Abstract
Metabolic-associated fatty liver disease (MAFLD) has become a considerable public health concern due to its elevated prevalence and its probability of progressing into hepatocellular carcinoma. Autophagy and pyroptosis are key cellular processes involved in MAFLD progression, with autophagy acting as a protective mechanism by inhibiting inflammasome activation. At the same time, excessive pyroptosis contributes to inflammation and tissue damage. Gasdermin D (GSDMD), a key executor of pyroptosis, plays a central role in this process by mediating inflammatory cell death and has emerged as a potential marker of liver injury severity. This study evaluates curcumin’s effects on liver fat accumulation, inflammatory cytokine levels, oxidative stress, and its potential to regulate autophagy and pyroptosis. Rats were allocated to three groups. Group I established a normal diet for twelve weeks. Group II was given a high-fat, high-fructose diet (HFFD) for twelve weeks. Group III received the HFFD diet for twelve weeks with curcumin supplementation from the 6th to the 12th week. Rats in Group III showed significant improvements in adiposity, lipid metabolism, oxidative stress, and inflammatory markers. Histopathological analysis confirmed the beneficial effects of curcumin, with notable improvements in liver structure and reduced Lipid-associated liver dysfunction severity. Furthermore, curcumin’s dual effects on autophagy through modulation of P62 protein and BECN1 gene and on pyroptosis through inhibition of GSDMD activation, were evident, supporting its protective role in preventing MAFLD progression to fibrosis and liver cancer.
Similar content being viewed by others
Introduction
Metabolic-associated fatty liver disease (MAFLD) is one of the most prevalent liver conditions worldwide, posing a significant public health concern due to its high occurrence and its association with an elevated risk of cirrhosis and hepatocellular carcinoma (HCC). This lipid-associated liver dysfunction is characterized by the excessive accumulation of triglycerides (TG) within hepatocytes in the absence of alcohol consumption. The disease spectrum ranges from simple steatosis and steatohepatitis to cirrhosis and, in severe cases, hepatocellular carcinoma1.
The “multiple hit” hypothesis describes metabolic fatty liver disease pathogenesis. The initial “hit” involves disrupted lipid metabolism and insulin resistance (IR), resulting in excessive fat deposition within hepatocytes (steatosis). Subsequent “hits” include imbalances in adipokines and cytokines, exposure to intestinal bacterial toxins, and increased oxidative stress. These factors collectively excite the activation of HSC (hepatic stellate cells) and Kupffer cells, ultimately contributing to liver damage2.
Although the exact mechanisms underlying the pathogenesis of such metabolic fatty liver disease are not clear, recent advances have improved understanding of the progression from steatosis to severe liver inflammation and fibrosis. Hepatic steatosis is strongly linked to chronic hepatic inflammation involving the NF-κB signaling pathway3. Moreover, inflammatory form as pyroptosis, of programmed cell death, has been implicated in liver diseases, where caspase-1 activation triggers membrane rupture, inflammatory cytokine release, and exacerbated tissue damage4
Furthermore, inflammasomes, including the NLRP3 ((NOD-, LRR-, and pyrin domain-comprising protein 3), have been concerned in liver diseases, where activation of this complex triggers’ caspase-1 activation, promoting pyroptosis and inflammatory cytokines release such as IL-1β and IL-18, further exacerbating liver damage5. The blocking of the NLRP3 inflammasome reduces intracellular lipid accumulation, suppresses inflammatory cytokines, and prevents hepatic fibrosis6.
A central effector of pyroptosis is Gasdermin D (GSDMD). This pore-forming protein is cleaved by activated caspase-1, leading to cell swelling, membrane rupture, and the release of pro-inflammatory contents. Elevated GSDMD expression has been associated with the progression of liver inflammation and fibrosis in metabolic liver diseases. Thus, GSDMD serves as a crucial marker for pyroptotic activity and a potential therapeutic target for mitigating inflammation-driven hepatocellular damage in MAFLD7.
Autophagy plays an essential role in mitigating these pathological processes by removing damaged organelles, including lipid droplets, and suppressing inflammasome activation. The activation of autophagy has been demonstrated to reduce oxidative stress, inhibit pyroptosis, and alleviate liver fibrosis, thereby decelerating the progression of MAFLD8. Key proteins such as P62 and Beclin-1 regulate autophagy and have emerged as important markers for disease progression, with P62 involved in both autophagy and inflammasome modulation9. Otherwise, BECN1, a key gene encoding the Beclin-1 protein involved in autophagy, has been described to show a role in liver fibrosis10,11.
Curcumin (Cur), the primary active compound in turmeric, has been demonstrated to modulate oxidative stress, inflammation12, and autophagy13. Evidence from both in-vitro and in-vivo studies suggests that curcumin benefits various metabolic fatty liver disease stages by reducing inflammation and fat accumulation. Additionally, curcumin has been displayed to prevent the progress of MAFLD to fibrosis state and lower the hazard of liver cancer13,14.
Despite the extensive studies supporting curcumin’s benefits in MAFLD, its precise mechanisms of action remain insufficiently discovered. Therefore, this study aims to explore curcumin’s role in modulating autophagy, pyroptosis, and inflammasome activity and try to understand the underlying mechanisms involved, focusing on its therapeutic potential in halting MAFLD progression.
Material and methods
Animals of the experiments
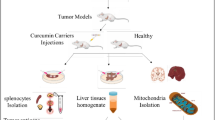
Eighteen male albino Sprague Dawley rats, around 180- and 200-g body weight were used for the research experimentations. They were provided by the animal facility at the Faculty of Pharmacy at the Delta University for Science and Technology (FPDUST) in Gamasa, Egypt. The rats were kept in plastic cages, (four animals/cage), in controlled environmental conditions: humidity ranging from 40 to 70%, a 12-h light/dark cycle, and temperature maintained at 20–22°C. The animals had unrestricted access to standard food and water throughout the study. The experimental protocol and procedures for animal handling were approved by the Institutional Animal Care and Use Committee (IACUC) of the Faculty of Pharmacy, Delta University for Science and Technology, Gamasa, Egypt (Approval Number: FPDU12/2023), which tracks the ARRIVE guidelines and the National Research Council’s Guide for the Care and Use of Laboratory Animals (Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011). Also, all methods were performed following these relevant guidelines and regulations.
Following a one-week acclimation period, the rats were randomly allocated into three groups, each containing six animals.
Chemicals used
-
Curcumin (Cur): cur was purchased from Sigma Aldrich (Cairo, Egypt), disolved in 0.5% carboxymethyl cellulose, and administered orally at a daily dose of 50 mg/kg.15.
-
Carboxymethyl cellulose (0.5%): Prepared by dissolving 0.5 g of carboxymethyl cellulose powder in 100 mL of distilled water.
Induction of MAFLD model
-
High-fat, high fructose diet (HFFD): MAFLD was induced using fat diet containing 45% fat in the basal diet and 20% fructose in drinking water (prepared by dissolving fructose (20 g / 100 mL of tap water) following previously established protocols16,17
-
Experimental groups
-
o
Group I (Control): Rats were fed a standard diet for twelve weeks.
-
o
Group II (HFFD): Rats received the highly-fat and fructose food for twelve weeks.
-
o
Group III (HFFD + Cur): Rats were fed the high-fat and fructose diet for twelve weeks, with cur supplementation (50 mg/kg via oral gavage, administered once daily) starting from the 6th week until the 12th week15.
-
o
The supplementation starting from the 6th week was chosen based on previous studies indicating that MAFLD-related pathological features (steatosis and inflammation) begin to manifest by this time. Intervention from the 6th to 12th week allows assessment of curcumin’s therapeutic rather than preventive effects15,17.
At the end of the study, rats were euthanized by cervical dislocation, and blood samples were collected from the heart via cardiac puncture. Blood samples were left to clot before centrifugation at 3000 rpm for 20 min, and the resulting serum was stored at -20°C for later biochemical analyses. Liver tissues were harvested, weighed, and divided into two portions. The liver wet weight was recorded to estimate the liver index employing the formula:
One portion of the liver was homogenized for the quantification of oxidative stress markers (reduced glutathione [GSH] and malondialdehyde [MDA]). The other portion was preserved in RNA later (Qiagen, Germany) at 4°C overnight and then stored at − 80°C for subsequent molecular analysis. Formalin-fixed liver samples were prepared for histological and immunohistochemical examination.
Body weight monitoring and weight gain
The body weight of each animal was documented at the beginning and terminal of the study. Weight gain was calculated using the formula:
Biochemical analysis
Fasting blood glucose and insulin:
Fasting blood glucose levels were recorded using an ACCU-CHEK glucometer. Serum insulin concentrations were measured by means of a commercial enzyme-linked immunosorbent assay (ELISA) kit (MyBiosource Inc., San Diego, CA, USA). Insulin resistance was determined using HOMA-IR (homeostasis model calculation of insulin resistance) by the formula:
Ref.18.
Lipid profile analysis
Total cholesterol, triglycerides, LDL cholesterol, and HDL cholesterol (TC, TG, LDL-C, HDL-C), respectively levels were analyzed using commercial kits obtained from Biodiagnostic Company (Giza, Egypt).
Liver function tests
Serum alanine transaminase (ALT) and aspartate transaminase (AST) levels were measured using clinical test kits from Elitech (UK) and analyzed spectrophotometrically.
Oxidative stress markers in liver tissue
Liver levels of malondialdehyde (MDA) and reduced glutathione (GSH) were measured using specialized kits (Biodiagnostic, Giza, Egypt).
Inflammatory markers (TNF-α and IL-1β)
ELISA kits (PeproTech, Rocky Hill, NJ, USA) were used to assess Tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) concentrations in liver homogenates according to the constructer’s instructions.
The hepatic concentration of Gasdermin D (GSDMD) was determined using a rat-specific ELISA kit (Cat # SEE529Ra, Cloud-Clone Corp., USA) according to the manufacturer’s protocol. Liver samples were homogenized and centrifuged to obtain clear supernatants. Absorbance was measured at 450 nm using a microplate reader, and GSDMD concentrations were calculated from the standard curve and expressed as ng/mg protein.
RNA extraction and quantitative real-time pcr (qrt-pcr) for becn1 gene expression
For the analysis of BECN1 gene expression, RNA was obtained using the Direct-zol RNA Miniprep Plus kit (Cat# R2072, ZYMO RESEARCH CORP., USA), and RNA purity and concentration were evaluated with a Beckman dual spectrophotometer (USA). Complementary DNA (cDNA) was synthesized using, the Takara SYBR Premix Ex Taq II (Bio-Rad, Hercules, CA, USA) on an Applied Biosystems, Darmstadt, Germany (ABI 7300 instrument). In the case of BECN1, reverse transcription and PCR were conducted in a single step utilizing the SuperScript IV RT-PCR kit (Cat# 12,594,100, Thermo Fisher Scientific, Waltham, MA, USA) on a StepOne real-time PCR system (Applied Biosystems, Foster City, USA).
Sangon Biotech Co. (Shanghai, China) created specific primers for BECN1 and housekeeping genes (detailed in Table 1). Amplification specificity was verified through melting curve analysis, and fluorescence detection was performed at 60°C. The PCR protocol included a step of denaturation at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 31 s. All qRT-PCR reactions were run in triplicate.
Relative gene expression was calculated using the 2^ − ΔΔCt method. For BECN1, the cycle threshold (Ct) standardised to the housekeeping gene GAPDH, and relative quantification (RQ) quantified by the same method.
Western blot analysis for P62
Total protein was extracted using a Bio-Rad protein extraction kit (Catalog #163–2086). Protein levels were determined by a Bradford Protein Assay Kit (Bio Basic Inc., Canada). Polyacrylamide gels were prepared using the TGX Stain-Free™ FastCast™ Acrylamide Kit (Bio-Rad Laboratories Inc., Catalog #161–0181). The membranes were blocked with tris-buffered saline containing Tween 20 (TBST) and 3% BSA for 1 h at room temperature. Primary antibodies against P62 (Proteintech, Catalog #18,420–1-AP) were incubated overnight, followed by Goat anti-rabbit IgG incubation, Novus Biologicals. Chemiluminescent signals were detected using a CCD camera-based imager, and band intensities were analyzed using ChemiDoc MP image software. Beta-actin was used as a loading control. The contrast in the displayed blot was not digitally manipulated beyond standard adjustments for clarity. No high-contrast modifications were applied that could alter the interpretation of the results. Full-length, unprocessed blots are provided in the Supplementary Information for transparency Supplementary figure S2.
Histopathological and immunohistochemical analysis for NLRP3, IL-1β, TNF-α and caspase-1
Liver samples were preserved in 10% neutral buffered formalin, dehydrated, and embedded in paraffin. Sections of 5 µm thickness were stained with hematoxylin and eosin (H&E) for routine histological evaluation, while Masson’s trichrome staining was used to detect collagen fibers.
For immunohistochemical staining, sections were treated with primary antibodies against NLRP3 (Invitrogen, Catalog No. PA5-115,661, 1:100 dilution), IL-1β, TNF-α and caspase-1 (Servicebio, Wuhan, China; dilutions of 1:800 and 1:600, respectively). Secondary antibodies were applied, and the stained slices were examined under a microscope. All photos were reserved with ICC50E Camera Module (Leica Microsystems AG) by LEICA DM 750 Microscope at the Department of Basic Medical Sciences, Faculty of Medicine, Ibn Sina University for Medical Sciences, Amman, Jordan.
Morphometric analysis
The percentage area of collagen in Masson’s trichrome-stained slices (400 × magnification) and the immunopositive areas for NLRP3, IL-1β, TNF-α, and caspase-1 (400 × magnification) were computed through ImageJ software (version 1.52a).
Statistical analysis
GraphPad Prism version 10.0 (GraphPad Software, San Diego, CA, USA) was used for creating bar graphs and visualizing group comparisons. . One-way ANOVA with post-hoc Tukey’s test was employed to assess differences among the groups, while an independent t-test was used to compare two groups. Statistical significance was defined as p < 0.05.
Results
Impact of curcumin on body weight, weight gain, liver weight, and liver index
The findings revealed no notable differences in the initial body weights across the experimental groups at baseline. Nevertheless, Group II experienced a marked elevation in body weight, weight gain, liver weight, and liver index compared to the control group (P < 0.001). Conversely, Group III demonstrated significantly (P < 0.001) reduced values for these parameters compared to Group II. The final measurements remained significantly (P < 0.01) higher in Group III relative to Group I, as presented in Fig. 1.
Impact of curcumin on serum biochemical measures
Group II (HFFD) exhibited significantly (P ≤ 0.001) elevated levels of fasting blood glucose (FBG), serum insulin, and HOMA-IR compared to Group I (control). The introduction of curcumin in Group III (HFFD + Curcumin) led to a significant (P ≤ 0.001) reduction in these parameters relative to Group II. Despite these reductions, FBG and HOMA-IR levels in Group III remained significantly higher than in Group I, however, serum insulin differences between Groups I and III were not statistically significant (P = 0.23).
Regarding lipid profiles, Group II showed significant increases in triglycerides (TG), total cholesterol (TC), and LDL-C (P ≤ 0.001), alongside a non-significant decrease in HDL-C (P = 0.86) compared to Group I. Curcumin supplementation in Group III significantly improved lipid parameters in comparison to Group II (P ≤ 0.001), with non-significant differences observed between Groups I and III (Fig. 2).
Impact of curcumin on fasting glucose, insulin, HOMA-IR, and lipid profile. Data are stated as mean ± SD, with P ≤ 0.05 statistical significance. Abbreviations: FBG = fasting blood glucose, HOMA-IR = homeostasis model assessment, TC = total cholesterol, TG = triglycerides, LDL = low-density lipoprotein, HDL = high-density lipoprotein. (n = 6 for each group). Group II induced metabolic dysfunction, whereas curcumin (Group III) improved glycemic and lipid parameters.
Impact of curcumin on liver function, oxidative stress, and inflammatory markers
Significant elevations (P < 0.001) in both serum ALT and AST levels were observed in Group II compared to Group I. In contrast, Group III exhibited notable reductions in both enzymes relative to Group II. No significant change was found between Groups I and III, indicating that curcumin effectively normalized enzyme levels, as shown in Fig. 3A. Group II had significantly (P < 0.001) increased tissue MDA levels and decreased tissue GSH levels compared to Group I, signifying heightened oxidative stress. Curcumin treatment in Group III significantly (P < 0.001) lowered MDA levels and elevated GSH levels compared to Group II; Group III still significantly differed from Group I, as shown in Fig. 3B. Tissue inflammatory markers IL-1β and TNF-α were significantly (P < 0.001) upregulated in Group II in comparison to Group I, indicating inflammation induced by the HFFD. Curcumin treatment in Group III significantly (P < 0.001) reduced these markers compared to Group II, with no significant differences detected between Groups I and III, suggesting effective mitigation of the inflammatory response as presented in Fig. 3C. Additionally, hepatic levels of Gasdermin D (GSDMD), were significantly elevated in Group II compared to the control (Group I) (P < 0.001), reflecting increased pyroptotic activity. Curcumin supplementation in Group III significantly (P < 0.01) decreased GSDMD levels relative to Group II. Although GSDMD expression in Group III remained slightly higher than Group I, the reduction was statistically significant, indicating curcumin’s ability to suppress pyroptosis. These results are depicted in Fig. 3D.
(A) Effect of curcumin supplementation on enzyme levels (ALT and AST) in rats fed a high-fat, high-fructose diet (HFFD). Group II (HFFD) showed significantly elevated enzyme levels compared to the control group, indicating hepatic injury. Curcumin treatment (Group III) significantly reduced these levels, reflecting hepatoprotective effects. Data are presented as mean ± SD (n = 6/group), analyzed using one-way ANOVA followed by Tukey’s test (P ≤ 0.05). ALT = alanine transaminase; AST = aspartate transaminase. (B) Hepatic levels of oxidative stress markers (malondialdehyde [MDA] and reduced glutathione [GSH]) across experimental groups. HFFD-fed rats (Group II) showed increased MDA and decreased GSH, indicating oxidative stress. Curcumin administration (Group III) ameliorated oxidative stress by lowering MDA and elevating GSH. Values are mean ± SD (n = 6); significance tested via ANOVA and Tukey’s post hoc test (P ≤ 0.05). (C) Effects of curcumin on pro-inflammatory cytokines (IL-1β and TNF-α) in liver homogenates. Group II showed elevated cytokine levels due to HFFD-induced inflammation. Curcumin (Group III) significantly reduced cytokine levels, indicating anti-inflammatory properties. Data are expressed as mean ± SD (n = 6); statistical comparisons used ANOVA with post hoc Tukey’s test (P ≤ 0.05). IL-1β = interleukin-1 beta; TNF-α = tumor necrosis factor-alpha. (D) Effects of curcumin on pyroptosis protein marker Gasdermin (D) in liver homogenates. Group II showed elevated levels due to HFFD-induced pyroptosis. Curcumin (Group III) significantly reduced Gasdermin D levels, indicating anti-pyroptotic properties. Data are expressed as mean ± SD (n = 6); statistical comparisons used ANOVA with post hoc Tukey’s test (P ≤ 0.05).
Impact of curcumin on BECN1 Gene and P62 protein expression
qRT-PCR analysis demonstrated a significant downregulation of BECN1 gene expression in Groups II and III in comparison to Group I (P ≤ 0.001). However, curcumin supplementation in Group III significantly upregulated BECN1 expression compared to Group II (P ≤ 0.001), although levels remained lower than in Group I, indicating partial recovery (Fig. 4A). Western blot results showed a significant elevation in P62 protein expression in Group II in comparable to Group I (P ≤ 0.001), reflecting impaired autophagy. Curcumin treatment in Group III significantly reduced P62 expression relative to Group II (P ≤ 0.001), though levels remained higher than in Group I, indicating partial restoration of autophagic activity (Fig. 4B). Supplementary Figure S2 shows the corresponding full-length blots.
(A) Quantitative RT-PCR analysis of BECN1 gene expression in liver tissues. A significant downregulation was observed in Group II, suggesting impaired autophagy. Curcumin (Group III) partially restored BECN1 expression, supporting its role in autophagy modulation. Data are mean ± SD (n = 6); *P < 0.05. (B) Western blot analysis of P62 protein levels in liver tissues. Increased P62 expression in Group II indicates autophagic flux impairment. Curcumin treatment (Group III) reduced P62 levels, suggesting autophagy restoration. Representative blots shown; quantitative data presented as mean ± SD. *P < 0.05. Data shown are from n = 2 biological replicates, with consistent results across replicates. Full-length, unprocessed blots are available in Supplementary Figure S2.
Histopathological analysis of liver tissues
Liver sections from Group I demonstrated normal hepatic structure, characterized by hepatocytes organized in cords radiating from the central vein and separated by distinct blood sinusoids. The hepatocytes appeared polyhedral with granular, acidophilic cytoplasm and large, rounded nuclei, some of which were binucleated (Fig. 5 (1A). The portal regions contained branches of the portal vein, bile ducts, and hepatic arteries (Fig. 5 (1B)).
Histological assessment of liver tissue structure and fibrosis. Figure 5 (1): Microscopic images of H&E-staining sections from Group I. (A) Normal hepatic architecture, featuring a central vein (star) surrounded by cords of hepatocytes, separated by distinct blood sinusoids (arrow). The cells exhibit granular, acidophilic cytoplasm with large, round vesicular nuclei, nearly are being binucleated (arrowhead). (B) Highlights the portal area (arrow), containing a branch of the portal vein (p), the hepatic artery (a), and the bile duct (b) (Magnification X400). Figure 5 (2): Microscopic images of the H&E-staining section of group II. (A) Dilated central vein surrounded by mononuclear cellular infiltration (star), large cytoplasmic vacuoles of many hepatocytes (arrow), and irregular shrunken hepatocytes with deeply stained pyknotic nuclei (arrowhead). (B) Dilated blood sinusoids (star) and heavy mononuclear cellular infiltration (arrow). (C) Hepatocytes with large cytoplasmic vacuoles (arrow) and others are shrunken and irregular with pyknotic nuclei (arrowhead). (D) Portal area with dilated branches of the portal vein (p), hepatic artery (a), and bile duct (b). Heavy mononuclear cellular infiltration (arrow). (Magnification X400). Figure 5 (3): Microscopic images of the H&E-staining section of group III. (A) Hepatocyte architecture nearly like group I (arrow). Notice mild dilation of the central vein and the blood sinusoids (stars). (B) Portal area elements almost like group I (arrow). (Magnification X400).
In contrast, liver tissues from Group II showed marked structural abnormalities, including dilated central veins, disrupted sinusoidal endothelial lining, and vacuolated hepatocytes. Several hepatocytes appeared shrunken with pyknotic nuclei, accompanied by dense mononuclear cell infiltration around the central veins and within portal areas (Fig. 5 (2A–C)). Additionally, many portal regions exhibited dilated, congested branches, with pronounced mononuclear infiltration (Fig. 5 (2D).
Liver sections from Group III displayed an architecture nearly comparable to the control group, with only slight dilation of the central vein and sinusoids in certain areas (Fig. 5 (3A–B).
Masson’s trichrome staining revealed minimal collagen deposition around the central veins and portal tracts in Group I (Fig. 6A,B). In contrast, Group II exhibited extensive collagen accumulation, revealing fibrosis (Fig. 6C,D). Curcumin treatment in Group III markedly reduced collagen deposition compared to Group II, with only a slight increase in collagen fibers relative to the control group (Fig. 6E,F).
Masson’s trichrome staining for collagen deposition in liver tissues. Masson’s trichrome-stained liver sections showing collagen deposition. Group I [Figure A, B] displayed minimal perivascular collagen (arrows). Group II [Figure C, D] exhibited extensive fibrosis (arrows), while Group III [Figure E, F] showed markedly reduced collagen accumulation (arrows) indicating curcumin’s anti-fibrotic effect. (Magnification: X400).
Immunohistochemical Examination
Immunohistochemical analysis was carried out to evaluate the expression of NLRP3, IL-1β, TNF-α, and caspase-1 across all experimental groups of liver tissue. In Group I, liver sections exhibited a faint positive cytoplasmic immunoreaction for all markers, indicating low basal expression levels (Fig. 7 (1 A & D, 2A & D,2G &J)) respectively. In contrast, liver sections from Group II demonstrated strong positive staining in the cytoplasm of hepatocytes for NLRP3, IL-1β, TNF-α, and caspase-1, reflecting significant stimulation of inflammatory pathways and pyroptosis due to HFFD diet-induced liver damage (Fig. 7 (1 B, E, 2 B, E, H & K) respectively. However, in Group III, moderate positive staining for all markers was observed, indicating that curcumin significantly reduced the inflammatory response and pyroptotic activity induced by the HFFD diet, though the expression levels of these markers did not fully refer to the basal levels seen in the group I (Fig. 7 (1 C, 7-F, 2 C, F, I & L) respectively.
Immunohistochemical staining for pyroptotic and inflammatory markers. Figure 7 (1). Immunohistochemical analysis of NLRP3 and IL-1β expression in liver tissues. HFFD-induced inflammation in Group II (B, C, D) increased immunoreactivity compared to Group I (A). Curcumin-treated rats (Group III) (E,F) showed reduced expression, suggesting attenuation of pyroptosis and inflammation. Bar graph (G) presents morphometric quantification, mean ± SD) (n = 6 rats per group). Immunoreactivity was analyzed using ImageJ and expressed as integrated optical density (IOD) per unit area. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test. * indicates a statistically significant difference compared to the control group (p < 0.05), # indicates a statistically significant difference compared to the NAFLD group (p < 0.05). Figure 7 (2). Immunohistochemical staining for TNF-α (A–F) and caspase-1 (G, H, I, J, K, and L) across experimental groups. Strong expression was noted in Group II (HFFD), while Group III showed reduced staining following curcumin intervention. (High magnification: X400, scale bar = 50 µm; Low magnification: X100, scale bar = 100 µm). Panel M quantifies immunopositive area percentages (mean ± SD) (n = 6 rats per group), indicating suppression of pyroptotic and inflammatory markers. Immunoreactivity was analyzed using ImageJ and expressed as integrated optical density (IOD) per unit area. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test. * indicates a statistically significant difference compared to the control group (p < 0.05), # indicates a statistically significant difference compared to the NAFLD group (p < 0.05).
Morphometric and Statistical Findings
Morphometric analysis was used to quantify the area percentage of positive staining for NLRP3, IL-1β, TNF-α, and caspase-1, as well as collagen deposition in liver tissue. The area percentage of NLRP3 and IL-1β was significantly elevated in Group II comparable to Group I. Curcumin treatment in Group III significantly reduced the expression of these markers compared to Group II (P ≤ 0.001). However, the expression of NLRP3 and IL-1β in Group III remained slightly higher than in Group I, though the difference was not significant, suggesting partial recovery (Fig. 7 (1 G).
The morphometric data for TNF-α and caspase-1 showed a similar trend. A significant (P ≤ 0.001) rise in the positive staining area percentage was detected in Group II in comparison to Group I. Curcumin treatment in Group III directed a significance decline in the expression of these markers compared to Group II (P ≤ 0.001), with a non-significant difference between Group III and Group I, indicating curcumin effectively normalized the levels of TNF-α and caspase-1 (Fig. 7 (2 M).
Furthermore, Masson’s trichrome evaluates the collagen deposition. In control group, liver sections displayed thin collagen fibers around the portal tracts and central veins (8.464 ± 0.583). In contrast, liver sections from Group II showed extensive collagen accumulation around the portal tracts and central veins, reflecting significant fibrosis (15.68 ± 1.07, P ≤ 0.001). Curcumin treatment in Group III markedly reduced collagen deposition compared to Group II, with a non-significant difference from the control group, indicating that curcumin prevented fibrosis progression and helped restore normal liver architecture.
Discussion
The growing prevalence of MAFLD and complications, as hepatocellular carcinoma, contributed to global disease burden19. Additionally, diabetes elevates the risk20. Notably, lipid buildup induces cellular stress, activates inflammasomes, and leads to apoptotic cell death21.
Liver disease progression is linked to inflammasome activation, despite mechanisms and effective treatments remain unclear22. This study investigates curcumin’s potential on HFFD- induced steatosis and its mechanism.
A 12-week HFFD resulted in significant weight gain, a rise in both liver weight and liver index. These observations are consistent with Mustika et al. (2023)23 and other studies24. In a previous study, curcumin exhibited less weight gain and lower body fat percentages25, supporting our findings.
The present study observed that HFFD led to hyperglycemia, hyperinsulinemia, and insulin resistance, aligning with findings from Cho et al.26. Curcumin is valid in lowering blood glucose, serum insulin levels, and HOMA-IR, corroborating results from previous researchs25,27. These improvements are likely by activating the Peroxisome proliferator-activated receptor (PPAR), enhancing glycolysis, inhibiting gluconeogenesis, and upregulating the expression of GLUT2, GLUT3 and GLUT4 genes28.
In the present study, the HFFD elevated TG, TC, and LDL-C, while HDL-C levels decreased. Curcumin improved the lipid profile, , consistent with Al-Saud27 andDing et al. (2016) who showed improvements in fasting blood glucose, insulin resistance, lipid profile, liver, and overall weight29.
Curcumin exhibited hepatoprotective properties by normalizing antioxidant enzyme activity (GSH) and decreasing MDA levels, consistent with prior study30, confirming its protective anti-inflammatory effects via nuclear factor-kappa B (NF-κB) regulation31.
The NLRP3 inflammasome is pivotal in intensifying liver inflammation, and pyroptosis32. by triggering the NF-κB pathway, which helps the release of TNF-α, IL-6, IL-1β, and caspase-1 as pro-inflammatory mediators33. In our study, HFFD markedly increased these inflammatory markers, suggesting inflammasome activation22. Previous research has shown that inflammasomes involve the sensor molecule (NLRP3), the adaptor protein ASC (apoptosis-allied speck-alike protein with a CARD domain), and pro-caspase-1 as the effector5, influencing IL-1β and IL-185,34.
Furthermore, Wang et al. (2021) demonstrated that inhibiting NLRP3 reduces steatosis and cytokine expression35. Moreover, NLRP3 inhibition limited macrophage and neutrophil infiltration, reduced caspase-1, and downregulated TGF-β along with its genes involved in collagen production and hepatic fibrosis progression6. These findings align with our study. Our results also correspond with Wu et al. (2023)36.
In this study also, curcumin significantly reduced hepatic GSDMD levels, indicating suppression of pyroptotic activity. These findings provide molecular evidence for curcumin’s anti-pyroptotic effect, supporting recent findings37. More to the point, Autophagy, maintains hepatocyte function by eliminating lipid dropletsand pro-inflammatory factors38. In this study, autophagy dysfunction was evidenced by decreased BECN1 gene expression and increased p62 levels in HFFD group39. Previous research has confirmed that impaired autophagy, reflected by elevated p62 and microtubule-associated protein 1 light chain 3 (unlipidated I and lipidated II ratio) (LC3II/I), is related to disease progression in patients suffering from NASH (non-alcoholic steatohepatitis) and HCC (hepatocellular carcinoma)9,40.
Curcumin restored autophagy in HFFD-fed rats by increasing BECN1 and reducing p62. BECN1 promotes autophagosome formation, and reduced p62 reflects restored autophagic flux. These effects may be mediated through AMPK/mTOR and PI3KC3 signaling41.
Curcumin supports antifibrotic effects via autophagy promotion. BECN1 knockdown reduces this effect. It also lowers ROS by activating PPAR-α and AMPK/mTOR42. Moreover, findings by Cao et al. suggest that downregulation of BECN1 enhances the anti-inflammatory activity of mesenchymal stem cells43.This study reveals curcumin’s dual regulation of autophagy and pyroptosis. Impaired autophagy worsens pyroptosis via ROS and NLRP3 inflammasome activation, while pyroptosis further inhibits autophagic flux44,45.46 Curcumin restores this balance, reducing hepatic injury and highlighting its dual-action therapeutic potential in MAFLD.Taken together, Curcumin exhibits dual regulation of autophagy and pyroptosis in MAFLD. Pyroptosis promotes inflammation via cytokines like IL-1β, while autophagy mitigates this by clearing damaged organelles. Impaired autophagy can worsen pyroptosis through ROS/NLRP3 activation. Curcumin’s upregulation of BECN1 and downregulation of P62 and caspase-1 suggests its protective effect via modulation of AMPK-mTOR and NF-κB pathways47,48,49.
Although TNF-α is not pyroptosis-specific, its elevated expression and histological evidence indicate pyroptosis in MAFLD. For clearer confirmation, GSDMD strengthens the mechanistic link between pyroptosis and hepatic injury.
50. The use of an animal model is a limitation, as it may not fully represent human MAFLD. Clinical studies are needed to validate curcumin’s therapeutic potential.
Curcumin’s clinical use is limited by poor bioavailability due to rapid metabolism and low solubility. Strategies like piperine co-administration, nanoparticles, or analogs may improve its absorption and therapeutic efficacy48.
P62 and BECN1 levels were used to assess autophagy, but they also function in other pathways. Lack of LC3-II/I ratio analysis limits assessment depth. Future studies should include dynamic autophagy flux assays and LC3-based analysis51,52,53,54
Conclusion
Our research indicates that curcumin is an effective natural therapeutic agent for MAFLD. It improves metabolic disturbances linked to HFFD consumption, enhances autophagy, and inhibits inflammasome activity, reducing liver inflammation and fibrosis. These findings further support curcumin’s therapeutic potential from a functional food perspective, emphasizing its dual regulatory role in pyroptosis and autophagy.
Data availability
Data are available upon reasonable request from the corresponding author Prof. Rania M. Khalil. The reference sequences used in this study are publicly available in the NCBI RefSeq database, which is part of the International Nucleotide Sequence Database Collaboration (INSDC). The BECN1 gene sequence is accessible under RefSeq accession number NM_001197217.1, and the housekeeping gene sequence is available under RefSeq accession number XM_063279674.1. These sequences can be retrieved from the NCBI Nucleotide database (https://www.ncbi.nlm.nih.gov/nucleotide/) by searching their respective accession numbers.
References
Ferro, D., et al., New Insights into the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Gut-Derived Lipopolysaccharides and Oxidative Stress. Nutrients, 12(9). (2020)
Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67(1), 328–357 (2018).
Auguet, T. et al. Relationship between IL-8 Circulating Levels and TLR2 Hepatic Expression in Women with Morbid Obesity and Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 21(11), 4189 (2020).
Wei, Y. et al. Pyroptosis-Induced Inflammation and Tissue Damage. J. Mol. Biol. 434(4), 167301 (2022).
Liu, T. et al. NOD-like receptor family, pyrin domain containing 3 (NLRP3) contributes to inflammation, pyroptosis, and mucin production in human airway epithelium on rhinovirus infection. J. Allergy Clin. Immunol. 144(3), 777-787.e9 (2019).
Torres, S. et al. The Specific NLRP3 Antagonist IFM-514 Decreases Fibrosis and Inflammation in Experimental Murine Non-Alcoholic Steatohepatitis. Front Mol. Biosci. 8, 715765 (2021).
Shi, J., Gao, W. & Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci 42(4), 245–254 (2017).
Shen, Q. et al. The pivotal role of dysregulated autophagy in the progression of non-alcoholic fatty liver disease. Front Endocrinol. (Lausanne) 15, 1374644 (2024).
Fan, G. et al. Natural-Product-Mediated Autophagy in the Treatment of Various Liver Diseases. Int. J. Mol. Sci. 23(23), 15109 (2022).
Mukhopadhyay, S. et al. Invariant BECN1 CXXC motifs bind Zn(2+) and regulate structure and function of the BECN1 intrinsically disordered region. Autophagy 20(2), 380–396 (2024).
Tan, C. T. et al. p62/SQSTM1 in liver diseases: the usual suspect with multifarious identities. Febs J. 290(4), 892–912 (2023).
Khalil, R. M. & Khedr, N. F. Curcumin Protects against Monosodium Glutamate Neurotoxicity and Decreasing NMDA2B and mGluR5 Expression in Rat Hippocampus. Neurosignals 24(1), 81–87 (2016).
Cicero, A. F. G. et al. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: a double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 59(2), 477–483 (2020).
Coban, D. et al. Dietary curcumin inhibits atherosclerosis by affecting the expression of genes involved in leukocyte adhesion and transendothelial migration. Mol. Nutr. Food Res. 56(8), 1270–1281 (2012).
Zakaria, S. S., ElBatsh, M. M. & Mowafy, S. E. Effects of Curcumin on Autophagy and Nrf2 signaling pathway in a Rat Model of High fructose diet induced Steatohepatitis %. J. Bull. Egypt. Soc. Physiol. Sci. 37(2), 208–226 (2017).
Mamikutty, N. et al. The establishment of metabolic syndrome model by induction of fructose drinking water in male Wistar rats. Biomed. Res. Int. 2014, 263897 (2014).
Jensen, V. S. et al. Dietary fat stimulates development of NAFLD more potently than dietary fructose in Sprague-Dawley rats. Diabetol. Metab. Syndr. 10, 4 (2018).
Okita, K. et al. Homeostasis model assessment of insulin resistance for evaluating insulin sensitivity in patients with type 2 diabetes on insulin therapy. Endocr. J. 60(3), 283–290 (2013).
Riazi, K. et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet. Gastroenterol. Hepatol. 7(9), 851–861 (2022).
Muzica, C. M. et al. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: A Bidirectional Relationship. Can. J. Gastroenterol. Hepatol. 2020, 6638306 (2020).
Powell, E. E., Wong, V. W. & Rinella, M. Non-alcoholic fatty liver disease. Lancet 397(10290), 2212–2224 (2021).
Yu, L. et al. The NLRP3 Inflammasome in Non-Alcoholic Fatty Liver Disease and Steatohepatitis: Therapeutic Targets and Treatment. Front Pharmacol. 13, 780496 (2022).
Mustika, S. et al. Impact of multiple different high-fat diets on metabolism, inflammatory markers, dysbiosis, and liver histology: study on NASH rat model induced diet. F1000Res 12, 180 (2023).
Saleh Aldayel, T. Apigenin attenuates high-fat diet-induced nephropathy in rats by hypoglycemic and hypolipidemic effects, and concomitant activation of the Nrf2/antioxidant axis. J. Funct. Foods 99, 105295 (2022).
Koboziev, I., et al., Effects of Curcumin in a Mouse Model of Very High Fat Diet-Induced Obesity. Biomolecules. 10(10). (2020).
Cho, I. J. et al. Allopurinol ameliorates high fructose diet induced hepatic steatosis in diabetic rats through modulation of lipid metabolism, inflammation, and ER stress pathway. Sci. Rep. 11(1), 9894 (2021).
Al-Saud, N. B. S. Impact of curcumin treatment on diabetic albino rats. Saudi J. Biol. Sci. 27(2), 689–694 (2020).
Na, L. X. et al. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 57(9), 1569–1577 (2013).
Ding, L. et al. Curcumin rescues high fat diet-induced obesity and insulin sensitivity in mice through regulating SREBP pathway. Toxicol. Appl. Pharmacol. 304, 99–109 (2016).
Mahmoudi, A., et al., Effect of Curcumin on Attenuation of Liver Cirrhosis via Genes/Proteins and Pathways: A System Pharmacology Study. Nutrients. 14(20). (2022).
Saadati, S. et al. Curcumin and inflammation in non-alcoholic fatty liver disease: a randomized, placebo controlled clinical trial. BMC Gastroenterol. 19(1), 133 (2019).
Swanson, K. V., Deng, M. & Ting, J. P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19(8), 477–489 (2019).
Guariglia, M., et al., Molecular Mechanisms of Curcumin in the Pathogenesis of Metabolic Dysfunction Associated Steatotic Liver Disease. Nutrients. 15(24). (2023).
Cunha, L. D. & Zamboni, D. S. Subversion of inflammasome activation and pyroptosis by pathogenic bacteria. Front Cell Infect Microbiol. 3, 76 (2013).
Wang, X. et al. NLRP3 inflammasome inhibitor CY-09 reduces hepatic steatosis in experimental NAFLD mice. Biochem. Biophys. Res. Commun. 534, 734–739 (2021).
Wu, J. et al. Curcumin Alleviates High-fat Diet-induced Nonalcoholic Steatohepatitis via Improving Hepatic Endothelial Function with Microbial Biotransformation in Rats. J. Agric. Food Chem. 71(27), 10338–10348 (2023).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535(7610), 153–158 (2016).
Ramos, V. M., Kowaltowski, A. J. & Kakimoto, P. A. Autophagy in Hepatic Steatosis: A Structured Review. Front Cell Dev. Biol. 9, 657389 (2021).
Tran, S., W.D. Fairlie, and E.F. Lee, BECLIN1: Protein Structure, Function and Regulation. Cells. 10(6). (2021).
Willy, J. A. et al. Function of inhibitor of Bruton’s tyrosine kinase isoform α (IBTKα) in nonalcoholic steatohepatitis links autophagy and the unfolded protein response. J. Biol. Chem. 292(34), 14050–14065 (2017).
Peng, X., et al., Curcumin Attenuates on Carbon Tetrachloride-Induced Acute Liver Injury in Mice via Modulation of the Nrf2/HO-1 and TGF-β1/Smad3 Pathway. Molecules. 23(1). (2018).
Kong, D. et al. Curcumin blunts epithelial-mesenchymal transition of hepatocytes to alleviate hepatic fibrosis through regulating oxidative stress and autophagy. Redox Biol. 36, 101600 (2020).
Cao, Y., Yang, H. & Wang, B. CircRNA_001373 promotes liver fibrosis by regulating autophagy activation in hepatic stellate cells via the miR-142a-5p/Becn1 axis. Hum. Exp. Toxicol. 43, 9603271241265104 (2024).
Zhou, R. et al. A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329), 221–225 (2011).
Lin, L. et al. Autophagy, Pyroptosis, and Ferroptosis: New Regulatory Mechanisms for Atherosclerosis. Front Cell Dev. Biol. 9, 809955 (2021).
Cong, L., Bai, Y. & Guo, Z. The crosstalk among autophagy, apoptosis, and pyroptosis in cardiovascular disease. Front Cardiovasc. Med. 9, 997469 (2022).
Yuk, J.M., P. Silwal, and E.K. Jo, Inflammasome and Mitophagy Connection in Health and Disease. Int. J. Mol. Sci., 21(13). (2020).
Bansal, S. S. et al. Controlled systemic delivery by polymeric implants enhances tissue and plasma curcumin levels compared with oral administration. Eur. J. Pharm. Biopharm. 80(3), 571–577 (2012).
Yu, J. et al. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc Natl. Acad. Sci. U. S. A. 111(43), 15514–15519 (2014).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526(7575), 660–665 (2015).
Liu, W. J. et al. p62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation. Cell Mol. Biol. Lett. 21, 29 (2016).
Lau, A. et al. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol. Cell Biol. 30(13), 3275–3285 (2010).
Duran, A. et al. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol. Cell 44(1), 134–146 (2011).
Karim, M. R. et al. Cytosolic LC3 ratio as a sensitive index of macroautophagy in isolated rat hepatocytes and H4-II-E cells. Autophagy 3(6), 553–560 (2007).
Funding
This research did not receive specific grants from any public, commercial, or non-profit funding organizations.
Author information
Authors and Affiliations
Contributions
Authorship Contribution Statement: Zienab Helmy Eldken and Noha Hammad Sakr: Methodology; Formal analysis; Funding acquisition; Investigation; Resources; Software; Writing – original draft. Ola Mohammed Youssef and Rania M. Khalil: Study concept and design; Data interpretation and manuscript revision. Amira Adly Mohammed Kassa: Conceptualization; Methodology; Supervision; Data interpretation and reviewed the manuscript to be in the final format. Asmaa Ramadan: Methodology; Formal analysis; Funding. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
Approval for this study was obtained from the Institutional Animal Care and Use Committee at FPDU (Reference Number: FPDU 12/2023).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Eldken, Z.H., Youssef, O.M., Khalil, R.M. et al. Curcumin’s dual effect on pyroptosis and autophagy in metabolic- associated fatty liver disease: a potential therapeutic strategy. Sci Rep 15, 34309 (2025). https://doi.org/10.1038/s41598-025-11135-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11135-2