Abstract
Tabata, which is involved 20 seconds of maximum-intensity exercise followed by 10 seconds of complete rest, repeated for 8 cycles totaling 4 minutes, has been identified to enhance energy expenditure and fat oxidation in humans. The study aims to find an optimal Tabata volume for weight loss. 32 male university students with overweight/obesity participated in three tests. Test I consisted of a single Tabata cycle, Test II consisted of two, and Test III consisted of three. Each cycle was separated by a 10-minute interval, and each test was was separated by 7 days. Gas exchange indices were monitored during the last Tabata cycle of the test and the 30-minute recovery period. Subsequently, fat and glucose oxidation amounts, rates, and energy expenditure were calculated. During the 10-minute recovery period, the fat oxidation amounts of Test II (0.80±0.26 g) and Test III (0.87±0.24 g) were higher than Test I (0.50±0.11 g, p<0.001). There was no significant difference between Test II and Test III. During the 20-minute and 30-minute recovery periods, Test II (3.21±0.50 g; 5.04±1.02 g) showed significantly higher fat oxidation amount than Test I (2.47±0.59 g, p<0.001; 4.41±0.98 g, p<0.05) and Test III (2.80±0.43 g, p<0.001; 4.11±0.96 g, p<0.001), there was no significant difference between Test I and Test III (p>0.05). No significant differences in energy expenditure were observed among the three tests during recovery periods (p>0.05). We conclude that two Tabata cycles show the highest fat oxidation amount with the same energy expenditure amount during the recovery period, which is the optimal Tabata volume for weight loss.
Similar content being viewed by others
Introduction
In recent years, obesity has risen globally. In 2023, the World Obesity Atlas Federation concluded that more than 50% of the world’s population would be overweight or obese within the next 12 years1. China faces a similar obesity crisis. According to the Report on Chinese Residents’ Nutrition and Chronic Disease Status (2020), over 50% of Chinese adults are overweight or obese2. Notably, the 20-39 age group demonstrated a relatively rapid increase in obesity rates3. In addition, from 2019 to 2021, China’s university students experienced a significant increase in overweight and obesity rates, with male students exhibiting an obesity prevalence of 27.92%, markedly higher than the 13.06% observed in female students‘4. This indicates that obesity has emerged as a significant factor effecting the physical and mental health of Chinese male college students.
Prospective cohort studies provide evidence that overweight and obesity are strongly associated with a higher risk of noncommunicable chronic diseases, including cardiovascular disease, type 2 diabetes, cancer, and premature mortality5. Research shows that obesity elevates the occurrence of cardiovascular diseases and the risk of mortality among cardiovascular patients6. The development of obesity is significantly influenced by genetic and environmental factors, which magnify genetic predisposition to diabetes7. Inflammation is a central and reversible mechanism through which obesity increases the risk and progression of cancer, with severe obesity being closely associated with alterations in fat tissue function, adipocyte death, and chronic inflammation, thereby highlighting the health risks that obesity poses to the body8. It is clear that obesity has many harmful effects. To tackle this issue, an effective exercise protocol is essential.
Weight management fundamentally depends on achieving an energy balance between caloric intake and expenditure, which explains why weight loss occurs when energy expenditure exceeds intake. Fat oxidation serves as a critical component of energy balance, where enhanced fat oxidation can increase total energy expenditure, thereby facilitating weight loss9. Enhanced fat oxidation demonstrates significant metabolic benefits beyond weight management. Robust evidence indicates that elevated fat oxidation rates correlate with improved insulin sensitivity, reduced cardiovascular disease risk, and attenuated inflammatory responses10,11,12. Obese individuals typically demonstrate impaired fat oxidation capacity, which pathophysiologically associates with three key abnormalities: diminished mitochondrial function in skeletal muscle, systemic insulin resistance, and metabolic dysregulation in adipose tissue13,14. Therefore, enhancing fat oxidation capacity is critically important for weight management in obese individuals. As a result, traditional weight loss exercise interventions, such as moderate intensity continuous training (MICT), including running, swimming, and cycling, have gained popularity and acceptance. MICT has been shown to increase energy expenditure and decrease fat accumulation15. However, these exercise protocols usually demand extended periods, which may not suit the time constraints faced by people nowadays.
High-intensity interval training (HIIT) is an exercise regimen characterized by alternating periods of high-intensity physical exertion and low-intensity recovery phases16, offering greater time efficiency and exercise-induced pleasure compared to MICT, which can enhance long-term adherence17,18. A notable HIIT variant, Tabata training, was developed by Japanese researcher Izumi Tabata and follows a strict protocol of 20-second all-out effort intervals interspersed with 10-second rest periods, repeated over 4 minutes19. Unlike traditional HIIT, which operates at 90-110% VO2max for 20-30 minutes, Tabata training demands extreme intensity (170% VO2max) in ultrashort sessions20. This design prioritizes rapid improvements in anaerobic capacity, muscular power, and metabolic rate21, making it particularly suited for time-constrained individuals like students or office workers, though beginners should approach it cautiously with modified intensities to avoid overexertion21,22. In contrast, traditional HIIT’s longer duration and moderate intensity better support cardiovascular endurance and sustained metabolic adaptations23. While both protocols are time-efficient, Tabata emphasizes maximized short-term results, whereas traditional HIIT balances intensity with endurance-focused outcomes.
Research demonstrates that Tabata training, like other forms of exercise (both aerobic and anaerobic), induces excess post-exercise oxygen consumption (EPOC), which is a physiological response characterized by elevated energy expenditure after exercise cessation. EPOC is a key mechanism of the delayed effects of Tabata training, as it signifies that the body continues to burn additional energy even after the set has ended, thus elevating the basal metabolic rate24,25. Furthermore, the enhanced fat oxidation observed following Tabata training is mediated through multiple metabolic pathways: Notably, post-exercise catecholamine concentrations increase significantly and these neurohormones stimulate lipolysis thus facilitating fat mobilization, with particular efficacy in reducing both subcutaneous and intramuscular fat26. In addition, Tabata training enhances fat oxidation through processes such as H+ and lactate clearance, as well as glycogen resynthesis26. Moreover, Tabata training significantly boosts interleukin-6 (IL-6) levels in muscles. This exercise-induced cytokine, secreted by fat tissue, activates brown fat thermogenesis (via UCP-1 protein) to enhance energy expenditure, while also providing vascular and neurological protection, collectively improving metabolic health27.
Current research on Tabata training primarily focuses on the acute effects of a single Tabata cycle (4-minute protocol). Studies investigating multiple consecutive Tabata cycles within a single training session remain limited. This gap is particularly significant for fat oxidation, which may respond differentially to accumulated Tabata volume. We hypothesize that fat oxidation rates increase with additional Tabata cycles, but reach an optimal threshold beyond which benefits plateau. This study systematically incorporates multiple Tabata cycles into one training session to identify the optimal training volume for maximizing both fat oxidation rates and the delay effect. If the right number of Tabata cycles in a single set are found, we can provide a more personalized training protocol for individuals.
Methods
This study employed an experimental research method, with 32 male university students with overweight/obesity as participants. After completing the preliminary measurements, including weight, height, body fat percentage, fat mass, lean body mass and resting heart rate, participants performed three Tabata training sessions with a one-week interval between each training session. During the last Tabata cycle of each training session and the following 30-minute recovery period, gas metabolism parameters were continuously measured. These data were then used to calculate the fat and glucose oxidation, and energy expenditure during the exercise and recovery periods, as well as the fat and glucose oxidation rates and energy expenditure rates at the 10th minute, 20th minute, and 30th minute after the completion of the exercise for the three different tests(with the basic data for energy expenditure during the exercise period in Test II coming from the second Tabata cycle and in Test III from the third one).
Participants
G*Power 3.1.9.7, with an effect size of 0.25, an alpha error probability of 0.05, and a power of 0.8, was used to estimate the sample size. Based on the calculation, the minimum sample size was estimated to be 86, indicating that each test consisting of 29 participants at least. This study utilized a convenience sampling method to recruit participants meeting the following criteria: male university students aged 18-24 years with \(BMI\ge 24\) (classified as overweight/obese according to Chinese standards28), no regular exercise habits in the past three months, and no history of smoking. Exclusion criteria included metabolic disorders (such as diabetes or dyslipidemia), hypertension, cardiovascular diseases, joint conditions, and students from sports academies. The final sample consisted of 32 overweight/obese male university students, which involved 8 overweight and 24 obese (means ± SD, age 20±1.25 years old; weight 96.06±13.06 kg; height 174.64±7.91 cm; BMI 31.50±4.07 \(\text {kg/m}^{2}\); body fat percentage 28.81±4.29%; fat mass 28.38±6.82 kg; lean body mass 67.68±7.73 kg). Participants were asked to avoid intense exercise, smoking, alcohol, caffeine containing drinks, or medication 48 hours before the test. Their diet for the week before the test was recorded, and they were asked to avoid high-sugar and high-fat diets. During the test, participants were required to maintain the same diet as the previous week to eliminate dietary influences. It was also suggested that they keep regular schedules and ensure enough sleep to minimize the potential effect of circadian rhythms and mental state. This study was approved by the ethical review of the Human Ethics Committee of Guangxi Normal University (20231126001). The studies were conducted in accordance with the local legislation and institutional requirements. Informed consent was obtained from all the participants.
Reproducibility testing
This study did not conduct Cosmed K5 reliability testing. However, the device’s measurement validity has been extensively documented in prior studies29,30,31. Although formal reproducibility assessments were not performed, our team implemented rigorous quality control protocols:
-
Strict adherence to manufacturer calibration guidelines;
-
Periodic validation using standardized gas mixtures;
-
All measurements conducted by a single certified operator;
-
Maintenance of consistent environmental conditions.
Experimental control
The tests were carried out in an indoor environment with a temperature of 24-\(26^{\circ }\)C and a relative humidity of 40−60%. Each participant performed the test at least 2 hours after lunch, and all tests started at 2:00 p.m. A uniform lunch was provided for each participant, containing rice, beef, vegetables, and a cup of yogurt.
Experimental procedures
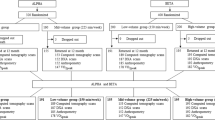
All 32 participants performed three Tabata training sessions of Test I, Test II, and Test III, with one-week between each session. At the start of the experiment, each participant engaged in 10-minutes of sedentary rest. During this period, the staff thoroughly explained and demonstrated to participants: 1) the specific exercises and training procedures included in the Tabata protocol; 2) guidelines for properly wearing the Cosmed K5 device; 3) participants were fitted with heart rate monitors to track exercise intensity. Before the formal training, a 1-minute warm-up which included dynamic stretching of the lower limbs and mobilization of the knees and ankles were done. Participants were then equipped with a cardiopulmonary testing device (Cosmed K5, Italy), which monitored gas exchange indices during the last Tabata cycle of the test and the 30-minute recovery period. A Tabata cycle consists of 4 sets of high leg raises and 4 sets of open and close jumps, totaling 8 sets. Each set lasted 20 seconds with a 10-second rest interval between sets, for a total training duration of 4 minutes. During Test I, participants completed one Tabata cycle. During Test II, they performed two cycles with a 10-minute rest period between each. Test III involved three cycles, also with a 10-minute rest interval between each cycle. (Fig. 1)
Preliminary measurement
Preliminary measurements included weight, height, body fat percentage, fat mass, lean body mass and resting heart rate. BMI was calculated by dividing body weight by the square of the height (\(\text {kg/m}^{2}\)). Body fat percentage, fat mass, lean body mass were measured using a body composition analyzer (InBody 770, Korea). Measurements of resting heart rate were taken 2 hours after meals. Maximum heart rates were calculated using the formula (206 - 0.7*age)32. Resting heart rate served as the baseline control measurement prior to the experiment, ensuring all participants began testing under standardized resting conditions. Maximum heart rates were used to evaluate fatigue recovery and exercise intensity. To balance Tabata’s intensity requirements with experimental safety, participants were required to reach 85% of their maximum heart rate during training.
Measure
Physiological indices measurement
During the exercise and recovery period, participants wore the Cosmed K5 to continuously measure VO2 (L/min) and VCO2 (L/min). The calculation formulas are as follows33,34:
-
Glucose oxidation rate (g/min) = 4.585 * VCO2 (L/min) - 3.2256 * VO2 (L/min);
-
Glucose oxidation amount (g) = Glucose oxidation rate (g/min) * Time (min);
-
Fat oxidation rate (g/min)= 1.695 * VO2 (L/min) - 1.701 * VCO2(L/min);
-
Energy expenditure rate (kcal/min)= 3.716 * VO2 (L/min) + 1.332 * VCO2(L/min);
-
Fat oxidation amount (g)= Fat oxidation rate (g/min) * Time (min);
-
Energy expenditure amount (kcal)= Energy expenditure rate (kcal/min) * Time (min).
HR was recorded every 1 minute throughout the test period. Rating of perceived exertion (RPE) was assessed using the Borg scale during exercise and at three key time points: at 10, 20, and 30 minutes after exercise to evaluate fatigue recovery35.
Statistical analysis
All data obtained from the experiment were analyzed using SPSS 26.0 software (Chicago, IL, USA), and results are presented as mean ± SD. The Shapiro-Wilk Test showed that the data were normally distributed. The Mauchly’s test of sphericity showed that the data satisfies the assumption of sphericity. A one-way repeated measures analysis of variance (ANOVA) was used to analyze all the data. If the ANOVA results showed significant differences, Tukey’s HSD Test was used to compare pairwise differences. Effect size was calculated as partial \(\eta ^{2}\) (\(\eta ^{2}\)p), and \(\eta ^{2}\)p equal to 0.01,0.06, 0.14 presented a small, medium, and large effect respectively36. A P<0.05 was considered statistically significant.
Results
Exercise period (Table 1)
During the exercise period, there was a significant difference in fat oxidation amount among the three tests (F2,62=45.8, p<0.001, \(\eta ^{2}\)p=0.596). As Tabata cycles performed in a single training set increased, a progressive trend in fat oxidation was observed across the three Tests (all p<0.01). (Fig. 2A)
During the exercise period, there was a significant difference in glucose oxidation amount among the three tests (F2,62=37.2, p<0.001, \(\eta ^{2}\)p=0.545). As Tabata cycles performed in a single training set increased, a progressive decline in glucose oxidation amount was observed across the three tests (all p<0.05). (Fig. 2B)
During the exercise period, there was no significant difference in energy expenditure among the three tests (F2,62=0.0427, p>0.05, \(\eta ^{2}\)p=0.001). (Fig. 2C)
Recovery period (Table 1)
10-minute recovery period (0-10min following exercise)
During the 10-minute recovery period, there was a significant difference in fat oxidation amount among the three tests (F2,62=28.6, p<0.001, \(\eta ^{2}\)p=0.480). Test I showed the lowest fat oxidation amount among the three tests (all p<0.001). There was no significant difference between Test II and Test III. (Fig. 3A)
During the 10-minute recovery period, there was a significant difference in glucose oxidation amount among the three tests (F2,62=31.9, p<0.001, \(\eta ^{2}\)p=0.507). Test I showed the highest glucose oxidation amount among the three tests (all p<0.001). There was no significant difference between Test II and Test III. (Fig. 3B)
During the 10-minute recovery period, there was no significant difference in energy expenditure among the three tests (F2,62=0.104, p>0.05, \(\eta ^{2}\)p=0.003). (Fig. 3C)
20-minute recovery period (0-20min following exercise)
During the 20-minute recovery period, there was a significant difference in fat oxidation amount among the three tests (F2,62=16.4, p<0.001, \(\eta ^{2}\)p=0.346). Test II showed the highest fat oxidation amount among the three Tests (all p<0.001). There was no significant difference between Test I and Test III. (Fig. 4A)
During the 20-minute recovery period, there was a significant difference in glucose oxidation amount among the three tests (F2,62=27.8, p<0.001, \(\eta ^{2}\)p=0.473). Test I showed the highest glucose oxidation amount among the three tests (all p<0.001). There was no significant difference between Test II and Test III. (Fig. 4B)
During the 20-minute recovery period, there was no significant difference in energy expenditure among the three tests (F2,62=0.792, p>0.05, \(\eta ^{2}\)p=0.025). (Fig. 4C)
30-minute recovery period (0-30min following exercise)
During the 30-minute recovery period, the difference in fat oxidation amount among the three tests was statistically significant (F2,62=7.89, p<0.01, \(\eta ^{2}\)p=0.203). Test II showed the highest fat oxidation amount among the three Tests (all p<0.05). There was no significant difference between Test I and Test III. (Fig.5A)
During the 30-minute recovery period, there was a significant difference in glucose oxidation among the three tests (F2,62=11.7, p<0.001, \(\eta ^{2}\)p=0.274). Test I showed the highest glucose oxidation amount among the three tests (all p<0.01). There was no significant difference between Test II and Test III. (Fig. 5B)
During the 30-minute recovery period, there was no significant difference in energy expenditure among the three tests (F2,62=0.382, p>0.05, \(\eta ^{2}\)p=0.012). (Fig. 5C)
Rates of substrate metabolism and energy expenditure after exercise (Fig. 6, Table 2)
At 10th minute after exercise
Fat oxidation rate had a significant difference among the three tests (F2,62=34.2, p<0.001, \(\eta ^{2}\)p=0.525). Test I showed the lowest fat oxidation rate among the three tests (all p<0.05).
Glucose oxidation rate also had a significant difference (F2,62=15.8, p<0.001, \(\eta ^{2}\)p=0.338). Test I showed the highest glucose oxidation rate among the three tests (all p<0.05).
Energy expenditure rate had no significant difference (F2,62=0.0880, p>0.05, \(\eta ^{2}\)p=0.003).
At 20th minute after exercise
Fat oxidation rate had a significant difference among the three tests (F2,62=28.4, p<0.001, \(\eta ^{2}\)p=0.478). Test II showed the highest fat oxidation rate among the three tests (all p<0.05)
Glucose oxidation rate had a significant difference (F2,62=19.2, p<0.001, \(\eta ^{2}\)p=0.382). Test II showed the lowest glucose oxidation rate among the three tests (all p<0.05).
Energy expenditure rate had no significant difference (F2,62=0.132, p>0.05, \(\eta ^{2}\)p=0.004).
At 30th minute after exercise
Fat oxidation rate had a significant difference (F2,62=12.5, p<0.001, \(\eta ^{2}\)p=0.287). Test II showed the highest fat oxidation rate among the three tests (all p<0.05).
Glucose oxidation rate had no significant difference among the three tests (F2,62=1.67, p>0.05, \(\eta ^{2}\)p=0.051).
Energy expenditure rate had no significant difference (F2,62=2.32, p>0.05, \(\eta ^{2}\)p=0.070).
Exercise heart rates and pre-test heart rates (Table 3)
No significant difference was observed among the 85% maximum heart rates of preliminary measurement and the heart rates during the three Tabata cycles (F3,93=0.592, p>0.05, \(\eta ^{2}\)p=0.622). It ensured that all participants have reached the prescribed exercise intensity targets.
No significant difference was observed among the resting heart rates of preliminary measurement and the pre-test heart rates of the three tests (F3,93=0.328, p>0.05, \(\eta ^{2}\)p=0.011). It ensured that all participants initiated testing under standardized resting conditions.
Rating of perceived exertion (Table 4)
No significant difference was observed among the RPE during exercise (F2,62=0.583, p>0.05, \(\eta ^{2}\)p=0.0182), at the 10th minute after exercise (F2,62=0.985, p>0.05, \(\eta ^{2}\)p=0.031) of the three tests.
The RPE at the 20th minute after exercise had a significant difference among the three tests (F2,62=3.45, p<0.05, \(\eta ^{2}\)p=0.100). There was no significant difference between Test I and Test II (P>0.05). There was a significant difference between Test I and Test III (P<0.05); There was a significant difference between Test II and Test III (P<0.05).
The RPE at the 30th minute after exercise had a significant difference among the three tests (F2,62=12.6, p<0.05, \(\eta ^{2}\)p=0.289). There was a significant difference between Test I and Test II (P<0.05). There was a significant difference between Test I and Test III (P<0.001); There was no significant difference between Test II and Test III (P>0.05).
VO2 and VCO2 during exercise and recovery periods (Fig. 7, Table 5)
During exercise
VO2 during exercise had a significant difference among the three tests (F2,62=7.33, p<0.01, \(\eta ^{2}\)p=0.191). There was no significant difference between Test I and Test II (P>0.05). There was a significant difference between Test I and Test III (P<0.001); There was a significant difference between Test II and Test III (P<0.001).
VCO2 during exercise had a significant difference among the three tests (F2,62=32.81, p<0.001, \(\eta ^{2}\)p=0.514). There was a significant difference between Test I and Test II (P<0.001); There was a significant difference between Test I and Test III (P<0.001); There was a significant difference between Test II and Test III (P<0.001).
The RER during exercise had a significant difference among the three tests (F2,62=74.57, p<0.001, \(\eta ^{2}\)p=0.706). There was a significant difference between Test I and Test II (P<0.001); There was a significant difference between Test I and Test III (P<0.001); There was no significant difference between Test II and Test III (P>0.05).
At 10th minute after exercise
VO2 at the 10th minute after exercise had a significant difference among the three tests (F2,62=3.87, p<0.05, \(\eta ^{2}\)p=0.111). There was no significant difference between Test I and Test II (P>0.05). There was a significant difference between Test I and Test III (P<0.01); There was no significant difference between Test II and Test III (P>0.05).
VCO2 at the 10th minute after exercise had a significant difference among the three tests (F2,62=5.53, p<0.01, \(\eta ^{2}\)p=0.151). There was a significant difference between Test I and Test II (P<0.05). There was a significant difference between Test I and Test III (P<0.001); There was no significant difference between Test II and Test III (P>0.05).
The RER at the 10th minute after exercise had no significant difference among the three tests (F2,62=1.669, p>0.05, \(\eta ^{2}\)p=0.051).
At 20th minute after exercise
No significant difference was observed among the VO2 (F2,62=0.395, p>0.05, \(\eta ^{2}\)p=0.013), VCO2 (F2,62=0.137, p>0.05, \(\eta ^{2}\)p=0.004) and RER (F2,62=2.26, p>0.05, \(\eta ^{2}\)p=0.068) of the three tests at the 20th minute after exercise.
At 30th minute after exercise
VO2 at the 30th minute after exercise had a significant difference among the three tests (F2,62=6.17, p<0.01, \(\eta ^{2}\)p=0.166). There was a significant difference between Test I and Test II (P<0.001). There was no significant difference between Test I and Test III (P>0.05); There was a significant difference between Test II and Test III (P<0.01).
VCO2 at the 30th minute after exercise had a significant difference among the three tests (F2,62=10.24, p<0.001, \(\eta ^{2}\)p=0.248). There was a significant difference between Test I and Test II (P<0.001). There was no significant difference between Test I and Test III (P>0.05); There was a significant difference between Test II and Test III (P<0.001).
The RER at the 30th minute after exercise had a significant difference among the three tests (F2,62=5.78, p<0.01, \(\eta ^{2}\)p=0.157). There was no significant difference between Test I and Test II (P>0.05); There was a significant difference between Test I and Test III (P<0.05); There was a significant difference between Test II and Test III (P<0.05).
Discussion
Substrate metabolism analysis during the exercise period
The study shows that when Tabata cycle increases, there is a progressive increase in fat oxidation amount and progressive decrease in glucose oxidation amount across the three tests during the exercise period, suggesting a positive effect of increased Tabata cycles on fat oxidation amount and negative effect on glucose oxidation amount. Regarding substrate metabolism, Tabata training triggers rapid glycogen depletion while simultaneously engaging both anaerobic and aerobic pathways to meet energy demands. During high-intensity intervals, the body initially relies on the phosphagen system (direct ATP-CP utilization) for immediate energy, then progressively recruits glycolytic and fatty acid oxidation pathways37. This unique metabolic profile enables Tabata to concurrently enhance fat oxidation and improve insulin sensitivity38. Research indicates that Tabata training relies more heavily on carbohydrates as the primary energy source compared to traditional HIIT, particularly during its high-intensity intervals. This metabolic characteristic enables Tabata to rapidly deplete glycogen stores within a condensed time frame20. This explains why fat oxidation progressively increases with additional Tabata cycles: as glycogen stores become depleted, the body gradually shifts toward fatty acid oxidation to meet ongoing energy demands39. In this process, fat acid is mobilized from adipose tissue into the bloodstream and oxidized by muscle cells, while insulin sensitivity increases and cortisol amount rises40, promoting the mobilization and oxidation of fatty acid. Additionally, the activity of enzymes involved in fatty acid oxidation, such as carnitine palmitoyl transferase and acyl-CoA oxidase, significantly increases41. These mechanisms indicate that multiple Tabata cycles will promote fat oxidation by increasing training intensity and total volume. The experimental data support this conclusion, demonstrating that multiple consecutive Tabata cycles significantly enhance fat oxidation efficiency compared to a single cycle.
Analysis of fat oxidation amount during the recovery period
The 10-minute post-exercise recovery period demonstrated progressively increasing fat oxidation rates with additional Tabata cycles, showing a clear dose-response relationship between training volume and lipid metabolism. These findings align with established research demonstrating that during the initial recovery period, HIIT significantly enhances fat oxidation rates compared to moderate-intensity continuous training (MICT). This suggests high-intensity exercise may preferentially stimulate fat oxidation in the post-exercise period42. Moreover, As exercise intensity increases (with higher peak power output), the fat oxidation rate correspondingly rises, indicating that high-intensity training can effectively enhance fat oxidation but requires greater training volume to sustain such high-intensity stimulation43. This study increased the number of tabata cycles, which increased training volume, so it is reasonable to expect an incremental increase in fat oxidation to occur.
During the 20-, 30-minute recovery period, the training volume corresponding to Test II enabled the body to achieve the best fat oxidation effect and the most significant fat reduction within this period, which meant excessive (Test I) or insufficient (Test III) training volumes were likely to reduce the fat oxidation effect. These results were special. Established research demonstrating a consistent dose-response relationship between training volume and fat oxidation (where greater volume typically yields higher fat oxidation)44; during high-intensity exercise, fat oxidation rates remain relatively low, yet post-exercise fat utilization significantly exceeds that of low-intensity training45. The distinctive characteristics of Tabata training, specifically its ultra-short duration and maximal-intensity nature, may explain the discrepancy between our findings and prior research. However, another study revealed a distinct pattern: high-intensity training increased fat oxidation rates by 43% during exercise, but elicited a 13% reduction during the recovery phase46, which suggested that while high-intensity training enhances fat oxidation capacity, its effects may vary across different physiological phases. This study may explain why excessive Tabata volume leads to reduced fat oxidation during recovery periods.
It is noteworthy that there was no significant difference in fat oxidation between Test I and Test III during the 20-minute and 30-minute recovery periods. This suggests that increasing Tabata volume will not result in higher fat loss efficiency.
In general, these results fully demonstrate that different training intensities and repetitions have a significant impact on post-exercise fat metabolism. An appropriate volume of Tabata training can effectively promote fat oxidation after exercise, while excessive or insufficient training volumes will lead to a reduction in fat oxidation efficiency.
Analysis of glucose oxidation amount during the recovery period
During the 10-, 20-, and 30-minute recovery period after exercise, Test I had significantly higher glucose oxidation amount compared to Test II and Test III indicating that glucose oxidation amount decreases with the increase in training volume. High intensity training exert significant effects on glucose metabolism. Research demonstrates that HIIT markedly alters post-exercise glucose-regulating hormones and metabolic responses. The active recovery group (ARG) showed a significant decrease in blood glucose levels and increase in insulin levels following maximal exercise, indicating enhanced glucose utilization. Furthermore, this research indicated that intermittent training augments muscular glucose uptake capacity, providing additional evidence that high-intensity training may promote glucose oxidation47. This is because after a single high-intensity exercise, the body initially relies on carbohydrates (such as glucose) as the primary energy source. As training volume increases (Test II and Test III), the body increasingly relies on fat and reduces glucose oxidation48. The decrease in glucose oxidation amount may reflect a metabolic shift, where the body gradually shifts from primarily re-lying on glucose for energy to relying more on fat49,50. This transition typically occurs after high-intensity or prolonged training when the body needs to conserve limited glycogen reserves51.
It is noteworthy that there was no significant difference in glucose oxidation between Test II and Test III. These findings align with previous research indicating that after high intensity exercise, the muscle glycogen depletion and reduced glucose oxidation may correlate with increased training volume52. Furthermore, while high-intensity training elevates muscle glycogen synthase activity, the glucose oxidation rate may decrease due to preferential utilization of muscle glycogen stores53.
In conclusion, these findings collectively suggest that different training intensities and volumes exert a significant influence on energy metabolism in the short term after exercise, particularly in substrate utilization, with clear trends of metabolic shifts from glucose to fat oxidation as training parameters vary.
Energy expenditure analysis during the exercise period and recovery period
During the exercise period and 30-minute recovery period, despite the increase in Tabata cycles, energy expenditure remained almost unchanged. These results were similar with previous researches. Research indicates that increased training volume typically elevates training load, potentially leading to substantially higher energy expenditure. However, post-exercise recovery energy expenditure may depend more on individual recovery mechanisms and metabolic states. For instance, although HIIT significantly boosts energy expenditure during exercise, post-training recovery energy expenditure can vary considerably among individuals54. On the other hand, HIIT reduces the accumulation of muscle metabolites (lactate and phosphocreatine), suggesting enhanced muscular recovery capacity. Nevertheless, this improved recovery may not significantly impact overall energy expenditure during the recovery period55. An explanation is that,, although the total exercise load increases with more Tabata cycles, the last Tabata cycle of each test has already maximally mobilized the body’s glycogen reserves and phosphocreatine, leading to stable energy expenditure during the last cycle. This is due to the metabolic demands and energy supply systems are already in a highly efficient and saturated state (a physiological condition where bodily functions achieve optimal equilibrium, representing peak metabolic and biomechanical efficiency)56. Adaptation to training intensity may lead to an optimized exercise economy, where the ratio of energy expenditure to exercise performance stabilizes, resulting in almost unchanged energy expenditure for each test57.
Delayed effects of substrate metabolism and energy expenditure after exercise
The study shows the changes in fat oxidation rates for three tests at 10 minutes, 20 minutes, and 30 minutes after exercise. The findings aligned with previous research reporting that fat oxidation rates significantly increase during the 2-hour post-exercise recovery period, reaching a peak within this timeframe. This suggests that while fat oxidation continues to rise during recovery, its rate of acceleration progressively declines58. Test I showed a gradual increase in the 30-minute recovery period, which is due to a delayed effect of fatty acid mobilization and oxidation59. The fat oxidation rate in Test II and Test III increased from 10 to 20 minutes and decreased again at 30 minutes. This shows that the limited enhancement of fat oxidation rates in the recovery period after increasing Tabata cycles, which may be due to the impact of the participants’ weak cardiorespiratory fitness60,61. Test II showed a more pronounced increase in fat oxidation rate throughout the recovery period, especially peaking at 20 minutes, showing that a single training set that includes two Tabata cycles most significantly promotes fat oxidation. Test III, having a relatively stable fat oxidation rate under high-intensity training, did not significantly surpass Test II, which reflects the limited potential for fat oxidation after high-intensity training, possibly related to increased metabolic stress and complexity in energy conversion. In summary, during the recovery period, Test II is most beneficial for fat oxidation, particularly peaking at 20 minutes. The fat oxidation rate of Test I, although increased in the later stages of recovery, is overall lower than Test II and Test III. The fat oxidation rate of Test III was almost the same as that of Test II at 10 minutes, but did not perform as well as Test II thereafter, which may be related to the metabolic load from high-intensity training.
The study also shows the changes in glucose oxidation rates for the three tests at 10 minutes, 20 minutes, and 30 minutes after exercise. All three tests showed gradual decreases in the 30-minute recovery period. The findings aligned with prior research: High-intensity training may increase glycogen synthase activity in muscles, yet glucose oxidation rates could decline due to preferential utilization of muscle glycogen stores. These data suggest that during the early post-exercise phase, participants exhibit robust glucose metabolism, which gradually tapers off as recovery progresses53. Our study showed that in the early stages after exercise, participants had enough intense glucose metabolism, which gradually reduced as recovery time extended. At 10 minutes, the glucose oxidation rate decreases with increasing Tabata cycles. At 20 minutes, the glucose oxidation rate was lower in Test II than in both Test I and Test III. At 30 minutes, there was no significant difference in glucose oxidation rate between the three tests. This shows that Test II was weaker in activating glucose oxidation, which is related to changes in exercise intensity62. Test I maintained a relatively high glucose oxidation rate throughout the recovery period, especially in the early stages. This shows that after low-volume exercise, the body relies more on rapid glucose metabolism for energy63. Test II had the lowest glucose oxidation rate at 20 minutes, indicating a possible earlier shift from glucose to fat metabolism, consistent with its higher fat oxidation rate, reflecting the characteristics of moderate-volume training to trigger metabolic conversion64. Test III maintained a relatively stable decreasing trend throughout the recovery period, indicating that high-volume training may maintain a higher metabolic amount over a longer time. In summary, different numbers of Tabata cycles significantly effected glucose oxidation rates during the recovery period. Test I showed higher initial glucose metabolism, which may be related to the body’s dependence on glucose after low-intensity. Test II shifted earlier to fat metabolism, significantly reducing glucose oxidation rates. These trends suggest that post-exercise metabolic pathways were significantly influenced by training volume.
The study showed that the energy expenditure rate of the three tests decreased between 10 and 30 minutes. The number of Tabata cycles had no effect on the energy expenditure rate.
Research limitations
The study’s exclusive focus on overweight/obese male university students, excluding females, other age groups, and individuals with exercise experience, which limits the generalizability of its findings. While previous studies have validated the reliability of the Cosmed K529,30,31, and our experimental protocol implemented standardized procedures to ensure data accuracy, the absence of reliability testing in this study may introduce potential measurement variability. Only 1-3 Tabata cycles were examined, leaving the effects of higher training volumes unexplored. The study solely examined acute metabolic responses (30-minute recovery period), failing to assess either the cumulative effects of long-term training on fat oxidation or associated body composition changes.
Future studies should expand participant diversity by including females, various age groups (e.g., adolescents, middle-aged/older adults), and individuals across fitness levels (e.g., trained vs. sedentary populations). Subsequent experimental designs could compare metabolic responses and fatigue accumulation across different Tabata cycle volumes (e.g., 1-3 vs. 4-6 cycles), while long-term intervention trials should track sustained outcomes including body fat percentage, lean mass, and resting metabolic rate.
Practical implications
Traditional viewpoint held that “greater exercise volume leads to more fat loss,” but this study revealed that excessive volume may paradoxically reduce fat oxidation efficiency. This study proposed an efficient, short-term exercise protocol for overweight/obese individuals: Just 2 Tabata cycles (8 minutes of high-intensity training with 10-minute intervals) could maximize fat oxidation, making it particularly suitable for time-constrained university students or working professionals. For general fitness enthusiasts, this protocol could serve as an effective fat-loss alternative, but required careful intensity management to prevent overexertion or injury.
Conclusion
This study found that the energy expenditure of the three tests had no significant difference, no matter during exercise or recovery periods. During the exercise period, the more Tabata cycles in a single training set, the more effective the fat oxidation during the exercise period. During the recovery period, two Tabata cycles in a single training set were found to be the most efficient for male university students with overweight/obesity in fat oxidation with the same energy expenditure amount, leading to the best fat oxidation. Regarding the delayed effect post-exercise, a single training set that includes two Tabata cycles showed the best delayed effect among the three tests. Therefore, considering the physical tolerance and time efficiency of male university students with overweight/obesity, a single training set that includes two Tabata cycles is an efficient and suitable exercise method for short-term weight loss for male university students with overweight/obesity.
Data availability
Data is provided within the manuscript or supplementary information files.
References
World Obesity Federation. World obesity atlas 2023 Tech. Rep, World Obesity Federation, (2023).
National Health Commission of the People’s Republic of China. Report on chinese residents’ nutrition and chronic disease status (2020). Tech. Rep., National Health Commission, Beijing, China (2020).
J, S., W, Z., T, G., D, Z. & Y, B. A retrospective study on association between obesity and cardiovascular risk diseases with aging in chinese adults. Sci. reports 8(1), 5086, https://doi.org/10.1038/s41598-018-24161-0 (2018).
Q, J. et al. Regional differences of physical fitness and overweight and obesity prevalence among college students before and after covid-19 pandemic since the “double first-class” initiative in china. Front. public health 11, 1252270. https://doi.org/10.3389/fpubh.2023.1252270 (2024).
ME, P., A, T. & JP, D. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 126, 1477–1500, https://doi.org/10.1161/CIRCRESAHA.120.316101 (2020).
Powell-wiley, T. et al. Obesity and cardiovascular disease: A scientific statement from the american heart association. Circulation 143, e984–e1010. https://doi.org/10.1161/CIR.0000000000000973 (2021).
Ruze, R. et al. Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 14, 1161521. https://doi.org/10.3389/fendo.2023.1161521 (2023).
Iyengar, N. M., Gucalp, A., Dannenberg, A. J. & Hudis, C. A. Obesity and cancer mechanisms: Tumor microenvironment and inflammation. J. clinical oncology : official journal Am. Soc. Clin. Oncol. 34, 4270–4276. https://doi.org/10.1200/jco.2016.67.4283 (2016).
J, A., M, G. & AE, J. Determination of the exercise intensity that elicits maximal fat oxidation. Medicine and science in sports and exercise 34(1), 92–97, https://doi.org/10.1097/00005768-200201000-00015 (2018).
SL, R., J, H., GS, F., ES, C. & GA, W. Maximal fat oxidation during exercise is positively associated with 24-hour fat oxidation and insulin sensitivity in young, healthy men. J. applied physiology (Bethesda, Md. : 1985) 18(11), 1415–1422, https://doi.org/10.1152/japplphysiol.00058.2015 (2015).
N, G.-M. et al. Loss of total and visceral adipose tissue mass predicts decreases in oxidative stress after weight-loss surgery. Obes. (Silver Spring) 17(3), 439–446. https://doi.org/10.1038/oby.2008.542 (2009).
B, S. et al. Serum oxidative status in people with obesity: Relation to tissue losses, glucose levels, and weight reduction. Antioxidants (Basel) 12(11), 1923, https://doi.org/10.3390/antiox12111923 (2023).
IA, C.-G., FJ, A.-G., A, R.-J. & JF, B. Toward exercise guidelines for optimizing fat oxidation during exercise in obesity: A systematic review and meta-regression. Sports medicine 53(12), 2399–2416, https://doi.org/10.1007/s40279-023-01897-y (2023).
TP, S., CM, M., RK, K., F, G. & JP, K. Effects of aging on basal fat oxidation in obese humans. Metabolism 57(8), 1141–1147, https://doi.org/10.1016/j.metabol.2008.03.021 (2009).
D, R., F, F., HJ, H., MF, N. & Y, Z. Effects of very low volume high intensity versus moderate intensity interval training in obese metabolic syndrome patients: a randomized controlled study. Sci. Reports 11, 2836, https://doi.org/10.1038/s41598-021-82372-4 (2021).
S, C., C, T., D, H. & MI, T. High-intensity interval training: a review of its impact on glucose control and cardiometabolic health. Diabetologia 60(1), 7–23, https://doi.org/10.1007/s00125-016-4106-1 (2017).
Jb, G. & Mj, G. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness?. Appl. Physiol. Nutr. And Metab. 39(3), 409–412. https://doi.org/10.1139/apnm-2013-0187 (2014).
JS, T., G, P., T, W. & TA, A. High-intensity interval training elicits higher enjoyment than moderate intensity continuous exercise. PLoS One 12(1), e0166299, https://doi.org/10.1371/journal.pone.0166299 (2017).
Tabata, I. Tabata training: one of the most energetically effective high-intensity intermittent training methods. The journal of physiological sciences: JPS 69, 559–572. https://doi.org/10.1007/s12576-019-00676-7 (2019).
Wang, Y. et al. A comparative analysis of energy expenditure and substrate metabolism in male university students with overweight/obesity: Tabata vs hiit and mict. Front. Endocrinology 15, 1323093. https://doi.org/10.3389/fendo.2024.1323093 (2024).
Y, L., HD, W., JS, B., Q, W. & S, Y. The effect of tabata-style functional high-intensity interval training on cardiometabolic health and physical activity in female university students. Front. physiology 14, 1095315, https://doi.org/10.3389/fphys.2023.1095315 (2023).
Ljubojevic, A., Gerdijan, N., Pavlović, R. & Šebić, L. Effect of tabata training program on body fat reduction in healthy inactive women. Pedagog. Phys. Cult. Sports 27, 198–207, https://doi.org/10.15561/26649837.2023.0303 (2023).
Nbj, V. & Rs, M. Research into the health benefits of sprint interval training should focus on protocols with fewer and shorter sprints. Sports medicine 47(12), 2443–2451. https://doi.org/10.1007/s40279-017-0727-x (2017).
Ohkawara, K., Tanaka, S., Ishikawa-Takata, K. & Tabata, I. Twenty-four-hour analysis of elevated energy expenditure after physical activity in a metabolic chamber: models of daily total energy expenditure. The Am. journal of clinical nutrition 87(5), 1268–1276. https://doi.org/10.1093/ajcn/87.5.1268 (2008).
Emberts, T. M., Porcari, J. P., Dobers-Tein, S., Steffen, J. & Foster., C. C. Exercise intensity and energy expenditure of a tabata workout. J. Sports Sci. Medicine 12(3), 612–613, https://jssm.org/volume12/iss3/cap/jssm-12-612.pdf (2013).
Maesaroh, S., Purwoto, S. & Novendri, D. Effects of tabata body weight training on body fat percentage in obese women. J. Maenpo: J. Pendidikan Jasmani Kesehatan dan Rekreasi 12, 118. https://doi.org/10.35194/jm.v12i1.2327 (2022).
E, M.-C. et al. Effect of hiit with tabata protocol on serum irisin, physical performance, and body composition in men. Int. journal of environmental research and public health 07(10), 3589, https://doi.org/10.3390/ijerph17103589 (2022).
Xiang, H., Yang, R., Tu, J., Guan, X. & Tao, X. Health impacts of high bmi in china: Terrible present and future. Int. journal of environmental research and public health 19, 16173. https://doi.org/10.3390/ijerph192316173 (2022).
DeBlois, J., White, L. & Barreira, T. Reliability and validity of the cosmed k5 portable metabolic system during walking. Eur. journal of applied physiology 121(1), 209–217. https://doi.org/10.1007/s00421-020-04514-2 (2021).
Faigenbaum, A. D. Sismes vii national congress. Sport Sci Heal. 11, 1–102. https://doi.org/10.1007/s11332-015-0234-0 (2015).
I, P.-S. et al. Accuracy and precision of the cosmed k5 portable analyser. Front. physiology 9, 1764, https://doi.org/10.3389/fphys.2018.01764 (2018).
Malchaire, J., D’Ambrosio Alfano, F. & Palella, B. Evaluation of the metabolic rate based on the recording of the heart rate. Ind. Heal. 55, 219–232. https://doi.org/10.2486/indhealth.2016-0177 (2017).
Trombold, J. R. et al. Postexercise macronutrient intake and subsequent postprandial triglyceride metabolism. Medicine and science in sports and exercise 46(11), 2099–2106. https://doi.org/10.1249/MSS.0000000000000333 (2014).
Frayn, K. N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 55, 628–634. https://doi.org/10.1152/jappl.1983.55.2.628 (1983).
A, B. G. Psychophysical bases of perceived exertion. Medicine and science in sports and exercise 14(5), 377–381 (1982).
Cohen, J. W. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates 2 (1988).
Haetami, M. et al. Effects of endurance training methods and mental toughness on vo2max. Int. J. Hum. Mov. Sports Sci. 12, 210–221. https://doi.org/10.13189/saj.2024.120122 (2024).
Yan, Y. & Chen, Q. Energy expenditure estimation of tabata by combining acceleration and heart rate. Front. Public Heal. 9, https://doi.org/10.3389/fpubh.2021.804471 (2022).
A, P. & M., R. A narrative literature review on the role of exercise training in managing type 1 and type 2 diabetes mellitus. Healthcare (Basel) 11(22), 2947, https://doi.org/10.3390/healthcare11222947 (2023).
Smith, U. & Kahn, B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Medicine 280, https://doi.org/10.1111/joim.12540 (2016).
Alghannam, A. F., Ghaith, M. M. & Alhussain, M. H. Regulation of energy substrate metabolism in endurance exercise. International journal of environmental research and public health 18, 4963. https://doi.org/10.3390/ijerph18094963 (2021).
S, T. et al. Effects of two workload-matched high-intensity interval training protocols on regional body composition and fat oxidation in obese men. Nutrients 13(4), 1096, https://doi.org/10.3390/nu13041096 (2021).
MM, A. et al. Effects of high-intensity interval training (hiit) and sprint interval training (sit) on fat oxidation during exercise: a systematic review and meta-analysis. Br. journal of sports medicine bjsports-2021-105181, https://doi.org/10.1136/bjsports-2021-105181 (2022).
WS, J. et al. Comparison of energy consumption and excess post-exercise oxygen consumption according to taekwondo taegeuk poomsae performance in taekwondo players. Phys. activity and nutrition 27(1), 41–46, https://doi.org/10.20463/pan.2023.0005 (2023).
C, L., de Glisezinski I, D, L., I, H. & C, M. Influence of acute and chronic exercise on abdominal fat lipolysis: An update. Front. physiology 11, 575363, https://doi.org/10.3389/fphys.2020.575363 (2022).
SA, A. The effect of moderate- and high-intensity interval training on substrate oxidation and nutrient preferences in obese men. J. Int. Soc. Sports Nutr. 11, P7, https://doi.org/10.1186/1550-2783-11-S1-P7 (2014).
AB, A. et al. Effects of recovery mode during high intensity interval training on glucoregulatory hormones and glucose metabolism in response to maximal exercise. J. athletic enhancement 7(3), 292, https://doi.org/10.4172/2324-9080.1000292 (2018).
Messonnier, L., Chatel, B., Emhoff, C.-A., Léo, B. & Oyono-Enguéllé, S. Delayed rebound of glycemia during recovery following short-duration high-intensity exercise: Are there lactate and glucose metabolism interactions?. Front. Nutr. 8, https://doi.org/10.3389/fnut.2021.734152 (2021).
Muscella, A., Stefàno, E., Lunetti, P., Capobianco, L. & Marsigliante, S. The regulation of fat metabolism during aerobic exercise. Biomolecules 10, 1699. https://doi.org/10.3390/biom10121699 (2020).
Holloszy, J. O., Kohrt, W. M. & Hansen, P. A. The regulation of carbohydrate and fat metabolism during and after exercise. Front. bioscience: a journal and virtual library 3, D1011–D1027. https://doi.org/10.1146/ANNUREV.NU.16.070196.001005 (1998).
carbohydrate and protein metabolism. Viru., A. A. Postexercise recovery period. Scand. J. Medicine & Sci. Sports 6, 2–14. https://doi.org/10.1111/j.1600-0838.1996.tb00063.x (1996).
van Loon LJ, PL, G., D, C.-T., WH, S. & AJ, W. The effects of increasing exercise intensity on muscle fuel utilisation in humans. The J. physiology 536 Pt 1, 295–304, https://doi.org/10.1111/j.1469-7793.2001.00295 (2001).
Garvick, A. Effect of high intensity intervals 24hr prior to a simulated 40 km time trial. Medicine & Sci. Sports & Exerc. https://api.semanticscholar.org/CorpusID:148938552 (2018).
AL, W., LA, G.-L., B, L., AJ, R. & KG, T. New approaches to determine fatigue in elite athletes during intensified training: Resting metabolic rate and pacing profile. PLoS One 12(3), e0173807, https://doi.org/10.1371/journal.pone.0173807 (2017).
D, B., J, E., C, T. & J, M. Effects of high-intensity training on muscle lactate transporters and postexercise recovery of muscle lactate and hydrogen ions in women. American journal of physiology. Regulatory, integrative and comparative physiology 295(6), R1991–R1998, https://doi.org/10.1152/ajpregu.00863.2007 (2008).
Langemann, D. & Rehberg, M. Unbuffered and buffered supply chains in human metabolism. J. biological physics 36, 227–244. https://doi.org/10.1007/s10867-009-9178-4 (2009).
Seiler, S. & Tønnessen, E. Intervals, thresholds, and long slow distance: the role of intensity and duration in endurance training. SPORTSCIENCE 13, 32–53, https://sportsci.org/2009/ss.htm (2009).
Honnor, M. & Herdsman, M. The effect of food timing on fat oxidation during exercise and resting recovery. Proc. Nutr. Soc. 71, https://doi.org/10.1017/S0029665112003278 (2012).
Dupuit, M. et al. Acute metabolic responses after continuous or interval exercise in postmenopausal women with overweight or obesity. Scand. J. Medicine & Sci. Sports 30(12), 2352–2363. https://doi.org/10.1111/sms.13814 (2020).
Pühringer, M. & Ring-Dimitriou, S. The influence of cardiorespiratory fitness level on the relationship between work rates at the aerobic threshold (aert) and the point of maximal fat oxidation (fatmax) in untrained adults. Front. sports and active living 6, 1321896. https://doi.org/10.3389/fspor.2024.1321896 (2024).
Jeppesen, J. & Kiens, B. Regulation and limitations to fatty acid oxidation during exercise. The J. of physiology 590, 1059–1068. https://doi.org/10.1113/jphysiol.2011.225011 (2012).
Blom, P. C. S., Vøllestad, N. K. & Costill., D. L. Factors affecting changes in muscle glycogen concentration during and after prolonged exercise. Acta physiologica Scand. Suppl. 556, 67–74, https://api.semanticscholar.org/CorpusID:30718471 (1986).
Newsom, S., Everett, A., Hinko, A. & Horowitz, J. A single session of low-intensity exercise is sufficient to enhance insulin sensitivity into the next day in obese adults. Diabetes care 36, 2516–2522. https://doi.org/10.2337/dc12-2606 (2013).
Edge, J., Bishop, D., Goodman, C. & Dawson, B. Effects of high- and moderate-intensity training on metabolism and repeated sprints. Medicine & Sci. Sports & Exerc. 37, 1975–1982. https://doi.org/10.1249/01.mss.0000175855.35403.4c (2005).
Acknowledgements
The authors thank all the participants volunteering in this study.
Funding
This research was funded by the National Social Science Foundation of China, grant number 21XTY018 and the Guangxi Higher Education Undergraduate Teaching Reform Project, grant number 2023JGZ110.
Author information
Authors and Affiliations
Contributions
ZY.C. helped in implement the study, data curation, writing-original draft. LX.L. helped in implement the experiment, writing-review. YB.H. helped in conceptualization, research design methodology. YB.W. helped in data curation and formal analysis. HL.W. helped in project administration, interpret the result, editing & revise manuscript. YM.H. helped in methodology, validation, editing & revise manuscript. All of the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, Z., Liu, L., Han, Y. et al. Two Tabata cycles in a single training set maximize fat oxidation after exercise in male college students with overweight/obesity. Sci Rep 15, 34011 (2025). https://doi.org/10.1038/s41598-025-13526-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-13526-x