Abstract
We aimed to compare overall survival (OS) and cancer-specific survival (CSS) between patients with chondroblastic osteosarcoma and fibroblastic osteosarcoma, and to identify prognostic risk factors for these subtypes. Clinical and demographic data from 723 patients with either chondroblastic osteosarcoma or fibroblastic osteosarcoma were extracted from the SEER database. Cox proportional hazards models were used to assess the association of these two types of osteosarcoma with OS, while Fine-Gray competing risk models evaluated CSS, with adjustments for covariates in both unweighted and inverse probability treatment-weighted (IPTW) samples. Subsequent univariate and multivariate analyses identified prognostic factors specific to each subtype. We did not observe any statistically significant differences in terms of the association of the two pathological types of osteosarcomas with OS and CSS using either unweighted or weighted samples. For chondroblastic osteosarcoma, OS was significantly associated with age ≥ 30 years, non-Hispanic Black race, first cancer, primary site, T2-4 stage, M1 stage, and having chemotherapy and surgery were significantly associated with the OS. CSS was associated with age ≥ 30 years, T2-4 stage, M1 stage, and having surgery were associated factors for CSS. In patients with fibroblastic osteosarcoma, OS was significantly associated with primary site, M1 stage, and having radiation and surgery. While CSS was associated with primary site, T2-T4 and TX stage, N1 and M1 stage, and having radiation and surgery. The present study demonstrated the survival profiles of patients with chondroblastic and fibroblastic osteosarcoma. Patients with fibroblastic osteosarcoma do not have better OS or CSS compared with those with chondroblastic osteosarcoma. Multiple risk factors for a poor prognosis were identified, and they can be used to refine the therapeutic approach.
Similar content being viewed by others
Introduction
Osteosarcoma represents one of the most common primary malignant sarcomas, characterized by its high invasiveness and predilection for affecting children and adolescents. Approximately 80% to 90% of osteosarcomas manifest in long tubular bones, with distal femur and proximal tibia being the most common sites of onset, followed by the proximal humerus1,2,3. The World Health Organization (WHO) 2020 classification of bone tumors prioritizes grading (high or low) over detailed histological subtypes for conventional osteosarcoma. Although traditional pathology identifies chondroblastic, fibroblastic, and osteoblastic variants, these are not primary diagnostic criteria in the modern system, which focuses on molecular characteristics and tumor biology for prognosis. Despite this, histological variants are still reported in clinical practice and can influence treatment decisions. At present, primary therapeutic approaches encompass surgical resection, chemotherapy, radiotherapy, and small molecule targeted therapy4,5. Perioperative chemotherapy has been shown to significantly enhance the 5-year survival rate and limb salvage rates in patients with osteosarcoma. However, the survival benefit conferred by chemotherapy has not seen a substantial improvement in recent years6. Furthermore, at the time of initial diagnosis, 10% to 15% of osteosarcoma patients present with metastases, most commonly to the lungs. The 5-year survival rate of such patients is only 20%7,8,9. Consequently, during the diagnostic and therapeutic processes, the primary objective is to improve patient prognosis and increase survival rates. Achieving this goal requires a thorough investigation of the risk factors associated with unfavorable disease outcomes. By focusing on these critical determinants, we aspire to make significant advancements in the survival outcomes for patients with osteosarcoma.
The prognosis and treatment of osteosarcoma are significantly influenced by complex genomic alterations and modifications in the tumor immune microenvironment10,11,12,13,14,15,16. Despite the considerable variability in the clinical manifestations of different osteosarcoma subtypes, current clinical practice tends to employ uniform treatment protocols for all patients. This approach leads to notable discrepancies in patient outcomes. Our article underscores the ongoing debate concerning the prognostic disparities between chondroblastic osteosarcoma and fibroblastic osteosarcoma patients17. One study suggested that patients with chondroblastic osteosarcoma exhibit a superior survival rate compared to those with osteoblastic osteosarcoma18. Conversely, another study reported that the 5-year overall survival rate for patients with fibroblastic osteosarcoma was significantly higher than that for patients with the other two osteosarcoma subtypes19,20. Chondroblastic osteosarcoma typically exhibits a less favorable response to chemotherapy relative to other osteosarcoma subtypes. This phenomenon is frequently linked to a reduced rate of tumor necrosis following chemotherapy, which correlates with a poor prognosis and an elevated risk of local recurrence and metastasis. Mirabello et al. notably observed that the 5-year survival rates of patients with chondroblastic and fibroblastic osteosarcoma varied among different age groups21. Nevertheless, the reliability of these findings is constrained by small sample size and inconsistent baseline characteristics of patients in the studies. Addressing these limitations through further research on survival rates in chondroblastic osteosarcoma and fibroblastic osteosarcoma patients is crucial for developing targeted clinical strategies to improve patient prognosis. Such investigations are essential for refining therapeutic approaches and ultimately enhancing patients’ quality of life.
We hypothesized that, after adjusting for clinicopathological factors, chondroblastic and fibroblastic osteosarcoma would show similar overall and cancer-specific survival rates. Differences seen in unadjusted analyses are likely due to demographic and clinical characteristic imbalances, not biological differences. Therefore, we employed the inverse probability of treatment weighting (IPTW) approach to counterbalance systematic disparities in baseline characteristics between the two cohorts (chondroblastic and fibroblastic osteosarcoma patients), thereby ensuring comparability of the baseline characteristics. This strategy facilitates an exhaustive comparison of the prognoses for chondroblastic osteosarcoma and fibroblastic osteosarcoma patients, providing substantial insights beneficial for clinical practice. Furthermore, we conducted comprehensive analysis to identify risk factors associated with a poor prognosis of these two types of osteosarcoma patients, which will enhance the identification of key determinants that influence survival.
Materials and methods
Data source
Patient data were collected from the 17 registries of the Surveillance, Epidemiology, and End Results (SEER) program administered by the United States National Cancer Institute, covering the period from 2000 to 2019. It comprises publicly accessible information devoid of personal identifiers. The SEER*Stat application (version 8.4.3) was employed to ascertain frequencies and variables of interest. The current study was conducted according to the Declaration of Helsinki. Ethical approval from the Institutional Review Board was waived because we had acquired a registered account with the approval for using patient data for research purposes from the SEER database. Informed consent from patients was not required for releasing data.
Data collection and study population
Site-specific codes were first used to identify all primary tumors that originated in the soft tissue, bones and joints; C400-C403, C408-C414, C418-C419, C470-C476, C478-C479, C490-C496, and C498-C499. Histologic International Classification of Diseases (ICD)−0–3 codes were then reviewed for all cases to identify the following histological subtypes; “chondroblast osteosarcoma” (9181/3) and “fibroblastic osteosarcoma” (9182/3).
Patient characteristics of interest included age, year of diagnosis, sex, race, tumor size, median household income, first malignant primary indicator, sequence number, grade of primary osteosarcoma, American Joint Committee on Cancer (AJCC) TNM stage, records of surgery, radiation and chemotherapy, survival months, vital status, and cause of death. Primary outcomes were defined as the 5-year overall survival (OS) from the time of initial diagnosis, and 5-year cancer-specific survival (CSS).
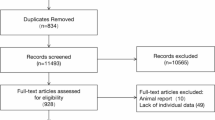
Due to the absence of specific classification for the diagnosis of AJCC TNM stage in the SEER database prior to 2004, all patients diagnosed before this year were excluded from the study. The search identified 735 patients with chondroblastic osteosarcoma or fibroblastic osteosarcoma diagnosed between 2004 and 2019. Given that OS and CSS were the primary endpoints, five patients with a survival time of zero were excluded. Furthermore, to ensure an optimal model fit, seven patients with missing information on TNM stage information were also excluded. Thus, we obtained complete data for 723 patients and enrolled them in the study, 570 patients with a diagnosis of chondroblastic osteosarcoma and 153 patients with fibroblastic osteosarcoma.
Data elements
We coded the covariates on the basis of the recognized cutoff values. Patients were divided into two groups by age, 0–29 years and ≥ 30 years. Race was categorized into non-Hispanic White, non-Hispanic Black, and other races. Annual household income was used as the economic status and categorized into less than $59,999, $60,000-$64,999, $65,000-$69,999, $70,000-$74,999, and more or equal to $75,000. Tumor grades were categorized into I well differentiated, II moderately differentiated, III poorly differentiated, IV undifferentiated, and unknown. In the present study, we combined I-III into one group. TNM classification were staged in accordance to the 6th edition AJCC Staging Manual.
Statistical analysis
Firstly, we conducted the descriptive analysis. Normality was checked for a continuous variable, that is, time of survival in our study. It shows a skewed distribution, so median and interquartile range were reported. Categorical variables were summarized as frequencies and percentages. To compare the baseline characteristics between the patients with chondroblastic osteosarcoma and those with fibroblastic osteosarcoma, we applied the Mann–Whitney U test for continuous variables with a skewed distribution, and Chi-square tests or Fisher’s exact Chi-square tests for categorical variables.
Secondly, we applied the univariate Cox regression analysis to select the variables that had a statistically significant association with OS and CSS. Furthermore, we assessed the association of the two types of osteosarcomas with OS using multivariate Cox proportional hazard models by establishing an unadjusted model (Model I) and two adjusted models (Model II and Model III). Model II was adjusted by age and sex, while Model III was additionally adjusted by variables selected in the univariate Cox regression model. We also assessed two types of osteosarcomas with CSS using multivariate Fine-Gray competing risk regression analyses by establishing an unadjusted model (Model I) and two adjusted models (Model II and Model III). Model II was adjusted for age and sex, while Model III was adjusted by variables selected in the univariate Cox regression model. After adjusting for confounding factors, we further evaluated the association between the two pathological types of osteosarcomas and OS and CSS within various subgroups. The results were visualized by a forest plot.
The IPTW method was used to mitigate confounding and selection bias by balancing the baseline characteristics (e.g., age and tumor stage) between the chondroblastic and fibroblastic groups. It calculates propensity scores to assign weight to patients, creating a balanced cohort where treatment assignment was based on random measured covariates. This helps isolate the true link between histological subtype and survival outcome while preserving sample size and statistical power. Following the application of weights, the balance of baseline covariates across diverse pathological groups was assessed using the Standardized Mean Differences (SMD) and P values, in order to detect any discrepancies at baseline. Next, we conducted the Cox Proportional Hazards Regression and the Fine-Gray model to evaluate the association of two types of osteosarcomas with OS and CSS in the weighted samples. Furthermore, we assessed the association between two pathological types of osteosarcomas and OS and CSS within various subgroups adjusted by covariates in the weighted sample and visualized the results via a forest plot.
Lastly, we applied univariate and multivariate analyses to identify the variables that had a statistically significant association with OS and CSS in patients with chondroblastic osteosarcoma and those with fibroblastic osteosarcoma. To evaluate OS, multivariate Cox proportional hazard models were used, while for evaluating CSS, Fine-Gray competing risk regression analyses were used.
All statistical analyses were conducted utilizing R software (version 4.3.0). Statistical significance was achieved when the P value was < 0.05.
Results
Table 1 presents the baseline characteristics of the study population. There are statistically significant differences between the patients with chondroblastic osteosarcoma and those with fibroblastic osteosarcoma in terms of age, T stage, receiving chemotherapy, and survival time (P < 0.05). Supplementary Figure S1 demonstrates trends in the case numbers of chondroblastic osteosarcoma and fibroblastic osteosarcoma from 2004 to 2019.
Supplementary Table S1 shows the results of univariate COX regression analysis to select the potential associated factors of OS and CSS. Table 2 presents results of multivariate regression analysis to assess the association of chondroblastic and fibroblastic osteosarcomas with OS and CSS. In the crude models, patients with fibroblastic osteosarcoma had a better OS compared with chondroblastic osteosarcoma patients (P = 0.022). Kaplan–Meier curves illustrate similar trends (see Supplementary Figure S2). As shown in Table 2, after adjusting for all the covariates selected from the univariate analysis, we did not observe any statistically significant differences in terms of the association of the two pathological types of osteosarcomas with OS or CSS. The loss of statistical significance after IPTW adjustment suggests that the initially observed survival advantage in fibroblastic osteosarcoma may be attributable to imbalances in baseline prognostic factors rather than inherent biological differences between subtypes.
Figure 1 is a forest plot demonstrating hazard ratios (HRs) and 95% confidence intervals (CIs) regarding associations between fibroblastic osteosarcoma and OS and CSS using chondroblastic osteosarcoma as a reference within various subgroups after adjusting covariates. Compared with chondroblastic osteosarcoma patients, patients aged 30 years or older with fibroblastic osteosarcoma had better OS (HR = 0.64, 95% CI: 0.42–0.98).
Multivariable Cox regression analysis of prognostic factors in chondroblastic vs. fibroblastic osteosarcoma (unweighted cohort). Forest plots display hazard ratios (HRs) with 95% confidence intervals (CIs) for overall survival (OS) with red horizontal lines and cancer-specific survival (CSS) with blue horizontal lines. Reference lines at HR = 1 (null effect) are shown as a vertical black line.
Supplementary Table S2 presents baseline characteristics of the patients before and after the IPTW adjustment using the Heman method. After adjustment, all the characteristics were balanced between the patients with chondroblastic and patients with fibroblastic, as indicated by the absolute value of the SMD less than 0.1 except for race and income. However, the differences between the two groups for all variables failed to reach statistical significance. Standardized mean differences before and after IPTW adjustment are presented in Supplementary Figure S3. Table 3 presents results of univariate and multivariate analysis of the association of the two pathological types of osteosarcomas with OS and CSS after IPTW adjustment. We did not observe a statistically significant difference. Figure 2 is a forest plot demonstrating HRs and 95% CIs in the weighted sample of associations between fibroblastic osteosarcoma and OS and CSS using chondroblastic osteosarcoma as a reference within subgroups after adjusting covariates.
Multivariable Cox regression analysis after inverse probability treatment weighting (IPTW). Forest plots show adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) in the IPTW-balanced cohort. Red horizontal lines are for overall survival (OS), and blue horizontal lines are for cancer-specific survival (CSS). Reference lines at HR = 1 (null effect) are shown as a vertical black line.
Supplementary Tables S3 and S4 show the results of univariate analyses to select potentially associated factors for OS and CSS from both groups. We included the variables with a statistically significance into the multivariate analyses (see Tables 4 and 5). In patients with chondroblastic osteosarcoma, age older than 30 years, non-Hispanic Black, first cancer, primary site, T2-4 stage, M1 stage, and having received chemotherapy and surgery were significantly associated with OS (all P values < 0.05). We found that factors influencing CSS as indicated by the multivariate analysis included age older than 30 years, T2-4 stage, M1 stage, and having surgery (all P < 0.05). In patients with fibroblastic osteosarcoma, the primary site, M1 stage, and having had radiation and surgery were significantly associated with OS (all P < 0.05), while for CSS, the primary site, T2-T4 and TX stages, N1 and M1 stages, and having radiation and surgery were associated factors as indicated by the multivariate analysis (all P values < 0.05).
Discussion
Osteosarcoma is the predominant type of malignant bone tumor in children and adolescents22. Chondroblastic and fibroblastic osteosarcoma are two of the major pathologic subtypes in osteosarcoma23. It remains controversial whether there are prognostic disparities between the chondroblastic and fibroblastic osteosarcoma patients18,19,20,21, and there is a paucity of information regarding the risk factors affecting the prognosis of patients with these conditions. In the present study, the prognoses of chondroblastic and fibroblastic osteosarcoma patients were compared using IPTW methods mitigating the differences between the two patient cohorts, based on one of the largest databases, SEER. We have identified multiple risk factors associated with the prognosis of chondroblastic and fibroblastic osteosarcoma by subgroup analyses.
The unadjusted Kaplan–Meier curves indicated that patients with fibroblastic osteosarcoma had significantly better OS and similar CSS compared with those with chondroblastic osteosarcoma. However, the baseline characteristics of the two patient cohorts were not comparable. Specifically, patients with chondroblastic osteosarcoma were younger, more likely to be at a T2 or higher stage, and more often had received chemotherapy compared with those with fibroblastic osteosarcoma. We can hypothesized from this that patients with fibroblastic osteosarcoma may have a better 5-year survival outcome than chondroblastic osteosarcoma patients. To test this hypothesis, we applied IPTW, a powerful statistical tool, to balance the disparities baseline characteristics. After IPTW adjustment, there were no disparities between the survival profiles of the two subtypes of osteosarcomas.
We also performed a subgroup analysis for patients with chondroblastic and fibroblastic osteosarcoma. Risk factors associated with each subtype of osteosarcomas were identified. For chondroblastic osteosarcoma, age, tumor size, N/M stage, radiation, chemotherapy, and surgery were prognostic factors that influenced both OS and CSS. For fibroblastic osteosarcoma, tumor size, M stage, radiation, and surgery were prognostic risk factors for both OS and CSS.
It is well-known that higher T/N/M stage suggests poorer prognosis. Therapeutic approaches, such as radiation, chemotherapy, and surgery, can greatly affect the survival of patients with osteosarcoma. It has been demonstrated that tumors located in the spine or pelvis leads to poorer prognosis than those located in the extremities, which means primary site also affects prognosis24. Interestingly, we found age older than 30 years and non-Hispanic Black race were two factors with a statistically significant association with the survival in patients with chondroblastic osteosarcoma. Lenka Ilcisin et al. showed that race and ethnicity were not associated with event-free survival or overall survival in pediatric nonmetastatic osteosarcoma patients25. However, in our study, that included OS patients for all ages, the results differed. It is known that OS primarily occurs in adolescents and elderly people. For elderly people with OS, which can also be classified to the group with their age older than 30 years in this study, the prognosis is usually poorer, compared with adolescents with OS26.
While both chondroblastic and fibroblastic subtypes are classified under conventional osteosarcoma in the WHO 2020 system due to their shared clinical behavior and treatment paradigms, they exhibit distinct histogenic features. Chondroblastic osteosarcoma has malignant cartilage matrix production (e.g., SOX9 +/COL2A1 + tumor cells), often with hyaline cartilage-like areas, while fibroblastic osteosarcoma is characterized by spindle-shaped tumor cells (vimentin +/fibronectin +) with collagen-rich stroma but minimal osteoid. Notably, these histological differences do not translate to significant survival disparities in our cohorts after multivariate adjustment, suggesting that microenvironmental factors (e.g., angiogenesis patterns) or treatment responsiveness may outweigh cellular phenotype in determining prognosis. Future studies integrating molecular profiling could further elucidate whether these subtypes harbor distinct driver alterations despite their prognostic equivalence.
While this study focused on resolving prognostic discrepancies between chondroblastic and fibroblastic subtypes, future multi-institutional studies with standardized pathology reviews are needed to compare these subtypes against classical osteoblastic osteosarcoma. Such efforts could clarify whether survival differences stem from intrinsic biology or treatment responsiveness across all three major conventional subtypes.
Strengths and limitations
This study utilizes the SEER database to address significant limitations inherent in previous single-center studies, such as limited sample sizes and inconsistent adjustment for confounding variables. Firstly, the population-based design, national scope, and standardized histological coding of SEER facilitate a robust comparison of these rare subtypes with adequate statistical power. This represents a distinct advantage over institutional cohorts, which are often susceptible to selection bias. Secondly, we used the IPTW method to balance the baseline characteristics that were significantly different between the two histologic groups of osteosarcomas in order to estimate the impacts of two types of osteosarcomas on survival outcomes by controlling confounding factors. Thirdly, we furthermore investigated the risk factors associated with overall survival and cancer specific survival in patient subgroups, which provided extensive information for better understanding the outcomes of these two conditions.
There are several limitations that should be addressed. First, all available data in the SEER database are maintained in a retrospective way, therefore the inherent constraints of a retrospective design were inevitable in this study. Second, some detailed information of the patients and treatments is missing from the database, such as patient comorbidities and the detailed techniques of tumor resection. However, most surgeons from different facilities in the United States treated their patients according to the current guidelines. Therefore, the interventions in this study are relatively well-defined. Meanwhile, in our study, the CSS and OS survival analyses show similar results, which makes our study results less susceptible to measurement errors in outcomes. We believe that the influence of information bias has been minimized. Third, while our study provides robust evidence regarding the prognostic equivalence of chondroblastic and fibroblastic osteosarcomas, we acknowledge that histological subtyping is becoming less central to risk stratification in the era of molecular classification. The WHO 2020 guidelines emphasize genetic alterations and tumor microenvironment features over traditional morphology for prognostic assessment. Our analysis was limited by the SEER database’s lack of molecular data, precluding evaluation of how these subtypes align with emerging genomic classifications. Future studies integrating histology with molecular profiling are needed to reconcile traditional pathology practice with evolving diagnostic paradigms.
Conclusion
The present study demonstrates the survival profiles of patients with chondroblastic and fibroblastic osteosarcomas. Patients with fibroblastic osteosarcoma do not have better OS or CSS compared with those with chondroblastic osteosarcoma. Multiple risk factors for a poor prognosis have been identified, which will enhance the understanding of survival outcomes for patients with osteosarcoma in order to refine the therapeutic approaches and eventually improve patients’ quality of life.
Data availability
The data supporting this study’s findings are available from the corresponding author, Dr. Wenhao Chen, upon reasonable request at whchenortho@zju.edu.cn.
References
Gill, J. & Gorlick, R. Advancing therapy for osteosarcoma. Nat. Rev. Clin. Oncol. 18(10), 609–624 (2021).
Belayneh, R., Fourman, M. S., Bhogal, S. & Weiss, K. R. Update on Osteosarcoma. Curr. Oncol. Rep. 23(6), 71 (2021).
Aran, V. et al. Osteosarcoma, chondrosarcoma and ewing sarcoma: Clinical aspects, biomarker discovery and liquid biopsy. Crit. Rev. Oncol. Hematol. 162, 103340 (2021).
Cersosimo, F. et al. Tumor-associated macrophages in osteosarcoma: From mechanisms to therapy. Int. J. Mol. Sci. 21(15), 5207 (2020).
Lee, J. A. et al. Osteosarcoma in adolescents and young adults. Cells 10(10), 2684 (2021).
Anderson, M. E. Update on survival in osteosarcoma. Orthop. Clin. North Am. 47(1), 283–292 (2016).
Chen, C. et al. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 500, 1–10 (2021).
Menendez, N., Epelman, M., Shao, L., Douglas, D. & Meyers, A. B. Pediatric osteosarcoma: Pearls and pitfalls. Semin. Ultrasound CT MR. 43(1), 97–114 (2022).
Wu, C. et al. A tumor microenvironment-based prognostic index for osteosarcoma. J. Biomed. Sci. 30(1), 23 (2023).
Gianferante, D. M., Mirabello, L. & Savage, S. A. Germline and somatic genetics of osteosarcoma - connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 13(8), 480–491 (2017).
Mai, P. L. et al. Risks of first and subsequent cancers among TP53 mutation carriers in the national cancer institute Li-Fraumeni syndrome cohort. Cancer 122(23), 3673–3681 (2016).
Rickel, K., Fang, F. & Tao, J. Molecular genetics of osteosarcoma. Bone 102, 69–79 (2017).
Smida, J. et al. Genome-wide analysis of somatic copy number alterations and chromosomal breakages in osteosarcoma. Int. J. Cancer 141(4), 816–828 (2017).
Yang, H., Zhao, L., Zhang, Y. & Li, F. F. A comprehensive analysis of immune infiltration in the tumor microenvironment of osteosarcoma. Cancer Med. 10(16), 5696–5711 (2021).
Sokratous, G., Polyzoidis, S. & Ashkan, K. Immune infiltration of tumor microenvironment following immunotherapy for glioblastoma multiforme. Hum. Vaccin. Immunother. 13(11), 2575–2582 (2017).
Tian, H. et al. Managing the immune microenvironment of osteosarcoma: the outlook for osteosarcoma treatment. Bone Res. 11(1), 11 (2023).
Jiang, Y. et al. Multi-omics analysis identifies osteosarcoma subtypes with distinct prognosis indicating stratified treatment. Nat. Commun. 13(1), 7207 (2022).
Junior, A. T. et al. Clinicopathological and immunohistochemical analysis of twenty-five head and neck osteosarcomas. Oral Oncol. 39(5), 521–530 (2003).
Bacci, G. et al. Neoadjuvant chemotherapy for high-grade central osteosarcoma of the extremity. Cancer 97(12), 3068–3075 (2003).
Al-Khan, A. A. et al. Fibroblastic subtype has a favourable prognosis in appendicular osteosarcoma of dogs. J. Comp. Pathol. 176, 133–144 (2020).
Mirabello, L., Troisi, R. J. & Savage, S. A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, epidemiology, and end results program. Cancer 115(7), 1531–1543 (2009).
Chen, W., Lin, Y., Jiang, M., Wang, Q. & Shu, Q. Identification of LARS as an essential gene for osteosarcoma proliferation through large-Scale CRISPR-Cas9 screening database and experimental verification. J. Transl. Med. 20(1), 355 (2022).
Mutsaers, A. J. et al. Modeling distinct osteosarcoma subtypes in vivo using Cre: lox and lineage-restricted transgenic shRNA. Bone 55(1), 166–178 (2013).
Chen, W. & Lin, Y. Nomograms predicting overall survival and cancer-specific survival in osteosarcoma patients (STROBE). Medicine (Baltimore) 98(26), e16141 (2019).
Ilcisin, L. et al. Poverty, race, ethnicity and survival in pediatric nonmetastatic osteosarcoma: a Children’s Oncology Group report. J. Natl. Cancer Inst. 116, djae103 (2024).
Chen, W., He, X., Yan, Z., Lin, X. & Bai, G. Predicting metastasis at initial diagnosis and radiotherapy effectiveness in patients with metastatic osteosarcoma. J. Cancer Res. Clin. Oncol. 149(12), 9587–9595 (2023).
Acknowledgements
We thank the Pediatric Evidence-based Medical and Clinical Research Laboratory, an internal institute of the National Clinical Research Center for Child Health, for the great support in terms of methodology and data analysis.
Funding
This work was supported by the grants from National Natural Science Foundation of China (82372668), the National Science Foundation for Young Scientists of China (82001312), Special Fund for the Incubation of Young Clinical Scientist, Children’s Hospital of Zhejiang University School of Medicine (CHZJU2022YS008) and the Medical Innovation Foundation of Fujian Province (2021CXA032).
Author information
Authors and Affiliations
Contributions
GB, LZ and WC contributed to the conception and design. YY, HF, JZ, JX and LS contributed to the development of methodology. GB, FH, JZ, LZ, and WC contributed to the writing, review, and/or revision of the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
We received acceptance of the data access agreement from the SEER administration. No Institutional Review Board approval was required for this study, since no personal-identifying information is included in our dataset.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bai, G., Fu, H., Zhang, J. et al. Survival profiles and associated factors for overall and cancer specific survival in patients with chondroblast and fibroblastic osteosarcoma. Sci Rep 15, 24310 (2025). https://doi.org/10.1038/s41598-025-10433-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-10433-z