Abstract
Immune-related factors are closely associated with tumor progression and responses to immunotherapy. A systematic analysis of the immunogenomic landscape and the identification of key immune-related genes (IRGs) can contribute to a better understanding of pancreatic cancer. To identify immune-related genetic prognostic characteristics (IRGPs) of pancreatic cancer, we first constructed an IRGP model containing multiple immune-related genes and determined the relative contributions of each gene using coefficients from the Least Absolute Shrinkage and Selection Operator (LASSO) Cox regression analysis. Subsequently, the prognostic value of the signature was validated through receiver operating characteristic (ROC) curve analysis and Kaplan-Meier survival analysis. Additionally, we explored the potential relationship between IRGPs and immune cell infiltration. Nine gene prognostic features were identified as the optimal IRGPs, which include six high-risk genes and three low-risk genes. Principal Component Analysis (PCA) demonstrated that this feature can effectively distinguish between high-risk and low-risk groups. The area under the curve (AUC) value indicated that IRGPs provide better prognostic clinical utility compared to existing TNM staging classifications. The median overall survival (OS) of high-risk patients was significantly shorter, and the infiltration levels of 24 immune cell types were lower. This study identified nine genes that have been identified as important prognostic biomarkers with immune-related characteristics for pancreatic cancer. Furthermore, we also explored the role of AIM2 in pancreatic cancer among the immune-related signature genes. AIM2 may influence the immune invasion and immunotherapy of pancreatic cancer by promoting the inflammatory environment of pancreatic cancer. AIM2 could be a new therapeutic target for pancreatic cancer.
Similar content being viewed by others
Introduction
Pancreatic cancer has the poorest prognosis among all solid malignancies, with a median survival time of 6 months1. Over the past decades, both the incidence rate and mortality of pancreatic cancer have been rising steadily2,3. It is estimated that by 2030, pancreatic cancer will become the second leading cause of cancer deaths in the United States4. This highly fatal malignant tumor is characterized by a lack of specific symptoms and no reliable early detection methods. Approximately 80-85% of patients are diagnosed with locally advanced disease or distant metastasis, which makes surgical intervention infeasible5,6. It is reported that only 15-20% of pancreatic tumors can be completely removed by surgery, and patients often experience local or distant recurrence within 2 years after surgery7,8,9,10. Systemic chemotherapy is currently the mainstay of treatment for advanced pancreatic cancer; however, due to the chemorefractory nature of the disease, most patients die within 12 months of diagnosis11. Therefore, there is an urgent need to explore reliable and non-invasive prognostic biomarkers to guide decision-making and treatment optimization. Although the current TNM staging system remains the standard for predicting the prognosis of patients with solid malignancies, including pancreatic cancer, patients with the same stage may have very different prognoses for the same specific disease, and disease outcomes in pancreatic cancer remain highly variable among patients with similar TNM stages12,13,14. Consequently, there is an urgent need to explore reliable prognostic biomarkers for pancreatic cancer, with the hope that such biomarkers can provide independent yet complementary prognostic factors to enhance the prognostic value of the TNM staging system. Currently, a wealth of data indicates that immune markers in the tumor microenvironment can serve as new independent prognostic indicators, with prognostic value comparable to or even surpassing that of the TNM staging system15,16,17,18.
Recently, immune checkpoint inhibitors (ICIs) have emerged as a promising treatment option for advanced cancers, including pancreatic cancer, by reversing the dysfunction of anti-tumor T cells and activating the immune system to recognize and eliminate cancer cells19,20,21. The therapeutic response is contingent upon immune checkpoint activation, which is closely related to tumor antigen-mediated immunity and the presence of a large number of activated immune cells in the tumor microenvironment, including CD8+ T cells, CD4+ T cells, Th1 cells, and cytotoxic cells22,23,24. Microsatellite instability has been utilized to guide the clinical management of pancreatic cancer25. However, this approach is only applicable to a small subset of patients. Therefore, there remains significant room for the development of biomarkers.
The immune regulatory signals within the tumor microenvironment modulate T cell responses, and the interplay between tumor genetics and tumor immune features plays a pivotal role in cancer pathogenesis, progression, and response to immune checkpoint inhibitor (ICI) treatment15,26. Consequently, we endeavored to identify promising prognostic factors and elucidate the molecular mechanisms underlying the interactions between tumor cells and immune cells within the tumor microenvironment (TME). Pancreatic cancer is characterized by a high degree of genomic heterogeneity, suggesting that the significance of a single genetic biomarker is limited27. The development of prognostic characteristics based on the analysis of the pancreatic cancer genome, combined with multiple genes, can significantly enhance the accuracy of prognosis prediction.
In studies exploring the relationship between cancer and inflammation, it has been established that inflammation plays a crucial role in the initiation, development, progression, angiogenesis, and invasion of cancer28. Inflammasomes are multimeric protein complexes formed within cells that play significant roles in host defense, autoinflammation, cancer, neurodegenerative diseases, cardiovascular diseases, obesity, and related metabolic syndromes29,30,31,32. The AIM2 inflammasome, as the prototype and best-characterized member of the ALR family, recognizes double-stranded DNA and recruits an adaptor protein called ASC (apoptosis-associated speck-like protein containing a CARD), thereby inducing caspase-1 activation. This activation leads to the maturation and release of inflammatory cytokines such as interleukin (IL)-1β and pro-IL-18, resulting in active inflammation33,34. A substantial body of evidence indicates that the AIM2 inflammasome is associated with tumor progression, treatment response, and prognosis34,35,36,37. Furthermore, the AIM2 inflammasome plays a key role in preventing tumorigenesis by regulating host immunity38. Therefore, the AIM2 inflammasome holds therapeutic potential in cancer treatment.
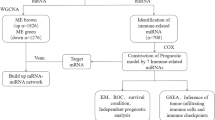
In this study, we aimed to construct an immune-related gene prognostic signature (IRGPs) for pancreatic cancer. We identified a nine-gene signature through a series of analyses, including univariate Cox proportional hazards regression, least absolute shrinkage and selection operator (LASSO) Cox regression, and multivariate Cox proportional hazards regression. We then validated the prognostic value of this signature using principal component analysis (PCA), receiver operating characteristic (ROC) curve analysis, and Kaplan-Meier survival analysis. Additionally, we analyzed the correlation between the prognostic signature and immune infiltration in pancreatic cancer. The development of a prognostic gene signature based on immune-related gene profiles could greatly assist in the prognostic stratification of pancreatic cancer. Furthermore, we examined the role of the AIM2 inflammasome in the occurrence and development of pancreatic cancer, suggesting that AIM2 may influence immune infiltration and immunotherapy in pancreatic cancer by promoting the inflammatory environment. AIM2 could potentially serve as a new therapeutic target for pancreatic cancer.
Results
Construction of immune-related genetic prognostic signatures
We identified 330 immune-related genes from MsigDB2 and performed univariate Cox proportional hazards regression analysis, which revealed that 35 genes were significantly associated with overall survival (OS) in pancreatic cancer patients. Additionally, we conducted LASSO Cox regression to determine the optimal weight coefficients for these 35 genes (Fig. 1A and B). Based on the LASSO Cox regression analysis, the coefficients of genes with relatively small contributions were set to zero. We then performed multivariate Cox proportional hazards regression analysis on the 15 genes with non-zero coefficients and identified a prognostic signature consisting of 9 genes as the best IRGPS from these 15 genes, including TNFRSF4, IL6R, AIM2, IL1R2, ACKR4, ERAP2, FYN, CDK6, and DEFB1. Risk coefficients and hazard ratios (HRs) along with their 95% confidence intervals (CIs) for the nine genes were derived from the multivariate Cox proportional hazards regression analysis (Supplementary Table 1).
Construction and validation of immune-related genetic prognostic signatures. (A-B) LASSO Cox regression analysis was performed on 35 immune-related genes to refine the selection. (C) Displays the distribution of risk scores among patients. (D) Illustrates the survival status of patients based on the prognostic model. (E) Presents the expression levels of the genes in the TCGA-PAAD cohort. (F-H) Principal Component Analysis (PCA) of the expression distributions for: (F) The prognostic signature genes. (G) The entire immune-related gene set. (H) All genes in the gene expression profile, comparing high-risk and low-risk groups. (I) Univariate Cox proportional hazard regression analyses were conducted with risk Score and various clinicopathological features, including T, N, and M stages, pathological stage, smoking status, gender, and age. (J) Multivariate Cox proportional hazard regression analyses were also conducted with risk Score and the same set of clinicopathological features. (K) The prognostic value of the signature was assessed using the ROC curve analysis. (L) Kaplan-Meier survival analysis of pancreatic cancer patients from the TCGA, stratified by the median risk score.
To stratify all samples into high or low immune risk groups, we calculated the individualized risk score for each patient based on the gene expression levels and the coefficients of each gene marker: Risk score = (-0.23280199× IL6R mRNA expression level) + (-0.20976× TNFRSF4 mRNA expression level) + (0.343246477× AIM2 mRNA expression level) + (0.11303796× IL1R2 mRNA expression level) + (0.19380208× ACKR4 mRNA expression level) + (0.17512558× ERAP2 mRNA expression level) + (-0.56166735× FYN mRNA expression level) + (0.53784218× CDK6 mRNA expression level) + (0.0959681× DEFB1 mRNA expression level). According to the contribution coefficients, the prognostic signature included six risk genes (AIM2, IL1R2, ACKR4, ERAP2, DEFB1, and CDK6) and three protective genes (TNFRSF4, IL6R, and FYN) (Fig. 1C). The TCGA-PAAD cohort patients were divided into high-risk and low-risk groups using the median risk score as the cutoff value. The number of deaths in the high-risk group was significantly higher than that in the low-risk group, and the expression levels of the 6 prognostic risk genes in the high-risk group were higher, while the expression levels of the 3 prognostic protective genes were lower (Fig. 1D and E). Additionally, PCA showed that the nine-gene prognostic signature (Fig. 1F) could better distinguish high-risk and low-risk groups than the 330 immune-related gene sets (Fig. 1G) and all genes in the gene expression profile (Fig. 1H). Thus, a pancreatic cancer IRGPS consisting of nine genes was constructed.
To confirm the prognostic value of the pancreatic cancer risk score, we performed both univariate and multivariate Cox proportional hazards regression analyses, taking into account the risk score along with other clinicopathological characteristics such as T, N, and M stages, pathological stage, smoking status, sex, and age. The results demonstrated that the risk score emerged as an independent prognostic factor (Fig. 1I and J). ROC curve analysis indicated that the IRGPS possessed good sensitivity and specificity for predicting the prognosis of pancreatic cancer, with an area under the curve (AUC) value of 0.826 (Fig. 1K). Kaplan-Meier survival analysis revealed that the median overall survival (OS) of patients in the high-risk group was significantly shorter than that of patients in the low-risk group (p < 0.0001; Fig. 1L). In conclusion, the nine-gene signature stands out as a valuable prognostic biomarker for pancreatic cancer.
IRGPs gene expression analysis and related analyses
We analyzed the expression of the aforementioned nine genes in both cancerous and adjacent normal tissues within the TCGA-PAAD cohort. The results revealed that the expression levels of TNFRSF4, IL6R, AIM2, IL1R2, ACKR4, ERAP2, FYN, and CDK6 were significantly upregulated in cancer tissues (P < 0.001), while DEFB1 expression was downregulated in cancer tissues (P < 0.001) (Fig. 2A). Subsequently, we examined the mutation profiles of these genes in pancreatic cancer and discovered that CDK6 had the highest mutation frequency, at 13%, followed by DEFB1 with a mutation frequency of 10%. The mutation frequencies of the remaining genes were as follows: AIM2 (9%), IL6R (7%), TNFRSF4 (5%), IL1R2 (5%), ACKR4 (4%), ERAP2 (3%), and FYN (2%) (Fig. 2B). The correlations among the nine genes in the IRGPS are depicted in a Circos plot (Fig. 2C), with the correlation coefficients detailed in Supplementary Fig. 1. Gene Ontology (GO) enrichment analysis indicated that these nine genes are involved in the regulation of cytokine secretion, T cell proliferation, and T cell activation in the biological process (BP) group. In the cellular component (CC) group, they were associated with extracellular components, inflammasome complexes, and the outer side of the plasma membrane. In the molecular function (MF) group, they were linked to growth factor receptor binding, cytokine binding, and cytokine receptor activity. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis revealed that these genes are primarily involved in the interaction of viral proteins with cytokines and cytokine receptors, the crosstalk between hematopoietic cell lineages, and the interaction of cytokines with their receptors (Fig. 2D).
Expression and correlation analysis of IRGPS Genes. (A) Differential expression analysis of the nine IRGPS genes in normal and cancer tissues within the TCGA-PAAD cohort. (B) A Circos plot displaying the inter-correlations among the nine immune genes. (C) Mutation data for the nine immune-related genes were retrieved from the cBioPortal website. (D) Signal pathway enrichment analyses were conducted on the nine genes to identify overrepresented pathways. (E-M) Kaplan-Meier survival analysis for each of the nine immune-related genes in the TCGA-PAAD cohort, assessing their prognostic significance individually.
Additionally, we analyzed the association of the nine genes with overall survival (OS) in pancreatic cancer. The findings indicated that patients with low expression levels of ACKR4 (Fig. 2E), AIM2 (Fig. 2F), CDK6 (Fig. 2G), DEFB1 (Fig. 2H), ERAP2 (Fig. 2I), and IL1R2 (Fig. 2J) exhibited a longer median OS. Conversely, for the other genes in the IRGPS, namely FYN, IL6R, and TNFRSF4, higher expression levels were associated with better OS in pancreatic cancer patients (Figs. 2K-M).
Identification of DEG-involved pathways between high-risk and low-risk groups
A total of 212 differentially expressed genes (DEGs) were identified between the high-risk and low-risk groups, comprising 53 upregulated genes and 159 downregulated genes (Fig. 3A and B). GO analysis of these DEGs revealed that in the biological process (BP) group, they were involved in regulating membrane potential, signal release, vesicle-mediated transport in synapses, multicellular biological signaling, synaptic vesicle trafficking, establishment of synaptic vesicle localization, synaptic vesicle localization, cardiac conduction, synaptic vesicle recycling, and cell communication involved in cardiac conduction (Fig. 3C). In the molecular function (MF) group, DEGs were associated with metal ion transmembrane transporter activity, cation channel activity, ion gated channel activity, gated channel activity, ion channel activity, substrate-specific channel activity, potassium ion transmembrane transporter activity, SNARE binding, synaptophysin binding, and synaptophysin-1 binding (Fig. 3D). For the cellular component (CC) group, DEGs were implicated in presynapses, neuronal cell bodies, transmembrane transport complexes, synaptic membranes, cation channel complexes, ion channel complexes, synaptic vesicles, synaptic vesicle membranes, and exocytic vesicle membranes (Fig. 3E). KEGG analysis indicated that DEGs were involved in pathways such as insulin secretion, dopaminergic synapses, cardiomyocyte adrenergic signaling, cAMP signaling pathway, myocardial contraction, calcium reabsorption regulated by endocrine factors, gastric acid secretion, aldosterone synthesis and secretion, proximal tubule bicarbonate recovery, and young adult diabetes (Fig. 3F). According to the results of GSEA analysis, several cancer-promoting signaling pathways were enriched in the high-risk group, including NOTCH signaling, MYC target signaling, protein secretion, epithelial-mesenchymal transition, and TGF-β signaling (Fig. 3G and Supplementary Fig. 2). Other enriched signaling pathways identified in GSEA analysis are presented in Supplementary Table 2.
Signal pathway enrichment analysis of differentially expressed genes between high-risk and low-risk groups. (A-B) Identification of differentially expressed genes between the high-risk and low-risk groups. (C) GO analysis focusing on the biological process group. (D) GO analysis focusing on the molecular function group. (E) GO analysis focusing on the cellular component group. (F) KEGG analysis to identify significant pathways. (G) GSEA performed between high-risk and low-risk groups based on the nine-gene signature.
Correlation analysis between IRGPs and tumor immune microenvironment
Patients in the TCGA-PAAD cohort were stratified into high-infiltration and low-infiltration groups based on the infiltration levels of 24 immune cells, including dendritic cells (DCs), immature DCs (iDCs), activated DCs (aDCs), plasmacytoid DCs (pDCs), B cells, cytotoxic cells, eosinophils, macrophages, mast cells, neutrophils, NK CD56bright cells, NK CD56dim cells, NK cells, CD8+ T cells, T cells, T helper cells, central memory T cells (Tcm), effector memory T cells (Tem), follicular helper T cells (Tfh), gamma-delta T cells (Tgd), Th1, Th2, Th17, and regulatory T cells (Treg). Comprehensive heatmaps were generated to illustrate the associations between immune infiltration of various immune cells and risk scores, age, sex, T stage, N stage, M stage, and pathological stage in both the high-infiltration and low-infiltration groups. The results indicated a significant correlation between the risk score and immune infiltration, with the low-infiltration group exhibiting a higher risk score; however, age, gender, T stage, N stage, M stage, and pathological stage were not significantly correlated with the risk score in the infiltration group (Fig. 4A). We identified differences in the abundance of 24 immune cells between the high-risk and low-risk groups of pancreatic cancer and found that the infiltration levels of T cells, Tem, Th2 cells, Tfh, CD8+ T cells, NK cells, and pDCs varied between the two groups. Except for Th2 cells, the infiltration levels of other immune cells in the high-risk group were lower (Fig. 4B). Further analysis of the relationships between 24 immune cells in the pancreatic cancer microenvironment revealed that CD8+ T cell infiltration was closely related to the response to immune checkpoint inhibitor (ICI) treatment. We observed positive correlations between CD8+ T cell levels in pancreatic cancer and NK cells, T helper cells, Th1 cells, and iDCs, and noted close relationships among DCs, Th1 cells, NK cells, CD8+ T cells, T cells, cytotoxic cells, and T helper cells (Fig. 4C). The correlation between IRGPs gene expression and the abundance of 24 types of immune cells was also studied. As shown in Fig. 4D, AIM2 and FYN exhibited the most significant positive correlation with immune cell infiltration, followed by TNFRSF4 and IL6R. Among the various immune cells, IL1R2 was only positively correlated with the infiltration level of Th2 cells, with a weak correlation (r = 0.27). DEFB1 showed weak to moderate correlations with the infiltration levels of Th17 cells (r = 0.28), mast cells (r = 0.26), macrophages (r = 0.42), neutrophils (r = 0.32), DCs (r = 0.21), iDCs (r = 0.35), CD8+ T cells (r = 0.29), Th1 cells (r = 0.20), and Tem cells (r = 0.20). CDK6 was closely related to the abundance of Th2 cells.
Association analysis between IRGPS and tumor immune microenvironment. (A) Patients of the TCGA-PAAD cohort were stratified into high-infiltration and low-infiltration groups based on immune cell infiltrate levels. (B) Detection of differences in the abundance of 24 immune cell types between the high-risk and low-risk groups. (C) Analysis of inter-correlations between the 24 immune cell types in the pancreatic cancer microenvironment. (D) Investigation of correlations between the expression of IRGPS genes and the abundance of 24 immune cell types using Pearson coefficients in pancreatic cancer.
AIM2 promotes the proliferation, migration and invasion of pancreatic cancer cells
We proceeded to further validate our analytical findings. Given that AIM2 and FYN exhibit the most significant positive correlation with immune cell infiltration in pancreatic cancer, we focused on these genes. FYN, classified as a favorable gene, is associated with longer median overall survival (OS) in patients with higher expression levels, possibly due to increased immune infiltration. Conversely, AIM2, a risk gene, has high expression levels that serve as a prognostic risk factor and are significantly positively correlated with immune cell infiltration. Further research is warranted to clarify the functional impact of AIM2 on the prognosis of patients with pancreatic cancer. Consequently, we selected AIM2 for additional validation.
To establish the functional link between AIM2 and pancreatic cancer, we constructed an AIM2 overexpression vector and transfected it into two pancreatic cancer cell lines, Panc-1 and BxPC-3, to verify its efficiency (Fig. 5A). We also designed three distinct AIM2 shRNAs and transfected them into the same pancreatic cancer cell lines. The results indicated that sh-AIM2-3 had the most pronounced reduction effect (Fig. 5B), leading us to choose sh-AIM2-3 for subsequent experiments.
AIM2 enhances proliferation, migration, and invasion of pancreatic cancer cells. (A) qRT-PCR assessment of AIM2 overexpression efficiency in pancreatic cancer cells. (B) qRT-PCR assessment of AIM2 knockdown efficiency in pancreatic cancer cells. (C) Scratch assay conducted in pancreatic cancer Panc-1 cells: Left panel: Representative image of the scratch assay. Right panel: Statistical results of the scratch assay following AIM2 overexpression. **, P < 0.01, experiment repeated three times. (D) Transwell invasion and migration analysis in pancreatic cancer Panc-1 and Bxcp-3 cells: Left panel: Representative images of the Transwell invasion and migration experiments. Right panel: Statistical results of the Transwell invasion experiment. **, P < 0.01, ***, P < 0.001, experiment repeated three times. (E) Colony formation assay in pancreatic cancer Panc-1 and Bxcp-3 cells: Left panel: Representative image of the colony formation assay. Right panel: Statistical results of the colony formation assay following AIM2 overexpression. **, P < 0.01, experiment repeated three times.
The scratch healing assay revealed that AIM2 overexpression enhanced the migration activity of Panc-1 and BxPC-3 cells, while AIM2 knockdown inhibited this activity (Fig. 5C and Supplementary Fig. 3A-3 C). Transwell cell migration assays demonstrated that AIM2 overexpression increased the migration ability of Panc-1 and BxPC-3 cells, whereas AIM2 knockdown reduced it. Similarly, Transwell cell invasion assays showed that AIM2 overexpression boosted the invasion ability of Panc-1 and BxPC-3 cells, and knockdown diminished this ability (Fig. 5D and Supplementary Fig. 3D-F). Furthermore, clone formation assays indicated that AIM2 overexpression increased the proliferation capacity of Panc-1 and BxPC-3 cells, while knockdown decreased it (Fig. 5E and Supplementary Fig. 3G). These results suggest that AIM2 may influence the progression of pancreatic cancer by promoting cell proliferation, migration, and invasion, aligning with the finding that high AIM2 expression is a prognostic risk factor.
AIM2 promotes pancreatic cancer progression via inflammasome
AIM2 exhibited a significant positive correlation with immune cell infiltration, including T helper cells, CD8+ T cells, Th1 cells, activated dendritic cells (aDCs), central memory T cells (Tcm), total T cells, B cells, and cytotoxic cells. As a member of the cytoplasmic pattern recognition receptors (PRRs), AIM2 recognizes double-stranded DNA (dsDNA) released from damaged host cells39,40,41,42. Activation of the AIM2 inflammasome in response to dsDNA results in the production of proinflammatory cytokines IL-18 and IL-1β, which drive the progression of sterile inflammation33,36,42,43. Thus, we hypothesized that AIM2 may promote the progression of sterile inflammation by enhancing the production of proinflammatory cytokines IL-18 and IL-1β. To test this hypothesis, we utilized ELISA kits to assess the impact of AIM2 on IL-18, HGMB1, and IL-1β levels. The ELISA results demonstrated that the secretion of IL-18, IL-1β, and HGMB1 by Panc-1 and Bxpc-3 cells increased following AIM2 overexpression and decreased after AIM2 knockdown (Fig. 6A and C). Given the crucial roles of NLRP3 and caspase-1 in inflammasome activation, we employed western blot to analyze their expression. The results revealed that overexpression of AIM2 in Panc-1 and Bxpc-3 cells upregulated the expression of NLRP3 and cleaved caspase-1, while knockdown of AIM2 downregulated these proteins (Fig. 6D). These findings suggest that AIM2 promotes inflammation.
AIM2 induces inflammatory responses. (A-C) ELISA kits were utilized to measure the levels of IL-18, HGMB1, and IL-1β: (A) Detection results for IL-1β. (B) Detection results for HGMB1. (C) Detection results for IL-18. (D) Western blot assay was conducted to assess the expression levels of AIM2, NLRP3, Pro-Caspase-1, and Cleaved-Caspase-1 proteins following AIM2 overexpression or knockdown, with GAPDH serving as an internal control.
To further elucidate the role of the AIM2 inflammasome in pancreatic cancer, we utilized the IL-converting enzyme/caspase-1 inhibitor VX-765 to block the inflammasome’s function44. ELISA results indicated that compared to the group treated with VX-765 alone, AIM2 knockdown or overexpression in VX-765 pre-treated cells had minimal impact on the levels of IL-18, IL-1β, and HGMB1 secreted by Panc-1 and Bxpc-3 cells (Fig. 7A and Supplementary Fig. 4A). The scratch healing assay, Transwell cell invasion, and Transwell cell migration assays revealed that compared to the group treated with VX-765 alone, AIM2 knockdown or overexpression in VX-765 pre-treated cells had negligible effects on the invasion and migration abilities of Panc-1 and Bxpc-3 cells (Fig. 7B and C, and Supplementary Fig. 4B). Additionally, clone formation experiments further demonstrated that compared to the group treated with VX-765 alone, AIM2 knockdown or overexpression in VX-765 pre-treated cells hardly altered the viability of Panc-1 and Bxpc-3 cells (Fig. 7D and Supplementary Fig. 4C). Collectively, these data provide evidence that AIM2 functions through inflammasome formation in pancreatic cancer cells. This also explains why high AIM2 expression, as a prognostic risk factor, is significantly positively correlated with immune cell infiltration.
AIM2 enhances pancreatic cancer progression via the inflammasome pathway. (A) ELISA kits were employed to measure the levels of IL-18, HGMB1, and IL-1β. (B) Scratch assay conducted in pancreatic cancer Panc-1 cells: Left panel: Representative image of the scratch assay. Right panel: Statistical results of the scratch assay. *, P < 0.05, experiment repeated three times. (C) Transwell invasion and migration analysis in pancreatic cancer Panc-1 and Bxcp-3 cells: Left panel: Representative images of Transwell invasion and migration experiments. Right panel: Statistical results of Transwell invasion and migration. **, P < 0.01, ns indicates not significant, experiment repeated three times. (D) Colony formation experiment in pancreatic cancer Panc-1 and Bxcp-3 cells: Presented is a representative image of the colony formation experiment. The experiment was repeated three times.
Discussions
Pancreatic cancer is known for its poor immunogenicity, characterized by a “cold” tumor microenvironment and the worst prognosis among all solid malignancies1. Immune-related factors are intimately linked to tumor progression, clinical outcomes, and responses to immunotherapy45. There is significant room for the development of immune biomarkers for pancreatic cancer prognosis. In this study, we constructed an IRGPS comprising nine immune-related genes from the TCGA-PAAD cohort and determined the relative contribution of each gene using coefficients from LASSO Cox regression analysis. We validated the prognostic value of this signature using ROC curve analysis and Kaplan-Meier survival analysis. Individual risk scores were calculated by multiplying the coefficients of the nine genes with their mRNA expression levels, and we found that high risk scores correlated with low immune infiltration. The nine genes identified were TNFRSF4, IL6R, AIM2, IL1R2, ACKR4, ERAP2, FYN, CDK6, and DEFB1. Compared to existing related studies, our study identifying multiple groups of prognostic immune-related genes for pancreatic adenocarcinoma offers several advantages. Firstly, we constructed a signature based on immune-related genes with prognostic significance, using LASSO Cox regression to screen out genes with greater contributions. The coefficients of each gene were optimized in multivariate Cox proportional hazards regression analysis. Our prognostic signature consists of 9 IRGPs, a suitable number for clinical application. The coefficients of the 9 genes are moderate, ranging from 0.096 to 0.56, which is more evenly distributed than in previous reports46,47. Additionally, we compared its prognostic clinical utility with the TNM staging system using ROC curve analysis. Secondly, we not only constructed an immune-related nine-gene prognostic signature with strong predictive power but also explored the relationship between tumor immune infiltration and risk score. Patients in the TCGA-PAAD cohort were divided into high-infiltration and low-infiltration groups based on the infiltration levels of 24 immune cells. Patients in the low-infiltration group had a higher prognostic risk score. The study found that the infiltration levels of T cells, Tem, Tfh, CD8+ T cells, NK cells, and pDCs were higher in the low-risk group. The results suggest that the prognosis of pancreatic cancer may be closely related to immune regulation and immune infiltration in the tumor microenvironment. Our immune-related nine-gene prognostic signature was shown to be an independent prognostic factor. In the era of immunotherapy, differentiating high-risk patients based on immune factors can guide decision-making and treatment optimization.
Kaplan-Meier survival analysis revealed that patients with higher expression levels of FYN, IL6R, and TNFRSF4 exhibited longer median overall survival (OS), which may be attributed to the association of these patients with high immune infiltration, such as T-cell and myeloid cell infiltration, leading to favorable outcomes. Interestingly, survival analysis indicated that high expression of AIM2 is a prognostic risk factor; however, AIM2 was significantly positively correlated with immune cell infiltration, including T helper cells, CD8+ T cells, Th1 cells, activated dendritic cells (aDCs), central memory T cells (Tcm), total T cells, B cells, and cytotoxic cells. AIM2, a member of the cytoplasmic pattern recognition receptors (PRRs), recognizes double-stranded DNA (dsDNA) released by damaged host cells. Activation of the AIM2 inflammasome in response to dsDNA results in the production of proinflammatory cytokines IL-18 and IL-1β, driving the progression of sterile inflammation33,36,41,48,49. This mechanism may explain the co-occurrence of high immune infiltration and AIM2 expression in pancreatic cancer tissues. Further studies are warranted to elucidate the functional impact of AIM2 on the prognosis of patients with pancreatic cancer.
As an important component of the inflammasome, AIM2 may play a key role in the progression of pancreatic cancer through multiple molecular interactions and signaling pathway activations. Existing studies have shown that AIM2, as a member of cytoplasmic pattern recognition receptors (PRRs), can recognize double-stranded DNA (dsDNA) released by damaged host cells50. The AIM2 inflammasome activated in response to dsDNA leads to the production of proinflammatory cytokines IL-18 and IL-1β, thereby promoting the progression of sterile inflammation51,52. In oral squamous cell carcinoma (OSCC), activation of the AIM2 inflammasome triggers pyroptosis and autophagy, while inhibiting p53 protein levels, thereby reducing apoptosis and promoting cancer cell proliferation53. Additionally, AIM2 activation induces the IL-1β/STAT1/NF-κB signaling pathway through an inflammasome mechanism, thereby enhancing the expression of programmed death ligand 1 (PD-L1) and contributing to improved radioresistance and migratory capacity of OSCC cells54. Studies in lung adenocarcinoma (LUAD) have also found that upregulated AIM2 expression is associated with poor patient prognosis. It can promote epithelial-mesenchymal transition (EMT) and PD-L1 expression by activating the NF-κB and STAT1 signaling pathways, leading to tumor metastasis and immune escape55.
To elucidate the functional impact of AIM2 on the prognosis of pancreatic cancer patients, we conducted experiments in pancreatic cancer cells and discovered that AIM2 promotes the proliferation, migration, and invasion of these cells, thereby affecting the progression of pancreatic cancer. This finding aligns with the observation that high expression levels of AIM2 are indicative of a prognostic risk factor. AIM2, a member of the cytoplasmic pattern recognition receptors (PRRs), recognizes double-stranded DNA (dsDNA) released by damaged host cells. The activation of the AIM2 inflammasome in response to dsDNA triggers the production of proinflammatory cytokines IL-18 and IL-1β, which in turn promotes the progression of sterile inflammation. Consequently, we hypothesize that AIM2 may enhance the progression of sterile inflammation by stimulating the production of proinflammatory cytokines IL-18 and IL-1β. Our results indicate that AIM2 promotes inflammation and that it enhances the proliferation, migration, and invasion of pancreatic cancer cells through inflammasome formation in these cells. This finding explains the significant positive correlation between AIM2, as a prognostic risk factor, and immune cell infiltration.
Based on the above research findings, we hypothesize that in pancreatic cancer, abnormal dsDNA generated by genomic instability in tumor cells can activate the AIM2 inflammasome. On one hand, this may activate signaling pathways such as NF-κB and MAPK; on the other hand, the activated AIM2 inflammasome mediates pyroptosis through the release of GSDMD (gasdermin D), thereby promoting the proliferation, invasion, and metastasis of tumor cells. Further validation and clarification of the specific molecular mechanisms of AIM2 in pancreatic cancer progression are needed in the future.
Our study reveals that the 9-gene immune risk signature directly captures impaired T-cell infiltration and activation in high-risk patients. Specifically, high-risk cases exhibit significantly reduced infiltration of CD8⁺ T cells, effector memory T cells (Tem), and follicular helper T cells (Tfh) (Fig. 4B), indicating compromised adaptive immunity. Mechanistically, key genes in our model regulate T-cell biology: (1) AIM2 (high-risk gene) demonstrates the strongest positive correlation with Treg infiltration (Fig. 4D) and drives IL-1β secretion (Fig. 6), promoting an immunosuppressive niche that exhausts cytotoxic T cells; (2) FYN (protective gene) correlates with cytotoxic cell abundance and enhances CD8⁺ T-cell activation through TCR signaling pathways; (3) IL1R2 (high-risk gene) weakly associates with Th2 polarization (r = 0.27), potentially skewing immunity toward pro-tumorigenic responses. Notably, the coordinated depletion of antigen-presenting cells (aDCs/iDCs) and CD8⁺ T cells in high-risk patients (Fig. 4B-C) reflects the “cold” tumor phenotype. Our signature thus provides a genomic framework for stratifying patients based on T-cell dysfunction, with high-risk tumors representing immune-desert microenvironments resistant to checkpoint blockade.
Comparative analysis with existing models: Existing studies integrated 1,793 immune-related genes (IRGs) from the ImmPort database, screened differentially expressed genes by combining four datasets, and finally identified seven genes to construct a risk signature through multiple steps including limma package analysis and intersection calculation56. Models reported in other literatures also analyzed immune-related genes from specific datasets (GSE71729 and GSE62452)57. In contrast, this study directly obtained 330 immune-related genes from MsigDB2, and determined a prognostic signature composed of nine genes through univariate Cox regression, LASSO Cox regression, and multivariate Cox proportional hazards regression. Compared with existing studies, our gene screening process features a more focused starting point and simpler workflow. Moreover, the model includes a larger number of genes, enabling a multi-dimensional evaluation of prognosis in pancreatic cancer. Unique advantages: By directly acquiring genes from professional databases, this study reduces interference from dataset differences, making the screened genes more targeted and representative. The novel nine-gene combination can more comprehensively reflect the relationship between the immune status and prognosis of pancreatic cancer patients, providing clinicians with richer molecular markers. Additionally, this study explores the relationship between tumor immune infiltration and risk scores.
However, our study is not without limitations. Firstly, the clinical information is derived from the TCGA-PAAD cohort, which has incomplete clinicopathological and survival data for some patients. The retrospective design of the study also introduces a certain degree of bias. Therefore, additional clinical samples and data are required to validate our findings. Future research should include large-scale, well-designed prospective clinical trials to facilitate the clinical application of IRGPs. Moreover, the external validity of our findings is constrained until further mechanistic studies are conducted to elucidate the molecular functions of the identified immune genes in pancreatic cancer progression and immune infiltration. While our model identifies distinct immune-related gene expression patterns predictive of prognosis, a limitation is the lack of direct analysis of key cytokine levels, such as IL-2 and IL-12, which are crucial for T-cell function and immune modulation. The TCGA dataset used primarily provides transcriptomic data, and comprehensive cytokine measurements were not available for systematic correlation with our risk groups or the identified IRGPs. Future studies incorporating multiplex cytokine profiling (e.g., in serum or tumor microenvironment) or spatial transcriptomics/proteomics alongside our risk score are warranted to elucidate the functional immune state, including the activity of these critical cytokine pathways, within the identified high- and low-risk patient subsets.KRAS, TP53, and SMAD4 are the most frequently mutated or deleted genes in PDAC. Studies have shown that oncogenic K-Ras activation and Smad4 deletion can mediate pancreatic cancer invasion through the EGFR/NF-κB signaling axis58. However, whether these genes are associated with the IRGPs identified in this study, such as AIM2, CDK6, IL6R, etc., remains unclear. This scientific question warrants further investigation.Additionally, the article lacks in vivo mouse experiments for further validation. In the future, it is feasible to construct AIM2 gene-knockout mouse models to compare the inflammatory responses, immune responses, and pathological changes in wild-type mice after stimulation by relevant pathogens. Adoptive transfer experiments of immune cells can also be performed to observe the functional performance of AIM2-deficient cells in immunodeficient mice.
In conclusion, we have established a prognostic signature comprising nine immune-related genes in pancreatic cancer patients, representing a promising independent prognostic model that is closely associated with tumor immune infiltration. Patients classified as high risk in our model exhibited a significantly shorter median overall survival and lower infiltration levels across 24 immune cell types. Further mechanistic studies on these nine genes will not only enhance our understanding of immune response dynamics within the pancreatic cancer microenvironment but also potentially reveal new avenues for the clinical application of immunotherapy in pancreatic cancer patients. Additionally, we observed that high immune infiltration in pancreatic cancer tissues coincided with AIM2 expression. AIM2’s role in promoting the proliferation, migration, and invasion of pancreatic cancer cells through inflammasome formation suggests a new therapeutic target for the immunotherapy of pancreatic cancer patients.
Methods
Data source
RNA-seq data of pancreatic cancer samples and their clinicopathological information were obtained from the TCGA-PAAD cohort in the TCGA database (https://portal.gdc.cancer.gov/). Clinical characteristics included patient age, gender, T stage, N stage, M stage, smoking status, TNM staging, and pathological grade.
Immune-related gene selection
Immune-related genes were obtained from the two immune gene sets IMMUNE_RESPONSE and IMMUNE_SYSTEM_PROCESS in the Molecular Signatures Database (MsigDB2)59,60. A total of 235 duplicates were removed, and the remaining 330 genes were subjected to further analyses. RNA-seq data for these genes were obtained from the TCGA-PAAD cohort.
Identification of IRGPS
The R package “survival” was utilized to perform univariate Cox proportional hazards regression analysis for screening immune-related genes that were significantly associated with overall survival (OS) in pancreatic cancer patients. These genes were then subjected to LASSO Cox regression analysis using the R package “glmnet” to calculate the contribution coefficients of each gene. Genes with coefficients that had relatively small contributions were assigned a value of zero. Subsequently, the genes with non-zero coefficients were subjected to multivariable Cox proportional hazards regression analysis to identify the optimal IRGPS60.
The individualized risk score for each patient was calculated based on the mRNA expressions and coefficients of these gene markers60,61. The risk score formula is as follows: Risk score = mRNA Expressiongene1 × Coefficientgene1 + mRNA Expressiongene2 × Coefficientgene2 +. mRNA Expressiongenen × Coefficientgenen. Revised version (Standardized): Risk Score=\(\:{\sum\:}_{i=1}^{9}(Coefi\times\:Expri)\) =(Coefficientgene1×mRNA Expressiongene1)+(Coefficientgene2×mRNA Expressiongene2)+. (Coefficientgene9×mRNA Expressiongene9).Patients were divided into high-risk and low-risk groups using the median risk score as the cut-off value. Principal component analysis (PCA) was conducted to analyze the expression distribution of all genes, the immune gene set, and the IRGPS for both the high-risk and low-risk groups. Kaplan-Meier survival analysis for the high-risk and low-risk groups was performed using the R package “survival”, and a ROC curve was constructed to evaluate the prognostic value of the signature.
Expression analysis and prognosis analysis on the IRGPS genes
Their inter-correlations of the IRGPS genes were evaluated by Pearson coefficients using R package “corrplot”, which were visualized in a Circos diagram (http://mkweb.bcgsc.ca/tableviewer/visualize/).
Differential expressions of the IRGPS genes between cancerous and normal tissues were analyzed using RNA-seq data from the TCGA-PAAD cohort. The mutations of these immune-related genes were retrieved from the cBioPortal website (https://www.cbioportal.org/). Kaplan-Meier survival analyses for these immune-related genes were conducted to predict pancreatic cancer prognosis.
Functional enrichment analyses of DEGs
The patients in the TCGA-PAAD cohort were divided into high-risk and low-risk groups based on the median risk score of the IRGPS. Differentially expressed genes (DEGs) between these high-risk and low-risk groups were identified using the R package “edgeR”, with an adjusted P-value of less than 0.05 and an absolute fold change greater than 1.50 as the thresholds.
The 212 DEGs were subjected to enrichment analysis including Gene Ontology (GO) analysis, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis and Gene Set Enrichment Analysis (GSEA). GO analysis was performed in the following aspects: biological process (BP), cellular component (CC), and molecular function (MF). GO and KEGG functional annotation of the DEGs were performed using R package “clusterProfiler”62. GSEA was performed using the gsea-4.0.3 software (https://www.gsea-msigdb.org/gsea/index.jsp) to reveal the signaling pathways in which DEGs were enriched between the high-risk and low-risk groups.
Tumor immune infiltration analysis
Based on the transcriptional profile of immune cell subpopulations in the tumor microenvironment (TME)63, the infiltration levels of 24 types of immune cells in pancreatic cancer were assessed using the TCGA-PAAD database with the ssGSEA algorithm. The 24 types of immune cells examined included dendritic cells (DCs), immature DCs (iDCs), activated DCs (aDCs), plasmacytoid dendritic cells (pDCs), B cells, cytotoxic cells, eosinophils, macrophages, mast cells, neutrophils, NK CD56bright cells, NK CD56dim cells, natural killer cells (NKs), CD8+ T cells, T cells, T helper cells, T central memory (Tcm), T effector memory (Tem), T follicular helper cells (Tfh), T gamma delta (Tgd), T helper 1 (Th1), Th2, T helper 17 cells (Th17), and T regulatory cells (Treg). Patients from the TCGA-PAAD cohort were categorized into high-infiltration and low-infiltration groups based on the levels of immune cell infiltrates. A comprehensive heatmap was generated to illustrate the associations between immune infiltrates of various immune cells and risk score, age, gender, T stage, N stage, M stage, and pathological stage. The differences in the abundance of the 24 immune cells were determined by t-tests between the high-risk and low-risk groups. Additionally, the correlations between the expression of IRGPS genes and the abundance of the 24 immune cell types were investigated using Pearson coefficients in pancreatic cancer.
Cell lines and cell culture
Human pancreatic cancer cell lines Panc-1 and Bxcp-3 were purchased from Wuhan Punosai Life Science Technology Co., Ltd. Both cell lines were cultured in DMEM medium supplemented with 10% fetal bovine serum (Gibco Life Technologies, NY, USA), penicillin (100 U/mL), and streptomycin (100 µg/mL) at 5% CO2 and 37 °C. All cell lines were tested for mycoplasma contamination.
Plasmid transfection
The human AIM2 coding sequence was cloned into the pcDNA3.1 vector, a construct prepared by Changsha Youbao Biotechnology Co., Ltd. Additionally, the AIM2 shRNA sequence was inserted into the pLKO.1-puro vector to generate the human PLKO.1-shAIM2 plasmid, also constructed by Changsha Youbao Biotechnology Co., Ltd., following the manufacturer’s protocol. Transfection of the gene plasmid was performed using Neofect DNA transfection reagent, supplied by Neofect Biotech, China.
Real-time quantitative polymerase chain reaction (qRT–PCR)
Total RNA was extracted from human breast cancer cell lines using TRIzol reagent (Invitrogen, MA, USA), following the manufacturer’s instructions. Reverse transcription of mRNA and miRNA was carried out with random primers and stem-loop primers, respectively. Quantitative real-time PCR (qRT-PCR) was conducted on a Bio-Rad CFX96 qPCR system, utilizing the SYBR Green PCR Master Mix kit (Vazyme, Nanjing, China). Fold changes were calculated using the relative quantitative 2^-ΔΔCT method, with GAPDH serving as an internal reference. The primers utilized in the study are listed in Supplementary Table 3.
Western blotting
Western blot analysis was conducted as previously described64. In brief, proteins were extracted from pancreatic cancer cell lines, and their concentrations were determined using a BCA protein quantification kit (Thermo Scientific, Rockford, IL, USA). The antibodies utilized in this analysis are detailed in Supplementary Table 4.
Colony formation, migration and invasion assays
Colony formation assays were conducted to assess cell proliferation capacity. Invasion ability was evaluated using Transwell assays, while cell migration ability was determined through wound healing assays. Detailed procedures for these assays have been described in our previous study64.
ELISA assay
The culture supernatant was collected, and ELISA kits from Kelu Biotechnology were utilized to detect IL-18, HGMB1, and IL-1β. A microplate reader along with SoftMax® Pro 7 software version 7.1.0 (Molecular Devices) was employed for the quantification of these cytokines. The experiments were carried out following the manufacturer’s instructions.
Statistical analysis
Statistical graphs were generated using GraphPad Prism 8 software. T-tests were employed to determine if there was a significant difference between any two groups, while one-way ANOVA was utilized to assess significant differences in data from more than two groups. All experiments were conducted with at least three replicates. A P-value less than 0.05 was considered indicative of a significant difference. The following notations represent the levels of significance: * for P < 0.05, ** for P < 0.01, *** for P < 0.001, and **** for P < 0.0001.
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author (yuanxia@hnca.org.cn).
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33 (2021).
Lin, Y. et al. Genome-wide association meta-analysis identifies GP2 gene risk variants for pancreatic cancer. Nat. Commun. 11, 3175 (2020).
Stoffel, E. M., Brand, R. E. & Goggins, M. Pancreatic cancer: changing epidemiology and new approaches to risk assessment, early detection, and prevention. Gastroenterology 164, 752–765 (2023).
Karamitopoulou, E. Emerging prognostic and predictive factors in pancreatic Cancer. Mod. Pathol. 36, 100328 (2023).
Balaban, E. P., Mangu, P. B. & Yee, N. S. Locally advanced unresectable pancreatic cancer: American society of clinical oncology clinical practice guideline summary. J. Oncol. Pract. 13, 265–269 (2017).
Conroy, T. et al. Pancreatic cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 34, 987–1002 (2023).
Versteijne, E. et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J. Clin. Oncol. 38, 1763–1773 (2020).
O’Reilly, E. M. & Ferrone, C. Neoadjuvant or adjuvant therapy for resectable or borderline resectable pancreatic cancer: which is preferred?? J. Clin. Oncol. 38, 1757–1759 (2020).
Huber, M. et al. The immune microenvironment in pancreatic Cancer. Int. J. Mol. Sci. 21, (2020).
Poyia, F., Neophytou, C. M., Christodoulou, M. I. & Papageorgis, P. The role of tumor microenvironment in pancreatic cancer immunotherapy: current status and future perspectives. Int. J. Mol. Sci. 25, (2024).
Zhang, W., Zhang, J., Liang, X. & Ding, J. Research advances and treatment perspectives of pancreatic adenosquamous carcinoma. Cell. Oncol. (Dordr). 46, 1–15 (2023).
Wu, S. et al. C-C motif chemokine 22 predicts postoperative prognosis and adjuvant chemotherapeutic benefits in patients with stage II/III gastric cancer. Oncoimmunology 7, e1433517 (2018).
Wang, L. et al. Comprehensive machine-learning survival framework develops a consensus model in large-scale multicenter cohorts for pancreatic cancer. Elife 11, (2022).
Tong, Z. et al. Development, validation and comparison of artificial neural network models and logistic regression models predicting survival of unresectable pancreatic Cancer. Front. Bioeng. Biotechnol. 8, 196 (2020).
Fan, J. Q. et al. Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma. Mol. Cancer. 19, 32 (2020).
Wang, H., You, S., Fang, M. & Fang, Q. Recognition of immune microenvironment landscape and immune-Related prognostic genes in breast Cancer. Biomed. Res. Int. 3909416, 2020. (2020).
Wang, Y. et al. Identification of prognostic immune-related gene signature associated with tumor microenvironment of colorectal cancer. BMC Cancer. 21, 905 (2021).
Bisheshar, S. K., De Ruiter, E. J., Devriese, L. A. & Willems, S. M. The prognostic role of NK cells and their ligands in squamous cell carcinoma of the head and neck: a systematic review and meta-analysis. Oncoimmunology 9, 1747345 (2020).
Esfahani, K. et al. Targeting the mTOR pathway uncouples the efficacy and toxicity of PD-1 Blockade in renal transplantation. Nat. Commun. 10, 4712 (2019).
Lin, X. et al. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer. 23, 108 (2024).
Chu, X., Tian, W., Wang, Z., Zhang, J. & Zhou, R. Co-inhibition of TIGIT and PD-1/PD-L1 in Cancer immunotherapy: mechanisms and clinical trials. Mol. Cancer. 22, 93 (2023).
Fontana, F. et al. Biohybrid vaccines for improved treatment of aggressive melanoma with checkpoint inhibitor. ACS Nano. 13, 6477–6490 (2019).
Wang, S. J., Dougan, S. K. & Dougan, M. Immune mechanisms of toxicity from checkpoint inhibitors. Trends Cancer. 9, 543–553 (2023).
Virassamy, B. et al. Intratumoral CD8(+) T cells with a tissue-resident memory phenotype mediate local immunity and immune checkpoint responses in breast cancer. Cancer Cell. 41, 585–601 (2023). e588.
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 Blockade. Science 357, 409–413 (2017).
Johnson, B. A. 3, Yarchoan, M., Lee, V., Laheru, D. A., Jaffee, E. M. & rd,, and Strategies for increasing pancreatic tumor immunogenicity. Clin. Cancer Res. 23, 1656–1669 (2017).
Biankin, A. V. & Maitra, A. Subtyping pancreatic Cancer. Cancer Cell. 28, 411–413 (2015).
Qin, Y. et al. Pan-cancer analysis of AIM2 inflammasomes with potential implications for immunotherapy in human cancer: A bulk omics research and single cell sequencing validation. Front. Immunol. 13, 998266 (2022).
Man, S. M. & Kanneganti, T. D. Regulation of inflammasome activation. Immunol. Rev. 265, 6–21 (2015).
Ta, A. & Vanaja, S. K. Inflammasome activation and evasion by bacterial pathogens. Curr. Opin. Immunol. 68, 125–133 (2021).
Hoffman, H. M. & Broderick, L. The role of the inflammasome in patients with autoinflammatory diseases. J. Allergy Clin. Immunol. 138, 3–14 (2016).
Deng, Z. et al. The roles of inflammasomes in cancer. Front. Immunol. 14, 1195572 (2023).
Lee, S. et al. AIM2 forms a complex with Pyrin and ZBP1 to drive PANoptosis and host defence. Nature 597, 415–419 (2021).
Sharma, B. R., Karki, R. & Kanneganti, T. D. Role of AIM2 inflammasome in inflammatory diseases, cancer and infection. Eur. J. Immunol. 49, 1998–2011 (2019).
Wang, Q. et al. AIM2 promotes renal cell carcinoma progression and Sunitinib resistance through FOXO3a-ACSL4 axis-regulated ferroptosis. Int. J. Biol. Sci. 19, 1266–1283 (2023).
Han, C. et al. The AIM2 and NLRP3 inflammasomes trigger IL-1-mediated antitumor effects during radiation. Sci. Immunol. 6, (2021).
Zhu, H. et al. The complex role of AIM2 in autoimmune diseases and cancers. Immun. Inflamm. Dis. 9, 649–665 (2021).
Karki, R., Man, S. M. & Kanneganti, T. D. Inflammasomes and Cancer. Cancer Immunol. Res. 5, 94–99 (2017).
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Wang, Y. et al. Overexpression of NAG-1/GDF15 prevents hepatic steatosis through inhibiting oxidative stress-mediated DsDNA release and AIM2 inflammasome activation. Redox Biol. 52, 102322 (2022).
Baatarjav, C. et al. dsDNA-induced AIM2 pyroptosis halts aberrant inflammation during rhabdomyolysis-induced acute kidney injury. Cell. Death Differ. 29, 2487–2502 (2022).
Hu, B. et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science 354, 765–768 (2016).
Zhang, M. et al. AIM2 inflammasome mediates Arsenic-induced secretion of IL-1 beta and IL-18. Oncoimmunology 5, e1160182 (2016).
Tezcan, G. et al. Therapeutic potential of Pharmacological targeting NLRP3 inflammasome complex in Cancer. Front. Immunol. 11, 607881 (2020).
Kovacs, S. A., Fekete, J. T. & Gyorffy, B. Predictive biomarkers of immunotherapy response with Pharmacological applications in solid tumors. Acta Pharmacol. Sin. 44, 1879–1889 (2023).
Bu, F. et al. A signature of 18 immune-related gene pairs to predict the prognosis of pancreatic cancer patients. Immun. Inflamm. Dis. 8, 713–726 (2020).
Wu, G. et al. Identification of prognostic Immune-Related genes in pancreatic adenocarcinoma and establishment of a prognostic nomogram: A bioinformatic study. Biomed. Res. Int. 1346045, 2020. (2020).
Hornung, V. et al. AIM2 recognizes cytosolic DsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Wang, B., Tian, Y. & Yin, Q. AIM2 inflammasome assembly and signaling. Adv. Exp. Med. Biol. 1172, 143–155 (2019).
Chew, Z. H. et al. Macrophage IL-1beta contributes to tumorigenesis through paracrine AIM2 inflammasome activation in the tumor microenvironment. Front. Immunol. 14, 1211730 (2023).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Wang, B. & Yin, Q. AIM2 inflammasome activation and regulation: A structural perspective. J. Struct. Biol. 200, 279–282 (2017).
Kondo, Y. et al. Overexpression of the DNA sensor proteins, absent in melanoma 2 and interferon-inducible 16, contributes to tumorigenesis of oral squamous cell carcinoma with p53 inactivation. Cancer Sci. 103, 782–790 (2012).
Chiu, H. W. et al. AIM2 promotes irradiation resistance, migration ability and PD-L1 expression through STAT1/NF-kappaB activation in oral squamous cell carcinoma. J. Transl Med. 22, 13 (2024).
Zheng, J. Q. et al. AIM2 upregulation promotes metastatic progression and PD-L1 expression in lung adenocarcinoma. Cancer Sci. 114, 306–320 (2023).
Wang, C. et al. Construction of immune-related signature and identification of S100A14 determining immune-suppressive microenvironment in pancreatic cancer. BMC Cancer. 22, 879 (2022).
Wang, C. et al. Immune-related signature identifies IL1R2 as an immunological and prognostic biomarker in pancreatic cancer. J. Pancreatol. 7, 119–130 (2024).
Bera, A., Zhao, S., Cao, L., Chiao, P. J. & Freeman, J. W. Oncogenic K-Ras and loss of Smad4 mediate invasion by activating an EGFR/NF-kappaB Axis that induces expression of MMP9 and uPA in human pancreas progenitor cells. PLoS One. 8, e82282 (2013).
Liberzon, A. et al. The molecular signatures database (MSigDB) hallmark gene set collection. Cell. Syst. 1, 417–425 (2015).
Chen, X. et al. Bioinformatics analysis finds immune gene markers related to the prognosis of bladder Cancer. Front. Genet. 11, 607 (2020).
Bao, Z. S. et al. Prognostic value of a nine-gene signature in glioma patients based on mRNA expression profiling. CNS Neurosci. Ther. 20, 112–118 (2014).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. ClusterProfiler: an R package for comparing biological themes among gene clusters. Omics: J. Integr. Biology. 16, 284–287 (2012).
Bindea, G. et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795 (2013).
Liang, L. et al. CircGSK3beta mediates PD-L1 transcription through miR-338-3p/PRMT5/H3K4me3 to promote breast cancer cell immune evasion and tumor progression. Cell. Death Discov. 10, 426 (2024).
Funding
This study was supported by grants from the Clinical Research Center For Gastrointestinal Cancer In Hunan Province [No.2021SK4016], the National Natural Science Foundation of China [No. 82203836], “Scientific Research Climbing Plan” of Hunan Cancer Hospital [No. YF2021003 and IIT2021006].
Author information
Authors and Affiliations
Contributions
LL, YZ, YZ performed the experiments. LL, SQ and ZY analyzed the data. HY and XY designed the study and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liang, L., Zhou, Y., Zhang, Y. et al. The immune-related prognostic gene AIM2 promotes pancreatic cancer progression via inflammasome. Sci Rep 15, 29733 (2025). https://doi.org/10.1038/s41598-025-12651-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12651-x