Abstract
Research indicates a U-shaped association between mortality and glycated hemoglobin (HbA1c) levels in patients receiving sulfonylurea or insulin. However, the relationship between glucose levels and cardiovascular events in patients on novel agents with a lower hypoglycemic potential remains unknown. This study was aimed to examine the association between cardiovascular events and HbA1c in patients with type 2 diabetes receiving drugs with different hypoglycemic potentials. This is an observational cohort study using a multicenter electronic medical record database. This study included patients who received a diagnosis of type 2 diabetes between 2009 and 2020 and received non-insulin antidiabetic drugs. These drugs were divided into drugs with a high-hypoglycemic-risk (sulfonylurea and meglitinides) and drugs with a low-hypoglycemic-risk (incretin mimetics, sodium-glucose cotransporter-2 inhibitors, thiazolidinediones, and acarbose). The events of interest were mortality and major adverse cardiovascular events (MACEs). A total of 6,789 patients were included, with 3,191 patients in low-hypoglycemic-risk drugs cohort and 3,598 patients in high-hypoglycemic-risk drugs cohort. Both cohorts exhibited a U-shaped association between HbA1c levels and the risk of mortality and MACEs. Among patients receiving low-hypoglycemic-risk drugs, HbA1c levels of 6.7% and 6.8% were associated with the lowest risk of mortality and MACEs, respectively. Similarly, in patients receiving high-hypoglycemic-risk drugs, the lowest risk of mortality and MACEs was observed at HbA1c levels of 6.8% and 7.2%, respectively. Both low and high HbA1c levels were associated with an increased risk of mortality and cardiovascular events, whereas intermediate levels were linked to the lowest risk. These findings support a U-shaped association between glycemic control and adverse outcomes in patients with type 2 diabetes receiving non-insulin-based therapies.
Similar content being viewed by others
Background
Diabetes is a major risk factor for both microvascular and macrovascular complications, and intensive glycemic control has long been hypothesized to reduce target organ damage and mortality. However, evidence from randomized controlled trials (RCTs) has challenged this assumption, with conflicting results regarding the cardiovascular benefits of near-normal glycemic targets.
For instance, the Action to Control Cardiovascular Risk in Diabetes trial found that intensive glycemic control (HbA1c < 6%) did not significantly reduce cardiovascular events in patients with type 2 diabetes compared to standard glycemic control (HbA1c 7–7.9%). Moreover, the trial reported an unexpected increase in mortality among patients in the intensive control group1. Similar findings were observe in the Veterans Affairs Diabetes Trial and the Action in Diabetes and Vascular Disease trial, where intensive glucose control was associated with a higher incidence of severe hypoglycemia2,3. Subsequent observational studies confirmed that severe hypoglycemia may contribute to an increased risk of mortality and cardiovascular events4,5. Notably, these RCTs primarily used metformin, sulfonylureas, and insulin as the cornerstone of glucose-lowering therapy.
Following these landmark trials, several novel antidiabetic agents have emerged, including dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and sodium-glucose cotransporter-2 (SGLT-2) inhibitors. Unlike insulin and insulin secretagogues, these newer agents have a lower risk of hypoglycemia, raising questions about whether the U-shaped association between HbA1c and cardiovascular outcomes observed in earlier studies also applies to these newer therapies. Furthermore, it remains unclear whether achieving near-normal HbA1c levels with these novel agents offers greater cardiovascular benefits compared to older treatment approaches.
To address this gap, this study aims to examine the association between HbA1c levels and cardiovascular outcomes in patients with type 2 diabetes treated with antidiabetic agents of different hypoglycemic potentials, utilizing real-world clinical data.
Methods
Study design and data sources
In this observational population-based cohort study, data were obtained from the Taipei Medical University Clinical Research Database (TMUCRD), a multicenter electronic medical record database managed by three medical centers. The TMUCRD contains extensive data on patient demographics; outpatient, emergency, and inpatient visits; medication records; diagnostic codes; and laboratory and examination reports. The TMUCRD ensures data reliability by adhering to a standardized data framework, conducting regular quality assessments, and performing validation processes to maintain data integrity and consistency6. This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Joint Institutional Review Board of Taipei Medical University (approval no. N202306045). Informed consent was waived by the Joint Institutional Review Board of Taipei Medical University due to the retrospective nature of this study.
Study cohort
The data period used in the study was from January 1, 2008 to December 31, 2021. Patients aged 30 years or above who received a diagnosis of diabetes and were first treated with non-insulin antidiabetic drugs between 2009 and 2020 were included in the study. A diagnosis of diabetes was established using International Classification of Diseases, Ninth Edition (ICD-9) code 250 or International Classification of Diseases, Tenth Edition (ICD-10) codes E08–E13. The date of the first prescription for a non-insulin antidiabetic drug was defined as the index date. Patients were classified into two cohorts based on the hypoglycemic risk of the medications they received. The high-hypoglycemic-risk drugs cohort included patients who received sulfonylureas or meglitinides, both of which stimulate insulin secretion irrespective of blood glucose levels, thereby increasing the risk of hypoglycemia. The low-hypoglycemic-risk drugs cohort included patients who received thiazolidinediones, acarbose, DPP-4 inhibitors, GLP-1 receptor agonists, or SGLT-2 inhibitors. These medications lower glucose levels without directly stimulating insulin secretion and do not increase the incidence of severe hypoglycemia, even when used in combination therapies7,8,9,10,11,12. Metformin was not included as an independent variable in our classification because many patients were already on metformin as background therapy (47.8% in the high-hypoglycemic-risk drugs cohort and 66.9% in the low-hypoglycemic-risk drugs cohort), making it non-discriminatory for stratification in our study.
Exclusion criteria
Patients meeting any of the following criteria were excluded from the study: (1) having type 1 diabetes; (2) having received non-insulin antidiabetic drugs for less than 180 days; (3) having received both high- and low-hypoglycemic-risk drugs simultaneously; (4) having received insulin; (5) dying before the index date or within 6 months after the index date; (6) having been hospitalized for MACEs or heart failure before the index date; (7) having incomplete laboratory data on HbA1c, triglyceride, low-density lipoprotein cholesterol, or creatinine levels within 180 days before the index date; or (8) having no HbA1c records within 180 days after the index date. eFigure 1 depicts the patient selection process.
Glycemic levels
Glycemic levels were represented by HbA1c values measured after the initiation of antidiabetic therapy (i.e., following the index date). Post-index mean HbA1c was defined as a single time-fixed variable for each patient. This variable was calculated as the average of all measurements made during follow-up. A sensitivity analysis was conducted to assess individual changes in HbA1c levels over time, using time-varying HbA1c for each patient. Time-varying HbA1c was sorted based on the date of the blood test and the HbA1c value would be carried forward until the next blood test. In the main and sensitivity analyses, the HbA1c values were ranked and divided into five quintiles based on the single mean values or time-varying HbA1c values.
Study outcomes
The events of interest were all-cause mortality and MACEs. MACEs were defined as a composite of cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke. Mortality records were obtained from the national database of the Ministry of Health and Welfare. Other outcomes were identified using ICD-9 or ICD-10 codes (listed in eTable 1).
Follow-up
Patients were followed up from the index date until the events of interest occurred, they discontinued their drugs, they switched from high- to low-hypoglycemic-risk drugs or vice versa, or the end of the study period was reached (December 31, 2021), whichever occurred first.
Comorbidities and medications
The severity of diabetes was evaluated using the Diabetes Complications Severity Index (DCSI), a research tool that reliably predicts the rates of mortality and hospitalization for patients with diabetes13. Patients were evaluated in terms of the extent and severity of organ damage caused by diabetes. A higher score indicated more severe diabetes-associated complications, with a maximum attainable score of 13.
Additionally, since health deterioration may be reflected by HbA1c levels and may affect clinical outcomes, we evaluated the degree of frailty by using the Multimorbidity Frailty Index, a research tool developed using real-world data and based on a cumulative deficit model involving ICD-9 and ICD-10 codes14,15. This index has been used as a reliable indicator of mortality and adverse health outcomes in older Taiwanese populations.
The disease diagnostic codes for the baseline comorbidities and the Anatomical Therapeutic Chemical codes for medications are listed in the supplementary materials.
Statistical analysis
Based on the post-index mean HbA1c distribution, each cohort was divided into five quintiles, with the middle quintile serving as the reference group. The baseline characteristics were compared across quintiles by using chi-squared tests and analysis of variance. Cox proportional-hazards models were used to estimate the risk of clinical events associated with HbA1c and other baseline covariates. Significant covariates were included in multivariate models for adjustment. A sensitivity analysis was conducted using time-varying Cox models to evaluate the risk of events related to HbA1c variations.
To assess association between HbA1c and clinical events and identify potential treatment targets, a Cox proportional-hazards model with a B-spline basis and a truncated power function basis was used to explore the nonlinear association between continuous HbA1c levels and clinical events. In addition, subgroup analyses were conducted for age, body mass index (BMI), and frailty to explore potential variations in associations.
A two-sided p value of less than 0.05 was considered significant. All analyses were conducted using SAS/STAT software version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
A total of 6,789 patients were included in this study. These patients were divided into groups of 3,191 patients on low-hypoglycemic-risk drugs and 3,598 patients on high-hypoglycemic-risk drugs. The mean follow-up period was 3.2 years and 3.9 years in the low-hypoglycemic-risk and high-hypoglycemic-risk drugs cohorts, respectively. As the primary objective of this study was not to compare the outcomes between the low-hypoglycemic-risk and high-hypoglycemic-risk drugs cohorts, the difference in follow-up times between these two cohorts does not introduce bias.
Table 1 and eTable 2 present the baseline characteristics of patients for the mortality and MACEs outcome, respectively, stratified by post-index mean HbA1c levels. In both cohorts, patients in the lower HbA1c quintiles tended to be older, frailer, have higher DCSI scores, and more comorbidities.
The impact of baseline characteristics on mortality and cardiovascular events is illustrated in eTables 3 and 4. In the low-hypoglycemic-risk drugs cohort, univariable analysis showed that patients with older age, male sex, certain comorbidities (such as atrial fibrillation), higher diabetic complication severity, moderate and severe frailty, more advanced CKD staging were associated with a higher risk of all-cause mortality and MACEs (eTable 3). Similarly, in the high-hypoglycemic-risk drugs cohort, older age, greater comorbidity burden, higher DCSI scores, more advanced CKD staging, and increased frailty were associated with a higher risk of all-cause mortality and MACEs (eTable 4). These significant baseline characteristics were adjusted for in the following analysis of clinical outcomes to account for potential confounding effects.
Risk of adverse events associated with HbA1c levels in patients on low-hypoglycemic-risk drugs
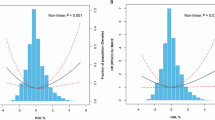
In patients on low-hypoglycemic-risk drugs, a U-shaped association was observed between HbA1c levels and the risk of all-cause mortality and MACEs. Figure 1 presents the results of a nonlinear model, indicating HbA1c levels of 6.7% and 6.8% were associated with the lowest risk of mortality and MACEs, respectively. The overall risk of mortality was low, with incidence rates ranging from 1.85 to 11.43 per 1,000 person-years. Compared with the reference quintile (quintile 3), the lowest and highest HbA1c quintiles were associated with a significantly higher risk of mortality, and the lowest HbA1c quintile was associated with a significantly higher risk of MACEs (Table 2). Sensitivity analysis using time-varying HbA1c revealed a similar U-shaped pattern for the two outcomes in patients on low-hypoglycemic-risk drugs (eTable 5). According to our subgroup analysis, this association between adverse events and HbA1c was consistent across all subgroups regardless of age, BMI, or degree of frailty (eTable 6).
Risk of cardiovascular events in relation to HbA1c in diabetic patients treated with low-hypoglycemic-risk drugs. In the low-hypoglycemic-risk drugs cohort, the relationship between post-index mean HbA1c and cardiovascular events displayed a U-shaped pattern. HbA1c levels of 6.73% and 6.78% were associated with the lowest risks of all-cause mortality (A) and MACEs (B), respectively.
Risk of adverse events associated with HbA1c levels in patients on high-hypoglycemic-risk drugs
In the patients on high-hypoglycemic-risk drugs, a similar U-shaped association was observed between HbA1c levels and the risk of adverse events (Fig. 2). In this cohort, HbA1c levels of 6.9% and 7.2% corresponded to the lowest risk of mortality and MACEs, respectively. These levels are slightly higher than those reported in patients on low-hypoglycemic-risk drugs. Compared with the reference quintile (quintile 3), higher (quintile 5) and lower (quintile 1) HbA1c quintiles were associated with a significantly higher risk of mortality, although the overall risk of mortality was low in this cohort (Table 3). Similarly, a higher (quintile 5) HbA1c quintile was associated with a significantly higher risk of MACEs compared with the reference quintile (quintile 3). When post-index HbA1c was used as a time-varying covariate, a U-shaped pattern was also observed for both mortality and MACE events (eTable 5). This U-shaped association was consistent across different subgroups, including those of older and younger patients and fit and nonfit patients (eTable 6).
Risk of cardiovascular events in relation to HbA1c in diabetic patients treated with high-hypoglycemic-risk drugs. In the high-hypoglycemic-risk drugs cohort, the relationship between post-index mean HbA1c and cardiovascular events displayed a U-shaped pattern. HbA1c levels of 6.85% and 7.15% were associated with the lowest risks of all-cause mortality (A) and MACEs (B), respectively.
Discussion
This study demonstrated a U-shaped association between HbA1c levels and the risk of mortality and MACEs in both low- and high-hypoglycemic-risk drugs cohorts. The lowest risk of mortality and MACEs was observed at HbA1c levels of 6.7% and 6.8% in the low-hypoglycemic-risk group, whereas in the high-hypoglycemic-risk group, the lowest risk was seen at HbA1c levels between 6.9% and 7.2%. These findings are consistent with prior studies that have reported nonlinear associations between glycemic control and cardiovascular risk, suggesting that both inadequate and excessive glucose lowering may be associated with adverse outcomes.
The U-shaped association between HbA1c and mortality has been widely reported in both RCTs and observational studies, reinforcing the complexity of glycemic control strategies16,17,18,19,20,21,22. Currie et al.22 demonstrated that, among patients receiving a combination of sulfonylurea and metformin, the lowest mortality risk was observed at an HbA1c level of 7.5%, while HbA1c levels approaching 6.5% were associated with increased mortality risk. Similarly, an early nested case-control study found that patients with HbA1c levels below 6% had a higher likelihood of experiencing cardiovascular events compared to those with HbA1c levels between 6% and 8%21. More recently, a prospective cohort study of community-dwelling patients with type 2 diabetes found a J-shaped association between baseline HbA1c levels and mortality risk, with the lowest risk observed at HbA1c levels between 6.5% and 7.0%18. These previous findings align with our study, which also demonstrated that both low and high HbA1c levels were associated with increased risks of mortality and cardiovascular events, with the lowest risk observed at intermediate HbA1c levels. This consistency supports the generalizability of the U-shaped association across diverse type 2 diabetes populations and treatment regimens.
Previous meta-regression analyses of RCTs on novel antidiabetic agents have reported a linear association between HbA1c reduction and lower cardiovascular risk, with most studies achieving an HbA1c level close to 7%23. However, our findings suggest that achieving near-normal HbA1c levels may not necessarily confer additional cardiovascular benefits, particularly among patients receiving low-hypoglycemic-risk drugs. This observation raises important questions about the optimal glycemic target in real-world settings, particularly in the context of modern antidiabetic therapies. Current clinical guidelines recommend an HbA1c target of < 7% for most patients while emphasizing the importance of individualized glycemic goals based on patient characteristics24. Our findings underscore the complexity of glycemic management and highlight the need for more nuanced approaches in defining optimal targets. Glycemic control should be contextualized within the broader clinical picture, taking into account comorbidities, functional status, and nutritional condition, particularly in patients with low HbA1c levels. Rather than applying uniform targets, individualized treatment strategies may be more appropriate. Further prospective studies and clinical trials are needed to refine optimal HbA1c targets for different patient subgroups and treatment strategies.
The mechanism underlying the lack of additional cardiovascular benefit from intensive glycemic control remains unclear. One potential explanation is the impact of hypoglycemia, which has been shown to impair cardiac autonomic function, promote platelet aggregation, and trigger inflammatory responses, all of which contribute to an increased risk of cardiovascular events25,26. However, hypoglycemia alone may not fully explain the observed U-shaped relationship between HbA1c levels and health outcomes. Emerging evidence suggests that low HbA1c levels do not always indicate optimal health; instead, in certain contexts, they may serve as a marker of vulnerability. Findings from the Action in Diabetes and Vascular Disease trial provides compelling support for this hypothesis27. This large-scale study identified a significant association between severe hypoglycemia and an increased risks of both cardiovascular and non-cardiovascular adverse events, suggesting that hypoglycemia may be a marker of underlying frailty or comorbid conditions that heighten susceptibility to poor clinical outcomes. Moreover, hypoglycemia is recognized as a predictor of frailty. Abdelhafiz et al. highlighted that frailty, characterized by weight loss, diminished physiological reserves, and reduced insulin resistance, can lead to normoglycemia or even hypoglycemia28. Additionally, hypoglycemia itself can contribute to the development of frailty, creating a cyclical relationship that underscores the importance of individualized diabetes management in this population. To account for the confounding effect of frailty, our study utilized the Multimorbidity Frailty Index, providing a more nuanced understanding of the relationship between HbA1c, hypoglycemia, and adverse clinical outcomes.
Notably, our study identified that an HbA1c level of 6.9% was associated with the lowest risk of mortality in patients receiving high-hypoglycemic-risk drugs. This HbA1c level is lower than those reported in prior observational studies, where the optimal HbA1c range was typically between 7% and 8%, particularly in populations where sulfonylurea use was predominant20,22. Several factors may contribute to these differences, including the younger age (mean: 57 years), lower burden of comorbidities, and lower overall mortality rate in our cohort compared with prior studies. These findings align with current clinical guidelines, which recommend lower HbA1c targets for patients with a longer life expectancy and fewer comorbidities24.
This study has several limitations. First, as an observational study, certain confounding factors could not be accounted for due to data availability, including BMI, smoking status, malnutrition, and anemia. Nutritional and smoking status data were unavailable in TMUCRD, and BMI and hemoglobin levels were not routinely measured for many participants. Malnutrition and certain nutrient deficiencies (e.g., iron and vitamin deficiency anemia) can reduce erythropoiesis and erythrocyte turnover, potentially leading to falsely elevated HbA1c levels29,30. Conversely, conditions such as protein-energy malnutrition, hemolytic anemia, and high red blood cell turnover can lower HbA1c levels, further complicating its interpretation31,32. Additionally, because TMUCRD is not a nationwide database, we could not determine the exact time from diabetes diagnosis to first medication use. To address this, we adjusted for baseline diabetes severity using the DCSI, which serves as a proxy for disease progression. Furthermore, as the use of DPP-4 inhibitors, SGLT2 inhibitors, thiazolidinediones, and GLP-1 receptor agonists is regulated by strict insurance reimbursement criteria in Taiwan, patients within the same cohort were likely prescribed these medications at similar time points, minimizing differences in diabetes duration. Second, the relatively short follow-up duration may limit the ability to fully capture long-term outcomes. Third, there is a potential loss to follow-up for MACE events if patients sought care outside our affiliated institutions. However, more than 90% of participants visited our institutions at least quarterly, reducing the risk of missing MACE events. Furthermore, the MACE event rate in our study was comparable to those reported in previous literature, supporting the reliability of our findings33,34. Fourth, the variability in HbA1c measurement frequency could affect the accuracy of the calculated post-index mean HbA1c values. To address this concern, we conducted a sensitivity analysis using time-varying HbA1c, which yielded consistent results with the primary analysis, supporting the robustness of our findings. Fifth, in the low-hypoglycemic-risk cohort, more than 70% of patients were prescribed DPP-4 inhibitors (eTable 7). Future research is needed to explore whether HbA1c associations with mortality or MACE risk vary with drugs offering confirmed cardiovascular benefits, such as SGLT-2 inhibitors or GLP-1 receptor agonists. Finally, our cohort, characterized by low event rates, represents a relatively low-risk population. Thus, findings may not fully generalize to higher-risk or more vulnerable groups. These limitations should be considered when interpreting the study findings.
Conclusions
In this cohort of patients with type 2 diabetes treated with non-insulin therapies, both elevated and low HbA1c levels were associated with increased risks of mortality and cardiovascular events, with the lowest risk observed at intermediate HbA1c levels. This U-shaped association between glycemic control and adverse outcomes underscores the complexity of glycemic management and highlights the need for future prospective studies to better define optimal HbA1c targets tailored to individual patient characteristics and treatment regimens.
Data availability
The data supporting the findings of this study are available from TMUCRD, but restrictions apply to the data availability as the data were used under license for this study and are therefore not publicly available. Data are however available from the authors upon reasonable request and with permission of Clinical Data Center, Office of Data Science, Taipei Medical University. Requests for data access can be directed to the corresponding author Chun-Yao Huang (email: cyhuang@tmu.edu.tw).
Abbreviations
- BMI:
-
Body mass index
- DCSI:
-
Diabetes Complications Severity Index
- DPP-4:
-
Dipeptidyl peptidase-4
- GLP-1:
-
Glucagon-like peptide-1
- HR:
-
Hazard ratio
- HbA1c:
-
Glycated hemoglobin
- ICD:
-
International Classification of Diseases
- MACE:
-
Major adverse cardiovascular event
- RCT:
-
Randomized controlled trial
- SGLT-2:
-
Sodium-glucose cotransporter-2
- TMUCRD:
-
Taipei Medical University Clinical Research Database
References
Action to Control Cardiovascular Risk in Diabetes et al. Effects of intensive glucose Lowering in type 2 diabetes. N Engl. J. Med. 358, 2545–2559 (2008).
Duckworth, W. et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl. J. Med. 360, 129–139 (2009).
Group, A. C. et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl. J. Med. 358, 2560–2572 (2008).
Hsu, P. F. et al. Association of clinical symptomatic hypoglycemia with cardiovascular events and total mortality in type 2 diabetes: a nationwide population-based study. Diabetes Care. 36, 894–900 (2013).
Khunti, K. et al. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. 38, 316–322 (2015).
Nguyen, P. A. et al. Taipei medical university clinical research database: a collaborative hospital EHR database aligned with international common data standards. BMJ Health Care Inf. 14;31(1):e100890 (2024).
Karagiannis, T., Paschos, P., Paletas, K., Matthews, D. R. & Tsapas, A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 344, e1369 (2012).
DeFronzo, R. A., Davidson, J. A. & Del Prato, S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes. Metab. 14, 5–14 (2012).
Davidson, M. A., Mattison, D. R., Azoulay, L. & Krewski, D. Thiazolidinedione drugs in the treatment of type 2 diabetes mellitus: past, present and future. Crit. Rev. Toxicol. 48, 52–108 (2018).
Campbell, L. K., White, J. R. & Campbell, R. K. Acarbose: its role in the treatment of diabetes mellitus. Ann. Pharmacother. 30, 1255–1262 (1996).
DeFronzo, R. A. et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on Metformin. Diabetes Care. 38, 384–393 (2015).
Mantsiou, C. et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 22, 1857–1868 (2020).
Glasheen, W. P., Renda, A. & Dong, Y. Diabetes complications severity index (DCSI)-Update and ICD-10 translation. J. Diabetes Complications. 31, 1007–1013 (2017).
Lai, H. Y., Huang, S. T., Chen, L. K. & Hsiao, F. Y. Development of frailty index using ICD-10 codes to predict mortality and rehospitalization of older adults: an update of the Multimorbidity frailty index. Arch. Gerontol. Geriatr. 100, 104646 (2022).
Wen, Y. C., Chen, L. K. & Hsiao, F. Y. Predicting mortality and hospitalization of older adults by the Multimorbidity frailty index. PLoS One. 12, e0187825 (2017).
Arnold, L. W. & Wang, Z. The HbA1c and all-cause mortality relationship in patients with type 2 diabetes is J-shaped: a meta-analysis of observational studies. Rev. Diabet. Stud. 11, 138–152 (2014).
Cavero-Redondo, I., Peleteiro, B., Alvarez-Bueno, C., Rodriguez-Artalejo, F. & Martinez-Vizcaino, V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open. 7, e015949 (2017).
He, Q. et al. Association between glycated hemoglobin and risk of all-cause mortality in community patients with type 2 diabetes: A prospective cohort study. J. Diabetes Investig. 15, 939–945 (2024).
Ying, D. G., Ko, S. H., Li, Y. C. & Chen, C. X. Association between intensive glycemic control and mortality in elderly diabetic patients in the primary care: A retrospective cohort study. Prim. Care Diabetes. 14, 476–481 (2020).
Chiang, H. H. et al. All-cause mortality in patients with type 2 diabetes in association with achieved hemoglobin A(1c), systolic blood pressure, and low-density lipoprotein cholesterol levels. PLoS One. 9, e109501 (2014).
Colayco, D. C., Niu, F., McCombs, J. S. & Cheetham, T. C. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care. 34, 77–83 (2011).
Currie, C. J. et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 375, 481–489 (2010).
Huang, C. J. et al. Blood glucose reduction by diabetic drugs with minimal hypoglycaemia risk for cardiovascular outcomes: evidence from meta-regression analysis of randomized controlled trials. Diabetes Obes. Metab. 20, 2131–2139 (2018).
American Diabetes Association Professional Practice C. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes-2024. Diabetes Care. ;47:S111-S25. (2024).
Adler, G. K. et al. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes 58, 360–366 (2009).
Wright, R. J. et al. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care. 33, 1591–1597 (2010).
Zoungas, S. et al. Severe hypoglycemia and risks of vascular events and death. N Engl. J. Med. 363, 1410–1418 (2010).
Abdelhafiz, A. H., Koay, L. & Sinclair, A. J. The effect of frailty should be considered in the management plan of older people with type 2 diabetes. Future Sci. OA. 2, FSO102 (2016).
Christy, A. L., Manjrekar, P. A., Babu, R. P., Hegde, A. & Rukmini, M. S. Influence of iron deficiency anemia on hemoglobin A1c levels in diabetic individuals with controlled plasma glucose levels. Iran. Biomed. J. 18, 88–93 (2014).
Kim, T. H. & Choi, S. H. Diagnosing diabetes with hemoglobin a1c: current debates and considerations for anemic patients. Diabetes Metab. J. 37, 340–342 (2013).
Kannan, S. et al. Impact of Anemia and red cell indices on the diagnosis of Pre-Diabetes and diabetes in Indian adult population: is there a Cut-off guide for clinicians?? Indian J. Endocrinol. Metab. 23, 91–96 (2019).
Klonoff, D. C. Hemoglobinopathies and hemoglobin A1c in diabetes mellitus. J. Diabetes Sci. Technol. 14, 3–7 (2020).
Group, A. S. C. et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl. J. Med. 379, 1529–1539 (2018).
Ogawa, H. et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 300, 2134–2141 (2008).
Acknowledgements
The authors would like to thank the Health Data Analytics and Statistics Center, Office of Data Science, Taipei Medical University, Taiwan, for their statistical and computational support.
Funding
This study was supported by Taipei Medical University Hospital, Taipei, Taiwan (112TMUH-P-03 and 113TMU-TMUH-13). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Y.C.L, C.W.C, Y.H.S and C.Y.H were responsible for the conception and design of the study. Y.C.L, L.Y.H, Y.H.S and C.Y.H were responsible for acquisition, analysis, and interpretation of data. L.Y.H and Y.H.S were responsible for the statistical analyses. Y.C.L, B.L.C, Y.H.S and C.Y.H supervised and provided administrative support. Y.C.L drafted the manuscript. C.W.C, L.Y.H, B.L.C, Y.H.S and C.Y.H critically reviewed the manuscript. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Joint Institutional Review Board of Taipei Medical University (approval no. N202306045). Informed consent was waived by the Joint Institutional Review Board of Taipei Medical University due to the retrospective nature of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, YC., Chen, CW., Huang, LY. et al. Glycemic levels and cardiovascular events in type 2 diabetes: A cohort study of drugs with different hypoglycemic potentials. Sci Rep 15, 24852 (2025). https://doi.org/10.1038/s41598-025-10215-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10215-7