Abstract
Recent studies have noted an unexpected inverse correlation between body mass index (BMI) and cerebral amyloid-beta (Aβ) deposition, suggesting a potential involvement of adipose tissue in AD pathology. Given the adipose-rich nature of salivary glands, we investigated whether Aβ uptake in the salivary glands is associated with the link between BMI and cerebral Aβ deposition using 18F-Florbetaben PET/CT. We analyzed data from 187 participants, classified into Aβ−cognitively normal (Aβ−CN), Aβ+ mild cognitive impairment (Aβ+MCI), and Aβ+ AD dementia (Aβ+D) groups. BMI, standardized uptake value ratio of salivary glands (SUVRSG), and brain (SUVRB) were quantified, and their associations were examined using Pearson correlation and multiple regression analyses. Higher BMI was correlated with increased SUVRSG (r = 0.231, p = 0.0019) and decreased SUVRB (r = -0.247, p = 0.0009). SUVRSG and SUVRB were negatively correlated (r = -0.188, p = 0.0102). Multiple regression analysis identified BMI as an independent predictor of composite brain SUVR (β = -0.0168, p = 0.0046), while SUVRSG was marginally significant (β = -0.0714, p = 0.0701). Regionally, both BMI and SUVRSG were significantly associated with lower SUVRB in the striatum, precuneus, and cingulate cortex. Consistent with previous studies, BMI exhibited an inverse correlation with SUVRB. Notably, SUVRSG was negatively associated with SUVRB while demonstrating a positive correlation with BMI. Interpreting SUVRSG as a fat-related marker, these findings suggest that Aβ sequestration in adipose tissue may contribute to lower cerebral Aβ accumulation. Additional research is necessary to elucidate the underlying mechanism.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder marked by cognitive deterioration and the pathological accumulation of amyloid-beta (Aβ) plaques in the brain1. The aggregation of Aβ is a key pathological hallmark of AD, contributing to synaptic dysfunction, neuroinflammation, and neuronal degeneration, thus making it a crucial focus in understanding AD pathogenesis2.
The relationship between body mass index (BMI) and AD has been extensively investigated, revealing intriguing temporal variations across the lifespan. While midlife obesity correlates with elevated AD risk3,4 and has been associated with enhanced Aβ deposition and neurodegeneration, the late-life BMI profile presents a contrasting pattern. Specifically, reduced BMI in older adults demonstrates a significant correlation with increased cerebral Aβ burden, particularly within the 1–2 years preceding positron emission tomography assessment5. This pattern is further complicated by the fact that increased visceral adiposity in the elderly corresponds with cortical thinning—a negative marker of AD progression—most prominently in the temporal, parietal, and cingulate regions6.
These findings collectively suggest that adipose tissue and related metabolic factors may substantially influence Aβ accumulation dynamics and neurodegenerative processes, though the precise mechanistic pathways underlying these relationships remain undefined. Studies on the association of adipose tissue metabolism with AD pathology indicate that glucose metabolism measured from visceral adipose tissue was significantly associated with cerebral Aβ burden, suggesting that metabolic dysfunction of adipose tissue could contribute to AD development7. Similarly, confirming Aβ accumulation within adipose tissue is expected to be crucial in elucidating mechanistic pathways, although not yet directly established.
Salivary glands (SGs) are a distinct type of adipose-enriched tissue that may provide valuable insights into the undefined adipose tissue-Aβ accumulation mechanism. SGs have demonstrated a potential metabolic link with BMI8 and MRI-based fat quantification studies reveal that fat content in the parotid and submandibular glands increases in proportion to BMI9. Furthermore, obesity has been linked to decreased salivary flow rates, emphasizing the broader metabolic implications of SG function10. Given their adipose-rich nature and metabolic activity, SGs may serve as a surrogate indicator of adipose tissue involvement in systemic amyloid dynamics.
The degree of brain amyloid accumulation can be assessed via molecular imaging techniques using positron emission tomography (PET) with various amyloid-specific radiotracers, including 11C-Pittsburgh compound B (PiB)11, 18F-florbetapir12, 18F-florbetaben13, and 18F-flutemetamol14. Notably, salivary glands, particularly the parotid glands, are within the field of view in amyloid PET scans, allowing for their assessment without additional imaging protocols or modifications. This accessibility makes them a convenient target for evaluating amyloid uptake in adipose tissue.
This study not only quantifies brain uptake but also evaluates amyloid uptake in the salivary glands using amyloid PET imaging. Our primary objective is to determine whether BMI is associated with amyloid retention in SGs and whether this uptake is linked to brain Aβ burden. Through this examination, we aim to verify the potential of salivary gland amyloid uptake as a surrogate for adipose tissue-Aβ accumulation mechanism, thereby enhancing our understanding of how adipose tissue influences brain amyloid deposition and AD pathology.
Materials and methods
Subjects
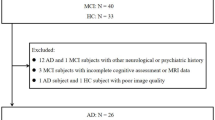
This study included participants who underwent 18F-Florbetaben PET/CT imaging at Ewha Womans University Mokdong Hospital between November 2018 and February 2023. Three diagnostic groups encompassing cognitively unimpaired individuals without amyloid deposition (Aβ−CN; NC group), individuals with mild cognitive impairment and amyloid deposition (Aβ+MCI; MCI group), and individuals with AD dementia (Aβ+D; AD group) were included in the analysis. The diagnostic criteria for MCI and AD were based on those proposed by the National Institute on Aging and Alzheimer’s Association (NIA-AA)15. Aβ status was determined based upon 18F- Florbetaben PET scan results16.
Demographic and clinical data were collected for all participants, including sex, age, years of education, weight, height, BMI, and APOE4 carrier status. BMI was calculated as weight (kg) divided by height squared (m²). Cognitive function was assessed using the Mini-Mental State Examination (MMSE) and the Clinical Dementia Rating (CDR) scale.
This study was conducted in accordance with the Declaration of Helsinki (2013 version) and approved by the Institutional Review Board (IRB) of Ewha Womans University Mokdong Hospital (EUMC IRB 2025-01-003), and the requirement for informed consent was waived by the IRB of Ewha Womans University Mokdong Hospital.
18F-Florbetaben PET/CT image acquisition
The radiotracer 18F-FBB (Neuraceq™) was produced by DuChemBio Co., Ltd. (Seoul, Korea) in compliance with the Korean Ministry of Food and Drug Safety (MFDS). After being delivered to our institutional PET center, all 18F-FBB PET/CT images were processed following our institution’s standardized protocol. PET images were acquired in three-dimensional (3D) list-mode using a specialized PET/CT scanner (Biograph mCT, Siemens) with 20-minute static scans obtained from 90 to 110 min after a bolus injection of 306.16 ± 17.85 MBq 18F-FBB. Additionally, a spiral CT scan of the brain was conducted with parameters of 120 kV, 30mAs, and a slice thickness of 1.0 mm. The resulting CT data were used for attenuation correction to the PET emission scans. The participant’s head was immobilized by head-holder and vacuum-based fixation equipment to reduce motion artifacts. The PET images were reconstructed into a 400 × 400 × 109 matrix (voxel 1.02 × 1.02 × 2.03mm3) using the integrated 3D ordered subset expectation maximization algorithm, configured with 4 iterations, 12 subsets, and a 5-mm Gaussian smoothing filter. The reconstructed data were then combined into single-frame images.
Analysis of 18F- Florbetaben PET/CT images
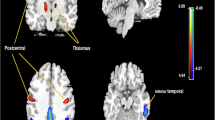
Brain standardized uptake value ratio (SUVRB) values were extracted using Neurophet SCALE PET 2.0 software (Neurophet Inc., South Korea), an artificial intelligence (AI)-based software, provides automated quantification by directly processing reconstructed DICOM (Digital Imaging and Communications in Medicine) PET images co-registered on T1w images17,18. Automatic quantitative analyses were performed using volumes of interest (VOIs) defined in each individual’s native T1w space, based on the Desikan-Killiany atlas19. For the SUVR quantification, we selected target regions, including cortical (frontal, precuneus, cingulate, lateral parietal, lateral temporal, and occipital cortices) and subcortical (striatum) regions, as well as a composite region encompassing all these target regions. The whole cerebellum was used as the reference region.
Salivary glands standardized uptake value ratio (SUVRSG) values were extracted according to the methodology described in a previous study20 with minor modifications. For each parotid gland, a sphere volume of interest (VOI) was manually delineated by the investigator, and the final VOI was determined using a 40% threshold of the maximum pixel value, which was automatically applied by MIM software (MIM Software Inc., Cleveland, OH). The mean SUV values from both glands were averaged for analysis. The relative uptake ratio (SUVRSG) was defined as the mean SUV of the salivary glands divided by the SUV of the cerebellum. The volume of each parotid gland (VolSG) was estimated using the target volume within the VOI, applying the 40% threshold.
Neuropsychological assessment
The Mini-Mental State Examination (MMSE) was performed for neuropsychological assessment. MMSE includes a set of questions that evaluate five areas of cognitive function: orientation, registration, attention and calculation, recall and language. The test has a maximum score of 30 points. A score of 24 or higher suggests normal cognitive function, while scores ranging from 19 to 23 indicate mild cognitive impairment. Scores between 10 and 18 are classified as moderate cognitive impairment, and scores below 9 reflect severe cognitive impairment.
The Clinical Dementia Rating (CDR) was also administered to assess the severity of Alzheimer’s disease. The CDR consists of semistructured interviews conducted with both the patient and an informant. During the interview, the informant rates the patient’s cognitive performance across six domains: Memory, Orientation, Judgment and Problem Solving, Community Affairs, Home and Hobbies and Personal Care. The CDR is scored on a five-point scale: 0 (none), 0.5(questionable), 1(mild), 2(moderate) and 3(severe), with higher scores indicating greater severity of cognitive impairment. CDR 0 means no dementia, while higher scores correspond to varying degrees of dementia, ranging from questionable to severe.
Statistical analysis
All statistical analyses were performed using Rex software (version 3.7.0.1, RexSoft, Seoul, Korea), with statistical significance set at p < 0.05 (two-tailed). Group differences in demographic characteristics and PET imaging features were assessed using one-way ANOVA for normally distributed variables and the Kruskal-Wallis test for non-normally distributed variables. Categorical variables were compared using chi-square tests. The relationships between BMI, SUVRB, SUVRSG, and salivary gland volume (VolSG) were analyzed using Pearson correlation coefficient. Multiple regression analysis was performed to evaluate the independent effects of BMI and SUVRSG with age as a covariate using the enter method. A Bonferroni-corrected threshold of p < 0.0071 (0.05/7) was applied for the regional SUVR analyses across seven target regions.
Results
Subjects
This study included 187 participants who underwent 18F-Florbetaben PET/CT imaging. Participants were categorized into three diagnostic groups: amyloid-negative cognitively unimpaired individuals (Aβ−CN, n = 114), amyloid-positive individuals with mild cognitive impairment (Aβ+MCI, n = 45), and amyloid-positive individuals with dementia (Aβ+D, n = 28). Demographic and clinical characteristics of each group are summarized in Table 1. The median age of participants was 72 years (interquartile range: 68–77), with no statistically significant difference among the groups (p = 0.1559). BMI was significantly different among the groups (p = 0.0292), with the Aβ−CN group having the highest median BMI (24.21, interquartile range: 22.43–27.08), whereas the Aβ+MCI (22.9, interquartile range: 21.33–24.84) and Aβ+D (23.27, interquartile range: 21.43–24.77) groups exhibited lower values. The prevalence of APOE4 carriers was notably higher in the Aβ+MCI (66.67%) and Aβ + D (71.43%) groups compared to the Aβ−CN group (11.4%) (p < 0.001). Cognitive function, assessed via MMSE and CDR scores, demonstrated significant group differences (p < 0.001), with the Aβ+D group exhibiting the lowest MMSE score (18.5, interquartile range: 15–20) and the highest CDR score (1.0, interquartile range: 1–1).
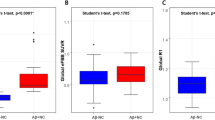
PET indices across diagnostic groups are presented in Table 2. SUVRB significantly increased across groups (p < 0.001, Fig. 1A), with the highest value observed in the Aβ+D group (1.67 ± 0.17). Regional SUVR values, including those for the frontal cortex (SUVRF), precuneus (SUVRPCu), cingulate cortex (SUVRCg), lateral parietal cortex (SUVRLP), lateral temporal cortex (SUVRLT), occipital cortex (SUVROC), and striatum (SUVRStr) also exhibited significant group differences (all p < 0.001), with higher uptake values observed in the Aβ+MCI and Aβ+D groups compared to the Aβ−CN group. In contrast, SUVRSG displayed a significantly different pattern across groups, with median values of 2.23 (1.97–2.58) in the Aβ−CN group, 2.04 (1.88–2.25) in the Aβ+MCI group, and 2.09 (1.78–2.3) in the Aβ+D group (p = 0.0049, Fig. 1B). VolSG did not show significant group differences (p = 0.6023).
Group-wise comparison between brain and salivary gland amyloid uptake. (A) The Standardized Uptake Value Ratio of the Brain (SUVRB) significantly differed among groups (p < 0.001), with the highest values observed in the Aβ+MCI and Aβ+D groups, indicating increased cerebral amyloid deposition in individuals with cognitive impairment. (B) The Standardized Uptake Value Ratio of the Salivary Glands (SUVRSG) also showed significant group differences (p = 0.0049), with the highest uptake in the Aβ−CN group. Data are presented as mean ± standard deviation, and statistical significance was determined using one-way ANOVA.
Correlation analysis
Pearson correlation analysis demonstrated significant associations between BMI, SUVRSG, and SUVRB. BMI showed a significant negative correlation with SUVRB (r = -0.247, p = 0.0009, Fig. 2A), indicating lower brain amyloid deposition in individuals with higher BMI. A significant positive correlation was found between BMI and SUVRSG (r = 0.231, p = 0.0019, Fig. 2B). SUVRSG was negatively correlated with SUVRB (r = -0.188, p = 0.0102, Fig. 2C). Additionally, BMI exhibited a strong positive correlation with VolSG (r = 0.469, p < 0.001, Fig. 2D).
Sex-stratified Pearson correlation analyses were performed. In both males and females, BMI was significantly negatively correlated with SUVRB (male: r = -0.372, p = 0.0061; female: r = -0.196, p = 0.0287) and positively correlated with VolSG (male: r = 0.533, p < 0.001; female: r = 0.458, p < 0.001). A significant positive correlation between BMI and SUVRSG was observed in females only (r = 0.285, p = 0.0013), while the correlation was not significant in males (r = 0.151, p = 0.2802). Likewise, SUVRSG was significantly negatively correlated with SUVRB in females (r = -0.249, p = 0.0046), but not in males (r = -0.066, p = 0.6192). Full statistical results are summarized in Supplementary Table S1, and scatter plots for each sex are shown in Supplementary Figure S1.
Correlations between BMI, SUVRB, SUVRSG, and VolSG. (A) Body mass index (BMI) was significantly negatively correlated with SUVRB (r = − 0.247, p = 0.0009), suggesting that individuals with higher BMI tend to have lower cerebral amyloid deposition. (B) BMI showed a significant positive correlation with SUVRSG (r = 0.231, p = 0.0019), indicating that increased peripheral adipose metabolism may be linked to higher amyloid uptake in the salivary glands. (C) SUVRSG was negatively correlated with SUVRB (r = − 0.188, p = 0.0102), supporting the hypothesis that amyloid sequestration in peripheral tissues may be associated with lower cerebral amyloid burden. (D) BMI exhibited a strong positive correlation with salivary gland volume (VolSG) (r = 0.469, p < 0.001), consistent with previous findings that salivary gland fat content increases with BMI. Pearson correlation analysis was used for statistical comparisons.
Multiple regression analysis
To assess the independent contributions of BMI and SUVRSG to brain amyloid burden with age as a covariate, multiple regression analyses were conducted for composite and regional brain SUVR values. The results are summarized in Table 3.
For composite brain SUVR, higher BMI was significantly associated with lower SUVRB (p = 0.0046), while SUVRSG showed a trend toward reduced SUVRB, though it did not reach statistical significance (p = 0.0701).
For regional SUVRs, after Bonferroni correction for multiple comparisons (adjusted threshold p < 0.0071), BMI remained significantly associated with lower SUVR in the frontal cortex (β = − 0.0181, p = 0.0037), precuneus (β = − 0.0212, p = 0.0061), cingulate cortex (β = − 0.0192, p = 0.0050), lateral temporal cortex (β = − 0.0151, p = 0.0051), and occipital cortex (β = − 0.0109, p = 0.0058). Associations in the lateral parietal cortex (β = − 0.0166, p = 0.0080) and striatum (β = − 0.0105, p = 0.0200) did not survive correction.
None of the associations between SUVRSG and regional SUVR values survived Bonferroni correction. However, nominal negative associations (p < 0.05) were observed in the frontal cortex (β = − 0.0812, p = 0.0498), precuneus (β = − 0.1300, p = 0.0120), cingulate cortex (β = − 0.1134, p = 0.0132), and striatum (β = − 0.0800, p = 0.0082). This inverse pattern did not reach the adjusted significance threshold and should be interpreted with caution.
Discussion
Higher BMI showed a significant association with reduced cerebral amyloid burden (SUVRB) while elevating salivary gland amyloid uptake (SUVRSG), revealing an inverse relationship between these two compartments. These findings align with earlier reports demonstrating that individuals with elevated BMI exhibit lower cerebral Aβ deposition across PET and CSF biomarkers21,22. Longitudinal evidence further corroborates this inverse relationship, with baseline Aβ levels predicting subsequent BMI decline23.
A striking paradox emerges from our data: while higher BMI correlates with reduced cerebral amyloid burden, obesity and visceral adiposity simultaneously increase Alzheimer’s disease risk, promote cortical thinning24 and drive neuroinflammation alongside insulin resistance25. This apparent contradiction suggests that adiposity influences AD pathogenesis through distinct, potentially competing pathways.
The peripheral sink hypothesis offers a compelling mechanistic framework for these observations26,27. Increased peripheral amyloid retention, as evidenced by elevated SUVRSG in our cohort, may actively sequester Aβ away from the brain, thereby reducing cerebral burden. Supporting this model, salivary gland fat content increases proportionally with BMI, while transgenic APP mouse models demonstrate Aβ accumulation in salivary and lacrimal glands28 establishing these peripheral tissues as functionally relevant amyloid reservoirs distinct from cerebral pathology.
Simultaneously, adipose-derived inflammatory mediators and metabolic dysfunction may accelerate neurodegeneration through amyloid-independent mechanisms29. This dual pathway model reconciles the seemingly contradictory protective effect of BMI on amyloid burden with its established role in AD risk.
Region-specific analyses revealed that BMI’s inverse association with SUVRB remained robust across frontal, precuneus, cingulate, lateral temporal, and occipital cortices after Bonferroni correction. While the SUVRB-SUVRSG association reached only nominal significance (p < 0.05) and failed to survive multiple comparison correction, this pattern nonetheless supports the peripheral retention hypothesis.
Particularly intriguing were the nominal inverse associations between SUVRSG and SUVRB within the frontal cortex, precuneus, cingulate cortex, and striatum—regions consistently implicated in both metabolic regulation and AD pathology. The precuneus serves as a critical site for early amyloid accumulation30 with visceral adiposity specifically linked to enhanced pathology in this region among middle-aged men31. The cingulate cortex mediates both cognitive function and metabolic control32,33 while the striatum bears significant impact from obesity and insulin resistance34 and demonstrates amyloid pathology in advanced AD stages35. Although these exploratory associations require cautious interpretation, they may reflect region-specific patterns in peripheral amyloid redistribution.
Sex-stratified analyses revealed that SUVRSG associations with both BMI and SUVRB achieved statistical significance exclusively in women. This pattern mirrors previous observations that women exhibit stronger microglial activation, a key component of neuroinflammation, independent of Aβ burden, particularly during early AD stages36. Additionally, visceral adiposity-related biomarkers including VAT, CRP, and PAI-1 demonstrate more pronounced associations with AD pathology in women37. However, the female-predominant sample composition (more than twice the male participants) may have influenced these sex-specific findings.
These results carry important implications for AD risk stratification and therapeutic development. The peripheral sink mechanism suggests that interventions targeting peripheral amyloid clearance might complement traditional brain-focused approaches. Furthermore, the sex-specific patterns indicate that metabolic interventions for AD prevention may require gender-tailored strategies.
The novel application of SUVRSG as a PET-based peripheral amyloid marker represents a key methodological advance. To our knowledge, this constitutes the first in vivo investigation examining salivary gland amyloid uptake in relation to cerebral Aβ burden. Integrating central and peripheral amyloid PET measures enabled a more comprehensive assessment of systemic Aβ dynamics, while region-specific SUVRB analyses provided spatially nuanced insights into adiposity’s influence on distinct cortical regions.
Nevertheless, several limitations warrant acknowledgment. The cross-sectional design precludes causal inference, necessitating longitudinal validation to establish temporal relationships. Although SUVRSG served as a proxy for peripheral amyloid retention, its specificity for adipose-related Aβ accumulation awaits histopathological confirmation. The female-predominant sample may have skewed interpretations beyond sex-stratified analyses. Finally, incomplete control for potential confounders including comorbidities, medications, and lifestyle factors may have introduced residual bias, which is inherent to our retrospective study design.
This investigation underscores the critical role of peripheral compartments in amyloid distribution and highlights metabolic factors’ complex influence on AD pathology. By incorporating SUVRSG into our analytical framework, we provide mechanistic insights into how adiposity may modulate brain Aβ burden through peripheral sequestration. Key questions remain for advancing our understanding of adiposity’s role in dementia. It is unclear whether this peripheral redistribution mechanism applies to other Aβ-associated conditions such as cerebral amyloid angiopathy or Down syndrome–related dementia, but doing so could uncover generalizable protective mechanisms across the Aβ spectrum. Integrating adiposity indices with accessible fluid biomarkers, including emerging plasma Aβ42/40 ratios and salivary amyloid measures, may help validate PET findings in more routine clinical contexts. Longitudinal studies that monitor peripheral and cerebral amyloid dynamics in parallel will be essential to confirm the peripheral sink hypothesis and explore its therapeutic potential.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
29 August 2025
The original online version of this Article was revised: In the original version of this Article the legends of Table 1 and 2 contained an error. In the legend of Table 1, “BMI body mass index, MMSE mini-mental state examination, CDR clinical dementia rating, Aβ−CN amyloid-negative cognitively unimpaired, Aβ+MCI amyloid-positive mild cognitive impairment, AD MCI, Aβ+D amyloid-positive dementia, ADD.” It now reads: “BMI body mass index, MMSE mini-mental state examination, CDR clinical dementia rating, Aβ−CN amyloid-negative cognitively unimpaired, Aβ+MCI amyloid-positive mild cognitive impairment, Aβ+D amyloid-positive dementia..” In the legend of Table 2,“…Aβ+MCI amyloid-positive mild cognitive impairment, AD MCI, Aβ+D amyloid-positive dementia, ADD.” It now reads: “Aβ+MCI amyloid-positive mild cognitive impairment, Aβ+D amyloid-positive dementia.” The original Article has been corrected.
References
Dubois, B. et al. Alzheimer disease as a clinical-biological construct—an international working group recommendation. JAMA Neurol. 81, 1304–1311 (2024).
Jack, C. R. Jr et al. Revised criteria for diagnosis and staging of alzheimer’s disease: alzheimer’s association workgroup. Alzheimer’s Dement. 20, 5143–5169 (2024).
Chuang, Y. F. et al. Correction: Midlife adiposity predicts earlier onset of alzheimer’s dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol. Psychiatry. 28, 4486–4486. https://doi.org/10.1038/s41380-023-02210-z (2023).
Gottesman, R. F. et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 317, 1443–1450. https://doi.org/10.1001/jama.2017.3090 (2017).
Lane, C. A. et al. Investigating the relationship between BMI across adulthood and late life brain pathologies. Alzheimers Res. Ther. 13, 91. https://doi.org/10.1186/s13195-021-00830-7 (2021).
Cho, J., Seo, S., Kim, W. R., Kim, C. & Noh, Y. Association between visceral fat and brain cortical thickness in the elderly: a neuroimaging study. Front. Aging Neurosci. 13, 694629 (2021).
Kim, S., Yi, H. A., Won, K. S., Lee, J. S. & Kim, H. W. Association between visceral adipose tissue metabolism and alzheimer’s disease pathology. Metabolites 12, 258 (2022).
Matczuk, J. et al. Insulin resistance and obesity affect lipid profile in the salivary glands. J. Diabetes Res. 2016 (8163474). https://doi.org/10.1155/2016/8163474 (2016).
Su, G. Y. et al. Effect of laterality, gender, age and body mass index on the fat fraction of salivary glands in healthy volunteers: assessed using iterative decomposition of water and fat with echo asymmetry and least-squares Estimation method. Dentomaxillofacial Radiol. 48 https://doi.org/10.1259/dmfr.20180263 (2018).
Hatipoglu, O., Maras, E., Hatipoglu, F. P. & Saygin, A. G. Salivary flow rate, pH, and buffer capacity in the individuals with obesity and overweight; A meta-analysis. Niger J. Clin. Pract. 25, 1126–1142. https://doi.org/10.4103/njcp.njcp_1760_21 (2022).
Yamin, G. & Teplow, D. B. Pittsburgh Compound-B (PiB) binds amyloid β‐protein protofibrils. J. Neurochem. 140, 210–215 (2017).
Johnson, K. A. et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in alzheimer’s disease dementia, mild cognitive impairment, and normal aging. Alzheimer’s Dement. 9, S72–S83 (2013).
Rowe, C. C. et al. Imaging of amyloid β in alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 7, 129–135 (2008).
Thal, D. R. et al. [18F] flutemetamol amyloid positron emission tomography in preclinical and symptomatic alzheimer’s disease: specific detection of advanced phases of amyloid-β pathology. Alzheimer’s Dement. 11, 975–985 (2015).
Jack, C. R. Jr et al. NIA-AA research framework: toward a biological definition of alzheimer’s disease. Alzheimer’s Dement. 14, 535–562 (2018).
Yoon, H. J. et al. Dual-phase 18F-florbetaben PET provides cerebral perfusion proxy along with beta-amyloid burden in alzheimer’s disease. NeuroImage: Clin. 31, 102773 (2021).
Lee, J. et al. Development of amyloid PET analysis pipeline using deep learning-based brain MRI segmentation—a comparative validation study. Diagnostics 12, 623 (2022).
Kim, S. et al. Development of a prediction model for cognitive impairment of sarcopenia using multimodal neuroimaging in non-demented older adults. Alzheimer’s Dement. 20, 4868–4878 (2024).
Lee, M. et al. Split-attention U-Net: a fully convolutional network for robust multi-label segmentation from brain MRI. Brain Sci. 10, 974 (2020).
Kang, S. Y. et al. Salivary gland uptake on 18F-FP-CIT PET as a new biomarker in patients with parkinsonism. Korean J. Radiol. 24, 690 (2023).
Vidoni, E., Townley, R., Honea, R., Burns, J. & Initiative, A. s. D. N. Alzheimer disease biomarkers are associated with body mass index. Neurology 77, 1913–1920 (2011).
Thirunavu, V. et al. Higher body mass index is associated with lower cortical amyloid-β burden in cognitively normal individuals in late-life. J. Alzheimers Dis. 69, 817–827 (2019).
Rabin, J. S. et al. Amyloid-beta burden predicts prospective decline in body mass index in clinically normal adults. Neurobiol. Aging. 93, 124–130 (2020).
Huang, X., Wang, Y. J. & Xiang, Y. Bidirectional communication between brain and visceral white adipose tissue: its potential impact on Alzheimer’s disease. EBioMedicine 84 (2022).
Herrero-Labrador, R. et al. Circulating insulin-like growth factor I is involved in the effect of high fat diet on peripheral amyloid β clearance. Int. J. Mol. Sci. 21, 9675 (2020).
Deane, R. et al. LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron 43, 333–344 (2004).
Deane, R., Sagare, A. & Zlokovic, B. The role of the cell surface LRP and soluble LRP in blood-brain barrier Aβ clearance in alzheimer’s disease. Curr. Pharm. Design. 14, 1601–1605 (2008).
Fukuchi, K. I., Li, L., Hart, M. & Lindsey, J. R. Accumulation of amyloid-β protein in exocrine glands of Transgenic mice overexpressing a carboxyl terminal portion of amyloid protein precursor. Int. J. Exp. Pathol. 81, 231–239 (2000).
Kern, P. A., Ranganathan, S., Li, C., Wood, L. & Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. physiology-endocrinology Metabolism. 280, E745–E751 (2001).
Cui, L. et al. Association of precuneus Aβ burden with default mode network function. Alzheimer’s & Dementia (2025).
Dolatshahi, M. et al. Alzheimer disease pathology and neurodegeneration in midlife obesity: a pilot study. Aging Disease. 15, 1843 (2024).
Bailly, M. et al. Precuneus and Cingulate Cortex Atrophy and Hypometabolism in Patients with Alzheimer’s Disease and Mild Cognitive Impairment: MRI and (18)F-FDG PET Quantitative Analysis Using FreeSurfer. Biomed Res Int 583931, (2015). https://doi.org/10.1155/2015/583931 (2015).
Yang, Y. et al. Perigenual anterior cingulate cortex and its structural covariance as predictors for future body fat gain in young adults. Obesity 31, 446–453 (2023).
Ter Horst, K. W. et al. Striatal dopamine regulates systemic glucose metabolism in humans and mice. Sci. Transl. Med. 10, eaar3752 (2018).
Beach, T. G. et al. Striatal amyloid plaque density predicts Braak neurofibrillary stage and clinicopathological alzheimer’s disease: implications for amyloid imaging. J. Alzheimers Dis. 28, 869–876. https://doi.org/10.3233/JAD-2011-111340 (2012).
Biechele, G. et al. Associations between sex, body mass index and the individual microglial response in alzheimer’s disease. J. Neuroinflamm. 21, 30 (2024).
Ramirez, M. F. et al. Sex differences in protein biomarkers and measures of fat distribution. J. Am. Heart Association. 13, e000223 (2024).
Acknowledgements
This research was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (RS-2024-00453521 and NRF-2020R1A5A2019210) and by a grant of the Korea Health Technology (RS-2025-02214254).
Author information
Authors and Affiliations
Contributions
K.R.K, K.M.K: Conception and design of study, acquisition of data, data curation, formal analysis. H.J.Y, J.Y.K: Conception and design of study, data curation, formal analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, K.R., Kim, K., Kim, JY. et al. Potential utility of salivary gland amyloid uptake as a surrogate marker for adipose tissue and amyloid-beta dynamics based on its association with BMI and cerebral amyloid burden. Sci Rep 15, 25513 (2025). https://doi.org/10.1038/s41598-025-11731-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-11731-2