
Sun, Y et al. Phosphorus partitioning contribute to phosphorus use efficiency during grain filling in Zea mays. Front. Plant Sci. 14; https://doi.org/10.3389/fpls.2023.1223532 (2023).
Ashraf, M & Harris, PJC. Photosynthesis under stressful environments: an overview. Photosynthetica 51(2), 163–190; https://doi.org/10.1007/s11099-013-0021-6 (2013).
Verlinden, MS et al. Phosphorus stress strongly reduced plant physiological activity, but only temporarily, in a mesocosm experiment with Zea mays colonized by arbuscular mycorrhizal fungi, Biogeosciences, 19, 2353–2364; https://doi.org/10.5194/bg-19-2353-2022 (2022).
Santana, LR et al. Arbuscular mycorrhizal fungi associated with maize plants during hydric deficit. Sci. Rep. 13(1519); https://doi.org/10.1038/s41598-023-28744-4 (2023).
Sandmann, G. Carotenoids and their biosynthesis in Fungi. Molecules 27(4), 1431; https://doi.org/10.3390/molecules27041431 (2022).
Baslam, M & Goicoechea, N. Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza 22(5), 347–359; https://doi.org/10.1007/s00572-011-0408-9 (2012).
Mathur, S., Sharma, M. P., & Jajoo, A. Improved photosynthetic efficacy of maize (Zea mays) plants with arbuscular mycorrhizal Fungi (AMF) under high temperature stress. J. Photochem. Photobiol. B: Biol.180, 149–154; https://doi.org/10.1016/j.jphotobiol.2018.02.002. (2018).
Smith, S, Jakobsen, I, Gronlund, M & Smith, F. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for Understanding and manipulating plant phosphorus acquisition. Plant Physiol. 156(1050), 1057; https://doi.org/10.1104/pp.111.174581 (2011).
Singh, A et al. Arbuscular mycorrhizal fungi efficiency on plant growth and nutrient acquisition: a comprehensive review. Microbiol. Res. J. Int. 34(8), 13–22; https://doi.org/10.9734/mrji/2024/v34i81466 (2024).
Pang, F et al. Soil phosphorus transformation and plant uptake driven by phosphate-solubilizing microorganisms. Front. Microbiol. 15; https://doi.org/10.3389/fmicb.2024.1383813 (2024).
Saia, S et al. Growth-promoting bacteria and arbuscular mycorrhizal fungi differentially benefit tomato and corn depending upon the supplied form of phosphorus. Mycorrhiza 30, 133–147; https://doi.org/10.1007/s00572-019-00927-w (2019).
Stoffel, SCG, Soares, CRFS, Meyer, E, Lovato, PE & Giachini, AJ. Yield increase of corn inoculated with a commercial arbuscular mycorrhizal inoculant in Brazil. Cienc. Rural 50(7), e20200109; https://doi.org/10.1590/0103-8478cr20200109 (2020).
Acknowledgements
The authors are grateful to National Council for the Improvement of Higher Education (CAPES), which provided graduate scholarships. We appreciate Dr. Cláudio Roberto Fonsêca Sousa Soares for providing the commercial mycorrhizal inoculant (Rootella BR ULTRA).
Author information
Authors and Affiliations
Contributions
Conceptualization, H.C.A.R.S., A.M.S.A and E.M.C.; methodology, H.C.A.R.S., A.M.S.A., R.W.F.N., G.N.M., M.H.F.D., A.P.M.S., A.C.F., M.R.L. and E.M.C.; software, H.C.A.R.S., A.P.M.S. and A.C.F.; validation, H.C.A.R.S., R.S.M., A.S.F.A. and E.M.C.; formal analysis, H.C.A.R.S., A.M.S.A., R.W.F.N., G.N.M., M.H.F.D. and M.R.L.; investigation, H.C.A.R.S., A.M.S.A., R.S.M., A.S.F.A. and E.M.C.; resources, R.S.M. and E.M.C.; data curation, H.C.A.R.S., A.P.M.S., A.C.F. and E.M.C.; writing-original draft preparation, H.C.A.R.S., A.C.F., A.M.S.A., and E.M.C; writing-review and editing, R.S.M., A.S.F.A. and E.M.C.; visualization, R.S.M., A.S.F.A.; supervision, E.M.C.; project administration, E.M.C.; funding acquisition, E.M.C. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The authors declare that this research was conducted ethically and responsibly, and all procedures were carried out according to relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Severo, H.C.d., de Santana Arauco, A.M., Nunes, R.W.F. et al. Co-inoculation of arbuscular mycorrhizal fungi and Bacillus subtilis enhances morphological traits, growth, and nutrient uptake in maize under limited phosphorus availability. Sci Rep 15, 25448 (2025). https://doi.org/10.1038/s41598-025-10038-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10038-6