Abstract
This study investigated the development and corrosion performance evaluation of Ni-P-Y2O3 nanocomposite coatings, synthesized through electroless deposition on A36 carbon steel with varying concentrations of Y2O3 nanoparticles (Y2O3NPs) (0.25 g/L, 0.50 g/L, and 0.75 g/L) within the Ni-P matrix. The incorporation of Y2O3 nanoparticles, along with the innovative use of patent commercialized products solutions Nichem 3010 A and Nichem 3010B where Nichem A and B are commercial electroless plating solutions, which introduces a novel approach to enhancing the corrosion resistance and mechanical properties of the coatings. Structural analyses confirm the formation of phase-pure coatings, with uniform dispersion of Y2O3NPs throughout the Ni-P matrix. Surface topographic analysis reveals an increase in surface roughness in Ni-P-Y2O3 nanocomposite coatings as Y2O3NP concentration increases to 0.75 g/L, attributed to the presence of insoluble and hard Y2O3NPs. Mechanical properties, as evaluated by Vickers microhardness and nanoindentation tests, demonstrated a marked improvement in hardness, with Y2O3NP (0.75 g/L) incorporation proved to have the highest microhardness (764.7Hv) and elastic modulus (6.52GPa), suggesting a synergistic enhancement due to grain refinement and dispersion effects. Electrochemical assessments, including Tafel analysis and Electrochemical Impedance Spectroscopy (EIS), reveal that incorporating 0.75 g/L of Y2O3NPs into the Ni-P structure significantly reduces the corrosion current density (icorr) while increasing polarization resistance (Rp) in comparison to other concentrations (0.25 g/L and 0.50 g/L). This result indicates superior corrosion resistance, with the Ni-P-Y2O3 nanocomposite coatings exhibiting an impressive corrosion resistance improvement of 82.76%. This enhanced resistance is attributed to the mechanism whereby Y2O3NPs occupy micropores, thereby reducing active corrosion sites within the Ni-P matrix. The findings strongly suggest that Ni-P-Y2O3 composite coatings offer a robust solution for corrosion mitigation, presenting them as promising candidates for application in aggressive and corrosive environments.
Similar content being viewed by others
Introduction
In this period of swift industrial advancement, contemporary enterprises are increasingly utilizing metallic components. Steel, a rising industrial material, is widely succeeding in the various sector1,2,3. Corrosion is a natural process that causes the deterioration of metallic and non-metallic structure over time, due to chemical or electrochemical reactions with their environment4. These metallic components undergo corrosion over time due to the interaction of corrosive ions, in addition to solid particulates, fostering a highly erosive and corrosive environment. Consequently, metallic components degrade over time due to exposure to various corrosive conditions and severe environments, leading to a shortened lifespan for steel in the oil and gas industry5,6,7,8,9. This can result in equipment failure, posing serious safety risks in industries like oil and gas, transportation and construction. In critical industries, downtime caused by corrosion-related failures can lead to substantial financial losses10. A material’s surface regulates properties like wettability, adhesion, corrosion resistance, and tribological performance by acting as a crucial interface between the bulk material and its surroundings11,12. According to previous literature, surface properties are frequently insufficient for industrial or biomedical applications without modification, so methods such as electrochemical deposition, plasma treatment, or nanoparticle reinforcement are required to customize surface characteristics11. As studied in the previous literature, the Ni-P-CrO3-ZrO2 nanocomposite layers studied, are an example of protective coatings that not only improve mechanical and anti-corrosion qualities but also prolong the service life of engineering components exposed to harsh environments12. Therefore, surface modifications are essential for bridging the gap between functional requirements and inherent material properties, guaranteeing optimal performance in practical applications. Corrosion also affects the reliability and efficiency of infrastructure, leading to increased energy consumption and operational inefficiencies in addition to the aesthetic degradation of structures and machinery which can diminish their value and usability13. Corrosion protection is vital for improving efficiency and extending the durability of metal components. Protective coatings, such as metallic, inorganic, or organic, serve as barriers to shield metal surfaces from corrosive agents6,8,14,15. Metallic coatings corresponding to Ni-P and Ni-B coatings, are widely popular in the oil and gas industry16,17,18. Nickel-based coatings, including Ni-P and Ni-B, are particularly valued for their excellent anti-corrosion properties and are known for their hardness, smoothness, excellent corrosion resistance, and ease of soldering. They are increasingly recognized as a suitable substitute for hard chromium coatings7,8,19,20,21. Electrodeposition coatings22Self-healing coatings23Cathodic protection24 are among the significant approaches used in this context. Additionally, designing corrosion resistance by selecting appropriate materials and employing corrosion-resistant alloys can significantly enhance durability. Ni-P coatings with low phosphorus content (< 5 wt%) demonstrate not as much resistance to corrosion in acidic or neutral circumstances compared to coatings with higher phosphorus content (> 8 wt%). The chemical uniformity and absence of imperfections, such as phase boundaries and dislocations, in amorphous coatings contribute a consistent protective layer on the surface8,25. Recent studies have shown that incorporating hard ceramic particles into a metallic coating can enhance its properties, as mentioned in prior literature17,26,27,28. Recent advancements have enabled the development of Ni-P coatings via electroless plating, which allow the deposition of a metallic coating onto a substrate without using an external electric current which offers improved adhesion properties, provided that appropriate surface treatments are applied and stringent quality control measures are maintained1,2,6,29. The incorporation of nanoparticles such as HfC1Al2O316, C3N4 30, ZrO231, BN32SiC33TiNi34SiO235, B4C36,37TiC2WC38 and TiO239 nanoparticles have also been utilized and exploited in previous literatures16,27,40 to create Ni-P-based coatings, ensuing in substantial improvements in corrosion resistance with wear characteristics. Likewise, the nanoscale size of these particles has led to remarkable advancements, as described in previous research. Si3N4 and CeO2 enhance corrosion resistance properties41,42. Yttria (Y2O3) nanoparticles offer numerous advantages when used as additives in coatings, particularly in Ni-P electroless coatings by improving hardness, wear resistance, corrosion protection, thermal stability, oxidation resistance, grain refinement, adhesion, toughness, and potential self-lubrication properties. In addition, the nanoparticle Y2O3, experimental research highlights the effectiveness of incorporating Y2O3 nanoparticles into Ni-based coatings to enhance their structural and functional properties. Studies on Ni–Y2O3 composite coatings show that the presence of Y2O3 leads to grain refinement, improved surface morphology, and a shift in preferred crystal orientation, all of which contribute to increased coating compactness and mechanical strength43. Pulse electrodeposition methods further optimize these effects by promoting finer grain structures, reducing surface defects, and achieving a more uniform nanoparticle distribution, which enhances both wear resistance and corrosion behavior44. Additionally, when Y2O3 is integrated into Ni–Mo alloy matrices, it contributes to improved microhardness and corrosion resistance without significantly altering the coating morphology, largely due to its role in suppressing defect formation and supporting homogeneous dispersion within the matrix45. These practical investigations, across different deposition systems and alloy combinations, consistently demonstrate the beneficial role of Y2O3 in advancing the durability, wear performance, and corrosion protection of Ni-based composite coatings, making it a reliable reinforcing phase for demanding engineering applications. Conversely, in this study, we optimized the integration of nanoscale yttrium oxide (Y2O3) as a nano additive in Ni-P electroless coatings. This was achieved by dispersing various concentrations of Y2O3 nanoparticles (0.25 g/L, 0.50 g/L and 0.75 g/L) into the Ni-P plating bath and depositing the composite coatings onto steel substrates. This approach significantly enhances the corrosion resistance and mechanical properties of the coatings, as the nanoparticles create a uniform and dense microstructure, resulting in a harder and more resilient surface. The nanoindentation results, including hardness and elastic modulus, were analyzed to correlate with and support the findings from electrochemical corrosion testing. The enhanced hardness and improved elastic modulus observed in the Ni-P-Y2O3 nanocomposite coatings is attributed to the incorporation of Y2O3 nanoparticles resulting in superior corrosion resistance. Higher hardness values indicate a more robust and compact coating structure, which acts as a barrier against corrosive species, reducing the likelihood of localized corrosion initiation. Similarly, the increased elastic modulus suggests better resistance to deformation under stress, further enhancing the coating’s ability to withstand corrosive environments1,3,16,18,46. These mechanical properties align with the electrochemical corrosion testing results, which demonstrated lower corrosion current densities and higher polarization resistances for the nanocomposite coatings. This correlation underscores the synergistic relationship between mechanical strength and corrosion performance, validating the effectiveness of the developed coatings in protecting the substrate from degradation. This unique integration has not been explored in previous studies, offering a new avenue for enhanced coating performance in addition to improved mechanical and corrosion properties.
Experimental
Preparation of steel substrates and coating deposition process
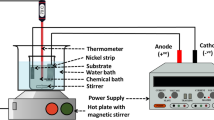
A36 Carbon steel substrates were prepared as 30 × 30 × 2 mm3 coupons for the deposition of Ni-P and Ni-P-Y2O3 nanocomposite coatings. The chemical composition of the carbon steel is detailed in Table 1. Initially, the steel substrates underwent a polishing process using SiC abrasive paper, with grit sizes ranging from 60 to 2000, to achieve a mirror-like surface devoid of any corroded layers. Following polishing, the substrates were degreased in acetone for 15 min and subsequently rinsed with distilled water. To restrict deposition on both sides, epoxy tape was applied to the one side of each substrate. The substrates were then immersed in an alkaline bath at 65 °C for 15 min to ensure thorough surface cleaning. The composition of the alkaline bath is provided in Table 2. After the alkaline treatment, acid pickling was performed in HCl (15 wt%) for 30 s to activate the substrate surface, facilitating the embedding of Y2O3 nanoparticles (Y2O3NPs) within the coating layer. Immediately following this activation step, the substrates were rinsed in deionized (DI) water and then placed into a chemical bath for the electroless deposition process, maintained at 92 ± 2 °C for 2 h. The electroless deposition was conducted with varying Y2O3NP concentrations of 0.25 g/L, 0.50 g/L, and 0.75 g/L to assess the effect of these concentrations on corrosion inhibition performance of Ni-P-Y2O3 nanocomposite coatings. The chemical bath used for the deposition process included Nichem 3010 A and Nichem 3010B, both standard commercial-grade products from Atotec Inc., Berlin, Germany. Table 3 provides a comprehensive breakdown of the bath composition, and the specific conditions required for electroless deposition. A schematic illustration of the coating process is presented in Fig. 1 for reference.
Characterization of electroless deposited steel substrates
Scanning Electron Microscopy (SEM) and EDS (Energy-Dispersive X-ray Spectroscopy) from Nova Nano-450 (ThermoFischer Scientific, Eindhoven, Netherland), was utilized to analyze the surface morphology and microstructural characteristics of the composite coating. A thickness gauge (PosiTector 6000, DeFelsko, Proctor Avenue Ogdensburg, NY, USA) was also employed to assess the thickness of composite coatings30. To be able to confirm the incorporation of the Y2O3NPs in the Ni-P matrix the XRD (X-ray diffraction) machine was utilized with 2θ ranging from 20° -100° at the 0.02° scanning rate for the structural and phase evaluation of the developed nanocomposite coatings2,47. Vickers microhardness testing (FMARS9000, USA) was used with the applied load of 100 g and dwell time of 10 s to assess the mechanical properties of the coatings, benefiting from its extensive load capacity and widespread use in material science research30. The formula applied to calculate corresponding Hv value of each Ni-P-Y2O3 with varying concentration can be seen in Eq. (1), where Hv is the Vickers hardness number, F is the applied force (load) in kgf, and d is the average diagonal length of the indentation in millimeters. Similar equation used can be seen in the previous literatures48,49 .
.
Additional characterization involves nanoindentation testing with the MFP-3D nano indenter head in conjunction with Atomic Force Microscopy (AFM) equipment. This setup operates at a scan rate of 200 N/s, applying a force of 1mN for a dwell time of 10 s during both the loading and unloading phases3. Additionally, Electrochemical Impedance Spectroscopy (EIS) was utilized to investigate the corrosion behavior of blank steel substrate, steel substrate with Ni-P, Ni-P-Y2O3 (0.25 g/L, 0.50 g/L, and 0.75 g/L ) nanocomposite coatings. This analysis is performed using a GAMRY 600 potentiostat/galvanostat/ZRA (Warminster, PA, USA), set up in a three-electrode cell configuration with electrode gap of 15 cm and the working electrode Ni-P coated sample and Ni-P-Y2O3 with varying concentrations had an exposed area of 2.86 cm², the counter electrode was graphite (25 × 25 × 5 mm), and the interelectrode gap was approximately 15 cm. All tests were conducted at room temperature (25 ± 2 °C).In this setup, the coated steel sample acts as the working electrode, Ag/AgCl serves as the reference electrode, and for the counter electrode graphite sheet is utilized, all subjected to immersion in 3.5 wt% NaCl solution. For the EIS measurements, perturbation of 10 mV is applied over a frequency range of 10− 2 to 105 Hz. Potentiodynamic polarization analysis was also done in addition to the EIS analysis for Ni-P and Ni-P-Y2O3 nanocomposite coatings in 3.5 wt% NaCl solution. The voltage range was set from − 0.25 V to 0.25 V, with scan rate of 0.167 m.s− 128,30,40.
Results and discussion
Structural and phase analysis
To examine the structure and phase of the synthesized Ni-P-Y2O3 nanocomposite coatings, XRD analysis was done. Figure 2 represents the XRD spectrum for Ni-P and Ni-P-Y2O3 nanocomposite coatings. For instance, illustrated in the Figure, all coatings displayed a visible peak at 2θ ranging from 40° to 50°, demonstrating the formation of an amorphous structure primairly due to the presence of phosphrous content, as confirmed by EDS/XPS analysis through the observed phosphorus weight%. An obvious peak around 45.59° is associated with the Ni (111) lattice structure, which is also an indication of face centered cubic structure in all Ni-P coatings. Moreover, additional peaks at 2θ values of approximately 26.38° and 82.44° correspond to the (222) and (662) planes of Y2O3, respectively, indicating its face-centered cubic (FCC) crystal structure. (reference code ICDD: 98-019-1794). Additionally, the increase in intensity of the peak associated with increasing concentration Y2O3NPs, demonstarted the effective incorporation of the Y2O3 nanoparticles into the Ni-P matrix50,51,52. This phenomenon suggests the formation of a hybrid (or mixed) amorphous + crystalline nanocomposite structure, where the nanoparticles are not merely dispersed but are strongly bonded within the Ni-P matrix. The presence of overlapping peaks between Y2O3 and Ni phases indicates the coexistence of both components, to confirm the nanoparticle integration additional characterization techniques SEM and EDX mapping were implemented to confirm dispersion and matrix interaction. This strong bonding is crucial as it indicates that the nanoparticles are embedded at the atomic level within the Ni-P matrix, rather than being loosely attached. The formation of the amorphous structure is also ascribed to the existence of phosphorus atoms within the nickel crystalline lattice, causing lattice distortion. This distortion obstructs the orderly propagation of nickel atoms, leading to the creation of a face-centered cubic structure in addition to the phosphorus concentration in the coating plays a critical role in shaping its structural characteristics, facilitating the development of amorphous, crystalline, or mixed-phase Configurations. This is also validated and endrosed form the previous published studies1,5,31,53,54. Such interaction contributes to improved mechanical properties, including hardness and wear resistance, while also enhancing the corrosion resistance of the coating. The shift and broadening of peaks in certain regions further support the presence of nano-sized crystallites and the strain induced by the incorporation of Y2O3 nanoparticles, which are typical features of nanocomposite materials. These findings align with previous studies, confirming the successful synthesis of a semi-amorphous nanocomposite coating with exceptional structural integrity, mechanical performance, and corrosion resistance. The XRD results thus provide compelling evidence for the advanced properties of the Ni-P-Y2O3 nanocomposite coating, underscoring its potential for applications requiring high durability and protection against corrosive environments2,7,8,32,38,55,56,57.
X-ray photoelectron spectroscopy (XPS) analysis
The X-ray photoelectron spectroscopy (XPS) analysis was conducted to investigate the compositional changes in the Ni-P matrix resulting from the incorporation of Y2O3 nanoparticles (YONPs). Figure 3 presents the survey spectrum of Ni-P-Y2O3 nanocomposite (NC) coating containing 0.75 g/L YONPs. The analysis reveals the presence of key elements such as nickel (Ni), phosphorus (P), yttrium (Y), and oxygen (O), confirming the successful integration of Y2O3 nanoparticles into the Ni-P matrix. The XPS results provide valuable insights into the chemical bonding and surface composition, highlighting the role of Y2O3 in modifying the structural and functional properties of the nanocomposite coating. This integration is critical for enhancing the mechanical, thermal, and corrosion-resistant properties of the coating, as demonstrated by the shifts in peak positions and the formation of new chemical states in the Ni-P-Y2O3 system. The survey XPS spectrum (Fig. 3) of the Ni-P-Y2O3 composite coating provides an overview of the elemental composition present on the material’s surface. The most prominent peak observed in the spectrum corresponds to the Ni 2p region, located around 850–880 eV, confirming the presence of nickel as a primary component. This peak suggests the presence of both metallic Ni (Ni0) and oxidized Ni (Ni2+). Additionally, a distinct peak at approximately 130 eV corresponds to the P 2p binding energy, confirming the incorporation of phosphorus within the Ni-P matrix. The presence of yttrium is evident from the Y 3d peak in the range of 150–160 eV, which indicates the successful incorporation of Y2O3 in the composite. The O 1s peak at around 530 eV confirms the presence of oxygen, likely due to the formation of oxides, including Y2O3, as well as possible oxidation of nickel and phosphorus58. A C 1s peak is also observed near 285 eV, which is typically associated with surface contamination from atmospheric hydrocarbons. The XPS analysis confirms the successful deposition of the Ni-P-Y2O3 coating, which is expected to enhance properties such as corrosion resistance, wear resistance, and thermal stability. These findings demonstrate the effective integration of Y2O3 nanoparticles into the Ni-P matrix, contributing to the enhanced structural and functional properties of the nanocomposite coating. It can be noticed from Fig. 4a that the Ni2p spectra can be nominated into two main dominated peaks of Ni2p3/2 at 853.4 eV and a peak of Ni 2p1/2 at 869.8 eV, respectively, which are corresponding to the metallic nickel1,16,32. Additionally, the satellite peaks of Ni 2p3/2 at 858.4 eV and Ni 2p1/2 at 874.8 eV are associated with Ni+ 2, which would be representative of NiO or Ni(OH)2. Intriguingly, the predominant and satellite peaks for Ni 2p exhibit a shift of 0.5 eV toward higher binding energy values from their nominal positions after the incorporation of 0.75 g/L Y2O3 nanoparticles (YONPs), as shown in Fig. 4(a). This shift indicates changes in the chemical environment of nickel within the Ni-P matrix, likely due to interactions with Y2O3 nanoparticles52. Additionally, the intensity of the satellite peaks increases significantly, which can be attributed to the enhanced formation of oxide and hydroxide phases36,52. This phenomenon suggests that the presence of Y2O3 nanoparticles promotes the oxidation of nickel, leading to the formation of a more complex surface chemistry. These findings highlight the role of Y2O3 in modifying the electronic structure and surface properties of the Ni-P coating, which is critical for improving its functional performance, such as corrosion resistance and mechanical stability. The P2p spectra in Fig. 4b can be deconvoluted into four distinct peaks corresponding to binding energies of 129.2 eV, 129.9 eV, 131.2 eV, and 133.4 eV. The peaks positioned at 129.2 eV and 129.9 eV are assigned to elemental phosphorus (P) as P 2p₁/2 and to the Ni-P alloy, respectively. The intensity of the phosphorus signal diminishes with its combination with nickel, indicating the formation of the Ni-P alloy phase. The central peak at 131.2 eV can be ascribed to the first or third valence state of phosphorus, likely originating from residual hypophosphite used in the electroless deposition process. Additionally, the peak located at 133.4 eV is attributed to a mixture of phosphorus oxide (POx) chemical states, such as P2O3 and P-OH, which are typically observed at binding energies close to 134.5 eV59. In the case of Ni-P-Y2O3 nanocomposite coatings, the presence of Y2O3 nanoparticles (Fig. 4c, d) may further influence the chemical states of phosphorus, potentially leading to slight shifts in binding energies or changes in peak intensities. This could be due to interactions between Y2O3 and the Ni-P matrix, which may alter the local chemical environment of phosphorus60. These findings provide valuable insights into the chemical bonding and oxidation states within the Ni-P-Y2O3 coating system, highlighting the complex interplay between the matrix and the incorporated nanoparticles. The Ni-P-Y2O3 nanocomposite coatings exhibit a compact and defect-free structure at the interface between the substrate and the coating. This dense and uniform microstructure not only improves the mechanical properties, such as hardness and wear resistance, but also enhances the corrosion resistance by preventing the penetration of corrosive agents55. The incorporation of Y2O3 nanoparticles thus plays a significant role in optimizing the coating’s structural integrity and functional performance. Further details results showing the binding energy, full width at half maximum (FWHM), and atomic concentration (wt%) of elements present in the Ni-P-Y2O3 composite coating can be found in Table 4.
Morphological and topographical studies of developed Ni-P-Y2O3 nanocomposite coatings
SEM/EDX analysis
To analyze the surface morphology of the coated steel samples, FESEM was employed. Figure 5 displays the surface morphology of Ni-P and the effect of increasing Y2O3 concentration from 0.25 g/L to 0.75 g/L. The images (Fig. 5a) reveal a smooth and homogeneous surface for Ni-P, characterized by the presence of small or negligible spherical nodules19,28,52. The incorporation of Y2O3 into the Ni-P matrix is evident in Fig. 5 (b, c, d). In addition, the as-electroless-deposited coatings have a thickness of 25 ± 3 μm. Additionally, the nanoparticles are distributed evenly and uniformly throughout the Ni-P matrix, which is highly desirable as it ensures consistent and homogeneous properties throughout the coating process. The introduction of 0.25 g of Y2O3 nanoparticles improved the morphology, leading to the formation of more spherical structures. As the Y2O3 nanoparticle concentration in the plating solution increased to 0.50 and 0.75 g/L, the resulting electroless plated coatings became rougher and denser, with notable changes in the dimensions of the nodules. This effect can be attributed to the adsorption of Y2O3 nanoparticles on the carbon steel surface, which acted as nucleation centers for the deposition of the Ni-P alloy1,17,20,34,40. In addition, to study micro/nano defects high FESEM have also been added which can be seen in Figure 6. No significant or appreciable evidence of any defects is prevalent or observed. To confirm the deposition of Y2O3 into the Ni-P matrix EDX analysis was employed, its results can be found in Fig. 7. In Fig. 7a-d, the EDX mapping results demonstrate the successful integration of Y2O3 particles (yttria, oxygen) into the Ni-P matrix. The presence of yttria in the EDX mapping (Fig. 7) clearly confirms the incorporation of Y2O3, alongside Ni and P particles16.In addition the corresponding wt% of each composition can be found in Table 5.
AFM analysis
Atomic Force Microscopy (AFM) was employed to analyze the surface topography and roughness of the coated nanocomposite layers17. Figure 8a-d present tribological images illustrating the transition from pure Ni-P coatings to Ni-P-Y2O3 nanocomposite coatings at varying concentrations of Y2O3 nanoparticles (NPs). The results reveal that the Ni-P coatings exhibit a relatively uniform and smooth surface compared to the Ni-P-Y2O3 nanocomposite coatings. This difference in surface morphology can be attributed to the incorporation of Y2O3 NPs, which introduces microstructural changes and alters the growth mechanism of the coating. Table 6 provides a detailed evaluation of the surface roughness parameters for both Ni-P and Ni-P-Y2O3 nanocomposite coatings. The average surface roughness (Ra) increases significantly approximately two-folds the concentration of Y2O3 NPs rises from 0.25 to 0.75 g/L. This increase in roughness is indicative of the denser and more heterogeneous morphology resulting from the incorporation of Y2O3 NPs into the Ni-P matrix. The presence of Y2O3 (NPs) leads to the formation of larger nodules and a more textured surface, which is consistent with previous studies reporting similar trends in nanocomposite coatings. The observed changes in surface roughness and morphology can be explained by the adsorption of Y2O3 NPs on the carbon steel substrate during the electroless deposition process. These NPs act as nucleation sites for the Ni-P alloy, promoting the growth of a more complex and irregular surface structure. Additionally, the presence of Y2O3 NPs creates physical barriers that hinder the smooth movement of the AFM cantilever probe tip, further contributing to the measured increase in surface roughness. This phenomenon has been documented in prior investigations, which have shown that the incorporation of nanoparticles into a coating matrix can significantly alter its tribological and mechanical properties9,61 and it also hinder the movement and generate a hurdle for the AFM cantilever probe tip for its smooth movement on the coated steel surface1 as shown in Fig. 9 as well.
Mechanical properties
To investigate the microhardness of the electroless deposited Ni-P-Y2O3 nanocomposite coatings, the Vickers microhardness test was employed, with results represented in Fig. 10a. The graph shows that the hardness values for Ni-P and Ni-P with 0.25, 0.50, and 0.75 g/L Y2O3NPs are 525 HV, 645.37 HV, 685.67 HV, and 764.7 HV, respectively. The addition of Y2O3NPs within the Ni-P matrix has significantly enhanced its mechanical properties, with the highest microhardness value of 764.7 HV observed for 0.75 g/L Y2O3NPs, representing an approximate 45.65% increase over the blank Ni-P coating. The improvement in the microhardness of the modified developed nanocomposite coatings is attributed to a phenomenon known as dispersion hardening1,5,17,52. The hard and insoluble Y2O3 nanoparticles within the Ni-P matrix act as obstacles to dislocation movement, thereby blocking further deformation. Additionally, Y2O3NPs serve as barriers, forcing dislocations to alter their paths, anticipated to the dispersion hardening mechanism. Furthermore, the dislocation motion at grain boundaries is hindered by the strength provided through the grain refinement process. These phenomena of dispersion hardening and grain refinement strengthening are in accordance with the previous literature1,2,19,52. Concurrently, the difference in microhardness values shown in Fig. 10a indicate some level of compositional non-uniformity in the structure of electroless Ni–P–Y2O3 coatings. Variations of this type have been previously documented for electroless Ni–P coatings with ceramic additions of HfC1TiC2or B4C36. Such variations are usually a result of non-uniform distribution of particles due to their agglomeration, gravitational settling, and low dispersion stability during the deposition process. In this work, continuous magnetic stirring was applied, but the lack of ultrasonic agitation may have decreased the uniformity of the particle distribution, causing microstructural regions with different reinforcement content. Scatter in the values of hardness is most probably the result of heterogeneities in local concentration of reinforcement, which is known to be sensitive to the local concentration of reinforcements. Suggested future work includes the use of surfactants or ultrasonic-assisted deposition for more stable dispersion during coating to improve uniformity of composition. Additionally, a nanoindentation test was conducted using the nanoindenter head (Berkovich diamond indenter tip) connected to AFM which uses the maximum 1mN indentation force at loading and unloading rate of 200 µN/s and having dwell time of 5s. The load-displacement curves of NiP and NiP-Y2O3NPs composite coatings, having various load-displacement curves to evaluate the mechanical characteristics of Ni-P and Ni-P-Y2O3 nanocomposite coatings at the nanometer scale. A line graph of the process of loading and unloading of force for Ni-P and Ni-P-Y2O3 nanocomposite coatings is shown in Fig. 10b. Further analysis indicates that the area beneath the line graph for Ni-P is larger than Ni-P-Y2O3, and this area decreases consistently with increasing concentrations of Y2O3NPs (0.25, 0.50, 0.75 g/L), suggesting that Ni-P-Y2O3 nanocomposite coating (0.75 g/L) has higher hardness and are resistant against deformation suggesting that Ni-P-Y2O3NPs are more robust compared to Y2O3 free nanoparticles. Additionally, the final indentation depth of Ni-P nanocomposite coatings is approximately ≈ 129.1 nm, whereas for Ni-P-Y2O3 nanocomposite coatings the depths are approximately ≈ 103.03 nm, ≈ 101.12 nm, and ≈ 96.39 nm for 0.25, 0.50, and 0.75 g/L, respectively, with further results are represented in Table 7. This indicates superior resistance to indentation and improved mechanical properties62. Furthermore, both Ni-P and Ni-P-Y2O3 nanocomposite coatings exhibit pores-free and a multiphase structure, as indicated by the lack of visible kinks in the nanoindentation curves. This is advantageous because the presence of pores in coatings could lead to micro collapse beneath the indenter, resulting in variable stress-strain analysis distributions16. The reduction in holding time (dwell time) in loading/unloading curves with concentration increases of Y2O3 is primarily due to the rise in hardness of the Ni-P-Y2O3 nanocomposite coatings. Harder coating materials, like the ones with higher content of Y2O3, have greater elastic behavior, which leads to less plastic deformation under load. As a result, the stabilization time in the hold segment (dwell time) reduces. On the other hand, softer films such as pure Ni-P experience greater plastic deformation owing to their lower hardness, which is not completely reversible upon unloading. This results in higher dwell time, as the material takes longer to reach equilibrium17,50,56. Moreover, the hold segment in softer films could be a sign of creep behavior, observing their poor elastic recovery during unloading. But the creep effect is minimized by the introduction of Y2O3 nanoparticles, which indicates the enhanced elastic properties of the Ni-P-Y2O3 coatings. The enhancement implies that Y2O3 increases the material’s resistance to permanent deformation and enhances its ability to elastically recover, which indicates its enhanced mechanical performance.
Corrosion behavior
The corrosion behavior of the Ni–P and Ni–P–Y2O3 coatings is not only influenced by composition and passive film formation, but also significantly affected by the grain size and microstructure of the coating. The broad XRD patterns indicate an amorphous or nanocrystalline structure, especially at higher phosphorus contents. Such microstructures are known to reduce the density of grain boundaries, which typically act as pathways for corrosion initiation. Additionally, the uniform dispersion of Y2O3 nanoparticles likely contributes to a more compact and homogeneous matrix, further enhancing the barrier effect. These structural features collectively improve corrosion resistance by minimizing localized electrochemical activity and hindering corrosive species from penetrating the coating. These factors affect the material’s electrochemical behavior and exposure to corrosion63. To assess the corrosion behavior of Ni–P-Y2O3 nanocomposite coatings with different concentrations of Y2O3NPs, electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PP) analysis were employed. The corrosion evaluations were performed in a 3.5 wt% NaCl solution at room temperature 25°C1,2. To improve the clarity of the corrosion process, Fig. 11 illustrates a schematic representation of the overall corrosion mechanism for both the cross-section of the coating (b-c) and the uncoated substrate (a). This diagram provides a detailed visualization of corrosion progression, highlighting the differences between the coated and uncoated regions.
Electrochemical impedance spectroscopy (EIS)
To analyze the corrosion performance of Ni-P and Ni-P-Y2O3 nanocomposite coatings, the EIS method was employed in a 3.5 wt% NaCl solution (35 g/L). To assess the impact of Y2O3 on the corrosion resistance of the coatings, OCP, EIS, and Tafel polarization techniques were used, with results compared to the bare substrate and plain Ni-P coating. The OCP plots in Fig. 12 illustrate that over a period of 4000 s in a 3.5 wt% NaCl solution, the Ni-P coating, serving as a protective barrier, reached a final OCP value of -0.45 mV, indicating the onset of corrosion. However, the OCP value for the reinforced Y2O3NPs gradually increased, reaching − 0.3,-0.34 and − 0.24mV for concentration of 0.25, 0.50 and 0.75 g/L respectively after 4000 s. This highlights the beneficial role of Y2O3 in enhancing the chemical stability of Ni-P coatings. However, the Ni-P-0.75Y2O3 coatings exhibited lower OCP values of -0.24 mV suggesting reduced corrosion resistance compared to the plain Ni-P coating − 0.45 mV. This decline is attributed to the inferior surface quality of coatings produced with higher Y2O3 concentrations27,28 The EIS Bode plots for carbon steel, Ni-P, and Ni-P-Y2O3 nanocomposite coatings are illustrated in Fig. 13. Initially, the coated samples experienced open circuit potential (OCP) for 4000 s, with an exposed area of 2.86 cm², in 3.5 wt% NaCl solution. To fit the results for Ni-P and Ni-P-Y2O3 nanocomposite coatings a two-time constant equivalent circuit model was applied and one-time constant equivalent circuit model for Carbon steel was utilized, as depicted in Fig. 14. Several parameters were determined, including the charge transfer resistance (Rct), pore resistance (Rpo), and solution resistance (Rs), along with the constant phase elements (CPE1 and CPE2), using Eq. (2) as an alternative of a capacitor for the electroless plated substrate, based on the EIS plot data7.
where Q denotes admittance (the CPE constant), ω represents the angular frequency of the AC signal whereas j represents the imaginary unit as well as n is the exponent of the CPE. When n equals 1, the element functions as a pure capacitor; when n equals zero, it acts as a pure resistor; and for 0 < n < 1, it signifies a deviation from ideal behavior. In addition, Protection Efficiency (PE%) is determined through the Eq. (3), where Rct2 represents the charge transfer resistance of Ni-P and Ni-P-Y2O3 coatings and Rct1 represents the charge transfer resistance of blank steel substrate. This protective efficiency evaluates the relative enhancement in corrosion resistance, offering a quantitative assessment of the protective intervention’s efficacy. As shown in Fig. 13, it was found that carbon steel has the lowest corrosion resistance at approximately 448 Ωcm2. In contrast, the Ni-P coatings demonstrate a significantly higher corrosion resistance, reaching around 1251 Ωcm2. This enhancement is accredited to the formation of a hypophosphite layer6,27,28,40. Moreover, the integration of Y2O3 NPs into the Ni-P matrix improves the corrosion resistance as shown by the increased in impedance value. The presence of durable, inert, and non-reactive Y2O3NPs reduces active corrosion sites, leading to higher impedance values in nanocomposite coatings. This is evident from the impedance value for 0.75 g/L Y2O3 nanoparticles, which exceeds that of blank Ni-P coatings. As stated in Table 8, the charge transfer resistance (Rct) of Carbon steel is found to be 448 Ωcm² which then enhanced to 1251, 3525, 5045, 7699 Ωcm2 for Ni-P, Ni-P-Y2O3 (0.25 g/L,0.50 g/L,0.75 g/L) respectively. This phenomenon is ascribed to obstructing micropores inside Ni-P matrix due to the incorporation of Y2O3 nanoparticles. This blockage disrupts the conductive electrolyte routes, leading to observed improvements in Rct and hence, higher corrosion resistance as well as incorporation of Y2O3 within the Ni-P matrix reduces the conductive path hence increasing pore resistance(Rpo)64. CPE1 models the dielectric behavior of the coating surface, while CPE2 reflects the electrochemical response at the coating substrate interface. For the Ni–P coating, a relatively high CPE1 value of 108.2 × 10−6 F·cm−2·s(α−1)¹⁾ and low n 0.437 indicate a non-uniform, porous surface with poor capacitive performance. With Y2O3 addition, CPE1 increased slightly at 0.25 g/L due to surface area effects but then decreased significantly to 84.9 × 10−6 at 0.75 g/L, accompanied by improved n values, suggesting enhanced surface uniformity and reduced dielectric heterogeneity. Similarly, CPE2 increased from 11.52 × 10−6 (Ni–P) to 782.9 × 10−6 at 0.50 g/L Y2O3, indicating poor barrier properties at the substrate. At 0.75 g/L, CPE2 dropped to 190.0 × 10−6, likely due to the formation of a compact interface with fewer electroactive sites and thus better corrosion resistance. The relatively stable m values across samples 0.75–0.95 support consistent non-ideal capacitive behavior at the interface, Similar trend can be observed in the previous literature where after the addition of the nanoparticle the enhanced uniformity and reduced dielectric heterogeneity can be observed52. In addition, the increase in mechano-corrosion performance with increasing Y2O3 content can be correlated with some surface-related features. Firstly, the decrease in CPE1 values and increase of n exponents indicate a coating surface that is somewhat denser and uniform, resting local galvanic sites and the penetration of the electrolyte. Meanwhile, lower CPE2 and higher Rct values mean stronger interfacial barrier properties, so that limited charge transfer occurs and corrosion reactions are suppressed at the coating–substrate interface. In terms of mechanics, Y2O3 nanoparticle reinforcement improves microhardness of the coatings, undoubtedly through grain refinement and particle dispersion strengthening. These improvements lower the capacity for crack formation during loading. The coating layer, which is found to be more compact and adherent, also supports the behavior observed such as the decrease in dielectric activity and increase in Rpo represent the developing of a resistivity barrier to mechanical and corrosive attack. The synergic improvement of surface morphology, electrochemical behavior, and microstructural integrity enables increased mechano-corrosion2,20,40,52. The capacitive loops in Nyquist plots for carbon steel, Ni-P, and Ni-P-Y2O3 NPs are illustrated in Fig. 13c. The corrosion protection efficiency (PE) shown in Table 8 also demonstrated the excellent corrosion resistance behavior of the Ni-P-Y2O3 nanocomposite coatings. This PE is also observed to be increased by an increase in the concentration of the Y2O3NPs. To introduce clarity about the corrosion, process the uncoated normal carbon steel substrate, as indicated in Fig. 11 (a), is highly prone to corrosion due to the absence of any protective coating. However, Fig. 11 (b) indicates the cross-section of the Ni-P coating, which depicts long, and straight morphology of the grains deposited and the gravitational effect. These interfaces are the best path for corrosion propagation, and once the passive layer is destroyed, the process of corrosion goes on unchecked along these interfaces. However, the Ni-P-Y2O3 coating, as shown in Fig. 11 (c), displays a distinct net-like cluster morphology. The Y2O3 nanoparticles co-deposited act as effective barriers, disrupting the development of corrosion paths and inhibiting columnar growth. This structural change significantly inhibits the progression of corrosion and becomes increasingly harder for corrosive media to progress into the underlying substrate.
Potentiodynamic polarization (PP) analysis
Figure 15 represents the results of PP analysis for Ni-P and Ni-P-Y2O3 nanocomposite coatings, which were immersed in a 3.5 wt% NaCl of volume 400mL solution at room temperature. Table 9 displays various results, including Ecorr (corrosion potential), icorr (corrosion current density), βc (Cathodic Tafel slope), βa (anodic Tafel slope), Rp (polarization resistance), and PE% (protection efficiency) attained after fitting data from PP analysis of the nanocomposite coatings. To validate the parameters, the following equations were incorporated:
To gain a comprehensive understanding of this phenomenon, it is crucial to grasp the significance of icorr1 and icorr2. icorr1 refers to the corrosion current density value of carbon steel, while icorr2 represents the corrosion current density of Ni-P and Ni-P-Y2O3 nanocomposite coatings. Carbon steel serves as a reference point for comparison to determine the protection efficiency. Note that the highest value of icorr (corrosion current density) for carbon steel is 43.00 µA/cm². A significant decrease in icorr for Ni-P (23.6 µA/cm²), and an even further reduction is observed after the addition of Y2O3 nanoparticles into the Ni-P matrix (ranging from 15.3 µA/cm² to 7.41 µA/cm²). This validates the improved corrosion resistance. The enhanced corrosion properties can be attributed to the decrease in defects within the metallic coatings, which otherwise serve as active sites for initiating corrosion65. Lastly, a significant change can be observed in both the anodic and cathodic Tafel slopes, which consistently decrease. This validates the reduction of the hydrogen cathodic reduction reaction and the anodic metal dissolution reaction. However, as observed Ni-P coated samples exhibit poor corrosion protection efficiency (PE%), which is approximately 45.11%. This confirms the formation of corrosion concentration cells due to the phenomenon of pitting corrosion at the nodule boundaries of Ni-P. This occurs due to the absorption of aggressive Cl− ions, which triggers the formation of NiCl2 ions66. The increase in corrosion protection efficiency after incorporating hard, insoluble Y2O3 nanoparticles into the Ni-P matrix can be attributed to the limited adsorption of aggressive hydrated Cl− ions. This reduction in adsorption decreases the number of active sites available for the formation of corrosion concentration cells, thereby enhancing corrosion resistance52,57,67.
Conclusion
Ni-P-Y2O3 nanocomposite coatings with varied Y2O3 nanoparticle concentrations were successfully synthesized via electroless deposition, demonstrating that Y2O3 concentration plays a pivotal role in enhancing the structural, microstructural, and surface characteristics of the coatings. The incorporation of 0.75 g/L Y2O3 yielded optimal mechanical and corrosion-resistant properties, with a significant increase in hardness (~ 764.7 HV) and enhanced corrosion resistance (82.76%) when compared to unmodified Ni-P coatings. The substantial improvement in microhardness is attributed to grain refinement and the dispersion-strengthening effect imparted by the uniformly distributed Y2O3 nanoparticles. Furthermore, the superior corrosion resistance observed in the 0.75 g/L Ni-P-Y2O3 coating can be linked to the effective blockage of micropores and reduction in the active surface area, as the inert Y2O3 particles serve to impede corrosive pathways within the Ni-P matrix. Lastly, the findings confirm that Ni-P-Y2O3 nanocomposite coatings have promising structural, mechanical and anticorrosive properties which make them viable for application in industry .
Data availability
The data that has been used is confidential. The corresponding author Dr. Abdul Shakoor should be contacted in case of any request for data.
References
Farhan, M., Fayyaz, O., Nawaz, M., Radwan, A. B. & Shakoor, R. A. Synthesis and properties of electroless Ni–P-HfC nanocomposite coatings. Mater. Chem. Phys. 291, 126696 (2022).
Farhan, M. et al. Mechanical and corrosion characteristics of tic reinforced Ni-P based nanocomposite coatings. Mater. Today Commun. 36, 106901 (2023).
Shakoor, R. A., Kahraman, R., Gao, W. & Wang, Y. Synthesis, characterization and applications of electroless Ni-B coatings-A review. Int. J. Electrochem. Sci. 11, 2486–2512 (2016).
Ahmad, S. et al. Modified Ni-Al layer double hydroxides as nanoparticles for self-healing anti-corrosion composite coating. Surf. Coat. Technol. 476, 130172 (2024).
Li, B. & Zhang, W. Microstructural, surface and electrochemical properties of pulse electrodeposited Ni–W/Si3N4 nanocomposite coating. Ceram. Int. 44, 19907–19918 (2018).
Fayyaz, O., Farhan, M., Bagherifard, S. & Shakoor, R. A. Impact of calcium zirconate nanoparticles on the mechanical and anti-corrosion properties of Ni-P coatings. Mater. Chem. Phys. 318, 129294 (2024).
Ahmadi Ashtiani, A., Faraji, S., Amjad Iranagh, S. & Faraji, A. H. The study of electroless Ni–P alloys with different complexing agents on Ck45 steel substrate. Arab. J. Chem. 10, S1541–S1545 (2017).
Shahzad, K. et al. Effect of concentration of tic on the properties of pulse electrodeposited Ni–P–TiC nanocomposite coatings. Ceram. Int. 47, 19123–19133 (2021).
Momenzadeh, M. & Sanjabi, S. The effect of TiO 2 nanoparticle codeposition on microstructure and corrosion resistance of electroless Ni-P coating. Mater. Corros. 63, 614–619 (2012).
Fayomi, O. S. I., Akande, I. G. & Odigie, S. Economic impact of corrosion in oil sectors and prevention: an overview. J. Phys. Conf. Ser. 1378, (2019).
Mozetič, M. Surface modification to improve properties of materials. Materials. 12, (2019).
Rasooli, A. et al. Enhanced tribomechanical and electrochemical performance of the Ni–P electrodeposits reinforced by duplex Cr2O3 and ZrO2 ceramic nanoparticles. J. Mater. Res. Technol. 35, 4566–4577 (2025).
Ossai, C. I., Boswell, B. & Davies, I. J. Pipeline failures in corrosive environments - A conceptual analysis of trends and effects. Eng. Fail. Anal. 53, 36–58 (2015).
Habib, S., Fayyed, E., Shakoor, A., Kahraman, R. & Abdullah, A. Improved self-healing performance of polymeric nanocomposites reinforced with Talc nanoparticles (TNPs) and urea-formaldehyde microcapsules (UFMCs). Arab. J. Chem. 14, 102926 (2020).
Nawaz, M. et al. Development and properties of polymeric nanocomposite coatings. Polym. (Basel). 11, 852 (2019).
Hu, R. et al. Deposition process and properties of electroless Ni-P-Al2O3 composite coatings on magnesium alloy. Nanoscale Res. Lett. 13, 2–9 (2018).
Shakoor, R. A. et al. Novel electrodeposited Ni-B/Y 2 O 3 composite coatings with improved properties. Coatings 7, 1–10 (2017).
Badihehaghdam, M., Mousavi Khoie, S. M., Khast, F. & Safarzadeh khosrowshahi, M. Mechanical properties and electrochemical behavior of electroless Ni–P-AlN nanocomposite coating. Met. Mater. Int. 28, 1372–1385 (2022).
Yusuf, M. M. et al. Synthesis and characterisation of Ni–B/Ni–P–CeO2 duplex composite coatings. J. Appl. Electrochem. 48, 391–404 (2018).
Shakoor, R. A., Kahraman, R., Waware, U., Wang, Y. & Gao, W. Properties of electrodeposited Ni-B-Al2O3 composite coatings. Mater. Des. 64, 127–135 (2014).
Yang, Y., Chen, W., Zhou, C., Xu, H. & Gao, W. Fabrication and characterization of electroless Ni–P–ZrO2 nano-composite coatings. Appl. Nanosci. 1, 19–26 (2011).
Ogihara, H., Wang, H. & Saji, T. Electrodeposition of Ni-B/SiC composite films with high hardness and wear resistance. Appl. Surf. Sci. 296, 108–113 (2014).
Zhang, Y., Yu, M., Chen, C., Li, S. M. & Liu, J. H. Self-Healing coatings based on Stimuli-Responsive release of corrosion inhibitors: A review. Front. Mater. 8, 1–14 (2022).
Ferraris, F., Parvis, M., Angelini, E. & Grassini, S. Measuring system for enhanced cathodic corrosion protection. In 2012 IEEE I2MTC - Int. Instrum. Meas. Technol. Conf. Proc. 1583–1587 https://doi.org/10.1109/I2MTC.2012.6229305 (2012).
Lin, J. D. & Chou, C. T. The influence of phosphorus content on the microstructure and specific capacitance of etched electroless Ni-P coatings. Surf. Coat. Technol. 368, 126–137 (2019).
Barati, Q. & Hadavi, S. M. M. Electroless Ni-B and composite coatings: A critical review on formation mechanism, properties, applications and future trends. Surf. Interfaces. 21, 100702 (2020).
Luo, H., Leitch, M., Zeng, H. & Luo, J. L. Characterization of microstructure and properties of electroless duplex Ni-W-P/Ni-P nano-ZrO2 composite coating. Mater. Today Phys. 4, 36–42 (2018).
Luo, H., Wang, X., Gao, S., Dong, C. & Li, X. Synthesis of a duplex Ni-P-YSZ/Ni-P nanocomposite coating and investigation of its performance. Surf. Coat. Technol. 311, 70–79 (2017).
Das, A. K. & Kumar, R. Investigation on wear behaviour of TiC/Co/Y2O3 metal matrix composite coating developed on AZ91D Mg alloy by plasma transferred Arc cladding process. Mater. Lett. 355, (2024).
Fayyad, E. M. et al. Effect of electroless bath composition on the mechanical, chemical, and electrochemical properties of new NiP–C3N4 nanocomposite coatings. Surf. Coat. Technol. 362, 239–251 (2019).
Wang, Y. et al. Duplex Ni-P-ZrO2/Ni-P electroless coating on stainless steel. J. Alloys Compd. 630, 189–194 (2015).
Liang, Y., Zhang, B., Liu, Z. & Liu, W. Electroless deposition surface engineering of Boron nitride sheets for enhanced thermal conductivity and decreased interfacial thermal resistance of epoxy composites. Int. J. Heat. Mass. Transf. 174, 121306 (2021).
Farzaneh, A., Mohammadi, M., Ehteshamzadeh, M. & Mohammadi, F. Electrochemical and structural properties of electroless Ni-P-SiC nanocomposite coatings. Appl. Surf. Sci. 276, 697–704 (2013).
Fayyad, E. M. et al. Novel electroless deposited corrosion — resistant and anti-bacterial NiP–TiNi nanocomposite coatings. Surf. Coat. Technol. 369, 323–333 (2019).
Sadeghzadeh-Attar, A., AyubiKia, G. & Ehteshamzadeh, M. Improvement in tribological behavior of novel sol-enhanced electroless Ni-P-SiO2 nanocomposite coatings. Surf. Coat. Technol. 307, 837–848 (2016).
Fayyaz, O., Yusuf, M. M., Bagherifard, S., Montemor, M. F. & Shakoor, R. A. Probing into the properties of B4C reinforced nickel phosphorus-based nanocomposite coating. J. Mater. Res. Technol. 20, 2323–2334 (2022).
Kilicarslan, A., Toptan, F. & Kerti, I. Electroless nickel-phosphorus coating on boron carbide particles. Mater. Lett. 76, 11–14 (2012).
Liu, Y. Y. et al. Synthesis and tribological behavior of electroless Ni-P-WC nanocomposite coatings. Surf. Coat. Technol. 201, 7246–7251 (2007).
Chen, W., Gao, W. & He, Y. A novel electroless plating of Ni-P-TiO2 nano-composite coatings. Surf. Coat. Technol. 204, 2493–2498 (2010).
Shahzad, K. et al. Corrosion and heat treatment study of electroless nip-ti nanocomposite coatings deposited on Hsla steel. Nanomaterials 10, 1–19 (2020).
Wang, S., Huang, X., Gong, M. & Huang, W. Microstructure and mechanical properties of Ni-P-Si 3 N 4 nanowire electroless composite coatings. Appl. Surf. Sci. 357, 328–332 (2015).
Zhou, X. W., Shen, Y. F. & Jin, H. M. Study on the preparation and anti-crossion performance of Ni-P/n-CeO2 composite coatings. Gongneng Cailiao/Journal Funct. Mater. 42, 305–309 (2011).
Tian, L. & Xu, J. Electrodeposition and characterization of Ni-Y 2 O 3 composite. Appl. Surf. Sci. 257, 7615–7620 (2011).
Zhang, Q. et al. Influence of Y2O3 content on the properties of Ni-Y2O3-MgO nanocomposite coatings prepared by pulse electrodeposition. Int. J. Electrochem. Sci. 19, 100769 (2024).
Fathi, M., Safavi, M. S., Mirzazadeh, S., Ansariyan, A. & Ahadzadeh, I. A. Promising horizon in mechanical and corrosion properties improvement of Ni-Mo coatings through incorporation of Y2O3 nanoparticles. Metall. Mater. Trans. Phys. Metall. Mater. Sci. 51, 897–908 (2020).
Meshram, A. P., Punith Kumar, M. K. & Srivastava, C. Enhancement in the corrosion resistance behaviour of amorphous Ni–P coatings by incorporation of graphene. Diam. Relat. Mater. 105, 107795 (2020).
Tian, M., Jian, Z., Chang, F. & Hai, R. Properties of electroless thick nickel–phosphorus coating on sicp/al composite surface in acidic conditions. J. Mater. Sci. 58, 1886–1904 (2023).
Jenila, R. M., Rajasekaran, T. R. & Charles, J. B. Influence of Mg 2 + on the mechanical properties of ammonium tetra fluoro antimonates. 3, 1–10 (2012).
Crawford, R. J. Microhardness testing of plastics. Polym. Test. 3, 37–54 (1982).
Jayasankar, K., Pandey, A., Mishra, B. K. & Das, S. Evaluation of microstructural parameters of nanocrystalline Y2O3 by X-ray diffraction peak broadening analysis. Mater. Chem. Phys. 171, 195–200 (2016).
Li, X. et al. One-step synthesis and characterization of Y2O3 nanoparticles via emulsion detonation method. Ceram. Int. 50, 27995–28003 (2024).
Bahgat Radwan, A. et al. Properties enhancement of Ni-P electrodeposited coatings by the incorporation of nanoscale Y 2 O 3 particles. Appl. Surf. Sci. 457, 956–967 (2018).
Wojewoda-Budka, J. et al. Microstructure characteristics and phase transformations of the Ni-P and Ni-P-Re electroless deposited coatings after heat treatment. Electrochim. Acta. 209, 183–191 (2016).
Agredo Diaz, D. G. et al. Effect of a Ni-P coating on the corrosion resistance of an additive manufacturing carbon steel immersed in a 0.1 M NaCl solution. Mater. Lett. 275, 1–4 (2020).
Chaudhari, A. K., Singh, V. B. & Singh, B. Structural features and functional properties of Y2O3 reinforced nickel matrix nanocomposites. ChemistrySelect 5, 11573–11582 (2020).
Wang, J. et al. Effects and influence of Y2O3 addition on the microstructure and mechanical properties of binderless tungsten carbide fabricated by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 71, 167–174 (2018).
Shakoor, R. A. & Bahgat Radwan, A. Remarkable improvement in properties of NI-B coatings by the addition of mixed oxide nanoparticles. Mater. Sci. Technol. Conf. Exhib. MS T 2015 1, 171–178 (2015).
Liu, M. et al. Yttrium oxide as a Q-switcher for the near-infrared erbium-doped fiber laser. Nanophotonics 9, 2887–2894 (2020).
Fayyaz, O. et al. Enhancement of mechanical and corrosion resistance properties of electrodeposited Ni–P–TiC composite coatings. Sci. Rep. 11, 1–16 (2021).
Song, J. B., Kim, J. T., Oh, S. G. & Yun, J. Y. Contamination particles and plasma etching behavior of atmospheric plasma sprayed Y2O3 and YF3 coatings under NF3 plasma. Coatings 9, 1–8 (2019).
Kumar, A., Singh, V. P., Singh, R. C., Chaudhary, R. & Kumar, D. Enhancing microstructural, tribological and corrosion responses of Al–Zn–Mg–Cu alloy via nano-/micro-Al2O3 particulates. J. Mater. Sci. 59, 7235–7257 (2024).
Temam, H., Ben, Chala, A. & Rahmane, S. Microhardness and corrosion behavior of Ni-SiC electrodeposited coatings in presence of organic additives. Surf. Coat. Technol. 205, 161–164 (2011).
Chen, J., Zou, Y., Matsuda, K. & Zhao, G. Effect of Cu addition on the microstructure, thermal stability, and corrosion resistance of Ni–P amorphous coating. Mater. Lett. 191, 214–217 (2017).
Sliem, M. H. et al. Enhanced mechanical and corrosion protection properties of pulse electrodeposited NiP-ZrO2 nanocomposite coatings. Surf. Coat. Technol. 403, 126340 (2020).
Zou, Y. et al. Ultrasonic-Assisted electroless Ni-P plating on dual phase Mg-Li alloy. J. Electrochem. Soc. 162, C64–C70 (2015).
Song, Y. W., Shan, D. Y. & Han, E. H. Comparative study on corrosion protection properties of electroless Ni-P-ZrO2 and Ni-P coatings on AZ91D magnesium alloy. Mater. Corros. 58, 506–510 (2007).
Sharifalhoseini, Z. & Entezari, M. H. Enhancement of the corrosion protection of electroless Ni–P coating by deposition of sonosynthesized ZnO nanoparticles. Appl. Surf. Sci. 351, 1060–1068 (2015).
Acknowledgements
The authors express their gratitude to the Central Laboratory Unit (CLU), at Qatar University for providing microstructural analysis services (FE-SEM/EDS).
Funding
This research was funded by the Qatar Research Development and Innovation Council (QRDI), grant number ARG02-0413-240176.
Author information
Authors and Affiliations
Contributions
Contributor’s Roles: Muhammad Ahmad: Methodology, Formal analysis, Investigation, Writing – original draft. Ahmad Saadi Samra: Methodology, Conceptualization, Validation. Sehrish Habib: Review & editing.Ramazan Kahraman: Resources, Formal analysis, review & editing.Bilal Mansoor: Resources, Formal analysis. R. A. Shakoor: Supervision, Writing – review & editing, Project administration, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ahmad, M., Samra, A.S., Habib, S. et al. Mechanical properties and corrosion resistance of electroless deposited Ni-P-Y2O3 nanocomposite coatings for industrial applications. Sci Rep 15, 26707 (2025). https://doi.org/10.1038/s41598-025-12319-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-12319-6