Abstract
Abdominal obesity (AO) exhibits unique endocrine and metabolic characteristics in women, yet its neural mechanisms remain unclear. The aim of this study was to investigate the neural mechanisms of AO in females by examining functional connectivity (FC) alterations across brain networks. Resting-state functional MRI (rs-fMRI) data were collected from 38 female AO patients and 24 healthy controls (HCs). Independent component analysis (ICA) was utilized to evaluate FC and functional network connectivity (FNC) within and between brain networks. FC analysis revealed significant alterations within the default mode network (DMN), ventral attention network (VAN), and visual network (VN) (p < 0.05, FDR corrected). In the DMN, increased FC was observed in the left posterior cingulate gyrus and left angular gyrus, while decreased FC was found in the left precuneus and right posterior cingulate gyrus. The VAN and VN showed reduced FC in regions associated with attention control and visual processing, including the left inferior parietal gyrus, left inferior frontal gyrus, triangular part, and left calcarine fissure and surrounding cortex. Internetwork analysis identified significantly higher FNC between the DMN and left frontoparietal network in female AO patients (p < 0.001). This study revealed significant abnormalities in brain functional connectivity in female AO patients, particularly in networks related to self-referential processing, attention, and decision-making. These findings provide a neural basis for cognitive and emotional dysregulation in AO and highlight the potential for targeted interventions to improve self-regulation and inform prevention and treatment strategies.
Similar content being viewed by others
Introduction
Abdominal obesity (AO), characterized by excessive visceral fat accumulation, poses significant health risks and unique challenges, particularly for women1,2,3. Unlike generalized obesity, AO in females is closely linked to sex-specific endocrine and metabolic factors, including hormonal fluctuations and heightened susceptibility to systemic inflammation4,5. These factors not only exacerbate cardiovascular and metabolic risks but also make brain regions involved in emotional regulation, cognitive control, and decision-making particularly vulnerable to dysfunction6,7. Given its profound and long-term impact on both physical and mental health, female AO represents a critical but understudied area of research requiring urgent attention.
Although neuroimaging research has revealed that obesity is associated with alterations in brain networks, such as the prefrontal cortex, insula, amygdala, and striatum—regions responsible for cognitive control, reward processing, and appetite regulation—these studies largely focus on generalized obesity, with limited attention to AO8,9,10,11. Recent findings suggest that women with obesity exhibit unique neural adaptations in brain regions such as the dorsal striatum and dorsolateral prefrontal cortex which are crucial for habitual and goal-directed behaviors, which differ significantly from those in men12,13. These adaptations, particularly within the prefrontal and limbic systems, are linked to changes in reward processing, emotional regulation, and cognitive control, potentially contributing to maladaptive eating behaviors and emotional dysregulation14,15. Despite these findings, the specific impact of AO on brain function in women, especially in networks related to emotional regulation and cognitive control, remains poorly understood. Given the pronounced sex differences in fat distribution, hormonal influences, and metabolic responses16, understanding the neural mechanisms of female AO is essential to fill this critical research gap.
Independent component analysis (ICA) is a powerful, data-driven neuroimaging method that can decomposes complex neural signals into independent functional networks, providing insights into spatially independent components, enabling the identification of functionally distinct brain networks without requiring prior assumptions about specific regions of interest17. Compared to seed-based analysis, which relies on predefined seed regions and may overlook broader network dynamics, or graph-theoretical approaches that emphasize topological features18, ICA is particularly advantageous for capturing large-scale FC patterns across the whole brain. Previous ICA studies have revealed altered functional connectivity (FC) in obesity, such as weakened connectivity between the basal ganglia (BG) and dorsolateral prefrontal cortex (DLPFC), underscoring the BG network’s role in reward processing and inhibitory control19. Furthermore, dynamic FC analyses have shown prolonged alterations in connectivity states involving the salience network (SN) and the default mode network (DMN) connectivity, which are closely associated with body mass index (BMI) and may reflect sustained dysregulation in attention and self-referential processing20. In this study, ICA was employed to analyze resting-state fMRI (rs-fMRI) data from female patients with AO. This approach enabled a comprehensive assessment of both within- and between-network FC alterations, offering deeper insights into the specific neural adaptations associated with female AO and advancing the understanding of its underlying brain mechanisms.
This study aims to examine alterations in both intranetwork and internetwork FC in female AO patients compared to healthy controls (HCs). We hypothesize that AO in women involves disruptions in brain networks critical for emotional and cognitive regulation, particularly the DMN. Additionally, we explore correlations between FC changes and obesity-related indicators, such as waist circumference (WC) and BMI. By identifying the neural basis of cognitive and emotional dysregulation in female AO patients, this study provides valuable insights into the pathophysiology of AO and highlights potential targets for prevention and therapeutic interventions tailored to women.
Materials and methods
Study design
This study utilized baseline data from a clinical neuroimaging trial (Supplementary material). Written informed consent was obtained from all participants before study-related assessments, and the study was approved by the Medical Ethics Committee of the Sports Trauma Specialist Hospital of Yunnan Province (Approval No. 2021–01). The study protocol was registered with the Chinese Clinical Trial Registry (ChiCTR2100048920) on July 19, 2021, and the first participant enrolled on August 1, 2021. All methods were performed in accordance with relevant guidelines and regulations by our approved protocol.
Participants
A total of 40 female AO patients and 25 HCs were recruited from the Second Affiliated Hospital of Yunnan University of Chinese Medicine, the Sports Trauma Specialist Hospital of Yunnan Province, and local communities in Kunming, China. The rs-fMRI data were collected from them and ICA was utilized to evaluate FC and functional network connectivity (FNC) within and between brain networks. The inclusion criteria for female AO patients: (1) Diagnostic following American Association of Clinical Endocrinologists (AACE) Board of Directors and American College of Endocrinology (ACE) guidelines21: ① BMI ≥ 28 kg/m2; ② WC ≥ 80 cm; (2) Right-handed, aged 18–40 years; (3) At least 6 years of formal education. Exclusion criteria: (1) BMI ≥ 40 kg/m2; (2) Secondary obesity due to endocrine diseases (e.g., hypothalamic, gonadal, pituitary diseases, thyroid diseases, etc.) or medications (e.g., antipsychotics, glucocorticoids); (3) Pregnancy or breastfeeding; (4) Chronic diseases, such as diabetes or cardiovascular disorders; (5) Psychiatric symptoms, including depression, insomnia; (6) Contraindications for MRI, such as metal implants or claustrophobia; (7) Significant brain lesions or cranial asymmetry; (8) History of obesity surgery. Age– and sex-matched HCs with a BMI of 18.5–23.9 kg/m2 were selected. Their exclusion criteria mirrored those of the AO group.
Sample size calculation
The primary objective of our study was to detect differences in FC between resting-state networks (RSNs). The sample size was calculated with reference to a prior obesity-related FC study22, which reported significant group differences in FNC between the SN and the frontoparietal network (FPN), with t-values of − 3.937 (SN-lFPN, p = 0.0002) and − 2.953 (SN-rFPN, p = 0.0043) in a sample of 35 obese and 35 normal-weight participants, corresponding to an average effect size of Cohen’s d = 0.83. Conservatively adopting Cohen’s d = 0.8, PASS software (https://www.ncss.com/software/pass) was used to estimate the required sample size, assuming a two-tailed test, α = 0.05, and power = 0.9. This calculation yielded approximately 21 participants per group (total = 42). To ensure adequate power and allow for potential data exclusions, we included 40 AO patients and 25 HC participants in the current study.
MRI data acquisition
MRI data were performed on a GE MRI Discovery 750w 3.0 T scanner. The scans were performed outside of the menstrual period. Participants lay supine with earplugs, eyes closed, and were instructed to stay awake and minimize head motion. The parameters for the high-resolution 3D T1- weighted BRAVO images were as follows: repetition time (TR) = 7.7 ms, echo time (TE) = 3.6 ms, matrix = 228 × 228, field of view (FOV) = 250 mm × 250 mm, 230 axial slices, and acquisition time = 6 min and 53 s. The rs-fMRI data were collected using a gradient-echo echo-planar imaging (GRE-EPI) sequence: 240 functional images. 30 slices, slice thickness = 4.0 mm, gap = 1.2 mm, TR = 2000 ms, TE = 30 ms, matrix = 64 × 64, FOV = 220 × 220 mm, flip angle = 90°. Scans were conducted by a trained technician. Participants reporting discomfort could stop the procedure at any time.
Data processing
Preprocessing
The r-sfMRI data were preprocessed was performed using the GRETNA toolbox (v2.0.0, http://www.nitrc.org/projects/gretna) in MATLAB R2018b (MathWorks Inc.). The preprocessing steps included (1) Conversion of DICOM to NIFTI format; (2) Removal of the first 10 time points to ensure signal stabilization; (3) Slice-timing and head-motion realignment, with exclusion of participants exhibiting > 2 mm translation or > 2° rotationcorrection (exclusion threshold: head motion > 2 mm; (4) Spatial normalization to Montreal Neurological Institute (MNI) space (voxel size = 3 × 3 × 3 mm); (5) Spatial smoothing with an 8 mm full-width at half-maximum (FWHM) Gaussian kernel; (6) Band filtering (0.01 Hz–0.08 Hz) to reduce low-frequency drift and high-frequency noise; and (7) Ruisance signal regression to remove non-neural signals. Nuisance regressors included mean signals from white matter (WM) and cerebrospinal fluid (CSF), extracted using masks derived from MNI templates, and head motion parameters modeled with the Friston 24-parameter approach (six motion parameters, their first derivatives, and squared terms). Global signal regression (GSR) was not applied to preserve intrinsic FC patterns, as GSR may remove neural-related signals and introduce artifacts23. Framewise displacement (FD) was calculated using the six-dimensional timeseries method24,25, with mean FD values of 0.21 ± 0.08 mm for AO patients and 0.19 ± 0.07 mm for HCs. All participants had > 90% valid volumes. Two female AO patients and one HC were excluded due to poor image spatial alignment, resulting in a final sample of 38 female AO patients and 24 HCs for analysis (Fig. 1).
Independent component analysis (ICA)
Group-level spatial ICA was performed using the GIFT toolbox (http://ictb.sourceforge.net/) in MATLAB R2018b (MathWorks Inc.). Based on the minimum description length (MDL) criterion, the number of independent components (ICs) was automatically estimated to be 22. The data were reduced by principal component analysis (PCA), and ICA was conducted using the infomax algorithm, repeated 100 times in ICASSO (http://research.ics.tkk.fi/ica/icasso/) for stable ICs. Participant-specific spatial IC maps and time series were reconstructed using the GICA inverse method.
Identification of resting-state networks (RSNs)
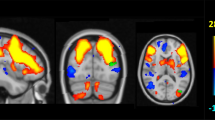
Non-neuronal ICs (e.g., cerebrospinal fluid, motion artifacts, vascular-induced hyperactivity) were excluded. RSNs were identified by correlating IC spatial maps with Stanford’s Functional Imaging in Neuropsychiatric Disorders (FIND) Laboratory template, with the highest spatial correlation coefficients used for selection. Manual inspection verified the eight RSNs: auditory network (AUN), DMN, dorsal and ventral attention networks (DAN and VAN), left and right frontoparietal networks (FPN_L and FPN_R), sensorimotor network (SMN), and visual network (VN) (Fig. 2). Two researchers independently reviewed the selection.
Functional brain network components. AUN auditory network; DMN anterior default network; DAN dorsal attention network; VAN ventral attention network; FPN_L left frontoparietal network; FPN_R right frontoparietal network; SMN sensorimotor network; VN visual network. The color bar represents T-values, indicating statistical significance (range: 0–15.3, thresholded at p < 0.05). Coordinates (x, y, z) are in MNI space.
Functional connectivity analysis
Intranetwork FC and internetwork FNC analyses were performed to assess connectivity alterations in AO. For intranetwork FC: Group-level templates for network comparisons were created using SPM12 (MATLAB). One-sample t-tests were performed to generate group templates, and two-sample t-tests with two-tailed testing (FWE-corrected, voxel p < 0.001, cluster p < 0.05) compared FC between groups, with age as a covariate. For internetwork FNC: Time series of RSNs were extracted, using the Mancovan module in the Group ICA of fMRI Toolbox (GIFT, v4.0b, http://mialab.mrn.org/software/gift). These time series were Fisher z-transformed and correlated to construct individual FNC matrices. Group differences in FNC were assessed using two-sample t tests, with false discovery rate (FDR) correction (p < 0.05, Benjamini–Hochberg method26) applied to control for multiple comparisons across RSN pairs. FDR was chosen for FNC to balance statistical sensitivity and control of false discoveries in ROI-to-ROI connectivity analyses. All analyses were based on the final sample of 38 AO patients and 24 HCs.
Statistical analysis
Statistical analyses were performed using SPSS 27.0 (SPSS Inc., Chicago, IL, USA) Continuous variables were expressed as mean ± standard deviation, and categorical variables as numbers and percentages. An independent t-test was used for normally distributed variables, and the Mann–Whitney U test was applied for non-normally distributed variables. Imaging data were compared using two-sample t-tests (p < 0.05, FDR-corrected). Controlling for age as a covariate, correlation analysis assessed between the clinical variables (e.g., body weight (BW), WC, BMI, appetite visual analogue scale (VAS) scores) and the differential FC/FNC within and between RSNs. Bonferroni correction was applied to control for multiple comparisons (p × n < 0.05).
Results
Demographics and clinical characteristics
The demographic and clinical characteristics of the study population are presented in Table 1. There were no significant differences in age between the AO and HC groups (p > 0.05). However, the AO group exhibited significantly higher BW, WC, BMI, and appetite VAS scores compared to the HC group (p < 0.01).
FC/FNC within and between RSNs
Pairwise correlation patterns
Pearson correlation coefficients were calculated for eight RSNs: AUN, DMN, DAN, VAN, FPN_L, FPN_R, SMN, and VN, to construct the FNC matrix for both groups. These coefficients were used to construct the FNC matrices for both groups.
Intranetwork FC variations
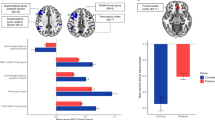
Significant FC differences within RSNs were observed between the AO and HC groups (p < 0.05, FDR corrected) (Table 2 and Fig. 3). In the DMN, female AO patients showed increased FC in the left posterior cingulate gyrus (PCG.L) and left angular gyrus (ANG.L). Conversely, they exhibited significantly decreased FC in the left precuneus (PCUN.L) and right posterior cingulate gyrus (PCG.R). For the VAN, female AO patients demonstrated reduced FC in the left inferior parietal gyrus (IPL.L), right temporal pole: middle temporal gyrus (TPOmid.R), and left inferior frontal gyrus, triangular part (IFGtriang.L). In the VN, significant decreases in FC were identified in the left calcarine fissure and surrounding cortex (CAL.L).
Differences in FC within resting-state networks and correlation analysis. The DMN, VAN, and VN exhibit significant FC differences. The color bar represents t-statistics, with warmer colors indicating greater statistical significance (p < 0.001, FDR-corrected). Within the AO group, VN FC shows a positive correlation with WC (r = 0.551, p = 0.001 × 4 < 0.05), reflecting within-group compensatory changes rather than group-level comparisons with HC. DMN anterior default network; VAN ventral attention network; VN visual network; FC functional connectivity; PCG posterior cingulate gyrus; ANG angular gyrus; PCUN precuneus; IPL inferior parietal gyrus; TPOmid temporal pole: middle temporal gyrus; IFGtriang inferior frontal gyrus, triangular part; CAL calcarine fissure and surrounding cortex; WC, waist circumference.
FNC variation between RSNs
Group comparisons revealed significant differences in FNC between the AO and HC groups (p < 0.05, FDR-corrected) (Fig. 4a,b). The AO group exhibited significantly higher FNC values between the DMN and FPN_L compared to the HC group (95% CI, − 0.30 to − 0.11, p < 0.001) (Fig. 4c).
Functional network connectivity differences between AO and HC groups. (a) Ring diagram illustrating increased connectivity between the DMN and FPN_L in the AO group compared to the HC group. The color bar represents log-transformed p-values (log10), with positive values indicating stronger connectivity in the AO group. (b) Matrix plot showing significant FNC differences between RSNs. Yellow squares represent significant FNC differences (p < 0.05, FDR-corrected), with warmer colors indicating increased connectivity in the AO group. (c) FNC between the DMN and FPN_L is significantly higher in the AO group (95% CI, − 0.30 to − 0.11, p < 0.001). (d) In the HC group, FNC between the DMN and FPN_L is positively correlated with BMI (r = 0.6066, p = 0.0017 × 4 < 0.05), but no such correlation is observed in the AO group (r = 0.0472, p > 0.05). DMN default mode network; FPN_L left frontoparietal network; FNC Functional network connectivity; AO abdominal obesity; HC healthy control; BMI body mass index.
Correlation analysis
Intranetwork FC Correlation: Within the AO group, FC in the VN was positively correlated with WC (r = 0.551, p = 0.001 × 4 < 0.05) (Fig. 3). Internetwork FNC correlation: In the HC group, FNC between DMN and FPN_L showed a significant positive correlation with BMI (r = 0.6066, p = 0.0017 × 4 < 0.05), while no significant correlation was observed between BMI and FNC in the AO group (r = 0.0472, p > 0.05) (Fig. 4d). Further analysis revealed no significant correlations between DMN-FPN_L FNC and BW (r = − 0.179, p = 0.282) or WC (r = − 0.313, p = 0.056) in the AO group after Bonferroni correction (p × 4 > 0.05).
Discussion
The This study revealed significant alterations in both intranetwork and internetwork FC in female patients with AO, providing novel insights into the neural mechanisms underlying AO. These findings, particularly within networks associated with self-referential processing, attentional control, and decision-making, highlight the critical impact of AO on cognitive and emotional regulation.
FC abnormalities within the DMN: compensation and imbalance
The DMN, crucial for self-referential thought, introspection, and social cognition27 exhibited both increased and decreased FC in female AO patients. Increased FC was observed in the PCG.L and ANG.L, regions integral to sensory integration, self-evaluation, and emotion regulation28. This enhancement likely reflects a compensatory response to heightened emotional and cognitive demands, such as body image concerns and food-related cues29,30,31. In contrast, decreased FC was identified in the PCUN.L and PCG.R, which are the key hubs for decision-making and integration of internal and external stimuli32,33. Reduced connectivity in these regions may impair self-awareness and decision-making, contributing to maladaptive behaviors such as emotional eating and poor dietary choices34,35,36. These findings suggest possible deficits in attention or decision-making, as supported by previous studies linking DMN alterations to cognitive impairments in obesity35,37. However, due to the absence of cognitive testing such as Clinical Assessment for Attention (CAT)38, Paced Auditory Serial Additions Task (PASAT)39 Clinical Assessment for Attention or Trail making test (TMT)40 in our study, this interpretation remains speculative and should be addressed in future research.
Our analysis revealed contrasting FC changes within the DMN in female patients with AO compared to HCs, specifically increased FC in PCG.L and decreased FC in the PCG.R. These hemispheric differences highlight a functional imbalance within the DMN, contributing to cognitive and emotional dysregulation in AO41,42. Neurobiological evidence indicates that the PCG exhibits functional lateralization: the left PCG is primarily associated with emotional regulation and self-referential processing, while the right PCG supports attention allocation, spatial navigation, and integration of internal and external stimuli43,44. In female AO patients, the enhanced FC in PCG.L likely represents a compensatory response to the heightened demands for self-evaluation and emotion regulation in female AO patients45. In contrast, the decreased FC in PCG.R suggests impairments in attentional control and decision-making, consistent with diminished interhemispheric coordination in lateralized brain regions43,46. While the left hemisphere may partially compensate for right-hemisphere deficits, such mechanisms may not fully restore network efficiency, leading to persistent cognitive and emotional dysregulation. Future studies should explore specific connectivity patterns to further elucidate these lateralized effects.
FC abnormalities within the VAN and VN: attention and visual deficits
The VAN, which plays a critical role in attentional control and cognitive flexibility47, exhibited decreased FC in the IPL.L, TPOmid.R, and IFGtriang.L in female AO patients. These disruptions may underlie common cognitive control deficits in obesity48,49,contribute to impulsive decision-making and difficulties in resisting food-related stimuli50. Similarly, the VN, exhibited decreased FC in the CAL.L, a region central to visual perception and stimulus integration51,52. These deficits may reflect difficulties in visual processing and attentional focus, exacerbating maladaptive eating behaviors driven by visual food cues53. Interestingly, within the AO group, VN FC was positively correlated with WC (Fig. 3), suggesting a compensatory increase in VN activity in individuals with greater abdominal fat. However, this apparent contradiction with Table 2 (showing reduced FC in AO vs. HC) can be explained by compensatory mechanisms: AO patients with larger WC may exhibit increased FC in specific VN regions to cope with heightened visual stimulus demands, possibly influenced by metabolic or hormonal factors. This enhanced VN activity may reflect increased sensitivity to food cues and their reward value, aligning with previous findings of heightened visual cortex activation and attentional bias to food in obese individuals. Despite this compensatory pattern, overall VN dysfunction remains a key feature of AO’s neurocognitive profile54,55. For example, obese individuals show enhanced attentional bias to food cues, with increased visual cortex activation and altered connectivity to salience and reward networks53,54. Additionally, morbid obesity is associated with disrupted visual plasticity, indicating broader VN dysfunction55. These alterations may amplify the salience of food cues, contributing to maladaptive eating behaviors. Metabolic and hormonal factors, such as insulin or leptin dysregulation, likely influence VN FC indirectly through reward and salience processing, though direct pathways remain speculative and require further investigation. Despite this compensation, overall VN function remains lower than in HC.
Altered DMN-FPN_L connectivity: compensatory but inefficient adaptation
This study identified significantly increased FNC between the DMN and the FPN_L in female AO patients compared to HCs. The DMN supports self-referential processing, while the FPN_L facilitates goal-directed tasks and executive control. Enhanced DMN-FPN_L connectivity may represent a compensatory mechanism to address self-regulatory challenges in AO, such as difficulties in appetite control and decision-making45. Alternatively, this hyperconnectivity could indicate inefficient neural communication between these networks, potentially contributing to dysfunction56,57. Notably, in HCs, a positive correlation between DMN-FPN_L connectivity and BMI was observed, suggesting that mild increases in BMI may require greater neural coordination to maintain cognitive control. DMN-FPN_L FNC did not show significant correlations with BMI, BW, or WC the AO group. The absence of this correlation in AO patients highlights neural circuit disruptions potentially caused by prolonged metabolic dysregulation22.
To systematically interpret these abnormalities, we adopted the triple network model, which emphasizes the SN as a dynamic regulator of DMN-FPN interactions58. In AO, altered DMN-FPN_L connectivity may reflect SN-mediated disruptions in switching between internally directed and externally oriented processes, leading to cognitiveemotional dysregulation. Although our study did not directly assess SN connectivity, recent evidence suggests SN dysfunction in obesity-related conditions, including altered dynamic connectivity in comorbid depression and obesity59,60. For instance, enhanced DMN-FPN connectivity in overweight/obese individuals with depression highlights network imbalances relevant to AO60. These observations align with the triple network model, widely used to explain cognitive-affective deficits in neuropsychiatric disorders61. However, the lack of direct SN analysis limits our conclusions, and future studies should employ seed-based or ICA approaches to examine SN’s role in AO.
Sex-specific considerations: the role of endocrine factors
The findings of this study align with evidence that women with obesity exhibit greater alterations in brain regions related to emotional regulation, potentially making them more vulnerable to emotionally driven and compulsive eating behaviors. These differences may be influenced by hormonal changes, such as estrogen fluctuations, which are known to modulate the brain’s reward and emotional regulation circuits, including the DMN and VAN. For instance, brain estrogen synthesis in the amygdala has been negatively correlated with BMI and positively associated with self-control62, while estradiol and progesterone have been shown to influence reward processing and impulse control63. In contrast, men with obesity tend to exhibit more prominent changes in brain regions related to visceral sensations, such as hunger and satiety, indicating a greater reliance on metabolic regulation13,64. However, as sex hormone levels were not assessed in our study, we could not directly examine their relationship with FC alterations—a key limitation. Future research should incorporate endocrine measurements to clarify the role of sex hormones in shaping brain network connectivity in women with AO.
Clinical implications and therapeutic potential
Our findings underscore the translational potential of neuroimaging biomarkers derived from altered connectivity in the DMN, VAN, and DMN-FPN_L may serve as predictive features of cognitive and emotional dysregulation and can inform the development of individualized treatment strategies. Machine learning techniques may further enhance the predictive power of these biomarkers by integrating complex connectivity patterns with clinical profiles, as demonstrated in recent studies applying machine learning to predict obesity risk and improve diagnostic precision across metabolic and neurocognitive disorders65,66.
Therapeutic interventions such as cognitive-behavioral therapy (CBT), which targets self-regulation and attentional control, and neuromodulation approaches tailored to specific neurobiological vulnerabilities, may help improve network efficiency67,68. Given the observed sex-specific patterns—particularly in networks involved in emotion regulation—females with AO may benefit from more personalized approaches. Notably, interventions targeting hormonal regulation, including modulation of estrogen or insulin sensitivity, may enhance central nervous system function and metabolic balance in women69,70,71. Menopausal hormone therapy, when initiated in midlife, has shown time- and formulation-dependent effects on cognitive domains such as verbal memory72. Such hormonal interventions could further potentiate the effects of network-targeted therapies like CBT or TMS73. This integrated strategy—combining brain network modulation with hormonal regulation—reflects a precision medicine approach tailored to sex-specific neurobiological vulnerabilities in AO70,74. However, the unique neuroendocrine shifts across different reproductive stages in women demand further exploration to fully optimize female-specific interventions.
Several limitations should be considered. First, the exclusive focus on female AO patients limits the generalizability of the findings. Future research should include male participants to explore potential sex-specific differences. Second, the cross-sectional design prevents causal inferences regarding the relationship between AO and brain connectivity. Longitudinal studies are necessary to assess the progression and long-term impact of AO on brain function13. Third, the lack of cognitive testing (e.g., CAT, PASAT, or TMT) limits our ability to directly link FC changes to specific cognitive or emotional outcomes. Future research should integrate comprehensive neuropsychological assessments and hormonal measurements to better elucidate the impact of AO on brain networks.
Conclusion
In conclusion, this study highlights significant alterations in both intranetwork and internetwork FC in female AO patients, particularly within the DMN, VAN, and VN. These changes suggest disruptions in cognitive and emotional regulation, with increased DMN-FPN_L connectivity reflecting compensatory but inefficient adaptations. These findings underscore the importance of targeting brain network dysfunctions through cognitive-behavioral therapies and early neuroimaging-based interventions, providing a foundation for developing tailored prevention and treatment strategies for AO in women.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Fenton, A. Weight, shape, and body composition changes at menopause. J. Midlife Health 12, 187–192. https://doi.org/10.4103/jmh.jmh_123_21 (2021).
Powell-Wiley, T. M. et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 143, e984–e1010. https://doi.org/10.1161/cir.0000000000000973 (2021).
Joham, A. E. et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 10, 668–680. https://doi.org/10.1016/s2213-8587(22)00163-2 (2022).
Lazúrová, I. et al. Relationship between steroid hormones and metabolic profile in women with polycystic ovary syndrome. Physiol. Res. 68, 457–465. https://doi.org/10.33549/physiolres.934062 (2019).
Shorakae, S. et al. Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin. Endocrinol. (Oxf). 89, 628–633. https://doi.org/10.1111/cen.13808 (2018).
Chiba, I. et al. Visceral fat accumulation is associated with mild cognitive impairment in community-dwelling older Japanese women. J. Nutr. Health Aging 24, 352–357. https://doi.org/10.1007/s12603-020-1330-7 (2020).
Kundakovic, M. & Rocks, D. Sex hormone fluctuation and increased female risk for depression and anxiety disorders: From clinical evidence to molecular mechanisms. Front. Neuroendocrinol. 66, 101010. https://doi.org/10.1016/j.yfrne.2022.101010 (2022).
Zhang, Q. et al. Multimodal magnetic resonance imaging on brain structure and function changes in vascular cognitive impairment without dementia. Front. Aging Neurosci. 15, 1278390. https://doi.org/10.3389/fnagi.2023.1278390 (2023).
Li, G. et al. Brain functional and structural magnetic resonance imaging of obesity and weight loss interventions. Mol. Psychiatry 28, 1466–1479. https://doi.org/10.1038/s41380-023-02025-y (2023).
Val-Laillet, D. et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 8, 1–31. https://doi.org/10.1016/j.nicl.2015.03.016 (2015).
Ha, O. R. & Lim, S. L. The role of emotion in eating behavior and decisions. Front. Psychol. 14, 1265074. https://doi.org/10.3389/fpsyg.2023.1265074 (2023).
Betancourt-Núñez, A. et al. Emotional eating and dietary patterns: Reflecting Food choices in people with and without abdominal obesity. Nutrients 14, 1371. https://doi.org/10.3390/nu14071371 (2022).
Horstmann, A. et al. Obesity-related differences between women and men in brain structure and goal-directed behavior. Front. Hum. Neurosci. 5, 58. https://doi.org/10.3389/fnhum.2011.00058 (2011).
Yokum, S. & Stice, E. Relation of overweight/obesity to reward region response to food reward and the moderating effects of parental history of eating pathology in adolescent females. Nutrients 15, 2558. https://doi.org/10.3390/nu15112558 (2023).
Donofry, S. D. et al. Comparison of food cue-evoked and resting-state functional connectivity in obesity. Psychosom. Med. 82, 261–271. https://doi.org/10.1097/psy.0000000000000769 (2020).
Power, M. L. & Schulkin, J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br. J. Nutr. 99, 931–940. https://doi.org/10.1017/s0007114507853347 (2008).
Yi, Y., Billor, N., Ekstrom, A. & Zheng, J. CW_ICA: An efficient dimensionality determination method for independent component analysis. Sci. Rep. 14, 143. https://doi.org/10.1038/s41598-023-49355-z (2024).
Lo, C. Y., He, Y. & Lin, C. P. Graph theoretical analysis of human brain structural networks. Rev. Neurosci. 22, 551–563. https://doi.org/10.1515/rns.2011.039 (2011).
Tan, Z. et al. Alterations in functional and structural connectivity of Basal Ganglia network in patients with obesity. Brain Topogr. 35, 453–463. https://doi.org/10.1007/s10548-022-00906-z (2022).
Tan, Z. et al. Obese individuals show disrupted dynamic functional connectivity between Basal Ganglia and salience networks. Cereb. Cortex 31, 5676–5685. https://doi.org/10.1093/cercor/bhab190 (2021).
Garvey, W. T. et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. 22(Suppl 3), 1–203. https://doi.org/10.4158/ep161365.Gl (2016).
Ding, Y. et al. Altered interactions among resting-state networks in individuals with obesity. Obesity (Silver Spring) 28, 601–608. https://doi.org/10.1002/oby.22731 (2020).
Murphy, K. & Fox, M. D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage 154, 169–173. https://doi.org/10.1016/j.neuroimage.2016.11.052 (2017).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. https://doi.org/10.1016/j.neuroimage.2011.10.018 (2012).
Power, J. D. et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84, 320–341. https://doi.org/10.1016/j.neuroimage.2013.08.048 (2014).
Glickman, M. E., Rao, S. R. & Schultz, M. R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 67, 850–857. https://doi.org/10.1016/j.jclinepi.2014.03.012 (2014).
Yeshurun, Y., Nguyen, M. & Hasson, U. The default mode network: Where the idiosyncratic self meets the shared social world. Nat. Rev. Neurosci. 22, 181–192. https://doi.org/10.1038/s41583-020-00420-w (2021).
Shen, Y. et al. Functional connectivity gradients of the cingulate cortex. Commun. Biol. 6, 650. https://doi.org/10.1038/s42003-023-05029-0 (2023).
Reichelt, A. C., Westbrook, R. F. & Morris, M. J. Integration of reward signalling and appetite regulating peptide systems in the control of food-cue responses. Br. J. Pharmacol. 172, 5225–5238. https://doi.org/10.1111/bph.13321 (2015).
Rolls, E. T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 224, 3001–3018. https://doi.org/10.1007/s00429-019-01945-2 (2019).
Ma, S. T., Abelson, J. L., Okada, G., Taylor, S. F. & Liberzon, I. Neural circuitry of emotion regulation: Effects of appraisal, attention, and cortisol administration. Cogn. Affect. Behav. Neurosci. 17, 437–451. https://doi.org/10.3758/s13415-016-0489-1 (2017).
Cavanna, A. E. & Trimble, M. R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129, 564–583. https://doi.org/10.1093/brain/awl004 (2006).
Jiang, P. et al. The hierarchical organization of the precuneus captured by functional gradients. Brain Struct. Funct. 228, 1561–1572. https://doi.org/10.1007/s00429-023-02672-5 (2023).
Harikumar, A., Evans, D. W., Dougherty, C. C., Carpenter, K. L. H. & Michael, A. M. A review of the default mode network in autism spectrum disorders and attention deficit hyperactivity disorder. Brain Connect. 11, 253–263. https://doi.org/10.1089/brain.2020.0865 (2021).
Syan, S. K. et al. Dysregulated resting state functional connectivity and obesity: A systematic review. Neurosci. Biobehav. Rev. 131, 270–292. https://doi.org/10.1016/j.neubiorev.2021.08.019 (2021).
Park, B. Y., Lee, M. J., Kim, M., Kim, S. H. & Park, H. Structural and functional brain connectivity changes between people with abdominal and non-abdominal obesity and their association with behaviors of eating disorders. Front. Neurosci. 12, 741. https://doi.org/10.3389/fnins.2018.00741 (2018).
Xu, S. et al. Altered structural node of default mode network mediated general cognitive ability in young adults with obesity. Prog. Neuropsychopharmacol. Biol. Psychiatry 135, 111132. https://doi.org/10.1016/j.pnpbp.2024.111132 (2024).
Kato, M. The development and standardization of clinical assessment for attention (CAT) and clinical assessment for spontaneity (CAS). Higher Brain Funct. Res. 26, 310–319 (2006).
Gronwall, D. M. Paced auditory serial-addition task: A measure of recovery from concussion. Percept. Mot. Skills 44, 367–373. https://doi.org/10.2466/pms.1977.44.2.367 (1977).
Tombaugh, T. N. Trail making test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 19, 203–214. https://doi.org/10.1016/s0887-6177(03)00039-8 (2004).
Karolis, V. R., Corbetta, M. & Thiebaut de Schotten, M. The architecture of functional lateralisation and its relationship to callosal connectivity in the human brain. Nat. Commun. 10, 1417. https://doi.org/10.1038/s41467-019-09344-1 (2019).
Andrews-Hanna, J. R., Reidler, J. S., Sepulcre, J., Poulin, R. & Buckner, R. L. Functional-anatomic fractionation of the brain’s default network. Neuron 65, 550–562. https://doi.org/10.1016/j.neuron.2010.02.005 (2010).
Leech, R. & Sharp, D. J. The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32. https://doi.org/10.1093/brain/awt162 (2014).
Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38. https://doi.org/10.1196/annals.1440.011 (2008).
Steward, T. et al. A multimodal MRI study of the neural mechanisms of emotion regulation impairment in women with obesity. Transl. Psychiatry 9, 194. https://doi.org/10.1038/s41398-019-0533-3 (2019).
Agathos, J. et al. Differential engagement of the posterior cingulate cortex during cognitive restructuring of negative self- and social beliefs. Soc. Cogn. Affect Neurosci. 18, nsad024. https://doi.org/10.1093/scan/nsad024 (2023).
Dajani, D. R. & Uddin, L. Q. Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends Neurosci. 38, 571–578. https://doi.org/10.1016/j.tins.2015.07.003 (2015).
Kaisari, P. et al. Top-down guidance of attention to food cues is enhanced in individuals with overweight/obesity and predicts change in weight at one-year follow up. Int. J. Obes. (Lond). 43, 1849–1858. https://doi.org/10.1038/s41366-018-0246-3 (2019).
Legget, K. T., Wylie, K. P., Cornier, M. A., Berman, B. D. & Tregellas, J. R. Altered between-network connectivity in individuals prone to obesity. Physiol. Behav. 229, 113242. https://doi.org/10.1016/j.physbeh.2020.113242 (2021).
Yokum, S., Ng, J. & Stice, E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring) 19, 1775–1783. https://doi.org/10.1038/oby.2011.168 (2011).
Li, S. et al. Intrinsic functional connectivity alterations of the primary visual cortex in primary angle-closure glaucoma patients before and after surgery: A resting-state fMRI study. PLoS ONE 12, e0170598. https://doi.org/10.1371/journal.pone.0170598 (2017).
Tong, Y., Huang, X., Qi, C. X. & Shen, Y. Altered functional connectivity of the primary visual cortex in patients with iridocyclitis and assessment of its predictive value using machine learning. Front. Immunol. 12, 660554. https://doi.org/10.3389/fimmu.2021.660554 (2021).
Doolan, K. J., Breslin, G., Hanna, D., Murphy, K. & Gallagher, A. M. Visual attention to food cues in obesity: An eye-tracking study. Obesity (Silver Spring) 22, 2501–2507. https://doi.org/10.1002/oby.20884 (2014).
Kullmann, S. et al. Functional network connectivity underlying food processing: Disturbed salience and visual processing in overweight and obese adults. Cereb. Cortex 23, 1247–1256. https://doi.org/10.1093/cercor/bhs124 (2013).
Lunghi, C. et al. Altered visual plasticity in morbidly obese subjects. iScience 22, 206–213. https://doi.org/10.1016/j.isci.2019.11.027 (2019).
Manoliu, A. et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr. Bull. 40, 428–437. https://doi.org/10.1093/schbul/sbt037 (2014).
Yamashita, K. I., Uehara, T., Taniwaki, Y., Tobimatsu, S. & Kira, J. I. Long-term effect of acetylcholinesterase inhibitors on the dorsal attention network of Alzheimer’s disease patients: A pilot study using resting-state functional magnetic resonance imaging. Front. Aging Neurosci. 14, 810206. https://doi.org/10.3389/fnagi.2022.810206 (2022).
Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 15, 483–506. https://doi.org/10.1016/j.tics.2011.08.003 (2011).
Schimmelpfennig, J., Topczewski, J., Zajkowski, W. & Jankowiak-Siuda, K. The role of the salience network in cognitive and affective deficits. Front. Hum. Neurosci. 17, 1133367. https://doi.org/10.3389/fnhum.2023.1133367 (2023).
Zhang, Z. Q., Liao, D., Guo, Z. P., Song, S. S. & Liu, X. J. Aberrant dynamic network connectivity changes in comorbid depression and overweight/obesity: Insights from the triple network model. J. Neurosci. Res. 102, e70001. https://doi.org/10.1002/jnr.70001 (2024).
Menon, V., Palaniyappan, L. & Supekar, K. Integrative brain network and salience models of psychopathology and cognitive dysfunction in schizophrenia. Biol. Psychiatry 94, 108–120. https://doi.org/10.1016/j.biopsych.2022.09.029 (2023).
Biegon, A. et al. Relationship of estrogen synthesis capacity in the brain with obesity and self-control in men and women. Proc. Natl. Acad. Sci. U S A. 117, 22962–22966. https://doi.org/10.1073/pnas.2006117117 (2020).
Hilz, E. N. & Lee, H. J. Estradiol and progesterone in female reward-learning, addiction, and therapeutic interventions. Front. Neuroendocrinol. 68, 101043. https://doi.org/10.1016/j.yfrne.2022.101043 (2023).
Kilpatrick, L. A., An, H. M., Pawar, S., Sood, R. & Gupta, A. Neuroimaging investigations of obesity: A review of the treatment of sex from 2010. Curr. Obes. Rep. 12, 163–174. https://doi.org/10.1007/s13679-023-00498-0 (2023).
Saberi-Karimian, M. et al. Potential value and impact of data mining and machine learning in clinical diagnostics. Crit. Rev. Clin. Lab. Sci. 58, 275–296. https://doi.org/10.1080/10408363.2020.1857681 (2021).
Harris, C., Olshvang, D., Chellappa, R. & Santhanam, P. Obesity prediction: Novel machine learning insights into waist circumference accuracy. Diabetes Metab Syndr. 18, 103113. https://doi.org/10.1016/j.dsx.2024.103113 (2024).
Forcano, L., Mata, F., de la Torre, R. & Verdejo-Garcia, A. Cognitive and neuromodulation strategies for unhealthy eating and obesity: Systematic review and discussion of neurocognitive mechanisms. Neurosci. Biobehav. Rev. 87, 161–191. https://doi.org/10.1016/j.neubiorev.2018.02.003 (2018).
Solier-López, L. et al. A program for the comprehensive cognitive training of excess weight (TRAINEP): The study protocol for a randomized, controlled trial. Int. J. Environ. Res. Public Health 19, 8447. https://doi.org/10.3390/ijerph19148447 (2022).
Albert, K. M. & Newhouse, P. A. Estrogen, stress, and depression: Cognitive and biological interactions. Annu. Rev. Clin. Psychol. 15, 399–423. https://doi.org/10.1146/annurev-clinpsy-050718-095557 (2019).
Mazza, E. et al. Obesity, dietary patterns, and hormonal balance modulation: Gender-specific impacts. Nutrients 16, 1629. https://doi.org/10.3390/nu16111629 (2024).
Yuen, E. Y., Wei, J. & Yan, Z. Estrogen in prefrontal cortex blocks stress-induced cognitive impairments in female rats. J. Steroid Biochem. Mol. Biol. 160, 221–226. https://doi.org/10.1016/j.jsbmb.2015.08.028 (2016).
Andy, C. et al. Systematic review and meta-analysis of the effects of menopause hormone therapy on cognition. Front. Endocrinol. (Lausanne) 15, 1350318. https://doi.org/10.3389/fendo.2024.1350318 (2024).
Hofmann, S. G., Asnaani, A., Vonk, I. J., Sawyer, A. T. & Fang, A. The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognit. Ther. Res. 36, 427–440. https://doi.org/10.1007/s10608-012-9476-1 (2012).
Rubinow, D. R. & Schmidt, P. J. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology 44, 111–128. https://doi.org/10.1038/s41386-018-0148-z (2019).
Acknowledgements
We would like to thank all the participants involved in the study, as well as to other staff for their support.
Funding
This study was supported by the ‘Liang Fanrong Expert Workstation’ of Yunnan Province-Yunnan Provincial Science and Technology Plan Project (202305AF150072), the Health Commission of Yunnan Province–Cultivation Objects of High-level Talents in Chinese Medicine in 2023: Acupuncture Discipline Leaders in Chinese Medicine, the Top-notch Young Talent Project of Yunnan Ten Thousand Talents (YNWR-QNBJ-2019-257) and the ‘Liu Zili Famous Doctor’ special talent program of the Yunnan Provincial Xing Dian Talent Support Program (Yunnan Party Talent Office [2022] No. 18).
Author information
Authors and Affiliations
Contributions
Q.L., G.H., and S.W.Z.: analyzed data and wrote a manuscript draft. T.G. Z.L.: funding, study concept and design, study supervision, technical support. Y.L., X.T.: analysis, critical revision of the manuscript for important intellectual content. S.M.Z., Z.C., X.P., R.Y., R.H., D.T., and C.X.: data interpretation, critical revision of the manuscript for important intellectual content. X.T.: statistical analysis. F.L., T.G., and Y.L.: contributed to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Q., Huang, G., Zhao, S. et al. Aberrant brain network connectivity related to cognitive and emotional regulation in women with abdominal obesity. Sci Rep 15, 24795 (2025). https://doi.org/10.1038/s41598-025-10134-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10134-7