Abstract
Colorectal cancer (CRC) is both a leading cause of cancer-related mortality and one of the most frequently diagnosed cancers. Previous studies have shown that zearalenone and theabrownin each exert anti-CRC effects. Here, we aimed to evaluate the anti-tumor properties of theabrownin and zearalenone mixture (TZ) and to assess whether supplementing TZ with 5-FU, a commonly used chemotherapeutic drug, could further suppress CRC tumorigenesis. Our results revealed that TZ significantly attenuated AOM/DSS-induced colorectal tumorigenesis. TZ improved survival rate, reduced tumor count, preserved colon length, and mitigated colonic inflammation in AOM/DSS mice. In addition, the concentration of pro-inflammatory cytokines IL-6, TNF-α and IL-17 A/F and proliferative PI3K/AKT were significantly reduced. Metagenomic analyses revealed that TZ modulated the gut microbiota and mycobiota composition and increased the fecal acetate and propionate levels. Furthermore, the enrichment of the bacterial Desulfovibrionaceae bacterium LT0009, Helicobacter sp. MIT 03-1616 and fungal Xylariaceae sp. FL0594 was associated with the reduction of tumor multiplicity and pro-inflammatory cytokines. No additional benefits were observed with combining TZ with 5-FU. Taken together, TZ presented remarkable inhibitory effects on colorectal tumorigenesis, indicating its potential as a novel therapeutic candidate for CRC.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) ranks as the second most prevalent c. cause of mortality among cancer-related deaths1. Colitis-associated colorectal cancer (CAC), a sub-type of CRC, accounts for approximately 2% of CRC cases and 10–15% of patients with inflammatory bowel disease. Colitis is a chronic inflammation condition that has an increasing prevalence, impacting over five million worldwide2. The persistent inflammation from colitis increases the risk of progression to CRC by promoting cancer-favorable conditions3. 5-Fluorouracil (5-FU) is a chemotherapeutic agent commonly used for CRC, impeding DNA replication and repair processes4,5. However, 5-FU administration is associated with toxicities and adverse side effects, including fever, fatigue, mucositis, stomatitis, nausea, vomiting, and diarrhea4,6,7. 5-Fu exacerbates the colitis symptoms and disrupts the gut microbiome, potentially further complicating the outcome and quality of life for patients with CAC8. The imbalance of gut microbiota would lead to chronic inflammation, disruption of host immunity and accumulation of virulence metabolites or toxins, fostering a diseases environment which promotes both the initiation and progression of CRC9,10.
Theabrownin (TB) and zearalenone (ZEA) have previously demonstrated anti-CRC properties11,12. TB is a fungal fermentation product derived from tea polyphenols extracted from Pu-erh tea13,14. During fermentation process, fungi species Aspergillus tubingensis, Aspergillus marvanovae, and Aspergillus fumigatus convert tea polyphenols, theaflavin and thearubigin, into TB. Previous studies demonstrated that TB attenuated CRC in the AOM/DSS model by suppressing the PI3K/Akt/mTOR pathway and increasing the abundance of SCFA-producing Prevotella and Alloprevotella11. Similarly, ZEA is a secondary metabolite from fungal species Fusarium and Gibberella15,16. They have previously been shown to suppress CRC in both in vivo and in vitro studies by regulating the Ras/Raf/ERK1/2 pathway and enriching SCFA-producing bacteria12,17,18,19.
Given their distinct mechanisms of action, it was hypothesized that TB and ZEA as a mixture could exert a synergistic effect against CAC through targeting oncogenic pathways, promoting gut microbiota composition balance. Furthermore, the anti-inflammatory effect and gut microbiota modulatory effect of TB and ZEA might counteract the adverse effects of 5-Fu. Subsequently, the study aimed to determine if the alone, mixture or in combination with 5-FU could inhibit AOM/DSS-induced colorectal carcinogenesis more effectively. Additionally, the study aimed to elucidate how TB and ZEA mixture affects CRC progression and the gut microbiome of AOM/DSS mice.
Methods
Chemicals and antibodies
Azoxymethane (AOM) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Dextran sodium sulphate (DSS) was acquired from TdB Labs (Uppsala, Sweden). TB (> 99.3% purity) was bought from PURENZHIZAO (Yunnan, China). ZEA was attained from Sigma-Aldrich (St. Louis, MO, USA). The primary antibodies were purchased from Abcam (Cambridge, UK) and CST (Massachusetts, USA). The secondary antibodies were bought from Biorad (Hercules, CA, USA).
Animals and experimental setup
Male BALB/c mice at 6 weeks old were obtained from Centre for Comparative Medicine Research (CCMR, HKU). Upon receipt, the animals were housed for one week of acclimation in a 12 h light/dark cycle with normal chow diet and drinking water given ad libitum. The mice were randomly allocated into (1) Healthy control (H), (2) AOM/DSS control (AOM/DSS), (3) 5-FU (F), (4) TZ and (5) TZ + 5-FU. Afterwards, the mice underwent one week of voluntary gel administration training, in which the chow diet was withdrawn overnight, and 0.20 g of MediGel® Sucralose gel (Maine, USA) was provided the next day in individual cages. Once the whole gel was consumed, mice were returned to their house cages with a restored chow diet. The process repeated for two more days without chow diet restriction. The AOM/DSS model was then performed as previously described20. At the beginning of the experiment, mice were injected intraperitoneally with AOM (10 mg/kg) except for the healthy control. After one week, drinking water was replaced with DSS (2.5%) for one week, followed by a week of regular drinking water for three weeks. Regular drinking water was given as control. At week 5, treatments of 5-FU, TZ and TZ + 5-FU began. For 5-Fu and TZ + 5-FU groups, 5-FU (35 mg/kg) was injected intraperitoneally every week21. PBS was injected to other groups as a control. For TZ and TZ + 5-FU, TB (225 mg/kg bw) and ZEA (10 mg/kg bw) were infused into MediGel® Sucralose gel (Maine, USA) and provided to each mouse at 0.20 g every other day until the end of the experiment. Dosage of TB and ZEA were determined from our previous studies11,12. MediGel® Sucralose gel without modification was given to other groups as control (Fig. 1). At week 12, all animals were euthanized via intraperitoneal injection of sodium pentobarbital (150 mg/kg). All animals were confirmed to be deeply anaesthetized and unresponsive, with the absence of reflexes, prior to tissue collection. Animals that reached humane endpoints requiring early euthanasia, as determined by animal facility veterinarians, were also humanely euthanized using the same method and dosage. Colon was harvested and cut open longitudinally to determine the tumor count and colon length. Colon samples were collected. One part of the colon was fixed in 10% formalin for histology analysis and the other was frozen at −80 °C for biochemical analysis. Fecal samples were obtained and frozen at –80 °C on the day of experimental endpoint. Liver samples were weighed as well. All animal experiments were conducted in accordance with institutional guidelines and approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong (CULATR No. 5584-20), and this study is reported in accordance with the ARRIVE guidelines.
Colon histological analysis
One part of the colon samples was fixed in formalin, embedded as paraffin blocks and sectioned on slides. After deparaffinization, Haematoxylin and eosin (H&E) staining was performed according to manufacturer’s manual (BASO, Wuhan, China). Similarly, according to manufacturer’s manual (Abcam, Cambridge, UK), Sirius red staining was carried out and the percentage the positively red-stained area over the entire area of specimens was calculated to quantify the degree of colonic collagen fibrosis.
Immunohistochemistry (IHC) staining
IHC staining was performed utilizing the heat-induced antigen retrieval method with sodium citrate (pH6) or Tris-EDTA (pH9). The inhibition of endogenous peroxidase activity was performed with 3% H2O2. CAS-block reagent (Invitrogen, Waltham, MA, USA) blocking was carried out for 1 h before the incubation with primary antibodies. Specimens were incubated with primary antibodies (1:100) at 4 °C overnight and with secondary antibodies (1:250) at room temperature for 1 h, followed by DAB (Abcam, Cambridge, UK) chromogen reaction and haematoxylin counterstaining. The percentage of positively stained areas was determined with ImageJ software (NIH, USA). By multiplying the percentage and the intensity of staining graded from non-stained, 0; weakly stained, 1; moderately stained, 2; and, strongly stained, 3, the histoscore was obtained22.
Cytokines ELISA analysis
Colonic protein extraction was performed with homogenization in RIPA buffer with protease and phosphatase inhibitor (Sigma-Aldrich, St. Louis, MO, USA). extracted protein was collected from centrifugation. The total protein content was measured using DC protein assay (Biorad, CA, USA). ELISA analysis of IL-6, TNF-α and IL-17 A/F were performed using Mouse ELISA MAX™ Set (BioLegend, CA, USA) according to the instructions of manufacturer. The absorbance was measured using SpectraMax iD3 microplate readers (Molecular devices, CA, USA).
Western blot analysis
Colonic protein was diluted and electrophorized in 10% SDS-PAGE gel. After being transferred to polyvinylidene fluoride (PVDF) membrane, it was blocked with either 5% non-fat milk or BSA. For primary antibodies incubation, the membrane was exposed under anti-PI3K (1:1000), anti-p-PI3K (1:1000), anti-Akt1/2/3 (1:1000), anti-p-AKT1/2/3 (1:1000), anti-cyclin D1 (1:10,000) (Abcam, Cambridge, UK) at 4 °C overnight. On the next day, goat anti-rabbit IgG (H + L) HRP conjugate or goat anti-mouse IgG (H + L) HRP conjugate (1:4000, Biorad, CA, USA) were used for secondary antibody exposure for 1 h at room temperature. Protein bands visualization was completed with enhanced chemiluminescence reagents (Biorad, CA, USA) under the ChemiDox XRS + imaging system (Biorad, CA, USA).
Metagenomics
Fecal DNA were collected at the end of the experiment. Using the QIAamp® PowerFecal® Pro DNA kit (Qiagen, Hilden, Germany), fecal microbial DNA was extracted according to manufacturer’s instructions. Extracted DNA was sent to BGI Genomics (Shenzhen, China) for whole genome shotgun sequencing. Upon receipt of clean data, low-quality and artificial adapter reads were filtered out by SOAPnuke23. Bowtie2 was utilized to exclude the mouse contamination (GRCm39) and phix genomes by mapping to their genomes. Taxonomic profiles were obtained using Kaiju with parameter “−e 5” and the latest release (2023-05-10) of NCBI nr database including archaea, bacteria, viruses, fungi, and microbial eukaryotes24. Taxa either presented in less than 10% samples or with a relative abundance of less than 0.01% were removed from the downstream analysis. After aggregating the raw abundance table into species-level counts per million (CPM) table by R package phyloseq alpha and beta diversity were calculated through R package vegan25,26. To test the overall composition difference between groups, dunn test and PERMANOVA (via adonis2 from the R package vegan) were carried out for alpha diversity and beta diversity respectively. ANCOM-BC was used to compare the abundance of microorganisms between AOM/DSS with and without treatment27. Linear discriminant analysis (LDA) was also used to lessen the bias present in the various differential methods28,29. Only the microorganisms with fold change ≥ 2 or ≤ 1/2, LDA score (1og10 transformed) ≥ 2 and FDR ≤ 0.05 were considered differentially abundant. HUMAnN3(v3.7) was applied to concatenated paired-end read files for gene family and metabolic Metacyc pathway abundance estimate30. By using the HUMAnN3 regroup table function, Kyoto Encyclopedia of Genes and Genomes (KEGG)31,32 ontology terms (KO), KEGG module and Metacyc pathway profiles were obtained. To test differential abundances (DA) of KO and metabolic pathway abundance, ANCOM-BC was applied to the raw profiles27. DA KO and DA pathway were estimated at FDR value ≤ 0.1.
Short chain fatty acids (SCFAs) analysis on fecal content
According to previous studies, fecal SCFAs content was measured with gas chromatography-mass spectrometry (GC–MS)33,34. Fecal samples homogenization was conducted in 0.005 M sodium hydroxide with internal standard (10 µg/mL acetic acid-d4). By centrifugation at 13,200 × g at 4 °C for 20 min, the supernatant was collected and mixed with 0.5 mL of 1-propanol/pyridine (3:2, v/v) and 0.1 mL of propyl chloroformate for 1 min under 60 °C incubation in order to derivatize the SCFAs. Followed by the addition of 0.5mL hexane, vortexing and centrifuging at 2000 × g for 5 min. The top layer was collected for GC–MS analysis (Agilent 6890 N-5973 GC–MS, USA) based on the previous studies33. The concentration of SCFAs was attained from the calibration curves constructed using the response ratios of acetic acid, propionic acid and butyric acids against acetic acid-d4.
Statistical analysis
GraphPad Prism 10.2.2 (GraphPad software, San Diego, CA, USA) was used to perform statistical analysis, and the data in this study were presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test or the Kruskal–Wallis test followed by Dunn’s multiple comparisons test was employed. The correlation between two variables was calculated from the Spearman correlation coefficient. For multiple-testing corrections, a false discovery rate (FDR) (Benjamini–Hochberg) was used to adjust the p value. A p value < 0.05 was recognized as having statistical significance.
Results
Supplementation of TZ attenuated AOM/DSS-derived colorectal tumorigenesis and improved survival rate over 5-FU
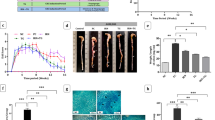
TZ and TZ + 5FU supplementation significantly improved survival outcomes compared to the AOM/DSS group (p < 0.05) (Fig. 2A). All treatment groups, including 5-FU, TZ and TZ + 5-FU, significantly reduced total tumor count (p < 0.05) (Fig. 2B, D). In addition, TZ supplementation significantly reduced the amount of the total adenoma count compared to AOM/DSS group (Fig. 3E). Colon shortening, a hallmark of colitis, was recovered in TZ group (p < 0.05) (Fig. 2C). Such a finding is consistent with the disease activity index, in which we found that the TZ group significantly decreased when compared to the AOM/DSS model (Fig. S1). Furthermore, AOM/DSS-induced enlarged caecum weight was reduced in both TZ and TZ + 5-FU groups (p < 0.05) (Fig. 2E). Compared to 5-Fu, which significantly reduced liver weight when compared to the healthy group, supplementation of TZ with 5-FU has preserved liver weight (Fig. 2F).
Histopathological analysis demonstrated significant reductions in colonic crypt depth and hyperplasia score (p < 0.05) in the TZ and TZ + 5-FU groups and inflammation score in the TZ group (p < 0.05) relative to the AOM/DSS group (Fig. 3A–E). The suppression in inflammation was supported by colonic inflammatory cytokine levels. TZ and TZ + 5-FU suppressed colonic IL-6 and IL-17 A/F concentration (p < 0.05) when compared to the AOM/DSS group. All treatment groups significantly reduced the concentration of TNF-α (p < 0.05) (Fig. 2G). In terms of collagen fibrosis, TZ supplementation significantly decreased the percentage of Sirius Red-positive staining, indicating significantly lower levels of fibrotic tissue in the colon compared to the AOM/DSS group (p < 0.05) (Fig. 3F–G).
TZ attenuated colorectal tumorigenesis, suppressed colonic inflammatory cytokines, and improved the survival rate of AOM/DSS mice over 5-FU. (A) Survival rate percentage. (B) Representative macroscopic pictures of dissected colon. (C) Total tumor counts. (D) Colon length. (E) Caecum weight. (F) Liver weight. (G) Colonic concentration of IL-6, IL-17 A/F and TNF-α. n = 6–10. Data are presented as mean ± SD (n = 6–10 per group). Comparisons were performed using one-way ANOVA followed by Tukey’s multiple comparisons test. No “*” indicates p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
TZ ameliorated colorectal AOM/DSS-induced histopathology. (A) Representative microscopic pictures of colonic sections under H&E staining. (B) Colonic crypt depth. (C) Hyperplasia score. (D) Inflammation score. (E) Adenoma counts categorized by microadenoma, low-grade macroadenoma and high-grade macroadenoma. (F) Representative microscopic pictures of colonic sections under Sirius red staining. (G) Sirius red positive percentage. Data are presented as mean ± SD (n = 6–10 per group). Comparisons were performed using one-way ANOVA followed by Tukey’s multiple comparisons test. No “*” indicates p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
TZ promoted apoptosis through suppressing proliferative PI3K/Akt pathway
IHC staining was performed to assess the proliferative and apoptotic status. All treatment groups, TZ, 5-FU and TZ + 5-FU, all promoted the H-score of the apoptotic marker, c-casp3 (p < 0.05), while only TZ significantly reduced the proliferative marker, Ki-67 (p < 0.05) when compared to AOM/DSS group (Fig. 4A–C). Western blotting analysis showed that TZ suppressed the phosphorylation of PI3K and Akt (p < 0.05), as well as their downstream transcription factor cyclin D1 (p < 0.05) (Fig. 4D–G). Meanwhile, TZ + 5-FU only reduced the phosphorylation of Akt and the level of cyclin D1 (p < 0.05) (Fig. 4D–G). These results indicated the inhibitory effects of TZ on the PI3k/Akt pathway.
TZ promoted apoptosis by regulating the PI3K/Akt signaling pathway. (A) Representative microscopic pictures of colonic sections under IHC staining in 400x magnification. Immunohistochemistry analysis H-score of (B) Ki67, and (C) c-casp3. (D) Representative immunoblots of p-PI3K/PI3K; p-Akt1/2/3/Akt1/2/3 and cyclin D1. Quantitative analysis of the relative expression of (E) p-PI3K/PI3K, (F) p-Akt1/2/3/Akt1/2/3, and (G) cyclin D1. Data are presented as mean ± SD (n = 6–10 per group). No “*” indicates p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
TZ-induced changes in microbiota composition and SCFA levels
Short-chain fatty acids (SCFAs), gut microbial metabolites, have been shown to exert a protective effect against carcinogenesis. In this study, supplementation of TZ and TZ + 5-FU significantly elevated fecal acetate and propionate levels (p < 0.05) when compared to AOM/DSS group (Fig. 5A), suggesting a partial restoration of SCFA levels that were otherwise depleted under tumorigenic and inflammatory conditions. Acetate (r = −0.63, p = 0.00006) and propionate (r = −0.37, p = 0.027) levels were negatively correlated with tumor count, indicating that higher faecal acetate and propionate concentrations were associated with reduced tumor burden (Fig. S3A, B).
Given that SCFAs are primarily produced by gut microbiota, we then further investigated the effect of TZ on gut microbiota composition. Notably, the TZ + 5FU group showed a significantly higher observed species number when compared to the AOM/DSS group. However, no significant changes were observed in community diversity (Shannon index) (Fig. 5B). Moreover, Principal coordinates analysis (PCoA) based on PERMANOVA indicated that the microbiota structure differed among groups (R2 = 0.1567, P = 0.032) (Fig. 5C). We next examined the relative abundances of bacterial species across groups. TZ supplementation significantly enriched Helicobacter ganmani, Desulfovibrionaceae bacterium LT0009, Helicobacter sp. MIT 03-1616, and Candidatus Borkfalkia ceftriaxoniphila compared to the AOM/DSS group (all p < 0.05). TZ also suppressed Roseburia sp. CAG:303, Clostridium sp. MD294, Clostridium sp. CAG:510, and Mucispirillum schaedleri (p < 0.05) (Fig. 5D). These results suggest that TZ selectively targeted specific taxa rather than inducing significant shifts in overall community diversity. Notably, in the TZ + 5-Fu group, Lactobacillus johnsonii and Desulfovibrionaceae bacterium LT0009 were further enriched compared to the AOM/DSS group.
We further assessed the association of these bacterial changes with clinical markers, such as tumor count, cytokines, and SCFAs levels. Desulfovibrionaceae bacterium LT0009 negatively correlated with total tumor count and IL-6 levels (p < 0.05). Another TZ-enriched taxon, Helicobacter sp. MIT 03-1616 showed inverse correlations with total tumor count, IL-6 and TNF-α (p < 0.05), while positively correlating with all three major SCFAs, namely acetate, propionate and butyrate, and the colon length (p < 0.05). Similarly, Candidatus Borkfalkia ceftriaxoniphila positively correlated with propionate and butyrate (p < 0.05) (Fig. 5D). Overall, our findings the role of TZ in selectively modulating gut microbial profiles and restoring SCFA levels, which may contribute to reduced CRC tumorigenesis.
Furthermore, Metacyc pathway analysis was performed to predict the biosynthesis and metabolic functions by TZ. TZ supplementation upregulated PWY-7388: octanoyl-[acyl-carrier protein] biosynthesis (mitochondria, yeast) and PWY-7013: (S)-propane-1,2-diol degradation pathways, which are associated with the biosynthesis of anti-tumor lipoic acid and propionate, respectively (p < 0.05) (Fig. S2A). PWY-7013 was also positively correlated to the relative abundance of Desulfovibrionaceae bacterium LT0009 (p < 0.05), a bacterium significantly enriched by TZ (Fig. S2B).
TZ supplementation modulates short-chain fatty acid levels and gut microbiota composition. (A) Fecal short-chain fatty acid (SCFA) concentrations (acetate, propionate, butyrate). (B) Alpha diversity metrics. Observed species richness (left) and Shannon diversity index (right). (C) Principal coordinates analysis (PCoA) based on a Bray–Curtis dissimilarity matrix. (D) Heatmap of differentially abundant bacterial taxa (left) and their correlations (right) with endpoint parameters. Differential abundance based on ANCOM-BC score (FDR < 0.1, p < 0.05, Fold change > 2 or < 1/2, LDA score (log10) > 2) were labelled with “*”. Color intensity is proportional to log2 transformed fold change (Treatment/Control), while size is proportional to the value of -log10 transformed adjusted p (FDR-corrected). Data are presented as mean ± SD (n = 6–10 per group). No “*” indicates p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
TZ induced gut microbiota and mycobiota modulation promoted SCFA concentrations and anti-cancer metabolic functions
Fungi have been shown to influence tumour development in colon cancer through modulating the immune system and producing fungi-derived factors35. The result of the alpha diversity showed that there is an increase in observed species in TZ + 5-Fu group compared to AOM/DSS group, indicating an increase in microbial richness. However, no significant changes in the Shannon index were observed across groups (Fig. 6B). The PCoA combined with PERMANOVA (R2 = 0.1598, p = 0.0021) showed significant differences in overall fungal composition among the treatment groups (Fig. 6C).
In terms of fungal species, 24 species showed at least a 2-fold decrease in abundance while 11 species were increased. Notably, TZ administration significantly reduced Paramyrothecium foliicola, Hyaloscypha hepaticicola, Grosmannia clavigera, Hypoxylon sp. FL1150, [Candida] arabinofermentans, Actinomortierella wolfii, Monilinia vaccinii-corymbosi, Dentiscutata erythropus, Candolleomyces eurysporus, Roridomyces roridus, Batrachochytrium salamandrivorans, Geranomyces variabilis, Dimargaris verticillate, Penicillium egyptiacum, Batrachochytrium dendrobatidis, Piromyces sp. E2, Neofusicoccum parvum, Rozella allomycis, Morchella snyderi, Claviceps sorghi, Geosiphon pyriformis, Stachybotrys elegans, Coelomomyces lativittatus, and Mortierella sp. GBA39 (p < 0.05). Besides, TZ enriched Lachancea thermotolerans, Actinomortierella ambigua, Gamsiella multidivaricata, Podila humilis, Glomus cerebriforme, Xylariaceae sp. FL0594, Xylariomycetidae sp. FL0641, Pecoramyces sp. F1, Lactifluus volemus, Chytridium Lagenaria, and Aplosporella prunicola (p < 0.05) (Fig. 6D).
Spearman’s correlation with clinical markers, such as tumor count and cytokines, suggested that TZ-depleted Batrachochytrium salamandrivorans, Batrachochytrium dendrobatidis, Neofusicoccum parvum, Claviceps sorghi, and Mortierella sp. GBA39 are positively correlated to total tumor counts (p < 0.05). Coelomomyces lativittatus, and Mortierella sp. GBA39 were positively correlated to the colon shortening (p < 0.05).
Notably, Batrachochytrium dendrobatidis were positively correlated to all three detected pro-inflammatory cytokines IL-6, IL-17 A/F, and TNF-α (p < 0.05). Noticeably, Batrachochytrium salamandrivorans was correlated to total tumor count, and all three pro-inflammatory cytokines being measured (p < 0.05), while inversely correlated to colon length (p < 0.05). On the contrary, TZ enriched Xylariaceae sp. FL0594 were inversely correlated with total tumor count, and IL-6 level (p < 0.05) (Fig. 6D).
To clarify the interkingdom interactions and co-occurrence pattern of TZ-modulated gut microbiota and mycobiota, we constructed a co-abundance network of TZ-modulated bacterial and fungal taxa. TZ-enriched bacterial species, Desulfovibrionaceae bacterium LT0009, and Helicobacter sp. MIT 03-1616, were each negatively correlated to the tumor counts, while positively correlated (p < 0.05) with Xylariaceae sp. FL0594, a fungus inversely correlated to tumor counts. In contrast, TZ-depleted bacteria also appeared to favor the growth of fungal species that showed a positive correlation with CRC tumor count. Clostridium sp. MD294 was positively correlated to Claviceps sorghi, which positively correlated tumor counts (p < 0.05), while Roseburia sp. CAG:303 was negatively correlated to the beneficial Xylariaceae sp. FL0594 (p < 0.01). Such findings highlight the role of bacterial-fungal interactions in mitigating tumor burden (Fig. 6A).
TZ modulated gut mycobiota composition, induced bacterial–fungal Community Shifts. (A) Co-abundance network of microbial species in the TZ-treated group relative to the AOM/DSS group. Red nodes indicate taxa enriched by TZ, whereas blue nodes represent taxa depleted under TZ treatment. Edges correspond to significant Spearman correlations (p < 0.05): red edges denote positive correlations, and green edges signify negative correlations. (B) Alpha diversity metrics. Observed species richness (left) and Shannon diversity index (right). (C) Principal coordinates analysis (PCoA) based on a Bray–Curtis dissimilarity matrix. (D) Heatmap of differentially abundant bacterial taxa (left) and their correlations (right) with endpoint parameters. Differential abundance based on ANCOM-BC score (FDR < 0.1, p < 0.05, Fold change > 2 or < 1/2, LDA score (log10) > 2) were labelled with “*”. Color intensity is proportional to log2 transformed fold change (Treatment/Control), while size is proportional to the value of -log10 transformed adjusted p (FDR-corrected). Data are presented as mean ± SD (n = 6–10 per group). Comparisons were performed using one-way ANOVA followed by Tukey’s multiple comparisons test. No “*” indicates p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Discussion
Our study demonstrated that TB, in combination with ZEA, effectively attenuates colitis-associated colon cancer by significantly reducing tumour burden and improving survival outcomes. Although the combination of TZ and 5-FU resulted in a significant reduction in the total tumour count, TZ alone emerged as the most effective treatment across CRC-related parameters. Notably, all animals from both TZ and TZ + 5-FU groups survived throughout the experimental period without exhibiting severe pain or distress necessitating premature euthanasia, suggesting that TZ may also improve the survival outcome of 5-FU-treated mice. The AOM/DSS model typically induces an enlarged caecum and shortening of colon length, serving as an indication of inflammation and carcinogenicity36,37. Both TZ and TZ + 5-FU treatments alleviated caecal enlargement, however, only TZ prevented colon shortening. In contrast, 5-FU alone failed to improve either measure, but 5-FU reduced liver weight compared to the healthy group, consistent with its known hepatotoxicity effects, including inflammation, collagen fibrosis and apoptotic cell death, which collectively reduce liver functions and cause a decline in liver weight38,39,40. By contrast, TZ not only did not cause liver damage, but it also suppressed the liver damage from 5-FU when combined with 5-FU.
Chronic inflammation is a critical element in the progression of CRC, especially in the progression of AOM/DSS model41. Hyperplastic or serrated colonic epithelium and thicken collagen fibers were reported to be chronic inflammatory responses that stimulate tumor growth and promote angiogenesis in CRC42,43,44. Both TZ and TZ + 5-FU relieved the increased colonic crypt depth and hyperplasia, yet only TZ reduced the inflammation score and collagen fibrosis. Furthermore, TZ and TZ + 5-FU suppressed the concentration of pro-inflammatory cytokines, IL-6, TNF-α and IL-17 A/F, whereas 5-FU alone only suppressed TNF-α. Such findings are consistent with a previous study, in which 5-FU can be pro-inflammatory, thereby undermining its anti-tumor efficiency in AOM/DSS mouse study and humans45,46. TZ not only ameliorated the inflammatory conditions in AOM/DSS mouse models but also mitigated the inflammatory response associated with 5-FU when administered in combination with it.
Oncogenic pathways are pivotal in cancer development, driving tumor initiation and progression via genetic mutations that lead to uncontrolled cell proliferation47,48. In this study, TZ, TZ + 5-FU and 5 -FU all increased apoptosis, as evidenced by the elevation of the c-casp3 marker. The PI3K/Akt signaling pathway plays a critical role in CRC growth, proliferation, and apoptosis49,50,51. TZ supplementation significantly suppressed the phosphorylation of PI3K and Akt, as well as downstream proliferative markers, cyclin D1 and Ki67. Inhibiting the PI3K and Akt kinases, along with the cyclin D1, plays a role in limiting CRC progression52,53,54,55,56,57. By contrast, TZ + 5-FU only reduced the phosphorylation of Akt and cyclin D1 protein expression, whereas 5-FU alone did not affect the PI3K/Akt pathway or other proliferative markers in this study. Since the PI3K/Akt pathway contributes to 5-FU resistance through metabolic reprogramming, enhancing drug efflux and interacting with the tumor microenvironment to enhance cancer cell survival58,59, targeting the pathway could restore 5-FU sensitivity60, but our data indicate no such effect, with TZ only providing better efficacy.
Metagenomics analysis revealed that TZ modulated both gut microbiota and mycobiota. Gut microbiota, Desulfovibrionaceae bacterium LT0009 and Helicobacter sp. MIT 03-1616 were enriched by TZ, which correlates with reducing tumor number and lower pro-inflammatory IL-6. Desulfovibrionaceae bacterium LT0009, also known as Taurinivorans muris, respires taurine and produces hydrogen sulfide (H2S), potentially prevent pathogen colonization61. H2S has a biphasic dose-response curve in cancer cells, inducing cell death at both low and high doses62,63. Further investigations could investigate if increasing endogenous H2S production through supplementing Desulfovibrionaceae bacterium LT0009, serve as a potential therapeutic strategy for CRC. Whereas there was not much information about Helicobacter sp. MIT03-1616, except that the bacteria was previously identified from rodents64. In the current study, Helicobacter sp. MIT03-1616 were found to be positively correlated to short chain fatty acids. Consistent with these findings, fecal acetate and propionate were elevated in the TZ group. Previous studies demonstrated that elevated levels of acetate and propionate, whether administered individually or in combination, inhibit the proliferation of CRC cells65,66,67,68. Acetate was reported to exert anti-CRC properties, which was previously demonstrated to reduce CRC tumor size in a xenograft mouse model69. Similar to butyrate, propionate also functions as a histone deacetylase (HDAC) inhibitor, which may influence PI3K/AKT signaling pathways70,71. The downstream effects of HDAC inhibition and modulation of PI3K/AKT signalling are critical for inducing anti-tumour responses and may help overcome PI3K/AKT inhibitors72,73. In addition, propionate has been shown to inhibit CRC by promoting the proteasomal degradation of the euchromatic histone-lysine N-methyltransferase 2 (EHMT2) and inhibiting PRMT1-associated mTOR signaling68,74. It also enhances the surface expression of NKG2D ligands on CRC cells, thereby promoting improved immune recognition and surveillance75. In addition, Candidatus Borkfalkia ceftriaxoniphila was also positively correlated with propionate and butyrate levels. Candidatus Borkfalkia ceftriaxoniphilla, also known as Borkfalkia ceftriaxensis, is a newly identified, low-abundance commensal species implicated in restoring the gut microbiota community following antibiotic treatment76.
As to the fungal species, TZ enriched Xylariaceae sp. FL0594 which correlated to reduced tumor counts. Noteworthily, Xylariaceae sp. FL0594, a recently sequenced genome under the Xylariaceae family, was reported to produce secondary metabolites with antimicrobial, antioxidant, and anti-cancer effects77,78. These compounds including a group of sesquiterpenoids and cytochalasans, exert their effects by inducing cytotoxicity and inhibiting cell proliferation79,80. Furthermore, TZ depleted multiple species which were correlated to elevated tumor counts and pro-inflammatory cytokines, including Batrachochytrium salamandrivorans, Batrachochytrium dendrobatidis, Claviceps sorghi, and Mortierella sp. GBA39. Whereas these species were successfully identified, data regarding these fungi remain dispersed, particularly concerning their impact on CRC81. Claviceps sorghi produces ergot alkaloids which can cause poisoning effects in both human and livestock82,83,84,85,86. It was primarily understood that Batrachochytrium salamandrivorans, Batrachochytrium dendrobatidis, Neofusicoccum parvum and Coelomomyces lativittatus exhibit pathogenic characteristics in non-mammalian species but their interaction with mice and humans are not well-documented87,88,89,90,91. Despite data on bacterial-fungal interactions in cancer context remain limited, our result shows that bacterial Desulfovibrionaceae bacterium LT0009, Helicobacter sp. MIT 03-1616 and fungal Xylariaceae sp. FL0594 were correlated. Their negative association with tumor counts and pro-inflammatory cytokines IL-6 levels suggest their potential as the biomarkers of TZ anti-CRC effects.
Apart from identifying the microbiota and mycobiota changes that TZ modulated, MetaCyc analysis was used to predict the functional impact of these community shifts. The TZ-enriched pathways PWY-7388 and PWY-7013 are both associated with CRC suppression. PWY-7388 corresponds to the mitochondrial octanoyl-[acyl-carrier protein] biosynthesis in yeast, which produces octanoyl-acyl-carrier protein (acyl-ACP), a precursor for biosynthesis of lipoic acid (LA) in Saccharomyces cerevisiae92,93. LA has been shown to selectively induce cell death selectively to CRC cells, while sparing normal colonocytes94. Moreover, LA mitigated inflammatory responses in ulcerative colitis by inhibiting NF-κB signaling, reducing levels of TNF-α and IL-6, and demonstrating antioxidant scavenging properties95,96. Additionally, PWY-7013 corresponds to the (S)-propane-1,2-diol degradation pathway which generates propionate97, a metabolite known to suppress colorectal cancer progression as discussed above.
Nonetheless, this study has several limitations. First, the in vivo CRC model used may not fully represent all forms of human colorectal cancer. Although the AOM/DSS model is widely used to mimic human CRC phenotypes, the findings derived from it may not be directly translatable to human diseases. Therefore, further validation using human CRC cell lines and clinical biopsies is warranted. Moreover, the experimental setup did not include separate TB-only and ZEA-only groups. Including these groups would help determine whether the observed effects of the TZ combination are synergistic or simply additive. In addition, the causal relationship between microbial changes and the tumour suppressive effects of TZ was not established in this study. The bacterial and fungal species identified as being correlated to TZ’s anti-CRC effects have only recently been sequenced and classified. As research on these species is still in its early stages, future studies could involve culturing and administering them individually in the AOM/DSS model to assess their direct contribution to CRC suppression. Our results also indicate that TZ + 5-FU did not produce additive or synergistic effects. TZ and TZ alone appeared to outperform the combination in several parameters. This may be due to the gut dysbiosis induced by 5-Fu, which could impair the microbiota modulatory efficiency of TZ and counteract its beneficial effects. Future research is warranted to explore this potential interaction. Lastly, while ZEA is often referred to as a mycotoxin, the dose used in this study (10 mg/kg) is close to the no-observed-effect level (NOEL) of 9.2 mg/kg body weight in mice72. However, the current study did not assess whether TB mitigates the potential adverse effects of ZEA, nor did it assess the toxicity profiles of the combined formulation. Comprehensive assessments of acute, sub-chronic, and chronic safety are essential before considering therapeutic application. Importantly, future translational studies must establish the long-term safety of TZ, particularly given the known estrogenic effects of ZEA.
Conclusion
The study demonstrated that the combination of theabrownin and zearalenone (TZ) significantly attenuated AOM/DSS-induced colorectal tumorigenesis. TZ demonstrated greater efficacy in alleviating CRC tumorigenesis than 5-FU, while the combination of TZ and 5-Fu did not yield additional or synergic benefits. TZ treatment improved survival rate, tumor count, colon length, colonic crypt depth, collagen fibrosis and colonic inflammation in AOM/DSS mice through the suppression of proliferative PI3K/AKT pathways and lowering pro-inflammatory cytokines. Moreover, TZ improved the gut microbiota and mycobiota, enhanced anti-CRC metabolic profile and increased fecal acetate and propionate concentration. The enrichment of the bacterial Desulfovibrionaceae bacterium LT0009, Helicobacter sp. MIT 03-1616 and fungal Xylariaceae sp. FL0594 were identified as potential microbial biomarkers for the suppression of CRC induced by TZ. Future translational studies should be performed to ensure the safety of TZ’s application in human, especially with the ZEA component. Overall, TZ displayed promising potential to be developed as a novel CRC therapeutic.
Data availability
Metagenomics sequencing data have been deposited at the NCBI GenBank Sequence Read Archive with accession numbers PRJNA1229795. The raw microbial taxa tables have been deposited in Zenodo (https://doi.org/10.5281/zenodo.15347314 ).
References
Jiang, Y. et al. Global pattern and trends of colorectal cancer survival: a systematic review of population-based registration data. Cancer Biol. Med. 19 (2), 175 (2022).
Le Berre, C., Honap, S. & Peyrin-Biroulet, L. Ulcerative colitis. Lancet, 402 (10401), 571–584 (2023).
Feagins, L. A., Souza, R. F. & Spechler, S. J. Carcinogenesis in IBD: Potential targets for the prevention of colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 6 (5), 297–305 (2009).
Vodenkova, S. et al. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 206, 107447 (2020).
Sethy, C. & Kundu, C. N. 5-Fluorouracil (5-FU) resistance and the new strategy To enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 137, 111285 (2021).
Lo, E. K. K. et al. Gut microbiota: Impact on 5-fluorouracil efficacy and toxicity. Curr. Opin. Toxicol. 26, 100423 (2023).
Tournigand, C. et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J. Clin. Oncol. 22 (2), 229–237 (2004).
Wu, C. et al. Berberine attenuates 5-fluorouracil-induced intestinal mucosal injury by modulating the gut microbiota without compromising its anti-tumor efficacy. Heliyon. 10(14), e34528 (2024).
Wong, S. H. & Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Revi. Gastroenterol. Hepatol. 16 (11), 690–704 (2019).
Torres-Maravilla, E. et al. Role of gut microbiota and probiotics in colorectal cancer: Onset and progression. Microorganisms 9 (5), 1021 (2021).
Leung, H. K. M., Lo, E. K. K. & El-Nezami, H. Theabrownin alleviates colorectal tumorigenesis in murine AOM/DSS model via PI3K/Akt/mTOR pathway suppression and gut microbiota modulation. Antioxidants 11 (9), 1716 (2022).
Leung, H. K. M. et al. Zearalenone attenuates colitis associated colorectal tumorigenesis through ras/raf/erk pathway suppression and SCFA-producing bacteria promotion. Biomed. Pharmacother. 164, 114973 (2023).
Wang, Q. et al. Bioconversion of tea polyphenols to bioactive theabrownins by Aspergillus fumigatus. Biotechnol. Lett. 36, 2515–2522 (2014).
Wang, Q. et al. Fungal isolates from a pu-erh type tea fermentation and their ability to convert tea polyphenols to theabrownins. J. Food Sci. 80 (4), M809–M817 (2015).
Bulgaru, C. V. et al. Zearalenone and the immune response. Toxins. 13 (4), 248 (2021).
Zheng, W. et al. Zearalenone promotes cell proliferation or causes cell death? Toxins. 10 (5), 184 (2018).
Lo, K. K. E. Investigation on the Involvement of Mycotoxin Zearalenone on colon Cancer Development and Progression: In Vitro and in Vivo Studies (HKU Theses Online (HKUTO), Pokfulam, 2020).
Kowalska, K., Habrowska-Górczyńska, D. E. & Piastowska-Ciesielska, A. W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Pharmacol. 48, 141–149 (2016).
Lagarde, F. et al. Non-monotonic dose-response relationships and endocrine disruptors: A qualitative method of assessment. Environ. Health. 14, 1–15 (2015).
Zhao, B. et al. Effect of Angelica sinensis root extract on cancer prevention in different stages of an AOM/DSS mouse model. Int. J. Mol. Sci. 18 (8), 1750 (2017).
Marjaneh, R. M. et al. Phytosomal curcumin inhibits tumor growth in colitis-associated colorectal cancer. J. Cell. Physiol. 233 (10), 6785–6798 (2018).
Fedchenko, N. & Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue–a review. Diagn. Pathol. 9 (1), 1–12 (2014).
Chen, Y. et al. SOAPnuke: A mapreduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 7 (1), gix120 (2018).
Menzel, P., Ng, K. L. & Krogh, A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat. Commun. 7 (1), 11257 (2016).
McMurdie, P. J. & Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of Microbiome census data. PloS ONE. 8 (4), e61217 (2013).
Oksanen, J. et al. Community ecology package. R package version, 2(0) (2020).
Lin, H. & Peddada, S. D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 11 (1), 3514 (2020).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, 1–18 (2011).
Nearing, J. T. et al. Microbiome differential abundance methods produce different results across 38 datasets. Nat. Commun. 13 (1), 342 (2022).
Beghini, F. et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with biobakery 3. Elife 10, pe65088 (2021).
Kanehisa, M. et al. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44 (D1), D457–D462 (2016).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28 (1), 27–30 (2000).
Zheng, X. et al. A targeted metabolomic protocol for short-chain fatty acids and branched-chain amino acids. Metabolomics 9, 818–827 (2013).
Cai, J. et al. Orthogonal comparison of GC–MS and 1H NMR spectroscopy for short chain fatty acid quantitation. Anal. Chem. 89 (15), 7900–7906 (2017).
Li, F. et al. Gut fungal mycobiome: A significant factor of tumor occurrence and development. Cancer Lett. 569, 216302 (2023).
Tian, Y. et al. Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. J. Nutr. Biochem. 57, 103–109 (2018).
Jeon, H. J. et al. Effect of vitamin C on azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced colitis-associated early colon cancer in mice. Nutr. Res. Pract. 12 (2), 101–109 (2018).
El-Sayyad, H. I. et al. Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int. J. Biol. Sci. 5 (5), 466 (2009).
Zeng, D. et al. Angelica polysaccharide antagonizes 5-FU-induced oxidative stress injury to reduce apoptosis in the liver through Nrf2 pathway. Front. Oncol. 11, 720620 (2021).
Gelen, V. et al. The protective effect of Rutin and Quercetin on 5-FU-induced hepatotoxicity in rats. Asian Pac. J. Trop. Biomed. 7 (7), 647–653 (2017).
De Robertis, M. et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 10, 9 (2011).
Shah, S. C. & Itzkowitz, S. H. Colorectal cancer in inflammatory bowel disease: Mechanisms and management. Gastroenterology 162 (3), 715–730 (2022). e3.
Onfroy-Roy, L. et al. Colon fibroblasts and inflammation: Sparring partners in colorectal cancer initiation? Cancers 13 (8), 1749 (2021).
Nissen, N. I. et al. Prognostic value of blood-based fibrosis biomarkers in patients with metastatic colorectal cancer receiving chemotherapy and bevacizumab. Sci. Rep. 11 (1), 865 (2021).
Sougiannis, A. et al. Impact of 5 fluorouracil chemotherapy on gut inflammation, functional parameters, and gut microbiota. Brain. Behav. Immun. 80, 44–55 (2019).
Abdellateif, M. S. et al. The prognostic significance of 5-fluorouracil induced inflammation and immuno-modulation in colorectal cancer patients. J. Inflamm. Res. 13, 1245–1259 (2020).
La Vecchia, S. & Sebastián, C. Metabolic Pathways Regulating Colorectal Cancer Initiation and Progression. In Seminars in Cell & Developmental Biology (Elsevier, Amsterdam, 2020).
Ahmad, R. et al. Emerging trends in colorectal cancer: Dysregulated signaling pathways. Int. J. Mol. Med. 47 (3), 14 (2021).
Leiphrakpam, P. D. & Are, C. PI3K/Akt/mTOR signaling pathway as a target for colorectal cancer treatment. Int. J. Mol. Sci. 25 (6), 3178 (2024).
Koveitypour, Z. et al. Signaling pathways involved in colorectal cancer progression. Cell. Biosci. 9 (1), 97 (2019).
Maharati, A. & Moghbeli, M. PI3K/AKT signaling pathway as a critical regulator of epithelial-mesenchymal transition in colorectal tumor cells. Cell. Commun. Signal. 21 (1), 201 (2023).
Lee, H. Y. et al. Bile acid regulates MUC2 transcription in colon cancer cells via positive egfr/pkc/ras/erk/creb, PI3K/Akt/IκB/NF-κB and p38/MSK1/CREB pathways and negative JNK/c-Jun/AP-1 pathway. Int. J. Oncol. 36 (4), 941–953 (2010).
Wang, L. et al. DIXDC1 targets p21 and cyclin D1 via PI3K pathway activation to promote colon cancer cell proliferation. Cancer Sci. 100 (10), 1801–1808 (2009).
Gao, F. et al. Inhibition of ERKs/Akt-Mediated c-Fos expression is required for piperlongumine-induced cyclin D1 downregulation and tumor suppression in colorectal cancer cells. OncoTargets Ther. 13, 5591–5603. (2020).
Tsukahara, T., Haniu, H. & Matsuda, Y. Cyclic phosphatidic acid induces G0/G1 arrest, inhibits AKT phosphorylation, and downregulates cyclin D1 expression in colorectal cancer cells. Cell. Mol. Biol. Lett. 20 (1), 38–47 (2015).
Song, S. et al. Preventive effect of genistein on AOM/DSS-induced colonic neoplasm by modulating the PI3K/AKT/FOXO3 signaling pathway in mice fed a high-fat diet. J. Funct. Foods. 46, 237–242 (2018).
Bishnupuri, K. S. et al. Reg IV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology. 130 (1), 137–149 (2006).
Dong, S. et al. ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 41 (1), 15 (2022).
Su, P. et al. Crosstalk between tumor-associated macrophages and tumor cells promotes chemoresistance via CXCL5/PI3K/AKT/mTOR pathway in gastric cancer. Cancer Cell Int. 22 (1), 290 (2022).
Li, T. et al. Emodin reverses 5-Fu resistance in human colorectal cancer via downregulation of PI3K/Akt signaling pathway. Am. J. Transl. Res. 12 (5), 1851 (2020).
Ye, H. et al. Ecophysiology and interactions of a taurine-respiring bacterium in the mouse gut. Nat. Commun. 14 (1), 5533 (2023).
Cao, X. et al. A review of hydrogen sulfide synthesis, metabolism, and measurement: Is modulation of hydrogen sulfide a novel therapeutic for cancer? Antioxid. Redox. Signal. 31 (1), 1–38 (2019).
Lin, H. et al. Implications of hydrogen sulfide in colorectal cancer: Mechanistic insights and diagnostic and therapeutic strategies. Redox Biol. 59, 102601 (2023).
Sheh, A., Shen, Z. & Fox, J. G. Draft genome sequences of eight enterohepatic Helicobacter species isolated from both laboratory and wild rodents. Genome Announc. 2 (6), 01218–01214. https://doi.org/10.1128/genomea (2014).
Scheppach, W., Bartram, H. & Richter, F. Role of short-chain fatty acids in the prevention of colorectal cancer. Eur. J. Cancer. 31 (7–8), 1077–1080 (1995).
Casanova, M. R. et al. Colorectal cancer cells increase the production of short chain fatty acids by Propionibacterium freudenreichii impacting on cancer cells survival. Front. Nutr. 5, 44 (2018).
Sahuri-Arisoylu, M. et al. Acetate induces growth arrest in colon cancer cells through modulation of mitochondrial function. Front. Nutr. 8, 150 (2021).
Ryu, T. Y. et al. Human gut-microbiome-derived propionate coordinates proteasomal degradation via HECTD2 upregulation to target EHMT2 in colorectal cancer. ISME J. 16 (5), 1205–1221 (2022).
Brody, L. P. et al. Cationic lipid-based nanoparticles mediate functional delivery of acetate to tumor cells in vivo leading to significant anticancer effects. Int. J. Nanomed. 12, 6677–6685 (2017).
Hou, H. et al. Gut microbiota-derived short-chain fatty acids and colorectal cancer: Ready for clinical translation? Cancer Lett. 526, 225–235 (2022).
Chen, Y. & Chen, Y. X. Microbiota-associated metabolites and related immunoregulation in colorectal cancer. Cancers. 13 (16), 4054 (2021).
Ropejko, K. & Twarużek, M. Zearalenone and its metabolites—general overview, occurrence, and toxicity. Toxins. 13 (1), 35 (2021).
Wu, D. et al. An acetyl-histone vulnerability in PI3K/AKT inhibition-resistant cancers is targetable by both BET and HDAC inhibitors. Cell. Rep. 34 (7), 108744 (2021).
Ryu, T. Y. et al. Downregulation of PRMT1, a histone arginine methyltransferase, by sodium propionate induces cell apoptosis in colon cancer. Oncol. Rep. 41 (3), 1691–1699 (2019).
Høgh, R. I. et al. Metabolism of short-chain fatty acid propionate induces surface expression of NKG2D ligands on cancer cells. FASEB J. 34 (11), 15531–15546 (2020).
Hildebrand, F. et al. Antibiotics-induced monodominance of a novel gut bacterial order. Gut 68 (10), 1781–1790 (2019).
Suwannasai, N. et al. Exploring the Xylariaceae and its relatives. Bot. Stud. 64 (1), 15 (2023).
Franco, M. E. et al. Ecological generalism drives hyperdiversity of secondary metabolite gene clusters in Xylarialean endophytes. New Phytol. 233 (3), 1317–1330 (2022).
Becker, K. & Stadler, M. Recent progress in biodiversity research on the Xylariales and their secondary metabolism. J. Antibiot. 74 (1), 1–23 (2021).
Macías-Rubalcava, M. L. & Sánchez-Fernández, R. E. Secondary metabolites of endophytic xylaria species with potential applications in medicine and agriculture. World J. Microbiol. Biotechnol. 33, 1–22 (2017).
Wu, M. et al. Chitooligosaccharides prevents the development of colitis-associated colorectal cancer by modulating the intestinal microbiota and mycobiota. Front. Microbiol. 10, 2101 (2019).
Tsukiboshi, T., Shimanuki, T. & Koga, H. Claviceps sorghicola and C. africana, the ergot pathogens of sorghum, and their cultural control in Japan. Jpn. Agric. Res. Q. JARQ. 35 (4), 221–226 (2001).
Frederickson, D. & Mantle, P. The path of infection of sorghum by Claviceps sorghi. Physiol. Mol. Plant Pathol. 33 (2), 221–234 (1988).
Berraies, S. et al. Ergot of Cereals: Toxins, Pathogens and Management (Plant Pathology, New York, 2024).
Scott, P. Ergot alkaloids: Extent of human and animal exposure. World Mycotoxin J. 2 (2), 141–149 (2009).
Alderman, S. Ergot: Biology and Control (USDA-ARS National Forage Seed Production Research Center, Corvallis, 2006).
Sabino-Pinto, J. et al. Asymptomatic infection of the fungal pathogen batrachochytrium salamandrivorans in captivity. Sci. Rep. 8 (1), 11767 (2018).
Turner, A. et al. Temperature as a driver of the pathogenicity and virulence of amphibian Chytrid fungus batrachochytrium dendrobatidis: A systematic review. J. Wildl. Dis. 57 (3), 477–494 (2021).
Baskarathevan, J. et al. Genetic and pathogenic diversity of neofusicoccum parvum in new Zealand vineyards. Fungal Biol. 116 (2), 276–288 (2012).
Ettinger, C. L. et al. Genomes and transcriptomes help unravel the complex life cycle of the blastoclad fungus, Coelomomyces lativittatus, an obligate parasite of mosquitoes and microcrustaceans. Mycologia. 115 (5), 630–647 (2023).
Rahimlou, S., Quandt, C. A. & James, T. Y. Metabolic Constraints and Dependencies between Uncultivable Fungi and their Hosts, in Fungal Associations 33–57 (Springer, Cham, 2024).
Pietikäinen, L. P. et al. Genetic dissection of the mitochondrial lipoylation pathway in yeast. BMC Biol. 19, 1–15 (2021).
Hermes, F. A. & Cronan, J. E. The role of the Saccharomyces cerevisiae lipoate protein ligase homologue, Lip3, in lipoic acid synthesis. Yeast. 30 (10), 415–427 (2013).
Dörsam, B. et al. Lipoic acid induces p53-independent cell death in colorectal cancer cells and potentiates the cytotoxicity of 5-fluorouracil. Arch. Toxicol. 89, 1829–1846 (2015).
Moeinian, M. et al. Effects of alpha lipoic acid and its derivative andrographolid-lipoic acid‐1 on ulcerative colitis: A systematic review with meta‐analysis of animal studies. J. Cell. Biochem. 120 (4), 4766–4782 (2019).
Trivedi, P. & Jena, G. Role of α-lipoic acid in dextran sulfate sodium-induced ulcerative colitis in mice: Studies on inflammation, oxidative stress, DNA damage and fibrosis. Food Chem. Toxicol. 59, 339–355 (2013).
Ricci, S. et al. Progressive microbial adaptation of the bovine rumen and hindgut in response to a step-wise increase in dietary starch and the influence of phytogenic supplementation. Front. Microbiol. 13, 920427 (2022).
Acknowledgements
The high-performance computations were performed using research computing facilities offered by Information Technology Services, the University of Hong Kong. Figure 1 was created with BioRender.com.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Hoi Kit Matthew Leung: Methodology, Investigation, Writing – original draft, Writing – review & editing, Visualization. Emily Kwun Kwan Lo: Conceptualization, Methodology, Investigation, Writing – review & editing, Visualization. Congjia Chen: Writing – original draft, Writing – review & editing, Visualization. Fangfei Zhang: Writing – review & editing. Felicianna: Writing - review & editing. Marsena Jasiel Ismaiah: Writing - review & editing. Hani El-Nezami: Investigation, Writing – review & editing, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Leung, H.K.M., Lo, E.K.K., Chen, C. et al. Theabrownin combined with zearalenone suppresses colitis-associated colorectal cancer by inhibiting PI3K/AKT pathway and enhancing microbial propionate production. Sci Rep 15, 26386 (2025). https://doi.org/10.1038/s41598-025-11820-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11820-2