Abstract
Vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKIs) represent a class of targeted drugs that have changed the cancer therapy landscape. However, the anti-angiogenic effect of VEGFR-TKIs significantly increase the incidence of cardiovascular adverse events (AEs). In this study, we conducted a comprehensive analysis of VEGFR-TKI-related thromboembolic events (TEEs), using data from the FAERS database between the drug launch and the third quarter of 2023. Disproportionality analysis, including reporting odds ratio, proportional reporting ratio, bayesian confidence propagation neural network, and multi-item gamma poisson shrinker, were used to explore the correlation between VEGFR-TKIs and TEEs. The total number of reported TEEs was 2688, representing 2.98% of all AEs associated with VEGFR-TKIs. A total of 17 and 12 preferred terms of arterial and venous thromboembolic events were observed in 8 VEGFR-TKIs. Lenvatinib, a VEGFR-TKI with low selectivity and high potency, exhibited the strongest TEE signal. A tendency of an increased signal of disproportionate reporting of TEEs was observed in sorafenib, regorafenib, and sunitinib. Indication, gender, age, drug class, concomitant medication and AEs may influence the occurrence of VEGFR-TKI-related TEEs. This study illustrates that TEEs are highly associated with VEGFR-TKIs, emphasizing the importance of screening for the TEE risks during VEGFR-TKI therapy.
Similar content being viewed by others
Introduction
In recent years, angiogenesis-targeted therapies have solidified their position as a pivotal category in cancer therapeutics. Among these, vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKIs) epitomize an innovative class of targeted anticancer drugs. By competitively binding to intracellular tyrosine kinases (TKs), they disrupt downstream signal transduction and cellular regulation, ultimately inhibiting phosphorylation1. In addition to VEGFR, VEGFR-TKIs inhibit several other TK targets2. Sorafenib, the pioneering VEGFR-TKI that received FDA approval in 2005, was initially indicated for the treatment of advanced renal cell carcinoma3. After sorafenib’s debut, a series of VEGFR-TKIs have emerged. Depending on the specific binding to TKs, VEGFR-TKIs can be classified as selective and nonselective inhibitors4. Third-generation selective inhibitors, such as axitinib and tivozanib, mainly target the VEGFR family with high potency. In contrast, non-selective VEGFR-TKIs, such as sunitinib and sorafenib, exhibit a wider spectrum of interactions with diverse targets and display low potency. Lenvatinib is an exceptionally potent non-selective inhibitor, with an average IC50 of approximately 0.71–1.3 nM for VEGFR. VEGFR-TKIs show obvious inhibitory effects on VEGF, a pivotal determinant of the survival and proliferation of vascular endothelial cells (ECs)5.
Tumor development relies predominantly on the formation of new blood vessels and sufficient blood supply, a process primarily driven by VEGF. High VEGF levels are linked to increased tumor growth, invasion, metastasis, and recurrence, highlighting VEGFR as a crucial target for cancer therapy6,7. VEGFR-TKIs can impede tumor angiogenesis and arrest cancer progression8. It can also enhance the chemotaxis of T lymphocytes to tumors, thereby improving the response of tumor cells to other therapies such as immunotherapy1. The pan-tumor applicability of VEGFR-TKIs, coupled with the translatability of anti-angiogenic mechanisms across diverse cancers, drives their broad clinical utility3. On the one hand, these agents have been approved by the FDA as first- or second-line recommended monotherapy strategies for highly vascular tumors, such as renal cell carcinoma, hepatocellular carcinoma, and thyroid cancer9,10,11. On the other hand, the significant breakthroughs in immunotherapy have unlocked new frontiers for the combination of VEGFR-TKIs3. With ongoing research and development, the landscape of VEGFR-TKIs continues to expand with the emergence of novel agents.
However, With the widespread clinical use of VEGFR-TKIs, the issue of drug safety for these agents cannot be overlooked. Although these drugs show fewer cytotoxic side effects than chemotherapy, some cardiovascular adverse events (AEs), including hypertension, prolonged QT interval, heart failure and thromboembolic events (TEEs), are frequently observed. Abnormal vascular endothelial function can lead to vasoconstriction, vasospasm, increased inflammatory response, and a tendency towards thromboembolism12. Increasing evidence suggests that long-term systemic suppression of the vascular endothelium by VEGFR-TKIs is associated with increased TEEs, including arterial thromboembolic events (ATEs) and venous thromboembolic events (VTEs)6. Compared with placebo, patients with cancer receiving sorafenib or lenvatinib treatment had an approximately 6.14-fold or 3.82-fold increased risk of TEEs, respectively13,14. The mortality rate of tumor patients with VTEs is alarmingly high at 55%, with a 31.2-fold increased risk of death15. The 1-year survival rate of patients with tumor and VTEs was 12%, in stark contrast to that of patients with tumor alone15. In summary, TEEs significantly impact the quality of life and create a substantial financial burden for patients.
Stringent inclusion and exclusion criteria in clinical studies may result in underestimation of factual thromboembolic risk of VEGFR-TKIs in real world. Currently, there is limited data on thromboembolic risk of VEGFR-TKIs based on open access database. An analysis based on the Italian Spontaneous Reporting System showed a proportional reporting ratio (PRR) of 15.18 for bevacizumab-associated TEEs in patients with tumors, whereas no reports were found for antitumor VEGFR-TKIs16. Based on the FDA Adverse Event Reporting System (FAERS) database, this study aimed to comprehensively identify the real-world thromboembolism risk of antitumor VEGFR-TKIs, explore potential influencing factors, and ultimately provide references for clinical decision-making in these patients.
Results
Descriptive analysis
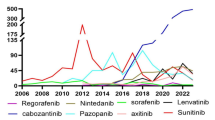
We conducted a thorough search of the FAERS database, spanning from drug launch to the third quarter of 2023, specifically targeting reports of TEEs associated with VEGFR-TKI treatment. The detailed data processing procedure is shown in Fig. 1. Subsequently, we excluded cases in which the primary suspect drug was not a VEGFR-TKI and with duplicate entries. Our analysis revealed that TEE reports constituted only a minor fraction of all reports related to VEGFR-TKIs, accounting for 2.98% (2688/90206). The incidence of TEEs varied across different VEGFR-TKIs, with lenvatinib exhibiting the highest incidence (N = 624, 4.60%), whereas tivozanib demonstrated the lowest incidence (N = 8, 0.73%). Notably, the incidence of ATEs was higher than that of VTEs for all VEGFR-TKIs (Fig. 2A and Supplementary Table S1). Furthermore, the proportion of VEGFR-TKI-related TEEs among all AEs was the highest in 2006 (N = 143, 7.04%), which gradually declined and stabilized in 2010 (1.85–3.39%) (Fig. 2B and Supplementary Table S2). Overall, the incidence of TEEs may be a non-negligible AE in patients receiving VEGFR-TKIs.
Statistics on the occurrence of VEGFR-TKI- TEEs reported in the FAERS database. (A) The number of TEEs and non-TEEs in VEGFR-TKIs AE reports for various treatment strategies in the FAERS database. The line chart shows the proportion of TEEs among all AE reports related to VEGFR-TKIs. (B) The number of TEEs and non-TEEs among VEGFR-TKIs AE reports in the FAERS database from 2006 to 2023. The line chart shows the proportion of TEEs among all AE reports related to VEGFR-TKIs.
After systematically searching for VEGFR-TKI-related reports, we identified all cases of VEGFR-TKI-related TEEs and subsequently provided a statistical description of their clinical characteristics (Table 1). The mean and median age of patients was 64.46 and 65 (interquartile range [IQR] 58–73) years, respectively. The patients were divided into four groups with ages 18, 65, and 75 years as dividing lines. Of the patients, 40.63% were over 65 years of age (N = 1092). The majority of reported cases were male (N = 1586, 59.00%) and renal cell carcinoma (N = 380, 15.47%), with the most reported cases originating from the United States (N = 1050, 39.06%), followed by Japan (N = 310, 11.53%) and France (N = 247, 9.19%). Among all the reported TEEs, cardiac disorders (N = 1019, 35.52%) and respiratory, thoracic and mediastinal disorders (N = 700, 24.40%) were the most common system organ classes (SOCs) involved (Fig. 3A and Supplementary Table S3). The primary clinical outcomes for the patients were hospitalization (N = 1595, 42.05%), and death (N = 577, 15.21%). For ATEs and VTEs, myocardial infarction (MI) and pulmonary embolism (PE) were the leading causes of death, respectively (Fig. 3B and Supplementary Table S4).
Analysis of VEGFR-TKI-related TEEs reports. (A) Anatomical diagram of the systems involved in VEGFR-TKI-related TEEs. The darker the color scale, the more TEE cases involved in this site. (B) The proportion of VEGFR-TKI-related TEEs leading to death outcomes. Among them, the AE case number less than 5 is classified as other. (C) The results of disproportionality analysis of VEGFR-TKI-related TEEs under different treatment strategies. 4 disproportionality analysis methods and thresholds were used, *, **, *** indicating that 2, 3, and 4 thresholds are met, respectively. NS indicates Not Significant. (D) The number of TEE reports associated with VEGFR-TKIs under different treatment strategies.
Disproportionality analysis
We conducted descriptive statistical analyses of TEE reports related to VEGFR-TKIs. Among all AEs, the top five preferred terms (PTs) for TEEs were MI (N = 672, 23.43%), PE (N = 598, 20.85%), deep vein thrombosis (DVT) (N = 230, 8.02%), transient ischemic attack (N = 187, 6.52%), and acute myocardial infarction (N = 178, 6.21%). To evaluate the magnitude of TEE linked to different VEGFR-TKIs, we conducted four types of disproportionality analysis algorithms including the reporting odds ratio (ROR), PRR, information component minus two standard deviations (IC-2SD), and the fifth percentile of the empirical bayesian geometric mean distribution (EB05). A signal was considered positive when its intensity significantly surpassed the background frequency of the entire database. Interestingly, after screening based on the criteria, we found that different VEGFR-TKIs were associated with different TEE signals of disproportionate reporting (SDRs). Lenvatinib presented the highest SDR as it fulfilled all four algorithm criteria (N = 590, ROR = 3.29 [3.03–3.57]; PRR = 3.28 [892.48], IC-2SD = 1.54, EB05 = 2.93). Sorafenib, regorafenib, and sunitinib failed to meet the criteria for all the algorithms, but their SDRs of TEEs cannot be disregarded (Fig. 3C and D and Supplementary Table S5).
Comparison between ATE and VTE subgroups
We stratified the reports of VEGFR-TKI-related TEEs in the FAERS database into ATE and VTE subgroups based on PTs belonging to different standard MedDRA queries (SMQs). The number of cases in the ATE group was higher than that in the VTE group. Further disproportionality analysis revealed significant differences in the clinical characteristics and SDRs among ATEs, VTEs, and TEEs. Although the primary outcomes of the VEGFR-TKI-related ATE and VTE reports were similar (p = 0.923), analyzing the differences between the two groups is important to explore the SDRs of different types of thromboembolism induced by VEGFR-TKIs and reduce hospitalization rates. There was no statistically significant difference in respect to sex between the ATE and VTE groups (p = 0.177), but patients with higher age appeared to be more likely to develop ATE (p < 0.001) (Table 1). Therefore, we need to pay more attention to VEGFR-TKI-related ATEs in elderly patients.
Subgroup analysis revealed varying SDRs of ATEs (Fig. 4A and Supplementary Table S6) and VTEs (Fig. 5A and Supplementary Table S7) among different VEGFR-TKIs. Only lenvatinib showed higher SDRs in both ATEs (N = 353, ROR = 3.03 [2.73–3.37], PRR = 3.02 [463.69], IC-2SD = 1.41, EB05 = 2.67) and VTEs (N = 242, ROR = 3.58 [3.15–4.07], PRR = 3.57 [437.73], IC-2SD = 1.62, EB05 = 3.10). Vandetanib had the lowest number of cases but showed the highest correlation with ATEs (N = 3, ROR = 21.88 [7.04–68.03], PRR = 21.83 [40.52], IC-2SD = 0.37, EB05 = 7.01). By contrast, regorafenib showed increased SDRs of VTEs without increasing the incidence of ATEs. Axitinib, pazopanib, sunitinib, sorafenib, and tivozanib exhibited SDR profiles similar to those of TEEs in all subgroups. PTs concurrently satisfying four algorithms were categorized as VEGFR-TKI-related TEEs, with 17 and 12 in the ATE and VTE groups, respectively (Figs. 4B and 5B). Interestingly, the SDRs of ATEs varied among different VEGFR-TKIs, with only four PTs associated with two kinds of drugs. Lenvatinib resulted in the highest number of positive PTs. Post-infarction angina showed the highest spike in lenvatinib-induced ATEs (N = 3, ROR = 515.38 [115.33–2303.00], PRR = 515.26 [610.20], IC-2SD = 0.22, EB05 = 65.99) (Fig. 4B and C and Supplementary Table S8). In the VTE subgroup, pazopanib, sunitinib, and sorafenib were all significantly associated with the occurrence of vena cava thrombosis, with pazopanib presenting the highest SDR (N = 17, ROR = 13.48 [8.34–21.81], PRR = 13.47 [180.04], IC-2SD = 2.29, EB05 = 8.16). Portal vein occlusion caused by lenvatinib had the highest SDR among positive PTs with only 3 cases reported (Fig. 5B and C and Supplementary Table S9). Supplementary Figure S1 and S2 showed the affiliation of PTs of VEGFR-TKI-related TEEs with other hierarchies in the MedDRA. The major SOCs were cardiac and nervous system disorders in ATEs, and respiratory, thoracic and mediastinal disorders in VTEs.
Mining of VEGFR-TKI-related ATEs based on the FAERS database. (A) The results of disproportionality analysis of VEGFR-TKI-related ATEs under different treatment strategies. 4 disproportionality analysis methods and thresholds were used, *, **, *** indicating that 2, 3, and 4 thresholds were met, respectively. NS indicates Not Significant. (B) The results of disproportionality analysis for 28 VEGFR-TKI-related ATEs (N ≥ 3) in the FAERS database. The color scale indicates the number of thresholds met, and the overall ATE outcome was based on the treatment strategy that met the most thresholds. (C) The number of reported cases of 17 kinds of VEGFR-TKI-related ATEs. Different colors represent the number and proportion of different treatment strategies.
Mining of VEGFR-TKI-related VTEs based on the FAERS database. (A) The results of disproportionality analysis of VEGFR-TKI-related VTEs under different treatment strategies. 4 disproportionality analysis methods and thresholds were used, *, **, *** indicating that 2, 3, and 4 thresholds were met, respectively. NS indicates Not Significant. (B) The results of disproportionality analysis for 12 VEGFR-TKI-related VTEs (N ≥ 3) in the FAERS database. The color scale indicates the number of thresholds met, and the overall VTE outcome was based on the treatment strategy that met the most thresholds. (C) The number of reported cases of 12 kinds of VEGFR-TKI-related VTEs. Different colors represent the number and proportion of different treatment strategies.
The influencing factors of VEGFR-TKI-related tees
We also examined the co-reported AEs and concomitant medications that could be potential influencing factors for TEEs. Among the 2688 VEGFR-TKI-related TEE cases, 66.37% of patients were accompanied by other AEs (Fig. 6A). In these cases, more than 50% of patients experienced vascular disorders (N = 1123, 62.95%), nervous system disorders (N = 1047, 58.69%), and cardiac disorders (N = 985, 55.21%) (Fig. 6B). Hypertension, diarrhea, and fatigue were the top three concomitant AEs in the ATE group. Cerebrovascular accident occurred in 4.21% of cases, whereas cardiac events accounted for 5.34% of all reported cases (cardiac failure: N = 27, 2.77%; cardiac disorder: N = 25, 2.57%) (Supplementary Figure S3). In contrast, dyspnea, fatigue, and asthenia were the most prominent concomitant AEs in the VTE group. Additionally, peripheral edema and chest pain were rated among the top 30, indicating vigilance against the occurrence of VTEs (Supplementary Figure S4). Concomitant medications among the top 30 agents comprised immune checkpoint inhibitors (ICIs), thyroid hormones, proton pump inhibitors, beta-blockers, diuretics, calcium channel blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, statins, glucocorticoids, and cytotoxic chemotherapies. Pembrolizumab predominated (N = 408, 7.07%), followed by levothyroxine (N = 245, 4.24%) and amlodipine (N = 175, 3.03%) (Fig. 6C). This pattern persisted in ATE and VTE subgroups (Supplementary Figure S5 and S6).
Factors influencing the SDR of VEGFR-TKI-related TEEs. (A) The proportion of cases with and without concomitant AEs in VEGFR-TKI-related TEE reports. (B) The number of SOCs containing PTs of concomitant AEs. (C) The number of top 30% concomitant medications of VEGFR-TKI-related TEEs. (D) Subgroup analysis results of VEGFR-TKI-related TEEs.
Subgroup analyses of SDR strength for VEGFR-TKI-related TEEs, ATEs, and VTEs revealed minimal variation across subgroups among positive signals. Sex-based differences showed higher SDRs in females for most drugs, except VTEs of sorafenib (ROR = 2.11 [1.84–2.43]) and sunitinib (ROR = 1.26 [1.07–1.49]). Age stratification indicated higher SDRs for lenvatinib-related TEEs, ATEs and VTEs in patients aged 18–64 years, while VTEs of regorafenib and sorafenib exhibited higher SDRs in those aged ≥ 75 years. By primary indication, lenvatinib demonstrated higher SDRs in thyroid cancer patients, whereas regorafenib, sorafenib, and sunitinib showed elevated SDRs in gastrointestinal stromal tumor (GIST) patients (Fig. 6D, Supplementary Figure S7, S8 and Table S10).
Discussion
Since VEGFR has emerged as a primary target for antitumor therapy, the continuous discovery of VEGFR-TKIs presents new opportunities for patients with cancer. However, drug-related AEs have become a significant barrier to sustainable cancer treatment. Previous studies on VEGFR-TKI-related TEEs have focused on clinical trials and meta-analysis, lacking comprehensive research on the expanded post-marketing population. The association between VEGFR-TKIs and TEEs in real-world settings remains poorly understood or insufficiently documented. We assessed the SDRs of VEGFR-TKI-related TEEs using four disproportionality analysis methods and identified 17 and 12 PTs of ATEs and VTEs, respectively. Additionally, we analyzed the clinical characteristics of these cases in conjunction with relevant reports and further explored the influencing factors for the occurrence of TEEs.
Admittedly, TEEs are significant but low-frequency AEs associated with VEGFR-TKIs. Consistent with previous findings, the FAERS database indicated that TEEs occurred in 2.98% (0.73–4.60%) of cases treated with VEGFR-TKIs from 2006 to 2023, with 1.73% ATEs and 1.25% VTEs17,18,19. Different VEGFR-TKIs carry varying risks of TEEs20. Statistical analyses of all reports showed that VEGFR-TKIs with lower selectivity, particularly those with higher potency (lenvatinib, N = 624, 4.60%), had a higher rate of TEEs. Among the VEGFR-TKI-related TEEs examined, cardiac disorders, nervous system disorders, and respiratory, thoracic and mediastinal disorders were the primary SOCs. Therefore, more attention should be paid to TEEs of multitarget VEGFR-TKIs in clinical practice, particularly in relation to cardiovascular and respiratory disorders.
AEs related to TEEs are commonly documented on drug labels of VEGFR-TKIs. A meta-analysis confirmed a 1.52-fold increased risk of ATEs in patients treated with VEGFR-TKIs (OR = 1.52, 95% CI 1.17–1.98)21. In contrast, previous research did not yield favorable findings regarding an increased incidence of VTEs, until a recent meta-analysis comprising 69 RCT studies revealed that VEGF-TKIs were associated with an increased risk (OR = 1.73, 95% CI 1.21–2.47)22,23. Regarding TEEs, variations were observed among different VEGFR-TKIs, with the highest risk associated with lenvatinib (OR = 3.12, 95% CI 1.13–8.61), sorafenib (OR = 1.54, 95% CI 1.10–2.16), and sunitinib (OR = 1.53, 95% CI 1.00–2.33)22. A recent study has demonstrated that cardiovascular AEs are reported more frequently with lenvatinib and sunitinib compared to other VEGFR-TKIs24. Similarly, we found that lenvatinib, a VEGF-TKI with high potency and low selectivity, have the strongest association with TEEs, ATEs, and VTEs, surpassing the thresholds of all four disproportionality analysis. Other pharmacovigilance studies have yielded similar outcomes24. Conversely, the VEGF-TKIs with moderate-to-high selectivity, such as axitinib, tivozanib, and pazopanib were associated with lower SDRs and probabilities of TEEs.
The primary cause may be the “off-target effect” of VEGFR-TKIs with low selectivity, which impacts a range of well-known vascular endothelial protective factors, including the platelet-derived growth factor receptor (PDGFR) family, fibroblast growth factor receptor (FGFR) family, epidermal growth factor receptor (EGFR), rearrangement during transfection (RET), and rapidly accelerated fibrosarcoma (RAF)25,26,27. The inhibition of PDGFR impairs the physiological regulation of vascular smooth muscle cell proliferation and migration, thereby inducing aberrant vascular wall remodeling and enhancing vulnerability to endothelial injury20,28. The compromised integrity of ECs results in exposure to subcutaneous collagen, increased levels of tissue factor (TF), activation of platelets and thrombin, and increased expression of adhesion molecules on leukocytes, which can lead to thromboembolism29. Furthermore, off-target inhibition of additional signaling pathways implicated in hemostasis, such as those governing anticoagulant molecule release or maintaining vascular endothelial homeostasis, may synergize with on-target effects to further augment the risk of thrombosis28. Another significant contributor to thrombosis is an imbalance between the endothelium-derived vasodilatory and vasoconstricting factors20. VEGFR-TKIs reduce the synthesis of the vasodilators nitric oxide (NO) and prostaglandin I2 (PGI2), and enhance the expression of the vasoconstrictors endothelin-1 (ET-1)/endothelin receptor B (ETB) pairs, thus antagonizing the effects of vasodilation and promoting platelet aggregation30,31,32. Finally, increased hematocrit and blood viscosity, decreased tissue-type plasminogen activator (t-PA), oxidative stress, and ferroptosis may also contribute to the occurrence of VEGFR-TKI-related TEEs31,32. Although some potential mechanisms have been highlighted, the underlying causes of thrombosis during VEGFR-TKI therapy remain poorly understood, and further research is needed.
Furthermore, we investigated the factors influencing VEGFR-TKI-related TEEs and observed a significantly higher proportion of cases in the elderly group (≥ 65 years) (p < 0.001). Subgroup analysis showed that VTEs associated with regorafenib and sorafenib had a higher SDR in patients aged ≥ 75 years. However, this finding does not represent the risk of TEEs in different age groups. It is conventionally believed that elderly patients with cancer are at an elevated risk of thromboembolism. The risk of ATEs escalates by 1.5 times per decade, and the relative risk surges by 1.2-fold for patients with tumor and above 60 years of age33,34. On the other hand, the incidence rate of TEEs was 3-fold in tumor patients aged ≥ 65 years35,36,37. Therefore, it is imperative to prioritize elderly patients and implement early intervention strategies to improve their prognosis. Although women demonstrate relatively higher SDRs in most VEGFR-TKI-related TEEs, males have been identified as a potential risk factor for major cardiac adverse events, with a 1.2-fold increased risk of ATEs (RR = 1.21, 95% CI 1.20–1.22)34,38. The influence of gender on VTE remains controversial due to the female-specific risk factors including pregnancy, postpartum and caesarean section, estro-progestin therapy, and assisted reproductive techniques35,39. Previous studies have not stratified patients according to gender and age, and large-scale clinical studies are needed to elucidate their relationship.
Based on our findings, the majority of TEEs were not independent events, with up to 66.37% concurrent with other AEs. AEs co-reported with VTEs, such as dyspnea, fatigue, chest pain, and peripheral edema, are the main symptoms of DVT and PE40,41. Although the etiology of VTEs cannot be elucidated, it provides an early indication that VTEs may be imminent. Nevertheless, hypertension is the predominant concomitant AE for ATEs and is widely recognized as a major risk factor for ATEs38. Additionally, 9.55% of patients experienced concomitant cardiovascular and cerebrovascular AEs, which could potentially have a mutually causal relationship with ATEs. VEGFR-TKIs induced hypertension and cardiovascular AEs may provide valuable insights into the mechanism of ATEs from a certain perspective.
The primary cancer site is a significant influencing factor for TEEs42. Thyroid cancer promotes thrombosis through tumor compression, angioinvasion, and prothrombotic states43,44. Patients with hyperthyroidism or hypothyroidism, complications of thyroid cancer, exhibit a significantly elevated risk of TEEs42. Conversely, TEEs rarely manifest clinically in GISTs, with only limited case reports suggesting a potential association44. This phenomenon may be explained by the later-line use of VEGFR-TKIs in advanced GISTs with increased TEE susceptibility42.
In addition to tumor type, cancer therapies and concomitant medications also contribute to thrombosis. Firstly, ICIs induce T-cell activation and endothelial inflammation, leading to release of neutrophil extracellular traps and increased risk of TEEs (HR = 4.98, p < 0.001)45. Second, glucocorticoids increase the risk of venous thromboembolism by 3.5-fold by elevating clotting factors VII, VIII, XI, and fibrinogen46,47. Thirdly, Chemotherapy increases the risk of VTEs in cancer patients by upregulating tissue factors on monocytes and reducing anticoagulants48. Previous studies have found that 42% of patients treated with gemcitabine-paclitaxel developed VTEs46. Although patient comorbidity information cannot be directly obtained from the FAERS database, the concomitant medication information justifies the belief that hypertension, hyperlipidemia, diabetes, and hypothyroidism are the most common comorbid diseases. These diseases have been widely proven to be high-risk factors for TEEs38,40,49,50,51,52.
The strength of this study is that the FAERS database provides a large amount of data for the investigation of rare VEGFR-TKI-related TEEs, while OpenVigil 2.1 provides clean data for subsequent analysis53,54. In addition, prior to our study, the SDRs of VEGFR-TKIs in TEEs had not been systematically investigated. This study aimed to address this critical knowledge gap and highlighted that personalized screening and monitoring are indispensable components of TEE management. We suggest to assess the factors such as tumor type, gender, age, drug class before prescribing VEGFR-TKIs. Moreover, it is necessary to conduct scale assessments and closely follow up on the symptoms and biomarkers for those specific patients to achieve individualized management.
However, this study has some limitations. First, the FAERS database is a spontaneous reporting system, featuring heterogeneity among different reporters and the lack of AE-related information, which may result in an increase in false positive rates. Second, as a retrospective study, the data on indications and co-reported drugs are incomplete, while the discontinuation/dechallenge data is missing. These issues may introduce confounding variables and bias. Third, OpenVigil, as a data mining tool, provides only partial transparency regarding its data cleaning and preprocessing methodologies. Finally, disproportionality analysis only represents a statistical correlation between the drugs and AEs. The causal relationship needs to be comprehensively evaluated in combination with clinical studies in the future.
Conclusions
Based on real-world data from the FAERS database, this study elucidates a significant correlation between TEEs and VEGFR-TKIs. We highlight the necessity of screening for the influencing factor of thromboembolism during VEGFR-TKIs therapy, including variables such as indication, gender, age, drug class, concomitant medication and AEs. However, further studies are required to validate our findings and identify effective strategies for mitigating the risk of TEEs.
Materials and methods
Data source
The FAERS serves as a comprehensive database for collecting and analyzing AE reports pertaining to drugs and biologics submitted by healthcare professionals, patients, and drug manufacturers55. OpenVigil 2.1 (http://h2876314.stratoserver.net:8080/OV2/search/) is a tool used to analyze pharmacovigilance data in FAERS56. It employs advanced data-mining techniques with highly customizable search criteria and output filters. Online disproportionality analysis facilitates signal detection, and the results can be viewed, sorted, filtered, and downloaded in real-time to enhance pharmacovigilance. We conducted a pharmacovigilance study of TEEs associated with VEGFR-TKIs using the FAERS database and OpenVigil 2.1 tool. All reported VEGFR-TKIs, including axitinib, lenvatinib, pazopanib, regorafenib, sunitinib, sorafenib, tivozanib, and vandetanib, were used as keywords to retrieve reports of VEGFR-TKIs in the FAERS database from launch to the third quarter of 2023 (September 30, 2023). The AEs recorded in the FAERS database were coded with PTs within the MedDRA term set, which encompasses five hierarchical levels: lowest-level terms (LLTs), PTs, high-level terms (HLTs), high-level group terms (HLGTs), and SOCs. These terms demonstrate multiaxiality, wherein a single PT may correspond to multiple SOCs57. The SMQ comprises a compilation of terms linked to a specific medical condition or area of concern, encompassing a cluster of related PTs. According to the SMQ embolic and thrombotic events, arterial (SMQ Code 20000082) and venous (SMQ Code 20000084), all PTs associated with TEEs were extracted and segregated into ATE (N = 157) and VTE (N = 97) subgroups to ensure a comprehensive representation of real-world drug-related TEEs.
Data processing procedure
AEs reports were extracted from OpenVigil 2.1 using the generic name of the drug, with the “Drug” drop-down menu set to “VEGFR-TKIs”, and designating the “Role of drug” as “primary suspect” while keeping other options at their default values. Subsequently, data from “Raw_data” and “Frequentist_methods” were retrieved and downloaded by counting records according to individual safety reports (ISR). The detailed screening steps are illustrated in Fig. 1. Deduplication process is conducted to minimize the impact of duplicate cases on the analysis according to the following criteria: (1) Reports sharing the same ISR code are considered as identical cases; (2) cases involving suspected drugs other than VEGFR-TKIs are excluded; (3) cases where the “Role of drug” is not “primary suspect” and is “interacting”, “concomitant”, and “secondary suspect” are also excluded; and (4) reports of TEEs with unclear culprit vessel. After de-reprocessing, all AE data for VEGFR-TKIs in the FAERS database were retrieved, including demographic and administrative information (DEMO), drug details (DRUG), reaction specifics (REAC), patient outcomes (OUTC), and indications for use/diagnosis (INDI)58.
Signal detection
Disproportionality analysis, including ROR59PRR60Bayesian confidence promotion neural network (BCPNN)61and multi-item gamma Poisson shrinker (MGPS)62have been widely used to detect AE signals in pharmacovigilance research. Disproportionality analysis identifies signals of AEs by examining the “imbalance” or “disproportionality” between the events of interest and other events in the database. Each method has its strengths and weaknesses63. ROR shows lower susceptibility to bias than that of other methods because it is less influenced by the quantity of reported drugs or AEs. Compared with ROR, PRR is more sensitive and susceptible to generate false positive signals. BCPNN excels in integrating data and cross-validation, enabling early detection of potential safety signals. MGPS can identify rare AEs and prevent excessive signal mining. We employed four methods simultaneously to maximize the benefits of multiple algorithms and minimize false positive rates. All disproportionality analysis algorithms are based on a 2 × 2 contingency table (Supplementary Table S11). In order to mitigate false positivity, only events satisfying all four algorithm criteria simultaneously were defined as SDRs in this study: the lower limit of 95% CI of ROR > 1, PRR ≥ 2 and χ2 ≥ 4, IC-2SD > 0, or EB05 > 264. In additon, we also discuss the events that meet at least one criteria to ensure a balance between methodological rigor and pragmatic vigilance. The specific calculation formulae and thresholds are listed in Table 2.
Statistical analysis
Reports of VEGFR-TKI-related TEEs obtained from the FAERS were classified into ATE and VTE subgroups based on the SMQ in the MedDRA dictionary. Descriptive analyses were performed on the characteristics of the reports, including sex, age, reporting country, year of receipt, outcome, and other clinical characteristics. The main outcomes were disability, hospitalization - initial or prolonged, life-threatening, and death. Further statistical analyses were conducted to compare differences in characteristics between the two groups. Continuous variables are presented as medians and quartiles, whereas categorical variables are presented as counts and percentages. Age differences between the VTE and ATE groups related to VEGFR-TKIs were compared using the log-rank test and nonparametric Mann–Whitney U test. Chi-square and Fisher’s exact tests were used to compare categorical variables, such as sex, age group, and outcome. All statistical tests were two-tailed. Human anatomical heat maps of the outcomes were obtained using the MOAHIT network tool (https://smuonco.shinyapps.io/MOAHIT/)65. Reports with missing values were excluded from the statistical analysis of relevant clinical characteristics. Statistical analyses and visualizations were performed using MedDRA (version 26.1, GE, CH), Microsoft Excel (version 2402, WA, USA), SPSS (version 25, IBM, NY, USA), Origin (version 10.1 SR1, MA, USA), and GraphPad Prism (version 8.0, CA, USA).
Data availability
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed at the corresponding authors.
References
Franczyk, B., Rysz, J., Lawinski, J., Cialkowska-Rysz, A. & Gluba-Brzozka, A. Cardiotoxicity of selected vascular endothelial growth factor receptor tyrosine kinase inhibitors in patients with renal cell carcinoma. Biomedicines 11, 181. https://doi.org/10.3390/biomedicines11010181 (2023).
Dobbin, S. J. H. et al. Toxicity of cancer therapy: what the cardiologist needs to know about angiogenesis inhibitors. Heart 104, 1995–2002. https://doi.org/10.1136/heartjnl-2018-313726 (2018).
Qin, S. et al. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J. Hematol. Oncol. 12 https://doi.org/10.1186/s13045-019-0718-5 (2019).
Fogli, S. et al. Optimizing treatment of renal cell carcinoma with VEGFR-TKIs: a comparison of clinical Pharmacology and drug-drug interactions of anti-angiogenic drugs. Cancer Treat. Rev. 84, 101966. https://doi.org/10.1016/j.ctrv.2020.101966 (2020).
Keefe, D. et al. Noncardiac vascular toxicities of vascular endothelial growth factor inhibitors in advanced cancer: a review. Oncologist 16, 432–444. https://doi.org/10.1634/theoncologist.2010-0271 (2011).
Li, J. & Gu, J. Cardiovascular toxicities with vascular endothelial growth factor receptor tyrosine kinase inhibitors in Cancer patients: A Meta-Analysis of 77 randomized controlled trials. Clin. Drug Investig. 38, 1109–1123. https://doi.org/10.1007/s40261-018-0709-2 (2018).
Liu, J. et al. Assessment and management of diarrhea following VEGF receptor TKI treatment in patients with ovarian cancer. Gynecol. Oncol. 150, 173–179. https://doi.org/10.1016/j.ygyno.2018.03.058 (2018).
Li, J. & Gu, J. Hand-foot skin reaction with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 119, 50–58. https://doi.org/10.1016/j.critrevonc.2017.09.016 (2017).
Li, J. & Gu, J. Risk of Gastrointestinal events with newly approved (after 2011) vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol. 73, 1209–1217. https://doi.org/10.1007/s00228-017-2299-y (2017).
Kato, T., Mizuno, R. & Miyake, H. Prevalence and management of proteinuria associated with vascular endothelial growth factor receptor-targeted tyrosine kinase inhibitor treatment in advanced renal cell carcinoma, hepatocellular carcinoma, and thyroid cancer. Int. J. Urol. 31, 465–474. https://doi.org/10.1111/iju.15409 (2024).
Das, A. et al. Bleeding with vascular endothelial growth factor tyrosine kinase inhibitor: A network meta-analysis. Crit. Rev. Oncol. Hematol. 157, 103186. https://doi.org/10.1016/j.critrevonc.2020.103186 (2021).
Ferroni, P., Formica, V., Roselli, M. & Guadagni, F. Thromboembolic events in patients treated with anti-angiogenic drugs. Curr. Vasc Pharmacol. 8, 102–113 (2010).
Escudier, B. et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 356, 125–134 (2007).
Schlumberger, M. et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl. J. Med. 372, 621–630. https://doi.org/10.1056/NEJMoa1406470 (2015).
Timp, J. F., Braekkan, S. K., Versteeg, H. H. & Cannegieter, S. C. Epidemiology of cancer-associated venous thrombosis. Blood 122, 1712–1723. https://doi.org/10.1182/blood-2013-04-460121 (2013).
Cutroneo, P. M. et al. Overview of the safety of Anti-VEGF drugs: analysis of the Italian spontaneous reporting system. Drug Saf. 40, 1131–1140. https://doi.org/10.1007/s40264-017-0553-y (2017).
Lee, N., Lee, J. L. & Lee, J. Y. Analysis of Anti-Angiogenesis-Related adverse events associated with vascular endothelial growth factor Receptor-Tyrosine kinase inhibitors (VEGFR-TKIs) in patients with metastatic renal cell carcinoma. Target. Oncol. 18, 247–255. https://doi.org/10.1007/s11523-023-00951-z (2023).
Qi, W. X., Shen, Z., Tang, L. N. & Yao, Y. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: an up-to-date meta-analysis. Crit. Rev. Oncol. Hematol. 92, 71–82. https://doi.org/10.1016/j.critrevonc.2014.04.004 (2014).
Liu, B., Ding, F., Zhang, D. & Wei, G. H. Risk of venous and arterial thromboembolic events associated with VEGFR-TKIs: a meta-analysis. Cancer Chemother. Pharmacol. 80, 487–495. https://doi.org/10.1007/s00280-017-3386-6 (2017).
Touyz, R. M., Herrmann, S. M. S. & Herrmann, J. Vascular toxicities with VEGF inhibitor therapies-focus on hypertension and arterial thrombotic events. J. Am. Soc. Hypertens. 12, 409–425. https://doi.org/10.1016/j.jash.2018.03.008 (2018).
Abdel-Qadir, H., Ethier, J. L., Lee, D. S., Thavendiranathan, P. & Amir, E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: A systematic review and meta-analysis. Cancer Treat. Rev. 53, 120–127. https://doi.org/10.1016/j.ctrv.2016.12.002 (2017).
Chen, Y. C., Chen, J. H. & Hsieh, F. I. Major adverse cardiovascular events of vascular endothelial growth factor tyrosine kinase inhibitors among patients with different malignancy: A systemic review and network meta-analysis. J. Chin. Med. Assoc. 87, 48–57. https://doi.org/10.1097/JCMA.0000000000001026 (2024).
Qi, W. X. et al. Risk of venous thromboembolic events associated with VEGFR-TKIs: a systematic review and meta-analysis. Int. J. Cancer. 132, 2967–2974. https://doi.org/10.1002/ijc.27979 (2013).
Goldman, A. et al. Cardiovascular toxicities of antiangiogenic tyrosine kinase inhibitors: A retrospective, pharmacovigilance study. Target. Oncol. 16, 471–483. https://doi.org/10.1007/s11523-021-00817-2 (2021).
Sun, Z., Kemp, S. S., Lin, P. K., Aguera, K. N. & Davis, G. E. Endothelial k-RasV12 expression induces capillary deficiency attributable to marked tube network expansion coupled to reduced pericytes and basement membranes. Arterioscler. Thromb. Vasc Biol. 42, 205–222. https://doi.org/10.1161/ATVBAHA.121.316798 (2022).
Choi, J. H. et al. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity 35, 819–831. https://doi.org/10.1016/j.immuni.2011.09.014 (2011).
Gupta, A. et al. SARS-CoV-2 infection- induced growth factors play differential roles in COVID-19 pathogenesis. Life Sci. 304, 120703. https://doi.org/10.1016/j.lfs.2022.120703 (2022).
Shyam Sunder, S., Sharma, U. C. & Pokharel, S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: pathophysiology, mechanisms and clinical management. Signal. Transduct. Target. Therapy. 8 https://doi.org/10.1038/s41392-023-01469-6 (2023).
Pantazi, D. & Tselepis, A. D. Cardiovascular toxic effects of antitumor agents: pathogenetic mechanisms. Thromb. Res. 213 (Suppl 1). https://doi.org/10.1016/j.thromres.2021.12.017 (2022). S95-S102.
Watson, N. & Al-Samkari, H. Thrombotic and bleeding risk of angiogenesis inhibitors in patients with and without malignancy. J. Thromb. Haemost. 19, 1852–1863. https://doi.org/10.1111/jth.15354 (2021).
Li, J. et al. Understanding Sorafenib-Induced cardiovascular toxicity: mechanisms and treatment implications. Drug Des. Dev. Ther. 18, 829–843. https://doi.org/10.2147/DDDT.S443107 (2024).
Neves, K. B., Montezano, A. C., Lang, N. N. & Touyz, R. M. Vascular toxicity associated with anti-angiogenic drugs. Clin. Sci. (Lond). 134, 2503–2520. https://doi.org/10.1042/CS20200308 (2020).
Grilz, E. et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica 103, 1549–1556. https://doi.org/10.3324/haematol.2018.192419 (2018).
Wang, J., Kim, Y. D. & Kim, C. H. Incidence and risk of various types of arterial thromboembolism in patients with Cancer. Mayo Clin. Proc. 96, 592–600. https://doi.org/10.1016/j.mayocp.2020.05.045 (2021).
Ren, X., Huang, Y., Ying, L. & Wang, J. Risk factors of venous thromboembolism for liver tumors: a systematic review and meta-analysis. HPB (Oxford). 26, 1–7. https://doi.org/10.1016/j.hpb.2023.09.008 (2024).
Akrivou, D., Perlepe, G., Kirgou, P., Gourgoulianis, K. I. & Malli, F. Pathophysiological aspects of aging in venous thromboembolism: an update. Med. (Kaunas). 58. https://doi.org/10.3390/medicina58081078 (2022).
Khorana, A. A., Francis, C. W., Culakova, E., Kuderer, N. M. & Lyman, G. H. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 110, 2339–2346. https://doi.org/10.1002/cncr.23062 (2007).
Virani, S. S. et al. AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 148, e9–e119. https://doi.org/10.1161/CIR.0000000000001168 (2023).
Faioni, E. M., Zighetti, M. L. & Vozzo, N. P. Sex, gender and venous thromboembolism: do we care enough? Blood Coagul Fibrinolysis. 29, 663–667. https://doi.org/10.1097/MBC.0000000000000773 (2018).
Konstantinides, S. V. et al. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 41, 543–603. https://doi.org/10.1093/eurheartj/ehz405 (2020).
Lyman, G. H. et al. American society of hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 5, 927–974. https://doi.org/10.1182/bloodadvances.2020003442 (2021).
Wang, Y., Ding, C., Guo, C., Wang, J. & Liu, S. Association between thyroid dysfunction and venous thromboembolism: A systematic review and meta-analysis. Medicine 102, e33301. https://doi.org/10.1097/MD.0000000000033301 (2023).
Ordookhani, A., Motazedi, A. & Burman, K. D. Thrombosis in thyroid Cancer. Int. J. Endocrinol. Metab. 16, e57897. https://doi.org/10.5812/ijem.57897 (2018).
Galeano-Valle, F., Del-Toro-Cervera, J. & Demelo-Rodriguez, P. Venous thromboembolism and Gastrointestinal stromal tumour: A rare association. Mol. Clin. Oncol. 12, 57–59. https://doi.org/10.3892/mco.2019.1942 (2020).
Gong, J. et al. Immune checkpoint inhibitors for cancer and venous thromboembolic events. Eur. J. Cancer. 158, 99–110. https://doi.org/10.1016/j.ejca.2021.09.010 (2021).
Oppelt, P., Betbadal, A. & Nayak, L. Approach to chemotherapy-associated thrombosis. Vasc Med. 20, 153–161. https://doi.org/10.1177/1358863X14568705 (2015).
Orsi, F. A. et al. Glucocorticoid use and risk of first and recurrent venous thromboembolism: self-controlled case-series and cohort study. Br. J. Haematol. 193, 1194–1202. https://doi.org/10.1111/bjh.17388 (2021).
Abdol RazakN. B., Jones, G., Bhandari, M., Berndt, M. C. & Metharom, P. Cancer-Associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers 10 https://doi.org/10.3390/cancers10100380 (2018).
Powers, W. J. et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 50, e344-e418, (2019). https://doi.org/10.1161/str.0000000000000211
Martinez, J. A., Qeadan, F., Burge, M. R. & Hypothyroidism Sex, and age predict future thromboembolic events among younger people. J. Clin. Endocrinol. Metab. 105, e1593–1600. https://doi.org/10.1210/clinem/dgz291 (2020).
Collet, J. P. et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European Heart Journal 42, 1289–1367, (2020). https://doi.org/10.1093/eurheartj/ehaa575 (2021).
Aboyans, V. et al. ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). European Heart Journal 39, 763–816, (2017). https://doi.org/10.1093/eurheartj/ehx095 (2018).
Tyagi, S. & Kumar, A. Safety of immune checkpoint inhibitors: An updated comprehensive disproportionality analysis and meta-analysis. Critical Reviews In Oncology/hematology 200, 104398, (2024). https://doi.org/10.1016/j.critrevonc.2024.104398
Sharma, A., Roy, S., Sharma, R. & Kumar, A. Association of antiviral drugs and their possible mechanisms with DRESS syndrome using data mining algorithms. J. Med. Virol. 95 https://doi.org/10.1002/jmv.28671 (2023).
Li, D. et al. Drug-Induced acute pancreatitis: A Real‐World pharmacovigilance study using the FDA adverse event reporting system database. Clin. Pharmacol. Ther. 115, 535–544. https://doi.org/10.1002/cpt.3139 (2024).
Böhm, R., Höcker, J., Cascorbi, I. & Herdegen, T. OpenVigil–free eyeballs on AERS pharmacovigilance data. Nat. Biotechnol. 30, 137–138. https://doi.org/10.1038/nbt.2113 (2012).
Brown, E. G. Using meddra: implications for risk management. Drug Saf. 27, 591–602 (2004).
Yang, X. et al. Thromboembolic events associated with epidermal growth factor receptor tyrosine kinase inhibitors: A pharmacovigilance analysis of the US FDA adverse event reporting system (FAERS) database. Clin. Drug Investig. 44, 199–207. https://doi.org/10.1007/s40261-024-01346-2 (2024).
Rothman, K. J., Lanes, S. & Sacks, S. T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 13, 519–523. https://doi.org/10.1002/pds.1001 (2004).
Evans, S. J., Waller, P. C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 10, 483–486. https://doi.org/10.1002/pds.677 (2001).
Bate, A. et al. A bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54, 315–321 (1998).
Dumouchel, W. Bayesian data mining in large frequency tables, with an application to the Fda spontaneous reporting system. Am. Stat. 53, 177–190. https://doi.org/10.1080/00031305.1999.10474456 (1999).
Jiang, Y. et al. Safety assessment of brexpiprazole: Real-world adverse event analysis from the FAERS database. J. Affect. Disord. 346, 223–229. https://doi.org/10.1016/j.jad.2023.11.025 (2024).
Shu, Y. et al. Version of FDA Adverse Event Reporting System. Clin. Epidemiol. 14, 789–802. https://doi.org/10.2147/CLEP.S365513 (2022). Disproportionality Analysis of Olaparib: Data Mining of the Public.
Chaozheng, Z. et al. MOAHIT: a web tool for visualizing tumor multi-omics data with human anatomy heatmaps. BioRxiv 2022.2009.2007.506938 https://doi.org/10.1101/2022.09.07.506938 (2023).
Acknowledgements
The authors thank the FAERS for providing the data for this study.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the China International Medical Foundation (No. Z-2021-46-2101-2023), Technology Innovation Project of Shapingba, Chongqing, China (grant number: 2024121), Chongqing Medical Scientific Research Project (Joint project of Chongqing Health Commission and Science and Technology Bureau, grant numbers: 2024QNXM016 and 2024ZDXM025), and Chongqing Medical Youth Top Talent Project (grant number: YXQN202456).
Author information
Authors and Affiliations
Contributions
Z.T. and H.S. wrote the manuscript; Z.T. designed the study; Z.T. and H.S. performed the study; L.P., M.Z., S.L., and W.L. analyzed the data; X.W. and W.C. contributed new reagents and analytical tools. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, Z., Song, H., Pan, L. et al. A pharmacovigilance study of thromboembolism events associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors based on FAERS database. Sci Rep 15, 25120 (2025). https://doi.org/10.1038/s41598-025-11067-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11067-x