Abstract
This study investigates the therapeutic potential of lycium barbarum glycopeptide (LbGp) in mitigating retinal ischemia-reperfusion injury (RIRI), a key pathological mechanism in glaucoma. RIRI involves inflammation, cellular senescence, and apoptosis, leading to retinal ganglion cell (RGC) degeneration and optic nerve damage. Using in vivo and in vitro models, the study evaluated LbGp’s anti-inflammatory, anti-senescence, and anti-apoptotic properties. In vivo, RIRI was induced in C57BL/6J mice by elevating intraocular pressure, followed by reperfusion, with LbGp administered orally. In vitro, R28 cells underwent oxygen-glucose deprivation/reoxygenation (OGD/R) to simulate RIRI, with LbGp added to cultures. Retinal structure and function were assessed using optical coherence tomography (OCT), OCT angiography (OCTA), and hematoxylin-eosin (HE) staining, while inflammatory markers (IL-6, IL-1β, TNFα), senescence markers (p21, p16), and apoptosis were evaluated via immunofluorescence, enzyme linked immunosorbent assay (ELISA), senescence-associated β-galactosidase (SA-β-gal) staining, terminal dUTP Nick End Labeling (TUNEL) assays, and flow cytometry (FCM). Results showed LbGp restored retinal blood flow, reduced retinal thinning, and improved RGC survival, while decreasing inflammatory, senescence and apoptosis markers. These findings suggest LbGp is a promising therapeutic candidate for glaucoma and ischemic retinal conditions, warranting further research to elucidate its mechanisms and clinical efficacy.
Similar content being viewed by others
Introduction
Glaucoma, a progressive and irreversible optic neuropathy leading to blindness, is characterized by visual field defects and progressive vision impairment1. This condition affects over 75 million individuals globally2. A critical pathophysiological mechanism in glaucoma involves retinal ischemia/reperfusion (I/R) events3. Sudden increases in intraocular pressure (IOP) lead to retinal capillary compression and vascular wall edema, triggering acute retinal ischemia and hypoxia. This results in retinal ganglion cell (RGC) degeneration and optic nerve impairment4,5,6. While reperfusion reestablishes ocular blood flow, it paradoxically induces excessive inflammatory mediator release and reactive oxygen species (ROS) generation. Rather than restoring retinal function, this process accelerates cellular aging, promotes cell death, and ultimately leads to permanent retinal dysfunction and optic nerve damage, collectively termed retinal ischemia reperfusion injury (RIRI)7,8,9. Current research focuses on mitigating RIRI through neuroprotective strategies, with particular emphasis on anti-inflammatory interventions, cellular senescence inhibition, and apoptosis prevention, representing both the challenges and objectives in glaucoma management and research.
Lycium barbarum holds significant importance in traditional Chinese medicine, with a history of use spanning nearly two millennia in China and other Asian nations10. Recently, it has garnered considerable interest from researchers globally due to its dual role as both a medicinal and nutritional resource11. The primary bioactive component of Lycium barbarum is Lycium barbarum polysaccharide (LBP), known for its antioxidant, anti-inflammatory, neuroprotective, and immunomodulatory properties12,13,14. Extensive research indicates that LBP can mitigate neurodegeneration, suppress apoptosis through the regulation of apoptosis-related genes and signaling pathways, reduce inflammatory responses, and slow down the aging process15,16,17. Furthermore, Lycium barbarum glycopeptide (LbGp), derived from further purification of LBP, is a glycoconjugate with enhanced biological activity. Studies have demonstrated that LbGp not only alleviates allergic airway inflammation but also reduces renal inflammatory responses18,19.
Current investigations into the role of LbGp in ophthalmic pathologies remain at a preliminary phase, with existing data being notably scarce. To address this gap, our research aims to establish RIRI models using murine subjects and RGC subjected to oxygen-glucose deprivation/reperfusion (OGD/R) conditions. Through these models, we intend to investigate the potential therapeutic effects of LbGp, specifically its anti-inflammatory, anti-aging, and anti-apoptotic properties, in cases of acute intraocular pressure elevation-induced RIRI. This study seeks to generate foundational experimental data supporting the potential clinical utilization of LbGp for retinal and optic nerve protection.
Results
LbGp exerts a protective effect on RIRI in mice
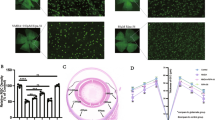
Optical coherence tomography angiography (OCTA) analysis revealed elevated blood flow density in both the retinal nerve fiber layer (RNFL) and ganglion cell complex (GCC) in RIRI mice compared to sham controls. However, LbGp administration resulted in reduced vascular perfusion density relative to untreated RIRI animals. (Fig. 1A, B). Structural evaluation through OCT demonstrated significant thinning of RNFL and GCC in RIRI mice, while LbGp treatment effectively restored these parameters. (Fig. 1C). Hematoxylin-eosin (HE) indicated substantial RGCs loss and vacuolization in RIRI mice compared to sham controls. Notably, LbGp intervention improved both RGC population and cellular morphology compared to untreated RIRI specimens. (Fig. 1D). These findings collectively demonstrate that RIRI induces structural and functional impairment of RGCs and associated neural complexes, while LbGp administration provides significant neuroprotective effects against such ischemic damage.
Effects of LbGp on the retina of RIRI mice. (A) OCTA demonstrates LbGp-mediated alterations in vascular density within the RNFL of RIRI mice and statistical analysis of blood vessel density in the RNFL layer of mice in each group. Scale = 400 μm. Values represent mean ± SD from triplicate experiments: **p < 0.01 vs. Sham group; #p < 0.05 vs. RIRI group. (B) OCTA demonstrates LbGp-mediated alterations in vascular density within the GCC of RIRI mice and statistical analysis of blood vessel density in the GCC layer of mice in each group. Scale = 400 μm. Values represent mean ± SD from triplicate experiments: **p < 0.01 vs. Sham group; #p < 0.05 vs. RIRI group. (C) OCT imaging reveals LbGp-mediated alterations in retinal thickness of RIRI mice and statistical analysis of GCC layer thickness values in each group of mice. Scale = 400 μm. Values represent mean ± SD from triplicate experiments: *p < 0.05 vs. Sham group; #p < 0.05 vs. RIRI group. (D) HE shows LbGp-mediated alterations in pathological of RIRI mice and statistical analysis the number of RGCs in each group of mice, Scale = 50 μm. Blue arrows point to vacuolated RGCs. Values represent mean ± SD from triplicate experiments: **p < 0.01 vs. Sham group; #p < 0.05 vs. RIRI group.
LbGp anti-inflammatory attenuates RIRI mice and OGD/R cells injury
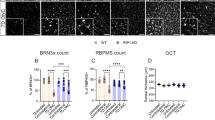
The Immunofluorescence staining (IF) analysis revealed a significant upregulation of interleukin-6 (IL-6) and interleukin-1β (IL-1β) expression levels in both RIRI model mice and OGD/R treated cells when compared to their respective controls (Sham group and Ctrl group). However, this inflammatory response was markedly attenuated in the LbGp-treated groups, as evidenced by reduced fluorescence intensities of these cytokines. (Fig. 2A-E). Consistent with these findings, enzyme linked immunosorbent assay (ELISA) quantification demonstrated elevated concentrations of pro-inflammatory mediators (IL-6, IL-1β, and tumor necrosis factor α (TNFα)) in both RIRI mouse serum and OGD/R cell culture supernatants. Importantly, LbGp administration resulted in substantial decreases in these inflammatory markers compared to untreated RIRI and OGD/R models. (Fig. 2F, G). These collective findings demonstrate that while both in vivo RIRI and in vitro OGD/R models induce robust inflammatory responses, LbGp exhibits significant anti-inflammatory properties in these experimental systems.
Anti-inflammatory effects of LbGp. (A) IF demonstrates LbGp-mediated modulation of IL-6 expression in the retinas of RIRI mice, scale = 50 μm. Dual fluorescence labeling reveals IL-6 (red) and nuclear DNA (blue) distribution patterns in retinal tissues. (B) IF demonstrates LbGp-mediated modulation of IL-1β expression in the retinas of RIRI mice, scale = 50 μm. Dual fluorescence labeling reveals IL-1β (red) and nuclear DNA (blue) distribution patterns in retinal tissues. (C) Quantitative analysis of IL-6 and IL-1β fluorescence intensity in mice. Values represent mean ± SD from triplicate experiments: **p < 0.01 vs. Sham group; #p < 0.05 vs. RIRI group. (D) IF demonstrates LbGp-mediated modulation of IL-6 and IL-1β levels in OGD/R cells, scale = 20 μm. Triple fluorescent labeling reveals the distribution pattern of IL-6 (red), IL-1 (green), and nuclear DNA (blue) in cells. (E) Quantitative analysis of cellular IL-6 and IL-1β fluorescence intensity. Values represent mean ± SD from triplicate experiments: **p < 0.01 vs. Ctrl group, #p < 0.05 vs. OGD/R group. (F) ELISA showing levels of IL-6, IL-1β and TNF-α in peripheral blood of mice. Data are expressed as mean ± SD (n = 6), **p < 0.01 vs. Sham group, #p < 0.05 vs. RIRI group. (G) ELISA showed the levels of IL-6, IL-1β and TNF-α in cell supernatants. Data are expressed as mean ± SD (n = 6), **p < 0.01 vs. Ctrl group, ##p < 0.01 vs. OGD/R group.
LbGp anti-senescence attenuates RIRI mice and OGD/R cells injury
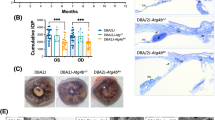
The senescence-associated β-galactosidase (SA-β-gal) analysis revealed significant optic nerve aging in RIRI model mice relative to sham group. Notably, LbGp administration attenuated this senescence phenotype in the LbGp group. (Fig. 3A). Quantitative IF demonstrated substantial upregulation of senescence markers p21 and p16 in both RIRI animal models and OGD/R cellular models compared to their respective controls. Conversely, LbGp intervention effectively suppressed the expression of these biomarkers in both experimental systems. (Fig. 3B-F). These findings collectively indicate that while RIRI and OGD/R conditions induce cellular senescence, LbGp exhibits potent anti-senescence properties in this pathological context.
Anti-senescence effects of LbGp. (A) SA-β-gal showing the state of optic nerve senescence in each group of mice. Red and blue coloring for healthy and aged tissues, respectively. (B) IF demonstrates LbGp-mediated modulation of p21 expression in the retinas of RIRI mice, scale = 50 μm. Dual fluorescence labeling reveals p21 (red) and nuclear DNA (blue) distribution patterns in retinal tissues. (C) IF demonstrates LbGp-mediated modulation of p16 expression in the retinas of RIRI mice, scale = 50 μm. Dual fluorescence labeling reveals p16 (red) and nuclear DNA (blue) distribution patterns in retinal tissues. (D) Quantitative analysis of p21 and p16 fluorescence intensity in mice. Values represent mean ± SD from triplicate experiments: **p < 0.01 vs. Sham group, #p < 0.05 vs. RIRI group. (E) IF demonstrates LbGp-mediated modulation of p21 and p16 levels in OGD/R cells, scale = 20 μm. Triple fluorescent labeling reveals the distribution pattern of p21 (red), p16 (green), and nuclear DNA (blue) in cells. (F) Quantitative analysis of cellular p21 and p16 fluorescence intensity. Values represent mean ± SD from triplicate experiments: **p < 0.01 vs. Ctrl group, ##p < 0.01 vs. OGD/R group.
LbGp anti-apoptosis attenuates RIRI mice and OGD/R cells injury
The terminal dUTP Nick End Labeling (TUNEL) assay revealed a significant increase in fluorescence intensity in RIRI model mice relative to the Sham group, while the LbGp group exhibited reduced fluorescence intensity compared to the RIRI group. (Fig. 4A, B). Parallel findings were observed in cellular experiments, where flow cytometry (FCM) analysis demonstrated an elevated apoptotic rate in OGD/R treated cells versus control group, with LbGp administration markedly attenuating this effect. (Fig. 4C, D). These collective findings indicate that both RIRI-induced renal injury and OGD/R-induced cellular stress trigger apoptotic pathways, while LbGp demonstrates significant anti-apoptotic properties in these pathological conditions.
Anti-apoptosis effects of LbGp. (A) TUNEL displays characteristic apoptotic patterns across experimental groups in retinal tissues, scale = 50 μm. Dual-color fluorescence demonstrates cellular viability, with green indicating apoptotic populations and blue marking viable cells. (B) Quantitative analysis of fluorescence intensity of apoptotic cells in mouse retina. Values represent mean ± SD from triplicate experiments: **p < 0.01 vs. Sham group, ##p < 0.01 vs. RIRI group. (C) Flow diagram of each group of cells. (D) Apoptosis rate in each group.Values represent mean ± SD from triplicate experiments: **p < 0.01 vs. Ctrl group, ##p < 0.01 vs. OGD/R group.
Discussion
RIRI leads to impaired retinal microcirculation and structural damage, accompanied by activation of inflammatory pathways, accelerated cellular aging, and programmed cell death. The administration of LbGp through oral route demonstrated remarkable therapeutic effects, including restoration of retinal hemodynamics, preservation of tissue integrity, and mitigation of pathological processes such as inflammation, senescence, and apoptotic cell death. These findings suggest that LbGp possesses multiple pharmacological properties, including inflammation suppression, senescence inhibition, and apoptosis prevention, making it a potential therapeutic agent for RIRI management.
First, in the RIRI mouse model, notable disruptions in retinal blood flow and tissue architecture were observed. LbGp demonstrated significant retinal protective properties in this context. Similar patterns of structural and functional impairment are evident in other I/R models. For instance, Middle cerebral artery occlusion/reperfusion (MCAO/R) rats exhibited elevated neurological deficit scores, disorganized neuronal arrays, and progressive neuronal necrosis20. Myocardial ischemia/reperfusion injury (MIRI) rats displayed myocardial tissue pallor or cyanosis, disrupted myocardial fiber alignment, and cytosolic edema21,22. Renal ischemia/reperfusion injury (RI/RI) mice showed renal tubular disarray, epithelial cell swelling, and cytoplasmic vacuolization23,24. Consistent with these findings, our study revealed increased retinal blood flow, reduced RNFL and GCC thickness, and marked RGC necrosis and vacuolation in RIRI mice. Following LbGp administration, significant improvements in retinal hemodynamics and structural integrity were noted, underscoring its protective efficacy in the RIRI mouse retina. Existing research indicates a limited understanding of the precise mechanisms through which LbGp exerts retinal protection. Current evidence demonstrates that LBP mediates retinal protection by activating the nuclearfactor erythroidderived 2-like 2/heme oxygenase-1 (Nrf2/HO-1) antioxidant pathway and preserving the blood-retinal barrier (BRB) in diabetic rat models via the rho-associated coiled-coil-containing protein kinase (ROCK) pathway25,26. Furthermore, studies have shown that Lycium barbarum can modulate the mitogen-activated protein kinase (MAPK) pathway to mitigate retinitis pigmentosa progression in murine models14,27. Based on these findings, we postulate that LbGp’s retinal protective effects may involve the modulation of multiple signaling pathways, including Nrf2/HO-1, ROCK, and MAPK, though this hypothesis requires empirical validation through further investigation.
Secondly, RIRI triggers a notable inflammatory reaction, in contrast to LbGP, which exhibits beneficial anti-inflammatory properties. In various I/R disease models, organs and tissues typically display an associated inflammatory response. For instance, in MCAO/R mice, the activation of NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammatory vesicles and microglia facilitates the release of pro-inflammatory cytokines like IL-1β and interleukin-18 (IL-18), thereby inducing neuroinflammatory reactions28,29,30. MIRI results in an exaggerated inflammatory response within cardiomyocytes, leading to the swift release of cytokines such as IL-6 and TNF-α, which in turn causes additional myocardial damage. The suppression of these pro-inflammatory factors can mitigate or prevent MIRI31,32. RI/RI provokes an inflammatory response and T-cell infiltration, culminating in kidney injury. Single-cell sequencing data indicate that inflammatory macrophage subpopulations serve as potential drug targets for RI/RI treatment33,34,35,36. Literature review indicates that IL-6 can directly lead to the loss, degeneration, and death of RGCs37,38,39, and its deficiency may also impair the structure and function of RGCs axons40. The interaction between Müller cells and microglia promotes the upregulation of pro-inflammatory factors such as IL-6, exacerbating retinal inflammation through a positive feedback mechanism41. Meanwhile, IL-1β can induce RGCs apoptosis via the NLRP3/caspase-1 pathway42, and its inhibition reduces RGCs loss43. Furthermore, IL-1β stimulates Müller cell gliosis and activates microglia to release TNF-α and ROS, directly damaging RGCs44,45,46. In addition, glaucoma patients showed high expression of IL-6 in atrial fluid47. Therefore, to validate the clinical findings, IL-6 and IL-1β were selected as inflammatory biomarkers of retinal disease in this study. And our findings align with previous studies on I/R-related pathologies, demonstrating elevated levels of pro-inflammatory mediators (IL6, IL1β, and TNFα) in both retinal tissues and serum samples from RIRI mice. Parallel observations were made in OGD/R cellular supernatants, confirming the induction of substantial inflammatory responses during RIRI. Notably, LbGp markedly attenuated these inflammatory manifestations, revealing its potent anti-inflammatory properties. Based on existing research, LbGp has been shown to modulate the inflammatory microenvironment and facilitate recovery in spinal cord injury (SCI) through suppression of the MAPK/nuclear factor kappa-B (NF-κB) signaling cascade48. Additionally, studies have demonstrated that LBP can mitigate inflammation triggered by corneal damage by downregulating TNF-α and interleukin-17 (IL-17) pathways49, ameliorate non-alcoholic fatty liver disease (NAFLD) caused by high-fat diets via the adenosine 5‘-monophosphate-activated protein kinase (AMPK)/peroxisome proliferators-activated receptor α (PPARα)/peroxisome proliferators-activated receptor γ coactivator l α (PGC-1α) axis50, and alleviate renal inflammatory damage through activation of the Keap1-Nrf2/antioxidant response element (ARE) pathway51. These findings suggest that in RIRI, the anti-inflammatory properties of LbGp might be mediated through similar molecular mechanisms, including the MAPK/NF-κB cascade, TNF/IL-17 signaling, AMPK/PPARα/PGC-1α axis, and Keap1-Nrf2/ARE pathway. However, further experimental validation is required to confirm these potential mechanisms.
Thirdly, RIRI induces cellular senescence, while LbGp exhibits a beneficial anti-senescence property. Cellular senescence represents a significant biological process in cells following RIRI. It is widely recognized that senescent cells can release numerous senescence-associated secretory phenotypes (SASPs), including pro-inflammatory factors, pro-fibrotic factors, chemokines, and growth factors, which subsequently intensify local inflammatory responses in tissues. Previous research has demonstrated that stress-induced senescence can be detected in renal tubular epithelial cells within hours after RI/RI onset52. Additionally, senescence worsens lung ischemia-reperfusion injury (LIRI) in mice due to heightened inflammation and compromised repair mechanisms53. SA-β-gal, a lysosomal enzyme, exhibits significantly elevated activity in senescent cells and serves as a well-established biomarker of cellular senescence54. In optic nerve injury models, SA-β-gal-positive cells are markedly increased in the GCL, correlating with RGCs apoptosis55. The cyclin-dependent kinase inhibitor p21, regulated by p53, is upregulated in response to DNA damage or oxidative stress, inducing G1 phase arrest and promoting senescence56,57. In optic nerve injury models, elevated p21 expression in RGCs is associated with cell cycle arrest and senescence58. Similarly, p16, a key driver of senescence, mediates irreversible cell cycle arrest by inhibiting CDK4/659,60. Its dysregulated expression impairs RGCs proliferation and contributes to senescence, which has been implicated in the pathogenesis of glaucoma61,62.These findings support the use of SA-β-gal, p21, and p16 as robust indicators of senescence in retinal diseases. In line with findings from various I/R models, our study demonstrated that RIRI in mice similarly triggered cellular aging processes. Notably, optic nerve degeneration was prominently observed, accompanied by elevated levels of senescence-related markers p21 and p16 in retinal tissues. Parallel results were obtained in OGD/R cellular models, which showed upregulated expression of these aging-associated proteins. Importantly, LbGp effectively mitigated these senescence-related changes in both RIRI animal models and OGD/R cellular systems, indicating its potent anti-aging properties. Existing studies indicate that Lycium barbarum exhibits significant anti-aging properties63. Research demonstrates that LBP components can mitigate cellular senescence caused by PM2.5 exposure64, while LbGp has been shown to upregulate key transcription factors including DAF-16/Forkhead box O (FoxO), SKN-1/Nrf2, and HSF-1, along with the nuclear receptor DAF-12, leading to enhanced longevity65. Based on these findings, we propose that in RIRI, the anti-senescence effects of LbGp may be mediated through similar molecular pathways involving these transcriptional regulators and nuclear receptors. However, the precise underlying mechanisms require further experimental validation.
Finally, RIRI induces apoptosis in retinal cells, whereas LbGp exhibits significant anti-apoptotic properties. Similar apoptotic mechanisms are observed across various I/R injury models. In the MCAO/R rat model, hippocampal neurons demonstrate elevated apoptosis rates, accompanied by reduced B-cell lymphoma-2 (Bcl-2)/BCL-2-associated X protein (Bax) mRNA expression and increased Caspase-3 protein concentrations66,67. The MIRI model presents with enhanced cardiomyocyte apoptosis, elevated serum markers indicative of myocardial damage, and reduced ATP production in cardiac cells, with cell death inhibitors showing therapeutic potential68,69,70. The RI/RI model similarly displays marked apoptotic responses, which are effectively mitigated through anti-apoptotic pharmacological interventions71,72,73,74,75. In line with findings from various I/R models, our study observed marked apoptotic activity in retinal cells of RIRI mice. Similarly, OGD/R cells exhibited a substantial increase in programmed cell death. Interestingly, in addition to the apoptosis of RGCs in the outer retina, apoptotic cells also appeared in the INL and ONL of the retina. We know that the IL contains mainly bipolar cells, horizontal cells and Müller cells, whereas the ONL consists of a dense arrangement of nuclei from photoreceptor cells. A review of the literature indicates that the appearance of apoptotic cells in the INL and ONL may be due to apoptosis of RGCs, followed by apoptosis of bipolar cells upstream of RGCs due to the loss of targeting connections76,77; it may also be due to the accumulation of glutamate triggered by high IOP, which over-activates the NMDA receptors of bipolar cells and triggers calcium overload and apoptosis78; or it may be that microglia activation releases TNF-α, IL-1β, which exacerbates photoreceptor-triggered apoptosis79. Notably, the administration of LbGp effectively attenuated apoptotic processes in both in vivo and in vitro models, indicating its potential as a therapeutic agent against cellular apoptosis.
A thorough examination of existing research reveals limited investigations into the precise anti-apoptotic mechanisms of LbGp. Current evidence indicates that LBP demonstrates cardioprotective properties by suppressing myocardial cell apoptosis in MIRI-induced rats through modulation of both Nrf2/HO-1 pathwas80,81. Additionally, LBP exhibits protective effects on corneal epithelial cells by targeting mitochondrial apoptotic pathways. Based on these findings, we postulate that in RIRI pathology, LbGp’s anti-apoptotic effects might involve similar molecular pathways, including Nrf2/HO-1 signaling, silent mating type information regulation2 homolog-3 (SIRT3)/Cyclophilin D (CypD) regulation, and mitochondrial function modulation82. However, these potential mechanisms require rigorous experimental validation to establish their precise roles in RIRI pathology.
In conclusion, LbGp demonstrates significant therapeutic potential in mitigating RIRI through its anti-inflammatory, anti-senescence, and anti-apoptotic properties. By restoring retinal function, reducing inflammation, and preventing cellular aging and death, LbGp emerges as a promising candidate for protecting retinal and optic nerve health in glaucoma and related conditions. However, we have to admit that this study ended at the level of phenotypic studies. We also agree that further mechanistic studies could provide deeper mechanistic insights. Therefore, at this stage, we are planning to continue to explore this issue using genomics and other technologies, which involves not only the specific mechanism of LbGp’s anti-inflammatory, anti-aging, and anti-apoptotic properties, but also comparative studies of LbGp with other neuroprotective agents. So, the main purpose of our current phenotypic study is to lay the foundation and provide preliminary data support for subsequent mechanistic studies, and then report more comprehensively on the underlying biological processes involved.
Methods
Animal culture and grouping treatment
A cohort of thirty male C57BL/6J mice, aged 8 weeks with body weights ranging from 20 to 25 g, were acquired from Spivey Biotechnology Co. (Animal Certification No. SCXK (Beijing) 2019-0010). These specimens were maintained at the Animal Research Facility of the Affiliated Hospital of Nanjing University of Chinese Medicine (Certification No. SYXK (Su) 2017-0069), where environmental parameters were strictly regulated, including a constant temperature of 24 ± 2℃, 45–55% humidity range, and standardized photoperiod conditions with equal light-dark intervals. We confirm that all experimental protocols were approved by the designated Ethics Committee of the Nanjing University of Chinese Medicine. All methods were carried out in accordance with relevant guidelines and regulation. All methods are reported in accordance with ARRIVE guidelines.
Using a randomized allocation protocol, the experimental cohort was divided into three distinct groups (n = 10 per group): sham-operated controls (Sham), retinal ischemia-reperfusion injury model (RIRI), and LbGp-treated RIRI subjects (LbGp). RIRI modeling was conducted on all mice except the Sham group. Tribromoethanol (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China; Cat. No.: XW017580906) administration via intraperitoneal route was utilized to achieve surgical anesthesia in all experimental subjects. The right eye was topically anesthetized with 0.4% ouabucaine hydrochloride eye drops (Benoxil; Santen Pharmaceutical Co., Ltd., Osaka, Japan; Cat. No.: HJ20215002) and the pupil was dilated using a compound tropicamide eye drops (Yongguang Pharmaceutical Co., Ltd., Hebei, China; Cat. No.: 0042218). A 33 G needle connected to a sterile saline bottle was inserted into the anterior chamber of the right eye via the temporal corneal limbus. The saline bottle was elevated to 150 cm to increase IOP to 110 mmHg (1 kPa = 7.5 mmHg). Successful retinal ischemia was confirmed by observing pale blood vessels in the corneoscleral rim and retina. After 90 min, the needle was withdrawn to restore blood flow, indicating reperfusion. The left eye remained untreated. Sham group animals received identical surgical interventions excluding the fluid administration protocol. To minimize postoperative complications, 0.3% tobramycin dexamethasone ophthalmic ointment (Alcon, Houston, TX, USA; Cat. No.: HJ20221126) was administered for infection prophylaxis and ocular surface hydration maintenance. Mice with cataracts, iris damage, hemorrhage, or anterior chamber leakage were excluded. Following successful modeling, the Sham and RIRI groups received saline via gavage, while the LbGp group was administered 100 mg·kg−1 LbGp (Tianren Gougou Biotechnology Co., Ltd., Ningxia, China; Cat. No.: 20231203) daily for 7 days, with doses adjusted for human-mouse equivalence83. IOP measurements were obtained with a portable Tonolab tonometer (TioLat, Finland) at baseline prior to surgical intervention and subsequently at 24-hour, 72-hour, and 1-week postoperative intervals.
Cell culture and grouping treatment
The R28 rat retinal precursor cell line was obtained from Kerafast (Boston, MA, USA). Cells were maintained in low-glucose DMEM (Servicebio, Wuhan, China; Cat. No.: G4520) supplemented with FBS (10% v/v; Gibco, NY, USA; Cat. No.: 11011 − 8611) and antibiotic-antimycotic solution (1% v/v; Solarbio, Beijing, China; Cat. No.: P1400). All cellular specimens were incubated under standard physiological conditions (37℃, 5% CO2) with maintained humidity levels.
The cells were categorized into three groups: control (Ctrl), oxygen-glucose deprivation/reoxygenation (OGD/R), and LBGp with OGD/R (LbGp). Ctrl cells were incubated for 24 h in nutrient-rich medium under standard atmospheric oxygen conditions (5% CO2, 95% O2). The other groups were subjected to a hypoxic setting (5% CO2, 95% N2) and a nutrient-deprived medium (lacking glucose and FBS) for 4 h to simulate hypoxic damage. Subsequently, the complete medium was reintroduced, and the cells were transferred to a normoxic chamber (5% CO2, 95% O2) for 18 h of reoxygenation. After 24 h, both Ctrl and Model group cells were kept in the complete medium, while the LbGp group was treated with the same medium supplemented with 100 µg·ml−1 LBP. Cells from all groups were harvested after 24 h for further analysis.
OCT/OCTA
Following the completion of the drug treatment protocol, ocular examinations were performed on the right eyes of all experimental mice using OCT/OCTA imaging technology. The preparatory procedures, including pupil dilation and anesthetic administration, were conducted following established protocols, with subsequent application of saline solution to maintain corneal hydration. For imaging acquisition, the subjects were positioned in a standardized supine orientation on the surgical platform, and retinal scans were obtained using an TowardPi BM-400 K BMizar OCT/OCTA (TowardPi Medical Technology, Beijing, China). Quantitative analysis of retinal parameters, specifically the thickness and vascular perfusion of both the retinal nerve fiber layer (RNFL) and the ganglion cell complex (GCC), was subsequently performed utilizing specialized image processing software (TowardPi analytical suite and Image J).
HE
Retinal specimens from the right eyes of experimental mice underwent standard histological processing. Following tissue dehydration and clearing, samples were embedded in paraffin. Tissue blocks were sectioned at 5 μm thickness using a microtome (Leica, Shanghai, China; machine model: RM2016). Sequential processing included deparaffinization, rehydration, and HE staining using a commercial kit (Servicebio, Wuhan, China; Cat. No.: G1076). After ethanol dehydration and xylene clearing, sections were mounted with neutral balsam. Microscopic examination was performed using a light microscope (Nikon, Japan; machine model: Eclipse E100) coupled with a nikon DS-U3 digital imaging system (Nikon, Japan). Quantitative analysis of retinal morphology, particularly RNFL and GCC thickness, was conducted using Image J software.
IF
Following standard deparaffinization and hydration procedures, murine right retinal paraffin sections underwent antigen retrieval and blocking steps. Primary antibodies targeting IL-1β, IL-6, P21, and P16 (all diluted at 1:500) were applied to the sections, followed by overnight incubation at 4 °C. Subsequently, species-matched fluorescent secondary antibodies (1:500 dilution) were introduced and allowed to react for 1 h at ambient temperature under light-protected conditions. Cell nuclei were stained with DAPI for 10 min under ambient temperature conditions, followed by application of anti-fade mounting medium. Fluorescence imaging was conducted using a nikon Eclipse C1 microscope (Nikon Corporation, Tokyo, Japan), followed by fluorescence quantification through Image J analysis.
In vitro cellular preparations were processed through sequential fixation-permeabilization steps, involving initial treatment with 4% paraformaldehyde (20 min, RT) and subsequent exposure to 0.3% Triton X-100 solution (10 min). Non-specific binding sites were blocked with immunostaining blocking solution for 30 min. Fluorescence staining protocols were maintained consistent with those employed for retinal tissue sections.
ELISA
Following the manufacturer’s protocol (AiFang, Hunan, China; Cat. No. AF-01880M1, AF-02446M1, AF-02415M1), serum samples or cellular supernatants from mice were analyzed by adding 10 µL aliquots to microplate wells. The quantification of inflammatory mediators (IL-1β, IL-6, TNF-α) was performed through spectrophotometric measurement at 450 nm wavelength.
SA-β-gal
The SA-β-gal staining procedure was performed following the supplier’s recommended experimental guidelines (Servicebio, Wuhan, China; Cat. No. GP1072). Briefly, mouse right optic nerve cryosections were initially treated with the fixation solution from the kit and maintained at 37℃ for 30 min. Following three PBS washes, the tissue sections were treated with the chromogenic solution and incubated for 16–18 h at physiological temperature (37℃). After subsequent PBS washing (three times), the tissue sections underwent dehydration and mounting procedures. Fluorescence imaging was conducted using a nikon Eclipse C1 microscope (Nikon Corporation, Tokyo, Japan). Quantitative analysis of optic nerve senescence was subsequently performed using Image J software.
TUNEL
Following the protocol provided in the TUNEL kit (Servicebio, Wuhan, China; Cat. No. GDP1043), retinal tissue sections from the right eyes of mice were processed through a series of steps. Initially, the sections were subjected to dewaxing and rehydration. Subsequently, they were treated with proteinase K solution (20 µg·mL-1, DNase-free) at 37℃ for 30 min. Following triple PBS washes, the samples were permeabilized using permeabilization solution with Triton X-100 for 30 min, followed by another series of PBS rinses. The sections were then exposed to 50 µL of reaction mixture for 60 min. After additional PBS washes, 50 µL of reaction solution was applied and maintained at 37℃ for 60 min under light-protected conditions. Post-incubation, the samples underwent further PBS washing and were counterstained with DAPI for 10 min. Finally, the sections were mounted using anti-fade mounting medium. Fluorescence imaging was performed using a Nikon Eclipse C1 microscope (Nikon Corporation, Tokyo, Japan). Quantitative analysis of apoptotic cells was conducted using Image J software following image acquisition.
FCM
According to the protocol provided by the Membrane Annexin V-FITC/PI Apoptosis Detection Kit (BD Pharmingen, USA; Cat. No. 556547), cellular samples were processed through a series of steps. Briefly, after centrifugation, cell pellets were reconstituted in 500 µL of binding buffer. Subsequently, 5 µL of Annexin V-FITC conjugate and 5 µL of propidium iodide solution were sequentially introduced into the cell suspension. The mixture was gently vortexed and subjected to a 15-minute incubation period at 37℃ under light-protected conditions. Cellular apoptosis was ultimately quantified through COULTER CytoFLEX flow cytometric analysis (BECKMAN; USA).
Statistical analysis
All statistical computations were conducted utilizing SPSS version 26.0 (IBM Corp., NY) and GraphPad Prism software (version 8.1; CA). Data are expressed as mean ± SD, with statistical significance defined as P < 0.05 (*) or P < 0.01 (**). Intergroup comparisons were analyzed using unpaired t-test for two-group analyses and one-way ANOVA for multi-group comparisons.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Weinreb, R. N., Aung, T. & Medeiros, F. A. The pathophysiology and treatment of Glaucoma A review. The J. Am. Med. Association. 311, 1901–11 (2014).
Bicket, A. K. et al. Minimally invasive Glaucoma surgical techniques for Open-Angle Glaucoma an overview of Cochrane systematic reviews and network Meta-analysis. JAMA Ophthalmology. 139, 983–989 (2021).
Gillipsie, M., Jyoti, S. & Nooruddin, K. Cellular stress response and immune signaling in retinal Ischemia–Reperfusion injury. Frontiers Immunology. 159, 56–65 (2016).
Pi, S. et al. Monitoring retinal responses to acute intraocular pressure elevation in rats with visible light optical coherence tomography. Neurophotonics. 6, 4 (2019).
Choi, M. et al. Analysis of microvasculature in nonhuman primate macula with acute elevated intraocular pressure using optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 62, 10 (2021).
Wang, S., Tong, S. & Jin, X. NaDang, pingxiusui, yangliu, yingwang, dajiang, Single-cell RNA sequencing analysis of the retina under acute high intraocular pressure. Neural Regen Res. 19, 2522–2531 (2024).
Zheng, X., Wang, M. & Liu, S. HaiqiaoLi, yifeiyuan, fayang, ludongqiu, suowang, hongweixie, zhixiang, mengqing, A lncRNA-encoded mitochondrial micropeptide exacerbates microglia-mediated neuroinflammation in retinal ischemia/reperfusion injury. Cell Death Dis. 14, 126 (2023).
Qin, Q. et al. Inhibiting multiple forms of cell death optimizes ganglion cells survival after retinal ischemia reperfusion injury. Cell. Death Dis. 13, 507 (2022).
Rahimi, M. et al. Impairments of retinal hemodynamics and oxygen metrics in ocular hypertension-induced ischemia-reperfusion. Exp Eye Res. 225, 109278 (2022).
Cao, Y. L., Li, Y. L., Fan, Y. F., Li, Z. & Liu, Z. J. Wolfberry genomes and the evolution of Lycium (Solanaceae). Communications biology. 4, 671 (2021).
Wenli, S., Shahrajabian, M. H. & Qi, C. Health benefits of wolfberry (Gou Qi zi, fructus barbarum L.) on the basis of ancient chineseherbalism and Western modern medicine. Avicenna J. Phytomedicine. 11, 109-119 (2021).
Tian, X. et al. Extraction, structural characterization, and biological functions of Lycium Barbarum polysaccharides: A review. Biomolecules. 9, 389 (2019).
Kim, J. E. Wolfberry (Lycium barbarum) Consumption with a Healthy Dietary Pattern Lowers Oxidative Stress in Middle-Aged and Older Adults: A Randomized Controlled Trial. Antioxidants. 10, 567 (2021).
Li, H. Y. et al. Lycium barbarum (Wolfberry) increases retinal ganglion cell survival and affects both microglia/macrophage polarization and autophagy after rat partial optic nerve transection. Cell Transplant. 28, 607–618 (2019).
Xue-Song et al. Lycium barbarum polysaccharides related RAGE and Aβ levels in the retina of mice with acute ocular hypertension and promote maintenance of blood retinal barrier. Neural Regen Res. V. 15, 178–186 (2020).
Bing, Q. et al. Lycium barbarum polysaccharides protect human Lens epithelial cells against oxidative Stress–Induced apoptosis and senescence. PLoS One. 9, e110275 (2014).
Zhu, S. et al. Lycium Barbarum polysaccharide protects HaCaT cells from PM2.5-induced apoptosis via inhibiting oxidative stress, ER stress and autophagy. Redox Rep. 27, 32–44 (2022).
Bai, M. et al. The use of metagenomic and untargeted metabolomics in the analysis of the effects of the lycium barbarum glycopeptide on allergic airway inflammation induced by Artemesia annua pollen. J. Ethnopharmacol. 337, 15 (2025).
Zhou, X. et al. Effects of Lycium barbarum glycopeptide on renal and testicular injury induced by di(2-ethylhexyl) phthalate. Cell Stress & Chaperones. 27, 257-271 (2022).
Sun, M. L. et al. Butylphthalide inhibits ferroptosis and ameliorates cerebral Ischaemia-Reperfusion injury in rats by activating the Nrf2/HO-1 signalling pathway. Neurotherapeutics 21, 12 (2024).
Chase, D., Eykyn, T. R., Shattock, M. J. & Chung, Y. J. Empagliflozin improves cardiac energetics during ischaemia/reperfusion by directly increasing cardiac ketone utilization. Cardiovasc. Res. 119, 2672–2680 (2023).
Lee, T. L., Lai, T. C., Lin, S. R., Lin, S. W. & Chen, Y. L. Conditioned medium from adipose-derived stem cells attenuates ischemia/reperfusion-induced cardiac injury through the microRNA-221/222/PUMA/ETS-1 pathway. Theranostics. 11, 3131–3149 (2021).
Zhi-yong, W. D. & Xie Li Zhang,Meng-jie Wang,Zhen-meng Xiao,Yu-hua Zhang,Wan-xin shi,ying huang,yan Yang,Cui-li li,lei Fu,Xing-chen Zhao,Rui-zhao Li,Zhi-lian Li,Yuan-han Chen,Zhi-ming Ye,Shuang-xin liu,zheng Dong,Xin-ling liang, NFAT inhibitor 11R-VIVIT ameliorates mouse renal fibrosis after ischemia-reperfusion-induced acute kidney injury. Actn Pharmcol Sin. 43, 2081–2093 (2022).
Wang, Q. et al. Empagliflozin protects against renal ischemia/reperfusion injury in mice. Sci Rep. 12, 19323 (2022).
Meihua, H. et al. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium Barbarum polysaccharides in the rodent retina after Ischemia-Reperfusion-Induced damage. PLoS One. 9, e84800 (2014).
Wang, J. et al. Protective effects of lycium barbarum polysaccharides on blood-retinal barrier via ROCK1 pathway in diabetic rats. American J. Translational Research. 11, 6304-6315 (2019).
Liu, F. et al. Wolfberry-derived Zeaxanthin dipalmitate delays retinal degeneration in a mouse model of retinitis pigmentosa through modulating STAT3, CCL2 and MAPK pathways. J. Neurochem. 158, 1131–1150 (2021).
Li, Y., Wang, R., Xue, L., Yang, Y. & Zhi, F. Astilbin protects against cerebral ischaemia/reperfusion injury by inhibiting cellular apoptosis and ROS-NLRP3 inflammasome axis activation. Int. Immunopharmacol. 84, 106571 (2020).
Zhang, L. T., Li, G. & Li, Y. TRIM59 suppresses the brain ischaemia/reperfusion injury and pyroptosis of microglial through mediating the ubiquitination of NLRP3. Sci. Rep. 14, 12 (2024).
Cheng, M., Yang, L., Dong, Z., Wang, M. & Zhang, X. Folic acid deficiency enhanced microglial immune response via the Notch1/nuclear factor kappa B p65 pathway in hippocampus following rat brain I/R injury and BV2 cells. Journal Cell. Mol. Medicine. 23, 4795-4807 (2019).
A, C. C. et al. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion through activating the AMPKα1/ULK1/FUNDC1/mitophagy pathway. Redox Biol. 52, 102288 (2022).
Zhuang, L. F., Zong, X., Yang, Q., Fan, Q. & Tao, R. Interleukin-34-NF-KB signaling aggravates myocardial ischemic/reperfusion injury by facilitating macrophage recruitment and polarization. EBioMedicine 95, 20 (2023).
Lau, A. et al. Dipeptidase-1 governs renal inflammation during ischemia reperfusion injury. Sci. Adv. 8, 10 (2022).
Tao, W. et al. Dexmedetomidine attenuates Ferroptosis-Mediated renal ischemia/reperfusion injury and inflammation by inhibiting ACSL4 via α2-AR. Frontiers Pharmacology. 13, 782466 (2022).
Yang, W. et al. Empagliflozin improves renal ischemia–reperfusion injury by reducing inflammation and enhancing mitochondrial fusion through AMPK–OPA1 pathway promotion. Cellular & Mol. Biology Letters. 28, 42 (2023).
Yao, W. et al. Single cell RNA sequencing identifies a unique inflammatory macrophage subset as a druggable target for alleviating acute kidney injury. Advanced Science. 9, 12 (2022).
Arfuzir, N. N. N., Agarwal, R., Iezhitsa, I., Agarwal, P. & Ismail, N. M. Magnesium acetyltaurate protects against endothelin-1 induced RGC loss by reducing neuroinflammation in Sprague Dawley rats. Exp Eye Res. 194, 107996 (2020).
Echevarria, F. D., Formichella, C. R. & Sappington, R. M. Interleukin-6 deficiency attenuates retinal ganglion cell axonopathy and Glaucoma-Related vision loss. Front. Neurosci. 11, 318 (2017).
Liang et al. Analysis of the Interleukin-6 (-174) locus polymorphism and serum IL-6 levels with the severity of normal tension Glaucoma. Ophthalmic Res. J. Res. Experimental & Clin. Ophthalmology. 57, 224-229 (2017).
Wareham, L. et al. Interleukin-6 promotes microtubule stability in axons via Stat3 protein-protein interactions. iScience 24, 103141 (2021).
Hu, X., Zhao, G. L., Xu, M. X. & Wang, Z. Interplay between Müller cells and microglia aggravates retinal inflammatory response in experimental glaucoma. J Neuroinflammation. 18, 303 (2021).
Ye, D., Xu, Y. & Huang, J. Anti-PANoptosis is involved in neuroprotective effects of melatonin in acute ocular hypertension model. Investig. Ophthalmol. Vis. Sci. 63, 2 (2022).
Namekata, K. et al. Drug combination of topical Ripasudil and brimonidine enhances neuroprotection in a mouse model of optic nerve injury. J. Pharmacol. Sci. 154, 326–333 (2024).
Liu, X. et al. IL-1β induces IL-6 production in retinal Müller cells predominantly through the activation of p38 MAPK/NF-κB signaling pathway. Exp. Cell Res. 331, 223–231 (2015).
Abcouwer, S. F. et al. Effect of IL-1β on survival and energy metabolism of R28 and RGC-5 retinal neurons. Invest. Ophthalmol. Vis. 49, 5581–5592 (2008).
Li, J. Q. et al. Discovery of Astragaloside IV against high glucose-induced apoptosis in retinal ganglion cells: bioinformatics and in vitro studies. Gene: Int. J. Focusing Gene Cloning Gene Struct. Function. 905, 148219 (2024).
Chen, K. H., Wu, C. C., Roy, S., Lee, S. M. & Liu, J. H. Increased interleukin-6 in aqueous humor of neovascular glaucoma. Investig. Ophthalmol. Vis. Sci. 40, 2627–2632 (1999).
Jiang, Z. et al. Lycium barbarum glycopeptide alleviates neuroinflammation in spinal cord injury via modulating docosahexaenoic acid to inhibiting MAPKs/NF-kB and pyroptosis pathways. J. Translational Med. 21, 1–14 (2023).
Liu, Q. et al. Exploring the role of Lycium barbarum polysaccharide in corneal injury repair and investigating the relevant mechanisms through in vivo and in vitro experiments. Molecules. 29, 49 (2024).
Li, D. D. et al. Supplementation of < i > lycium barbarum polysaccharide combined with aerobic exercise ameliorates High-Fat-Induced nonalcoholic steatohepatitis via AMPK/PPARα/PGC-1α pathway. Nutrients 14, 14 (2022).
Huang, Y., Shen, C. & Wang, H. Y. X. Iii, lbp reduces Theinflammatory injuryof kidney in septic rat and regulates the keap1-nrf2∕are signaling pathway 1. Acta Cirurgica Brasileira. 34, 1 (2019).
Lee, J., Ko, Y. S., Lee, H. Y., Yang, J. & Kim, M. G. The role of senescence of bone marrow cells in acute kidney injury. Kidney Res. Clin. Practice. 38, 25-32 (2019).
Hayasaka, K. et al. Aging exacerbates murine lung ischemia-reperfusion injury by excessive inflammation and impaired tissue repair response. American J. Transplantation: Official J. Am. Soc. Transplantation Am. Soc. Transpl. Surgeons. 24, 293–303 (2024).
Lee, B. Y. et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 5, 187–195 (2006).
Shi, Y. et al. Melatonin ameliorates retinal ganglion cell senescence and apoptosis in a SIRT1-dependent manner in an optic nerve injury model. Biochim. Et Biophys. Acta (BBA) - Mol. Basis Disease. 1870, 11 (2024).
Feng, C. et al. Cyclic mechanical tension reinforces DNA damage and activates the p53-p21-Rb pathway to induce premature senescence of nucleus pulposus cells. International J. Mol. Medicine. 4, 3316-3326 (2018).
Wang, B., Han, J., Elisseeff, J. H. & Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 25, 21 (2024).
Yao, Y. et al. Cellular senescence mediates retinal ganglion cell survival regulation post-optic nerve crush injury. Cell Prolif. 57, 12 (2024).
Afifi, M. M. et al. Steven D., Irreversible cell cycle exit associated with senescence is mediated by constitutive MYC degradation. Cell Reports. 42, 113079 (2023).
Kawamura, Y. et al. Cellular senescence induction leads to progressive cell death via the INK4a-RB pathway in naked mole-rats. EMBO J. 42, 19 (2023).
Liao, J. et al. Setanaxib mitigates oxidative damage following retinal ischemia-reperfusion via NOX1 and NOX4 Inhibition in retinal ganglion cells. Biomed Pharmacother. 170, 116042 (2024).
Thakur, N. Genetic association between CDKN2B/CDKN2B-AS1 gene polymorphisms with primary glaucoma in a North Indian cohort: an original study and an updated meta-analysis. BMC Med. Genomics. 14, 1 (2021).
Yanjie, G., Yifo, W., Yuqing, W., Fang, G. & Zhigang, C. Lycium barbarum: A traditional Chinese herb and A promising Anti-Aging agent. Aging Disease. 8, 778–791 (2017).
Shen, H. et al. Lycium barbarum polysaccharide’s protective effects against PM2.5-induced cellular senescence in HUVECs. Ecotoxicology Environ. Safety. 274, 116232 (2024).
Zheng, J. M. et al. <i > Lycium barbarum glycopetide prolong lifespan and alleviate parkinson’s disease in < i > caenorhabditis elegans</i >. Front. Aging Neurosci. 15, 11 (2023).
Kang, T. et al. Overexpression of olfactory receptor 78 ameliorates brain injury in cerebral ischaemia-reperfusion rats by activating Prkaca-mediated cAMP/PKA-MAPK pathway. J. Cell. Mol. Med. 28, 18 (2024).
Zhang, Q. et al. Isoflurane post-conditioning contributes to anti‐apoptotic effect after cerebral ischaemia in rats through the ERK5/MEF2D signaling pathway. Journal Cell. & Mol. Medicine. 25, 3803-3815 (2021).
Shen, S. C., He, F., Cheng, C., Xu, B. L. & Sheng, J. L. Uric acid aggravates myocardial ischemia–reperfusion injury via ROS/NLRP3 pyroptosis pathway. Biomed. Pharmacother. 133, 110990 (2021).
Zhu, Q. X. et al. Semaglutide inhibits ischemia/reperfusion-induced cardiomyocyte apoptosis through activating PKG/PKCe/ERK1/2 pathway. Biochem. Biophys. Res. Commun. 647, 1–8 (2023).
Luo, Y. et al. Therapeutic potentials of cell death inhibitors in rats with cardiac ischaemia/reperfusion injury. J. Cell. Mol. Med. 26, 2462–2476 (2022).
Xia, K. et al. Degradation of histone deacetylase 6 alleviates ROS-mediated apoptosis in renal ischemia-reperfusion injury. Biomed. pharmacotherapy = Biomedecine Pharmacotherapie. 165, 115128 (2023).
Güler, M. C., Akpinar, E., Tanyeli, A., Omakli, S. & Bayir, Y. Costunolide prevents renal ischemia-reperfusion injury in rats by reducing autophagy, apoptosis, inflammation, and DNA damage. Iranian J. Basic. Med. Sciences. 26, 1168-1176 (2023).
Cui, Y. et al. TREM2 deficiency aggravates renal injury by promoting macrophage apoptosis and polarization via the JAK-STAT pathway in mice. Cell Death Dis. 15, 401 (2024).
Alaaeldin, R. et al. Azilsartan modulates HMGB1/NF-κB/p38/ERK1/2/JNK and apoptosis pathways during renal ischemia reperfusion injury. Cells 12, 15 (2023).
Zhu, J. et al. Theaflavin pretreatment ameliorates renal ischemia/reperfusion injury by attenuating apoptosis and oxidative stress in vivo and in vitro. Biomed. Pharmacother. 171, 13 (2024).
Schmid, H., Renner, M., Dick, H. B. & Joachim, S. C. Loss of inner retinal neurons after retinal ischemia in rats. Invest. Ophthalmol. Vis. 55, 2777–2787 (2014).
Ling, B., Takae, K., Hongyan, L., Wang, S. W. & Steven, B. Birth of cone bipolar cells, but not rod bipolar cells, is associated with existing RGCs. PLoS One. 9, e83686 (2014).
Milla-Navarro, S. et al. Visual Disfunction due to the selective effect of glutamate agonists on retinal cells. Int J. Mol. Sci. 22, 6245 (2021).
Zhou, W., Zhou, Y., He, P. L. & Jing TREM2 deficiency in microglia accelerates photoreceptor cell death and immune cell infiltration following retinal detachment. Cell Death Dis. 14, 219 (2023).
Pan, H. et al. Lycium barbarum polysaccharide protects rats and cardiomyocytes against ischemia/reperfusion injury via Nrf2 activation through autophagy Inhibition. Spandidos Publications. 24, 778 (2021).
Gao, Y. et al. <i > Lycium barbarum polysaccharides (LBP) suppresses hypoxia/reoxygenation (H/R)-induced rat H9C2 cardiomyocytes pyroptosis via Nrf2/HO-1 signaling pathway</i >. Int. J. Biol. Macromol. 280, 12 (2024).
Du, S. et al. Lycium barbarum Polysaccharides Protect Rat Corneal Epithelial Cells against Ultraviolet B-Induced Apoptosis by Attenuating the Mitochondrial Pathway and Inhibiting JNK Phosphorylation. BioMed Res. Int. 2017, 1–10 (2017).
Kong, Q., Han, X. & Cheng, H. JiayuZhang, huijundong, tangrongchen, jiansuso, Kwok-FaiMi, xuesongxu, yingtang, shibo, Lycium barbarum glycopeptide (wolfberry extract) slows N-methyl-N-nitrosourea-induced degradation of photoreceptors. Neural Regen Res. 19, 2290–2298 (2024).
Acknowledgements
This study was supported by National Natural Science Foundation of China (No: 8217444; No: 82401826), The Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No: 24KJB320012), and Jiangsu Provincial Hospital of Chinese Medicine Outstanding Young Doctor Program (No: 2024QB039).
Author information
Authors and Affiliations
Contributions
C. Z. wrote the main manuscript text and prepared Figs; Y.Z. provided valuable assistance during animal imaging tests; X.L. contributed significantly to both study design and statistical analysis; W.S. contributed to financially support the study and commented the final versions of the manuscript. And all authors edited the manuscript and approved its final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
All methods were carried out in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, C., Lu, X., Zhao, Y. et al. Lycium barbarum glycopeptide mitigates retinal ischemia-reperfusion injury through its anti-inflammatory, anti- senescence, and anti-apoptosis properties. Sci Rep 15, 27806 (2025). https://doi.org/10.1038/s41598-025-10763-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-10763-y