Abstract
This systematic review aims to summarize the therapeutic benefits and safety profile of long-acting PEGylated recombinant human growth hormone (PEG‑rhGH) compared with daily recombinant human growth hormone (DGH) in pediatric growth hormone deficiency (PGHD) participants. We conducted an extensive literature review utilizing multiple databases, and evaluated change in height standard deviation score (∆Ht-SDS) as the primary outcome. Secondary outcomes included change in height velocity (∆HV) and incidence of total adverse events (AEs). The meta-analysis focused on comparing the standard dose of PEG‑rhGH (0.20 mg/kg/w) and DGH. The systematic review encompassed ten studies involving a total of 1,393 participants. In RCTs and cohort studies, ∆Ht-SDS at 6 months showed no significant difference between PEG-rhGH and DGH (RCTs: MD = 0.02, 95%CI: -0.02 to 0.07, p = 0.32; Cohort studies: MD = -0.02, 95%CI: -0.24 to 0.19, p = 0.82). However, PEG-rhGH had superior ∆Ht-SDS (MD = 0.19, 95%CI: 0.03 to 0.35, p = 0.02) at 12 months. Results from RCTs also showed that the incidence of total AEs was comparable for PEG-rhGH and DGH (OR = 1.12, 95%CI: 0.84 to 1.49, p = 0.45). PEG-rhGH showed superior efficacy to DGH at 12 months and comparable efficacy at other time points. The safety profiles were similar for the two treatments.
Similar content being viewed by others
Introduction
Growth hormone deficiency (GHD) is a clinical syndrome that can manifest either as isolated or commonly arises as a complication of various pituitary and hypothalamic disorders. Its manifestation depends on the timing of disease onset, and its primary clinical manifestation is growth disorder1, although other comorbidities, such as hypoglycemia, may occur as an indication of PGHD, even in the absence of significant impairment in length or height2. A meta-analysis comprising nine epidemiological studies conducted between 1974 and 2022 reported a prevalence range of 1 in 8,646 to 1 in 1,107 among children and adolescents3.
The administration of exogenous growth hormone has been recognized as the primary treatment option4,5. For the past three decades, recombinant human growth hormone (rhGH) has been used to treat PGHD, with the primary objective of improving linear growth6. Additionally, rhGH is also used in patients without significant hormonal deficiencies, such as those with idiopathic short stature and Turner syndrome7,8. Despite its efficacy and safety, conventional rhGH therapy requires daily injection, which often result in poor adherence and lower height gain9,10,11. However, guidelines recommend that PGHD treatment should last at least for 1–2 years for significant effectiveness, making it necessary to ensure good adherence during long-term treatment. To improve compliance and potentially optimize treatment efficacy and safety, weekly formulations that allow fewer injections have been developed. Four types of weekly growth hormone (GH) have been developed to treat PGHD: somapacitan, lonapegsomatropin, somatrogon, and Jintrolong12,13,14. PEGylated recombinant human growth hormone (PEG-rhGH, Jintrolong) is a long-acting growth hormone. In Jintrolong, rhGH is conjugated with polyethylene glycol to increase the molecular weight of rhGH and extend its elimination half-life15,16,17. In 2014, Jintrolong was approved by the National Medical Products Administration of China, becoming the only weekly administration PEG-rhGH used to treat PGHD in China so far15. Studies have shown that Jintrolong has comparable safety to DGH18,19. In the phase III non-inferiority trial, Jintrolong showed significantly greater height velocity (HV) increases than DGH injection20. In a previous meta-analysis, Mameli et al.19 conducted a sub-analysis regarding Jintrolong vs. Jintropin AQ (a daily injection of GH) by pooling the results of four randomized controlled trials (RCTs) and a cohort study, and found that HV (cm/y) showed no difference between Jintrolong and daily Jintropin AQ. However, that meta-analysis did not include studies published in Chinese. Since Jintrolong is mainly used in China, the objective of this study is to comprehensively review the therapeutic benefits and safety profile of Jintrolong in PGHD through a systematic review and meta-analysis of studies published in Chinese and English.
Methods
This systematic review and meta-analysis strictly adhered to the Cochrane Handbook method21 and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) reporting guideline22. The protocol of this systematic review was registered on the PROSPERO platform (https://www.crd.york.ac.uk/prospero/).
Search strategy and study selection
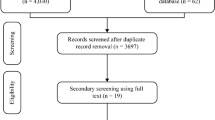
A comprehensive literature search was conducted in PubMed, Embase, Cochrane Library, and Web of Science for English-language publications and CNKI, Wanfang, VIP, and CBM for Chinese-language publications from their inception to April 26, 2024. Two reviewers independently screened search results in a two-step process: initially excluding studies that did not meet inclusion criteria or that met exclusion criteria based on the title and abstract, then conducting a thorough review of the full text of the included literature. All disagreements were resolved through discussion, with the involvement of a third reviewer if necessary. Finally, a PRISMA flowchart was created to illustrate the study selection process.
Eligibility criteria
The study inclusion criteria were: (1) The study sample was prepubertal PGHD; (2) the intervention utilized was marketed PEG-rhGH at a standard dose of 0.20 mg/kg/w; (3) the comparison was made with DGH at a dose of 25–50 µg/kg/day (0.075–0.15 IU/kg/day); (4) the outcomes included change in height standard deviation score (∆Ht-SDS), change in height velocity (∆HV), IGF-1 level, and total adverse events (AEs); (5) the studies were RCTs or cohort studies. Exclusion criteria were: (1) PEG-GH was administered alongside other hormone therapies; (2) control groups received either a placebo or a standard lifestyle intervention; (3) no primary or secondary outcome of interest was reported; (4) the literature was not written in Chinese or English, included only accessible abstracts without full-text articles, or consisted of case reports, reviews, letters, or editorials.
Data extraction
Two reviewers independently extracted data from each study using a standardized form, with disagreements resolved by a third reviewer. Authors were contacted to obtain detailed information if necessary. We extracted general information including the first author, publication year, sample size, study region, and research design; baseline characteristics of participants including country, disease type, age, and gender; details of interventions such as drug name, dose, frequency, and duration; and outcomes including ∆Ht-SDS, ∆HV, IGF-1 level, and total AEs.
Risk of bias assessment
We independently assessed the risk of bias in the included studies, using the Cochrane risk of bias tool23 for RCTs and the Newcastle-Ottawa Scale (NOS)24 for cohort studies. Disagreements were resolved through discussion with the involvement of a third party if necessary,.
Statistical analysis
Since phase II and phase III studies associated Jintrolong at a standard dosage of 0.2 mg/kg/w with a higher IGF-1 level, and this dosage was the lowest recommended starting point, our statistical analysis focused on comparing PEG-rhGH at the standard dose with DGH. The meta-analysis was performed using Review Manager version 5.3 software with the fixed-effect model due to the relative uniformity of the included population. We used odds ratios (ORs) with 95% confidence intervals (CI) to describe dichotomous results and mean differences (MDs) to describe continuous results. To identify potential heterogeneity, we assessed statistical heterogeneity by examining the forest plot. The magnitude of heterogeneity was quantitatively measured by I2 and Chi2, and heterogeneity was considered non-negligible when I2 ≥ 50% or p < 0.05. No priori subgroup analyses or sensitivity analyses were conducted in this study. Because of the limited number of included studies under each outcome indicator, we did not attempt to detect publication bias.
Results
Results of study selection
Following the initial search, a total of 750 articles were retrieved, 417 of which remained after removing duplicates. Through screening of titles and abstracts, 398 articles were excluded, with an additional ten articles excluded during a full-text review. Ultimately, nine eligible publications19,20,25,26,27,28,29,30,31 (ten studies) were included (Fig. 1).
Basic characteristics
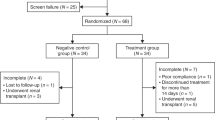
A total of ten studies with 1,393 participants (sample size: 46–363) were included in the systematic review and meta-analysis (Table 1). Six19,20,26,27,31 studies were RCTs and four25,28,29,30 were cohort studies, including three were retrospective cohort studies and one was prospective cohort study. One study reported the results of two clinical trials20. All studies focused on PGHD in China. One study28 reported outcomes after 3 months of treatment, five studies19,20,26,27,31 reported 6-month outcomes, three19,29,30 studies reported 12-month outcomes, and one25 study reported 24-month outcomes. Among the 1,393 participants, 907 were male, 481 were female, and five did not report their gender. The mean ages of the PEG-rhGH-treated children (n = 714) and DGH-treated children (n = 679) were 5.41–11.75 and 5.95–11.77 years, respectively.
Quality of assessment
Two studies did not detail the method of random sequence generation, six studies did not disclose the method of allocation concealment, and two studies lacked information on the blinding implementation. Consequently, we rated the risk of bias in these domains as unclear (Fig. 2). The NOS assessment indicated no obvious risk of bias in the cohort studies (Table 2).
Overall outcomes
∆Ht-SDS
Results from RCTs
Five RCTs20,26,27,31 compared the ∆Ht-SDS for participants receiving PEG-rhGH versus DGH after 6 months. Results revealed similar improvements in both groups (MD = 0.02 (95%CI: −0.02, 0.07), p = 0.32), with statistical heterogeneity (Fig. 3). Detailed results are provided in Table 3.
Results from cohort studies
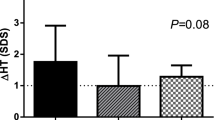
Two cohort studies25,29 compared the ∆Ht-SDS at 6 months between PEG-rhGH and DGH groups. Results showed similar improvements in both groups (MD = −0.02 (95%CI: −0.24, 0.19), p = 0.82), with some heterogeneity observed (Fig. 4). Three studies25,29,30 reported ∆Ht-SDS at 12 months and found that PEG-rhGH was superior to DGH (MD = 0.19 (95%CI: 0.03, 0.35), p = 0.02) (Fig. 5). One study25 reported an MD of 0.14 (95%CI: −0.19, 0.47, p = 0.41) for ∆Ht-SDS after 2 years. Detailed results are provided in Table 4.
∆HV (cm/y)
Three RCTs20,31 and two cohort studies25,28 compared the ∆HV at 6 months between PEG-rhGH and DGH treatments. Both RCTs (Table 3) and cohort studies (Table 4) found no significant difference between the groups (RCTs: MD = 0.30 (95%CI: −0.31, 0.91), p = 0.34; cohort studies: MD = −0.23 (95%CI: −0.98, 0.52), p = 0.55) (Figures S1 and S2). Three cohort studies25,29,30 reported ∆HV at 12 months (MD = 0.21, 95%CI: −0.22, 0.63, p = 0.34), and one study25 reported the follow-up result at 2 years (MD = 0.38, 95%CI: −0.30, 1.06, p = 0.28) (Table 4), all showing no statistical significance (Figures S3 and S4).
IGF-1-SDS
Two RCTs20,31 reported the results of IGF-1-SDS at 6 months (MD = 0.13 (95%CI: −0.14, 0.39), p = 0.34), showing comparable values between PEG-rhGH and DGH. However, in the two cohort studies25,29, the PEG-rhGH group exhibited higher IGF-1-SDS than the DGH group at 6 months (MD = 0.40 (95%CI: 0.04, 0.76), p = 0.03). Long-term follow-up results from a cohort study25 revealed no significant difference in IGF-1-SDS between the two groups at 12 months (MD = 0.17 (95%CI: −0.41, 0.75), p = 0.56), but the PEG-rhGH group had higher IGF-1-SDS than the DGH group at 24 months (MD = 0.57 (95%CI: 0.02, 1.12), p = 0.04).
Three RCTs20,26,31 compared ∆IGF-1-SDS between PEG-rhGH and DGH treatments at 6 months, finding a significant improvement (MD = 0.33 (95%CI: 0.16, 0.51), p = 0.0002). Two cohort studies25,29 reported greater ∆IGF-1-SDS for PEG-rhGH than for DGH at 6 months (MD = 0.39 (95%CI: 0.04, 0.74), p = 0.03) and 12 months (MD = 0.46 (95%CI: 0.10, 0.81), p = 0.01). One study25 found that after 2 years, participants receiving PEG-rhGH had greater ∆IGF-1-SDS than those receiving DGH (MD = 0.61 (95% CI: 0.09, 1.13), p = 0.02). In addition, one study30 reported a 12-month increase in ∆IGF-1 (ng/mL) of 20.54 (95% CI: 9.17, 31.91, p = 0.0004) for PEG-rhGH participants versus DGH participants. In the phase III study, only 7.5% and 4.3% of children in the Jintrolong and DGH groups had an elevated IGF-1 level above 2 SDS, respectively20. Similar findings were observed in a retrospective cohort study, where only one child in both the Jintropin group and the DGH group exhibited elevated IGF-1 level (5% vs. 3.3%)29. The studies by Du and Li both reported that all IGF-1 levels were in the normal range during the treatment period19,31.
Incidence of total AEs
In the included studies, adverse events included peripheral edema, thyroid dysfunction, hyperinsulinemia, headache, joint pain, muscle pain, upper respiratory tract infection, cough and fever, and bronchitis. Four RCTs20,26,31 reported total AEs for participants receiving PEG-rhGH versus DGH. The pooled OR was 1.12 (95%CI: 0.84–1.49, p = 0.45), indicating that total AEs were comparable for the two groups.
Discussion
This systematic review and meta-analysis comprehensively compared the efficacy and safety of Jintrolong (PEG-rhGH) at the standard dose of 0.2 mg/kg/w with DGH for treating PGHD. We found that Jintrolong had similar height gain to DGH at 6 months but showed a significant improvement at 12 months. The changes in HV were comparable between Jintrolong and DGH. No significant difference in total AEs was found between Jintrolong and DGH.
In the field of chronic diseases, there is consensus that long-acting drugs with longer dosing intervals significantly increase treatment compliance, with greater potential to optimize efficacy and safety32. For children with GHD, daily injection pose a major challenge for long-term treatment. Jintrolong was designed to for weekly use, significantly reducing pain, fear, and hospital visits among pediatric patients and thereby improving treatment compliance33. In this study, we used ∆Ht-SDS as the primary outcome to evaluate efficacy between Jintrolong and DGH in children with GHD. The 6- and 24-month analysis showed comparable efficacy. The similar efficacy of Jintrolong and DGH was also verified in another meta-analysis using Ht-SDS as the outcome indicator34. However, the 12-month analysis revealed that Jintrolong was more effective than DGH in terms of ∆Ht-SDS. Interestingly, in a recent real-world study of children with idiopathic short stature (ISS), ∆Ht-SDS was greater for Jintrolong than for DGH at higher dosages (0.2–0.3 mg/kg/w)33. These findings indicate that Jintrolong is not inferior and perhaps even slightly superior to DGH in achieving height gain in children with short stature. Further research is needed in the future to explore the real-world benefits of Jintrolong for pediatric patients, especially height improvement.
∆HV was also an important outcome in this study. The meta-analysis showed that children treated with Jintrolong had comparable ∆HV to those on DGH. In most studies, HV is the major endpoint used to compare the efficacy of different GHs for PGHD19,20,35,36. The phase III multicenter RCT demonstrated that children treated with Jintrolong experienced significantly greater increases in HV (from 2.26 ± 0.87 to 13.41 ± 3.72 cm/year) compared to those treated with DGH (from 2.25 ± 0.82 to 12.55 ± 2.99 cm/year) after 25 weeks (p < 0.05)20. A similar result was also observed in three cohort studies28,29,30. In the other four RCTs and one cohort study, the results of ∆HV were similar for Jintrolong and DGH19,25,26,27,31. Taken together, especially considering the results from the phase III multicenter RCT with the large sample size20, we believe that Jintrolong is not inferior and probably superior to DGH in terms of ∆HV. However, this finding needs to be further validated in strictly designed, prospective, larger-sample studies.
IGF-1 is important in the regulation of cell proliferation and in the growth and development of the bone system37,38. Changes in IGF-1 level are mainly regulated by GH39, making it a crucial indicator for clinically evaluating the severity of PGHD and its treatment19,20,35,36. In the present study, we also compared the influence of Jintrolong and DGH on IGF-1-SDS. All included studies showed that both treatments improved IGF-1 level, with PEG-rhGH being more effective than DGH at each observation point. We also found that the IGF-1 level generally remained in normal range, suggesting that Jintrolong and DGH both have little risk of increasing the IGF-1 level to over the upper limit. In Jintrolong’s phase I study, although pharmacodynamic analysis revealed that the cumulative areas under the concentration vs. time curve (AUC) of IGF-1 after a single injection of Jintrolong and after seven consecutive days of injections of short-acting drugs were similar, Jintrolong tended to exhibit a higher AUC40. The phase I study also confirmed that Jintrolong is more effective than DGH in stimulating the biological effects of physiological GH release. Therefore, a higher IGF-1 level within a safe range in patients treated with Jintrolong suggests greater therapeutic efficacy. The confirmed effective blood exposure of Jintrolong for 7 days in this phase I study indicates that it may have a sustained stimulating effect on GH to promote growth, resulting in higher IGF-1 level and a greater HV and HT-SDS41. We conclude that the superiority of Jintrolong to DGH over HV and Ht-SDS observed in this meta-analysis is unlikely to be due to chance; however, more rigorously designed studies are needed to confirm its long-term benefits in PGHD.
We found that the total AEs were comparable between Jintrolong and DGH across all included studies, and no serious AEs were observed during the study period. In addition, no GH antibodies were detected in the patients treated with PEG-rhGH. The adverse reactions (ADRs) associated with Jintrolong mainly include peripheral edema, hypothyroidism (or a simple decrease in T4 level), elevated fasting insulin, joint pain, and reactions at the injection site. Some patients experienced mild scoliosis without aggravation42. These ADRs were common among children treated with GH and have been reported in previous studies20,26,31,42. No patients developed diabetes or discontinued GH therapy due to ADRs. All ADRs were mild and transient and did not worsen when the PEG-rhGH dose was increased. In addition, a low dose of thyroxine can restore thyroid function in patients with hypothyroidism. Note that the longest follow-up period of the studies in this review was 2 years. A 3-year real-world study of Jintrolong also showed an acceptable safety profile42. Further evaluation is still needed to assess the long-term impact of Jintrolong on the risk of tumor occurrence and cardiovascular metabolic diseases. A long-term, large-scale, real-world follow-up study on Jintrolong’s safety is ongoing (NCT06110910), and it is anticipated to yield additional safety data in the future.
The present study has some strengths. Firstly, our meta-analysis represented an up-to-date and comprehensive analysis of Jintrolong’s efficacy and safety in treating PGHD patients. Secondly, this study included both RCTs and cohort studies, thus partially reflected real-world experience. However, it also had some limitations. Firstly, some unpublished studies were not included in the analysis. Secondly, some RCTs had performance bias or unclear selective or blinding bias. Thirdly, as no individual patient data was obtained, we did not perform a patient-level meta-analysis. Fourthly, because no data on patient compliance in the real world was extracted, we could not compare the compliance between patients receiving PEG-rhGH and those receiving DGH.
The results of our study provided evidence-based support for Jintrolong as the preferred treatment option for PGHD in China. In clinical practice, the recommended initial of Jintrolong for GHD is 0.2 mg/kg/w, due to its proven efficacy and acceptable safety profile. Optimizing the dose during treatment can enhance efficacy without increasing safety risks. We also advocate that future studies explore factors influencing Jintrolong’s efficacy and safety for PGHD, as well as the quality of life for children and their families, and the compliance of treatment.
Conclusion
This study offers up-to-date evidence showing PEG-rhGH’s superior efficacy to DGH at 12 months and comparable efficacy at other time points in treating PGHD. Moreover, the safety profiles were similar for PEG-rhGH and DGH, further validating the results observed in RCTs. The efficacy of Jintrolong can be ensured by administering the standard initial dose followed by dose optimization in clinical practice.
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Chinoy, A. & Murray, P. G. Diagnosis of growth hormone deficiency in the paediatric and transitional age. Best Pract. Res. Clin. Endocrinol. Metab. 30 (6), 737–747 (2016).
Boguszewski, M. C. S. Growth hormone deficiency and replacement in children. Rev. Endocr. Metab. Disord. 22 (1), 101–108 (2021).
Mameli, C. et al. Epidemiology of growth hormone deficiency in children and adolescents: a systematic review. Endocrine 85, 91–98 (2024). https://doi.org/10.1007/s12020-024-03778-4.
Grimberg, A. et al. Guidelines for growth hormone and Insulin-Like growth Factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary Insulin-Like growth Factor-I deficiency. Horm. Res. Paediatr. 86 (6), 361–397 (2016).
Richmond, E. & Rogol, A. D. Treatment of growth hormone deficiency in children, adolescents and at the transitional age. Best Pract. Res. Clin. Endocrinol. Metab. 30 (6), 749–755 (2016).
Collett-Solberg, P. F. et al. Growth hormone therapy in children; research and practice - A review. Growth Horm. IGF Res. 44, 20–32 (2019).
Gao, X. et al. First clinical study on Long-Acting growth hormone therapy in children with Turner sydrome. Horm. Metab. Res. 54 (06), 389–395 (2022).
Xie, L. et al. Effect of long-acting pegylated growth hormone for catch-up growth in children with idiopathic short stature: a 2-year real-world retrospective cohort study. Eur. J. Pediatr. 183 (10), 4531–4539 (2024).
López-Siguero, J. et al. Long-term safety and efficacy of the Recombinant human growth hormone Omnitrope® in the treatment of Spanish growth hormone deficient children: results of a phase III study. Adv. Ther. 28 (10), 879–893 (2011).
Li, J. et al. Metabolomic differential compounds reflecting the clinical efficacy of polyethylene glycol Recombinant human growth hormone in the treatment of childhood growth hormone deficiency. Front. Pharmacol. 13, 864058 (2022).
Coutant, R. et al. Yearly height gain is dependent on the truly received dose of growth hormone and the duration of periods of poor adherence: practical lessons from the French easypod™ connect multicenter observational study. Front. Endocrinol. (Lausanne). 12, 790169 (2021).
Miller, B. S. What do we do now that the long-acting growth hormone is here? Front. Endocrinol. (Lausanne). 13, 980979 (2022).
Zhu, J. et al. Long-acting growth hormone in the treatment of growth hormone deficiency in children: a systematic literature review and network meta-analysis. Sci. Rep. 14 (1), 8061 (2024).
Deal, C. L. et al. Efficacy and safety of weekly Somatrogon vs daily Somatropin in children with growth hormone deficiency: A phase 3 study. J. Clin. Endocrinol. Metab. 107 (7), e2717–e2728 (2022).
Jiang, Z. et al. Short-term efficacy and safety of a lower dose of polyethylene glycol Recombinant human growth hormone in children with growth hormone deficiency: A randomized, dose-comparison study. Front. Pharmacol. 13, 955809 (2022).
Wang, C. et al. The impact of pegylated Recombinant human growth hormone replacement therapy on glucose and lipid metabolism in children with growth hormone deficiency. Ann. Palliat. Med. 10 (2), 1809–1814 (2021).
Lundberg, E. et al. Broad variability in pharmacokinetics of GH following RhGH injections in children. Growth Horm. IGF Res. 40, 61–68 (2018).
Wu, W. et al. PEGylated Recombinant human growth hormone Jintrolong(®) exhibits good Long-Term safety in Cynomolgus monkeys and human pediatric growth hormone deficiency patients. Front. Endocrinol. (Lausanne). 13, 821588 (2022).
Du, H. et al. Evaluation of efficacy and safety of long-acting pegylated Recombinant human growth hormone (Jintrolong) for patients with growth hormone deficiency. J. Pediatr. Endocrinol. Metab. 35 (4), 511–517 (2022).
Luo, X. et al. Long-acting pegylated Recombinant human growth hormone (Jintrolong) for children with growth hormone deficiency: phase II and phase III multicenter, randomized studies. Eur. J. Endocrinol. 177 (2), 195–205 (2017).
Cumpston, M. et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst. Rev. 10 (10), pEd000142 (2019).
Moher, D. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6 (7), e1000097 (2009).
Higgins, J. P. et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. Bmj 343, d5928 (2011).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 (9), 603–605 (2010).
Qiao, Y. et al. Use of pegylated Recombinant human growth hormone in Chinese children with growth hormone deficiency: A 24-Month Follow-Up study. Int. J. Endocrinol. 2019, p1438723 (2019).
Sun, C. et al. Reduced effectiveness and comparable safety in biweekly vs. Weekly pegylated Recombinant human growth hormone for children with growth hormone deficiency: A phase IV Non-Inferiority threshold targeted trial. Front. Endocrinol. 12, 779365 (2021).
Wang, C. L. et al. Effects of short- and long-acting Recombinant human growth hormone (PEG-rhGH) on left ventricular function in children with growth hormone deficiency. Acta Pædiatrica. 100 (1), 140–142 (2011).
Chen, W. J. & Zhang, J. A comparative study on efficacy of long- and short-term Recombinant growth hormones in pediatric patients with growth hormone deficiency. J. Clin. Experimental Med., 18(2), 171–174 ( (2019). https://doi.org/10.3969/j.issn.1671-4695.2019.02.017.
Wan, N. J. et al. Study on the eficacy and safety of long-acting growth hormones in treating pediatric growth hormone deficiency patients. Chin. J. Child. Health Care. 29 (7), 755–758 (2021).
Yang, D. H. & Zhang, J. Impact of long -Acting Recombinant human growth hormone therapy on growth and IGF – 1 level of children with growth hormone deficiency. Clin. Med. Eng. 29 (8), 1079–1080 (2022).
Li, J. & Liu, D. Y. Efficacy, Safety and Compliance of Phase IV Clinical Trial of PEG-rhGh in the Treatment of Children with GHD (ANHUI MEDICAL UNIVERSITY, 2020).
Chaudhary, K., Patel, M. M. & Mehta, P. J. Long-Acting injectables: current perspectives and future promise. Crit. Rev. Ther. Drug Carrier Syst. 36 (2), 137–181 (2019).
Xie, L. et al. Effect of long-acting pegylated growth hormone for catch-up growth in children with idiopathic short stature: a 2-year real-world retrospective cohort study. Eur. J. Pediatr. 183, 4531–4539 (2024).
Mameli, C. et al. Efficacy, safety, quality of life, adherence and cost-effectiveness of long-acting growth hormone replacement therapy compared to daily growth hormone in children with growth hormone deficiency: A systematic review and meta-analysis. Pharmacol. Res. 193, 106805 (2023).
Luo, X. et al. Long-acting pegylated growth hormone in children with idiopathic short stature. Eur. J. Epidemiol 187(5), 709–718 (2022).
Miller, B. S., Blair, J. C. & Rasmussen, M. H. Weekly Somapacitan is effective and well tolerated in children with GH deficiency: the randomized phase 3 REAL4 trial. J. Clin. Endocrinol. Metab 107(12), 3378–3388 (2022).
Laron, Z. Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol. Pathol. 54 (5), 311–316 (2001).
Gow, D. J., Sester, D. P. & Hume, D. A. CSF-1, IGF-1, and the control of postnatal growth and development. J. Leukoc. Biol. 88 (3), 475–481 (2010).
Nicholls, A. R. & Holt, R. I. Growth hormone and Insulin-Like growth Factor-1. Front. Horm. Res. 47, 101–114 (2016).
Hou, L. et al. Comparative pharmacokinetics and pharmacodynamics of a pegylated Recombinant human growth hormone and daily Recombinant human growth hormone in growth hormone-deficient children. Drug Des. Devel Ther. 10, 13–21 (2016).
Thornton, P. S. et al. Weekly Lonapegsomatropin in Treatment-Naïve children with growth hormone deficiency: the phase 3 height. Trial 106 (11), 3184–3195 (2021).
Hou, L. et al. Long-term pegylated GH for children with GH deficiency: A large, prospective, Real-world study. J. Clin. Endocrinol. Metab. 108 (8), 2078–2086 (2023).
Acknowledgements
We would like to express our gratitude to Systematic Review Solutions Ltd. for their help in data collection and assembly as well as the editorial assistance.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Qiuli Chen and J. Z. designed the study protocol and supervision. S. G. and T. W. carried out formal analysis and data curation, J. Z. drafted the manuscript, and Q. C. and S. G. provided revisions. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors assert that there are no conflicts of interest present.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, J., Guo, S., Wang, T. et al. Comparison between long-acting pegylated and daily recombinant human growth hormone for pediatric growth hormone deficiency a systematic review. Sci Rep 15, 26746 (2025). https://doi.org/10.1038/s41598-025-10613-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10613-x
Keywords
This article is cited by
-
Adverse events with usage of human growth hormone: a review
Discover Medicine (2025)