Abstract
The electrocoagulation (EC) method, when combined with sonication and aeration, is an example of an advanced oxidation process (AOP) that may be used to treat a variety of wastewaters. The methods of sono (US), airlift (AL), EC, US/AL, AL/EC, US/EC, and AL/US/EC were used to investigate the decrease of color and chemical oxygen demand (COD) in landfill leachate wastewater (LLW). According to experimental findings, under the following optimal conditions of treatment time (TT) = 3 h pH = 7, current density (J) = 1 A dm−2, COD = 3200 mg L−1, concentration of electrolyte (ConElec) = 4 g L−1, electrode combination (EleCom) = Fe/Fe, aerated flow rate (AFR) = 25 L hr−1, sonication power (USp) = 100 Watts and inter-electrode spacing (IES) = 1 cm, the AL/US/EC method reduced the 100% of color and COD from LLW with consumption of power (CP) approximately 6.50 kWhrm−3. The values discovered were significantly higher than those obtained from the US, AL, US/AL, EC, US/EC, and AL/EC procedures. To determine the optimal operating conditions, the influence of several distinct control variables, TT = 0.5–3.5 h, J = 0.2–1.2 A dm−2, COD = 1600–6000 mg L−1, ConElec = 0.5–5 g L−1, AFR = 0–35 L hr−1, USp = 20–100 W, and IES = 1–4 cm on color and COD reduction was investigated. Enhancements in COD reduction effectiveness were seen with extended TT, elevated J, increased USp and AFR, reduced COD concentrations, and diminished IES when employing Fe/Fe electrode combinations. The synergy index between the AL and US/EC processes was analyzed and recorded. This study indicated that the AL/US/EC approach is highly effective for treating LLW.
Similar content being viewed by others
Introduction
The emergence of new consumption patterns and the growth in the global population have led to a significant increase in waste production, which is primarily disposed of in sanitary landfills1,2. The leachate produced by sanitary landfills has the potential to generate a variety of contaminants, both organic and inorganic and etc3,4.,. Leachate waste from sanitary landfills is thought to be a major source of pollutants due to its complex composition and resistance to degradation5. When landfill leachate wastewater (LLW) is released into the environment, it damages aquatic life, reduces soil fertility, causes cancer in people, and eventually upsets ecological balance6,7. It is very difficult to reduction of pollutants from the LLW to a standard level because of its extremely variable composition and high pollutant load. Consequently, identifying an efficient method for eliminating pollutants from wastewater is essential. Numerous techniques for the removal of contaminants from LLW have been discovered by earlier research, including hydrodynamic cavitation and activated carbon8coagulation-flocculation9ozone/Fenton10, electro-Fenton4electrochemical oxidation11combination of electrocoagulation (EC)12,13,14 with advanced oxidation processes (AOPs)15,16photo(UV)–Fenton, UV–H2O2 and Fenton reaction5ultrasound (US) irradiation17Fenton and ozonation (O3) systems6etc.,. Some of those techniques are successful, but a number of disadvantages, as an example, comparatively greater operating costs, the requirement for added chemicals, and complex treatment conditions, limit their practical application.
The electrocoagulation method typically uses metals as sacrificial anodes, such as iron (Fe) or aluminum (Al), resulting in the release of metal ions when current is applied between the cathodes and anodes18,19,20,21,22,23,24. The ions experienced hydrolysis, resulting in the formation of metal hydroxide and complexes, while contaminants in the solution were removed by adsorption, neutralization, and co-precipitation18,19,20. Consequently, the straightforward, effective, and economical electrochemical technique is optimally appropriate for wastewater treatment18,19,20. The primary EC reactors used in laboratories and industries are tanks with integrated electrodes that do not come into contact with coagulants or pollutant particles. Consequently, the mechanical mixer is employed to enhance the efficacy of the mixing process. However, the permanent disintegration of flocs and the resulting increased energy consumption may be the consequence of the high shear force generated by mechanical agitation25.
Numerous studies have effectively demonstrated the effectiveness of EC when used in combination with other treatments, including EC/magnetic field, aerated-EC26UV/EC27,28,29, EC/adsorption, US/EC30–32, EC/reverse osmosis, EC/chemical coagulation33alternating-current EC34,35peroxi/EC36O3 assisted EC32,37,38,39EC-nanofiltration and membrane filtration40,41,42and biological treatment with EC43 for the elimination of contaminants from wastewater. As a consequence of this, a number of researchers started combining EC with airlift (AL) reactors in order to develop airlift-electrocoagulation (AL-EC) reactors. These reactors proved to be extremely efficient in the reduction of dye, oil, fluorine, arsenic, and COD from wastewater26,44,45. The successful performance of AL-EC was further influenced by the incorporation of air. Aeration was beneficial in that it increased the oxidation process and decreased the amount of passivation that happened on the electrodes. This was one of the features that made aeration beneficial46. Aeration in the EC process has been demonstrated to increase the efficacy of arsenic reduction from contaminated groundwater as well as the decolorization and COD removal of dye wastewater26.
The utilization of aerated and modified electrical coagulation procedures has resulted in the achievement of an appropriate level of reduction efficiency47. Several studies have demonstrated that EC with aeration reduces COD more effectively than EC without aeration3,44. When aerated EC was used to remove oil, grease, and COD from car wash effluents, a higher reduction efficiency was observed48. Karimi et al. discovered that the combination of aerated EC and the adsorption of maghemite nanoparticles effectively removed COD from textile wastewater44. Anuar et al. discovered that during EC treatment, aeration enhances the removal of NH₃-N and color from leachate waste49. Rusdianasari et al. presented their findings after combining the EC and aeration methods with the addition of NaCl to treat the leachate50. Omar Khalifa et al. used an Al anode in EC aerated and non-aerated processes to treat synthetic oily wastewater. According to their findings, aerated cells had a reduction efficiency that was around 12% higher than that of non-aerated cells45.
Conversely, US-EC, which integrates US and EC, is a novel hybrid AOP for the elimination of contaminants from wastewater51,52. The use of ultrasound during the US-EC process can sometimes prevent insulating materials from being deposited on the electrode surface. As a consequence of this, the current efficiency and the mass transfer rate continue to increase throughout and during the operation. As a result, the amount of energy that is consumed decreases until the pollutants have been completely degraded53. Consequently, US/EC not only improves pollutant reduction efficiency but also extends electrode lifespan and decreases treatment costs. Özyonar et al.54 found that combining US and EC was more effective at removing color and COD from aqueous dye solutions than using either alone. Furthermore, when EC treatment was combined with US irradiation, electrode passivation was reduced compared to the EC technique. Prajapati55 examined the effectiveness and cost of reduction color and COD from biodigester effluent using US, EC, and US/EC. They discovered that, with just 0.58 kWhrm−3 of energy consumption, the US/EC approach significantly increased reduction efficiencies for both color (61.60% efficiency) and COD (99.10% efficiency) as compared to employing just the US and EC. Emerick et al. have demonstrated that the ECF and ECF/US technologies are practical approaches to wastewater treatment30. Oza et al.56, discovered that a promising approach to the elimination of hazardous compounds such as arsenic involved the integration of EC and US treatment procedures.
A thorough analysis of the literature indicated that combining AOPs with electrochemical techniques effectively removes pollutants from synthetic wastewater. A limited number of experiments have focused on the application of hybrid EC and AOPs for the treatment of actual wastewater. These investigations have been quite restricted in scope57,58. In order to remove pollutants from wastewater, the integrated methods consumption of power (CP) is crucial from a financial perspective. Furthermore, environmental engineers must develop simple, cost-effective, and efficient hybrid technologies to address the shortcomings of conventional treatment techniques.
The authors are unaware of any prior studies that have examined the integration of AL and US treatment with an EC approach to assess the CP associated with COD and color reduction from LLW. This research was conducted with the intention of developing and improving innovative treatment techniques for assessing CP while simultaneously eliminating pollutants from LLW. The primary goal of this research is to compare airlift (AL), sono (US), AL/US, electrocoagulation (EC), AL/EC, US/EC, and AL/US/EC technologies in terms of color and COD reduction, as well as to calculate the CP from LLW and select the best one. The influence of process factors such as treatment time, current density, COD, concentration of electrolyte and types, aerated flow rate, US power, and inter-electrode spacing on the COD reduction and CP of LLW treated with an AL/US/EC process was studied. A further investigation and documentation of the synergy index (SI) between the AL and US/EC processes was carried out.
Materials and methods
Collection and characterization of wastewater
For the purpose of collecting landfill leachate wastewater (LLW) from a municipal solid waste sanitary landfill site in Jimma, Ethiopia, a clean plastic sample container was utilized through the collection process. Subsequently, the wastewater was promptly transported to the laboratory and stored appropriately until it was required again. The wastewater has the following characteristics: temperature: 19.70–25 °C, electrical conductivity: 3.8 mS/cm, pH: 8.3–8.5, color: light green (4.05 Abs), Odor: stench ammonia, BOD: 2600–3000 mg L−1, COD: 4750–6250 mg L−1, NH4: 500 mgL−1, PO4: 450 mgL−1, Turbidity: 275–325 NTU, TDS: 3500–3750 mg L−1 and TSS: 135–175 mg L−1.
Materials and chemicals
All of the chemical reagents used in the studies, including HCl, NaOH, hexamethylene tetra amine ((CH2)6N44), K2Cr2O7, (NH4)2Fe(SO4)2), and etc., were obtained from Merck analytical supplies, and the commercially available version was used without being purified. Both the treated wastewater and wastewater that had been tested to color analysis using a UV/VIS spectrophotometer (PerkinElmer – Lambda 25) and a closed reflux method (Spectroquant ® TR320) for determining the concentration of COD.
Combined airlift-sono-electrocoagulation (AL/US/EC) setup and process
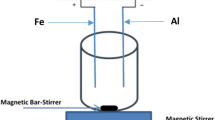
Figure 1 presents a diagrammatic representation of the AL/US/EC reactor that was developed for the purpose of this investigation. With an active working capacity of 1.0 L for wastewater, Perpexi glass was used in the construction of the 1100 mL electrochemical system. It also included an air generator, an airflow meter, and a stone diffuser. The specified air flow rate (AFR) was established on the air flow meter valve (aeration does not occur when the AFR is set to 0 L min−1). The iron (Fe) metal plate electrodes (both anode and cathode) were positioned vertically inside the cell. The electrode had a thickness of 0.1 cm and an effective surface area (width x height) of 10 cm by 15 cm, with spacing between electrodes ranging from 1 to 4 cm. The electrodes were connected to a DC power supply unit (0–5 A, 0–250 V, 50 Hz; AMETEK Model: EC 1000 S) in monopolar parallel mode. To remove the oxide film, the electrodes were sandpaper polished and rinsed with distilled water and a 1% HCl solution prior to each run. Using a pH meter (Elico: Model LI120), the pH of wastewater was measured and then adjusted to predetermined values using a diluted solution of 0.1 N HCl or NaOH. Dissolved oxygen (DO) concentrations were quantified with a multi-parameter water quality analyzer (PCD, OAKTON) that was equipped with a DO electrode (9500 DO2 meter, JENWAY).
Similar operating conditions as the airlift (AL)-EC method were used for the experiment; however, the reaction contents were further subjected to ultrasonic (US) radiation. Distilled water was added to the ultrasonic (US) water bath (model: D-7822 K, Elma Ultrasonics) until it reached the proper level. The distilled water in the US water bath was replaced prior to the commencement of each experiment. The airlift-electrochemical reactor setup was placed in an US water bath for the airlift-sono-electrocoagulation operation, which is known as the AL/US/EC process. After a few minutes, when the steady state was achieved, the power supply was turned on with the proper current and voltage settings. A multimeter is used to track the current and cell voltage during the EC, AL/EC, US/EC, and AL/US/EC processes. Samples were taken periodically from the AL/US/EC reactor and moved to an Erlenmeyer flask filled with Na2S2O3 solution in order to quench the reaction. The liquid supernatant is immediately analyzed for color and COD after the samples are centrifuged for 10 min at 15,000 rpm to separate the liquid from the particles.
Analysis
COD & color reduction efficiency, (%)
The solution’s COD and color reduction are calculated using an Eqs. (1 & 2).
Where,
The COD at starting and treatment duration (t) is denoted by COD1 and COD2, respectively.
Where,
The absorbance spectrum areas under the curves before and after treatment time t are denoted by A1 and A2, respectively.
Consumption of power (CP)
The combination of AL and US together with the EC approach results in a hybrid process that requires a significant amount of electrical energy. It is possible that the amount of power consumed constitutes a substantial operating expense59. Thus, consumption of power can be used to assess the economic viability of the hybrid process. Therefore, the total consumption of power was calculated using the following Eqs. (3–5) (\(\:{CP}_{AL/US/EC}\))60.
Where,
\(\:{CP}_{AL/US/EC}\),\(\:{CP}_{AL},\:\:{CP}_{US}\), and \(\:{CP}_{EC}\) are consumed electrical energy for AL/US/EC process, AL, US and EC process, respectively.
EC process
Where,
The variables U, I, t, and VR represent cell voltage (V), current (A), treatment time (hr), and VR wastewater volume (L), respectively.
AL and US process
Where,
Pet-rated power for the AL and US process, respectively, is expressed in kW.
Results and discussion
Process optimization for AL/US/EC
To improve the performance of the AL/US/EC process, the subsequent variables were thoroughly examined and addressed: treatment time (TT) = 0.5–3.5 h, J = 0.2–1.2 A dm−2, CODs = 1600–6000 mg L−1, concentration of electrolyte (ConElec) = 0.5–5 g L−1, aerated flow rate (ARF) = 0–35 L h−1, sonication power (USp) = 20–100 W, and inter-electrode spacing (IES) = 1–4 cm. In addition to that, the selection and modification of anode materials play a crucial role in enhancing the efficiency and effectiveness of the EC process61,62. The performance of EC is significantly influenced by the electrode materials, with Al and Fe being the most frequently employed63. To remove the COD using EC is preferred to use Fe electrodes due to an additional reduction efficiency is obtained in the removal efficiency in comparison to the other electrodes64.
After doing preliminary study, the operating parameter range that was mentioned above was identified for the AL/US/EC process that is used for the treatment of landfill leachate wastewater.
Influence of TT
When using a hybrid approach to treat wastewater, one of the most important factors to consider is the amount of time required for the treatment process65,66. The results of the AL/US/EC process with TT influence (0.5 to 3.5 h), while maintaining other parameters like COD = 3200 mg L−1, ConElec = 4 g L−1, pH = 7, EleCom = Fe/Fe, J = 1 A dm−2, USp = 100 W, ARF = 25 L hr−1, and IES = 1 cm are shown in Fig. 2. As shown in Fig. 2, the % of COD reduction and CP, has grown from 15.45 to 100%, ad 1.25 to 6.5 kWhr m−3, respectively, as the TT has increased from 0.5 to 3 h. Garca-Morales et al., have presented findings that are in agreement with these findings67. The extent of COD reduction from the wastewater is directly correlated with the quantity of ions produced by the electrodes. As the duration of treatment increases, more electrode ions and associated oxidizing species are produced65,68. As a result, the efficacy of COD reduction is enhanced by an extended treatment period. The formation of OH radicals is enhanced when the cell voltage is increased, which results in an improvement in the elimination of COD (6)66.
However, the effectiveness of COD reduction in AL/US/EC does not grow beyond a certain point (3 h) since there are enough flocs available at this time for COD reduction to finish the treatment process.
A CP is a significant measure that is used in AOPs and electrochemical processes for the reduction of COD from LLW. This metric is important from an application standpoint. The computation of the amount of CP was carried out with the help of Eqs. (3–5) that were based on the reduction of COD; the results are displayed in Fig. 2. The Fig. 2 shows that the CP increased from 0 to 7.95 kWhr m−3 while the TT increased from 0 to 3.5 h. As the TT increases, an equal amount of electrode ions and associated oxidizing species are produced. Enhanced COD reduction is the result of an increase in hydroxyl radicals caused by an increase in CP via cell voltage69. Furthermore, the optimal TT in this study was determined to be 3 h in order to achieve the maximum COD reduction with the least amount of CP required by employing an AL/US/EC method.
Impact of AFR
By varying the air flow rate (AFR) from 0 to 35 L hr−1 while keeping all other operating parameters constant (ConElec = 4 g L−1, J = 1 A dm−2, EleCom = Fe/Fe, pH = 7, USp = 100 W, TT = 3 h, COD = 3200 mg L−1, and IES = 1 cm), the tests evaluated the effect of the AFR on color and COD reduction efficiency. It has been demonstrated that aeration improves color and COD reduction efficiency as compared to a non-aeration method26,44. A properly increased AFR (0 to 25 L hr−1) can significantly improve the efficiency of COD (75.5 to 100%) nd color (80 to 100%) reduction (Fig. 3). Turbulence produced by the increasing air flow enhanced the mixing conditions of the solutions, raised the probability that the pollutants and flocs would collide, and improved the efficiency of the pollutants’ collection. Turbulence also improved ion movement between electrodes by reducing the passivation layer. At the same time, the electrode surfaces developed metalhydroxide flocs and the released gases accumulated, which increased the electrical resistance between the electrodes, causing poor performance and additional energy consumption during the EC process70. The air flow enhanced flow velocity, enhancing hydrodynamic scouring, while reducing the influence of air bubbles and deposits. Simultaneously, an increased AFR can increase the generation of specific reactive oxygen species accelerates pollutant breakdown and enhances efficiency71.
Further increasing the AFR from 25 to 35 L hr−1 resulted in a reduction in COD and color from 100 to 90% and from 100 to 94%, respectively. Excessive aeration may destabilize the aggregated flocs, causing pollutants absorbed during the EC process to be released back into the solutions, which might explain the decrease in elimination rate under high airflow conditions. Furthermore, this led to an increase in the concentration of particulates that were suspended in the water. In instances of diminished or absent AFR, the restricted oxidation rate of Fe3+ ions may lead to poor color and COD reduction efficiency. The optimal AFR for this investigation was determined to be 25 L hr−1 in order to attain the higher color and COD reduction from LLW by employing an AL/US/EC method.
Impact of treatment time (TT) on the % COD and color reduction, and consumption of power (CP) from LLW by combined AL/US/EC process (Current density (J) =1 A dm-2, Chemical Oxygen Demand (COD) = 3200 mg L-1, pH=7, concentration of electrolyte (ConElec) = 4 g L-1, electrode combination (EleCom) =Fe/Fe, aerated flow rate (AFR) = 25 L h-1, sonication power (USp)= 100 Watts, and inter-electrode spacing (IES =1cm)
Influence of US power
The process of sonolysis (US) results in the production of hydrogen atoms as well as OH radicals when applied to water. The recombination process, on the other hand, results in a large loss of •H and •OH species72,73.
When treating industrial wastewater with the US/EC process, changing the US intensities has a significant impact on pollutant elimination74. For LLW treatment, a number of experiments were conducted to investigate the effect of US power on the efficiency of color and COD reduction, as well as CP, in the AL/US/EC technique, which utilizes US power at a range of 20 to 100 W. Figure 4 displays the findings, by increasing the US power from 20 to 100 W, the color and COD reduction efficiency increased from 90 to 100% and 81.50 to 100%, respectively, and the CP increased from 5.1 to 6.5 kWhm−3. As the US intensity increased, the passive layer thinned, reducing resistance and increasing the AL/US/EC process’s energy efficiency. As a result, the ultrasonic approach effectively removes the passive coating, increasing the release of coagulant metal75,76. Li et al.56, observed similar results for the effectiveness of phosphate reduction when they tested the US and EC procedures alone and in combination. They found that the US/EC system outperformed the two systems together in terms of phosphate reduction efficiency. This demonstrates the positive outcome of merging the US and EC procedures77.
When performing AL/US/EC, ultrasound is utilized to eliminate insulating substances from the surface of the electrodes through the cavitation effect and/or micro-streaming. In other words, the sonication process ensures that the electrode surface is cleaned in-situ, resulting in a consistent rate of coagulant generation throughout the entire process65. The availability of metal ions (Mn+) is necessary for the elimination of organic contaminants because these metal ions, when combined with negative ions, pass through various organic functional groups and increase the effective size of particles that fall by gravity. Furthermore, the sonication process generates hydroxyl radicals, which accelerate the oxidation rate of pollutants. As a result, US and EC work better together to reduce pollutants65. In conclusion, the optimal US power was determined to be 100 Watts in order to achieve the highest COD reduction with the need for CP by treating LLW utilizing an AL/US/EC procedure.
Influence of J
The term “applied current density” (J) refers to the ratio of the amount of current that is applied to the area of the electrode. This is an important parameter for hybrid-EC processes because it determines the amount of metal dissolved in solutions, which influences the formation of coagulants78,79. Figure 5 illustrated the COD reduction rate with time in the AL/US/EC reactor at varying J (ranging from 0.2 to 1.2 A dm−2). The findings indicated that the COD reduction rate augmented with increasing J, as elevating the J from 0.2 to 1 A dm−2 enhanced the COD reduction efficiency from 36.20 to 100%. Faraday’s Law states that increasing the current will yield a greater production of metals and hydroxyl, facilitating the formation of coagulants and the reduction of contaminants. This would also result in additional H2 bubbles from the cathode, which would sequester contaminants while improving solution mixing and mass transfer near the electrode. The chemical oxidation reaction would be improved by more active species produced by the anode; nevertheless, some research has found that this might lead to an increase in byproducts and increased environmental issues. Excessive applied current may cause electrode passivation and polarization, increasing electrical energy consumption74. The calculated quantity of CP for 3 h of treatment at varying J is illustrated in Fig. 5. It shown that CP increased from 1.50 to 8.21 kWh m−3 when the J increased from 0.2 to 1.2 A dm−2. This could be due to the direct connection between the cell’s voltage and the J74. In subsequent experiments, a J of 1 A dm−2 was employed to balance the efficiency of COD reduction with CP.
Impact of COD
The experiment included a range of beginning COD levels, from 1600 to 6000 mg L−1, with a J of 1 A dm−2. The reduction of COD declined from 100 to 55%, whereas CP diminished from 11.39 to 3.25 kWhr m−3, respectively, when, as seen in Fig. 6, during a 3 h treatment period, the initial CODs were increased from 1600 to 6000 mg L−1. The reduction of COD has decreased as the initial concentration of solutions has increased, while the quantities of pollutant concentration eliminated have increased. Faraday’s law states that a fixed volume of Fe2+ passes to the solution to raise the initial COD concentration at a constant galvanostatic value of J and duration. Under same J and TT, the release rate of ●OH radicals and the number of flocs produced with Fe(OH)3 remained nearly constant38,80. The primary mechanism for pollutant elimination in the hybrid AL/US/EC process was ion adsorption onto iron hydroxide flocs; however, the adsorption capacity of the flocs was constrained. The fact that the initial COD content was higher indicates that the wastewater solution contained more pollutants from the start. As a result, hydroxide matrices have a better ability to collect and remove increased COD from the solution via sweep coagulation. On the other hand, as the initial COD content of the wastewater increases, the power consumption decreases because more COD is eliminated at constant J.
Supporting electrolyte types effects
The EC process is enhanced by the addition of a supporting electrolyte to increase the conductivity of the solution and reduce the Ohmic drop. This results in a reduction in power consumption and an improvement in the efficiency of pollutant reduction69,70. Thus, in order to examine the impact of supporting electrolyte type on color, COD reduction efficiency, and consumption of power, tests were conducted using NaCl, KCl, Na2SO4, and Na2CO3 as supporting electrolytes. The findings are displayed in Fig. 7 (a) and (b).
It displayed the electrolyte concentration performances at 1 A dm−2 J. It is evident that the reduction rate for NaCl and KCl is greater than that for Na2SO4 and Na2CO3, which obtained color % of 100, 100, 65, and 50% and COD % of 100, 95, 40, and 35%, respectively. The Cl− generated active chlorine species, which may take part in the oxidation of organic contaminants and ferrous ions, were responsible for the improved performance for NaCl and KCl. According to reports, iron hydroxides would react with high concentrations of Na2SO4 and Na2CO3, reducing the amount of coagulant and lowering the reduction effectiveness. Furthermore, the conductivity of NaCl and KCl was higher than that of Na2SO4 and Na2CO3 at the same ion intensity, and the required voltage was much lower, explaining why using Na2SO4 and Na2CO3 as supporting electrolytes consumed more power in Fig. 7 (a) and (b). The degradation byproducts may be more hazardous than the parent pollutants, even if adding Cl− can increase the oxidation capability. Furthermore, an increase in the corrosion pitting rate, which results in an excessive consumption of the Fe electrode, can be caused by an excess of Cl−83.
Impact of supporting electrolyte concentration
The conductivity of a solution is a crucial factor that directly affects the AL/US/EC process cost when considering energy usage. There is a correlation between the conductivity of the solution and the reduction in electrical consumption. Consequently, in order to improve process optimization, the effects of electrolyte (NaCl) concentration on the percentage reduction of COD and CP were investigated utilizing a range of NaCl values of 0–5 g L−1 at EleCom = Fe/Fe, AFR = 25 L h−1, COD = 3200 mg L−1, USp = 100 W, J = 1 A dm−2, TT = 3 h, pH = 7, and IES = 1 cm. Figure 8 depicts the findings that were obtained. It has been noted that the concentration of NaCl increases from 0 to 4 g/L, COD reduction increases from 53.50 to 100% and CP decreased from 30.5 to 6.50 kWh m−3, respectively with TT of 3 h. The lowest power usage (5.40 kWh m−3) was noted at the maximum NaCl content (5 g L−1). This might be because adding NaCl to the solution causes an anodic discharge of Cl2 and OCl−. Because the OCl− enhances the solution’s conductivity, the least amount of electrical energy is needed to finish the process65. Furthermore, OCl− is a powerful oxidant that effectively eliminates organic compounds from the LLW. Additional NaCl concentrations do not rise because excessive NaCl production produces excess OCl−, which might destabilize organic particles55,69.
Impact of inter-electrode spacing
The inter-electrode spacing (IES) in a hybrid-EC process is an essential operational parameter for achieving total pollutant reduction while lowering operating expenses31,36. The impact of IES on the AL/US/EC process was examined utilizing measurements from 1 to 4 cm under specified operating conditions: ConElec = 4 g L−1, COD = 3200 mg L−1, EleCom = Fe/Fe, pH = 7, AFR = 25 L h−1, J = 1 A dm−2, USp = 100 Watts, and TT = 3 h. Figure 9 illustrates that augmenting the IES from 1 to 4 cm decreased the % COD reduction efficiency from 100 to 50.45% and elevated the CP from 6.50 to 18.50 kWh m3. As IES increases, Fe2+ at the anode decreases as a result of anodic oxidation being inhibited by Ohmic losses linked to anode and cathode overvoltage and mass transfer resistance81. Consequently, both the adsorption of contaminants at a higher IES and the formation of coagulants in the middle will be slowed down81. Conversely, when the IES is kept to a minimum, the electrolytic process is aided by a lower resistance to current flow in solution, which increases COD reduction. In order to decrease the presence of CP and increase the effectiveness of COD reduction, the optimal inter-electrode distance is determined to be 1 cm.
The comparison of processes
Investigations were carried out in this particular region to compare the percentages of color and COD reduction with the necessary CP. Separate methods of EC, AL, and US as well as various combination processes, such as US/AL, AL/EC, US/EC, and AL/US/EC, were used to treat wastewater from LLW. Figure 10(a) and (b) illustrate the findings that were obtained. Figure 10(a) shows that when advanced oxidation and electrochemical processes are used to reduction of color and COD from LLW, the rankings are as follows: color and COD reduction of US < AL < US/AL < EC < US/EC < AL/EC < AL/US/EC. Figure 10(a) made it clear that the AL/US/EC, AL/EC, US/EC, and EC only processes had much greater percentages of color and COD reduction efficiency than the US only, AL only, and US/AL processes. The data given above indicate that the addition of AL to the US/EC procedure resulted in a considerable improvement in color and COD reduction percentages. The successful elimination of color and COD from LLW may result from the formation of parallel pathways that generate sufficient •OH radicals through AL, US, and EC activities31,55,65,82,83,84,85,86,87. The results of a comparison of the pollutant reduction efficiency achieved by combining the US/EC and AL/EC procedures with a variety of wastewater types are shown in Table 1. The conclusions of this comparison are displayed in the table. Based on the findings, it has been determined that the approach that combines the AL/EC and US/EC processes is more efficient in removing pollutants from all types of wastewater presented in Table 1. Nevertheless, there is a dearth of research on the reduction of pollutants and the calculation of the electrical energy consumed by LLW through the use of a hybrid AL/US/EC approach.
Electrical energy consumption affects the economic viability of coupling an EC process with an AL and US process. Figure 10 (b) displays the results of the computation, which was performed using Eqs. (3–5). Using a hybrid AL/US/EC method, it was necessary to consume 6.5 kWhr m−3 of electrical energy in order to totally remove color (100%) and COD (100%) from the leachate wastewater from the landfill. When compared to the AL/US/EC, other combinations and individual operations, such as US/AL, US/EC, AL/EC, and EC, AL, US process, needed a higher amount of electrical energy usage in order to eliminate better % of color and COD. Therefore, this hybrid approach (AL/US/EC) based on electrochemical processes and AOPs is appropriate for treating industrial effluents and wastewater.
Instrumental analysis
The UV/Vis-Spectra that were developed for the evaluation of LLW are illustrated in Fig. 11 (a). Each step was applied, and the results were compared to the original LLW, which was as: US treatment, AL, EC, as well as the combinations of US/AL, AL/EC, US/EC, and AL/US/EC. According to Fig. 10, the results from the UV/Vis spectra suggest that the AL/US/EC procedure is more effective than other approaches including US/EC, AL/EC, EC alone, US/AL, AL alone, and US alone in terms of decolorizing and degrading wastewater from landfills that contains leachate. The quantitative and qualitative comparison of landfill leachate wastewater treated with the AL/US/EC technology throughout the duration of time is depicted in Fig. 11 (b). It was determined that the color of the effluent could be eliminated by the treatment procedure.
Sludge analysis
To treat landfill leachate wastewater, AL and US were combined with EC techniques involving an iron (Fe) electrode combination. Sludge appeared as a consequence of the treatment process that was carried out. As a result of the sedimentation process, the sludge can be extracted from the reactor. The production of the sludge was accomplished by the utilization of processes that were either about US–40 mL, AL–20 mL, and EC–150 mL, as well as combinations of US/AL–75 mL, AL/EC–160mL, US/EC–190 mL, and AL/US/EC–200 mL, repectively. Furthermore, the sludge content varies with the sort of wastewater utilized and may be assessed using instrumental examination. By using the bomb calorimeter method, the heating value of sludge was found to be 5.50 MJ/kg. This sludge can be burnt in furnaces or incinerators, or it can be utilized to produce mixed fuel briquettes with other organic fuels. The bottom ash can be mixed with cement-like substances to make building materials after it has been burned. Setting and leaching studies on cementious mixtures have shown that bottom ash can be widely used in cement matrices without causing unacceptable cement setting delays or excessive heavy metal leaching from solidified products88. This technique of disposing of hybrid electrocoagulation residue recovers energy from the residues while chemically fixing iron and other dangerous elements contained in the sludge produced by the hybrid electrocoagulation process.
Characterization of Flocs
Using a US/EC reactor, FTIR was used to describe the flocs that were produced as a result of the process under both the aeration and non-aeration circumstance. The peak forms of FTIR for aerated and non-aerated flocs were highly comparable, having six typical peaks, as can be seen in Fig. 12. They were also quite similar. The Fe–O bond is shown by the distinctive peak at 542 cm−189, hence it may be concluded that iron oxide compounds are present in both aerated and non-aerated flocs. The C = O stretching bond is shown by the distinctive peak at 1080 cm−1, hence it may be concluded that primary alcohol compounds are present in both aerated and non-aerated flocs. From 1400 to 1650 cm−1, flocs exhibited peaks at 1415, 1563, and 1650 cm−1, which correspond to the characteristic peaks of O-H group90aromatic C = = C91 bond and C = = O, respectively. The strong and wide absorption bands near 3425 cm−1 are caused by the stretching vibration of –OH and water molecules92. This shows that the flocs contain hydroxide and water molecules. It was an indication that flocs were being formed. The FTIR results show that iron hydroxide flocs were formed during the EC process. Complexation and physical adsorption were the primary methods used to remove the color and COD, after which they precipitated together with flocs.
Synergy index (SI)
When developing an integrated approach for wastewater treatment, it is critical to take into account the synergistic index (SI), also known as the enhancement factor93. When two or more processes or variables are combined, their combined influence is larger than the sum of their individual effects when considered separately. This concept is known as the SI. Equation (6) can be employed to calculate the SI by comparing the pollutant reduction efficiency of the integrated process to the sum of the pollutant reduction rates of the individual processes.
Where, \(\:{k}_{AL/US/EC}\), \(\:{k}_{AL}\), and \(\:{k}_{US/EC}\) are the rate constants of the hybrid AL/US/EC process, the individual AL, and US/EC process, respectively. The color and COD removal rates of the AL, US, and EC processes demonstrated a first-order process that was exactly proportional to the COD level of the solution. The rate constant (k) was determined using the 1 st -order equation based on the COD removal rate for each process, and it was discovered that for the process of AL/US/EC of 1.475 min−1, US/EC of 1.150 min1, and AL of 0.19 min1, respectively. An enhancement factor of 9.75 was calculated for the reduction of COD, and it was discovered that the synergistic impact of AL and US/EC was SE > 1. When the SI value is positive, it indicates a positive synergistic effect. This is a sign that the impact is good. This integrated process is more efficient than the individual processes because of the following factors: (i) because there is no passive layer present on the surface of the electrode; (ii) enhanced capacity for mass transfer and activation of electrodes; and (iii) increased amounts of ●OH being released into the surroundings94,95,96.
Economic analysis
Like all electrochemical processes, the hybrid EC method significantly depends on cost estimation for economic analysis. The operational expenses include fixed costs such as electricity, chemicals, labor, maintenance, electrode replacement, and sludge disposal fees. Consequently, Eq. (14) can be employed to ascertain the operational cost97,98,99.
In this scenario, ECP stands for consumption of power (kWhm−3), ECEle for consumption of electrode (kgm−3), and ECChe for consumption of chemical (kgm−3). The ideal conditions, which include the following: J = 1 A dm−2, COD = 3200 mg L−1, ConElec = 4 g L−1, pH = 7, EleCom = Fe/Fe, USp = 100 Watts, TT = 3 h, AFR = 25 L h−1, and IES = 1 cm, It was found that consumption of power, electrode, and chemicals were 6.5 kWhm−3, 0.50 kgm−3, and 1.75 kgm−3, respectively. Equation (13) was employed to determine the operating cost under optimal circumstances, resulting in 5.5 US $ m−3.
Equation (15) was used to calculate the total cost (TC) per unit volume of treated landfill leachate wastewater, which accounts for operational expenditure (OPEX) normalized by annual volume of treated effluent and capital expenditure (CAPEX) normalized by volume of treated effluent added annually to the time, in years, of operation of the combined airlift, sono, and electrocoagulation techniques, as determined by Eq. (16).
Where, RCAPEX-normalized capital cost per volume of treated effluent (US$ m−3), n-operating period of the leachate treatment plant considered in years, VT - total volume of treated effluent (m3.
Table 2 presented the comparison of operating costs between the current study and other existing methods for treating landfill leachate wastewater. The results indicated that the current investigation was appropriate for the treatment of LLW in comparison to other methods.
Conclusion
In the context of this research, the treatment of wastewater derived from landfill leachate was investigated using a variety of individual procedures as well as a combination of advanced oxidation and electrochemical techniques, including US alone, AL alone, EC alone, and combinations of US/AL, AL/EC, US/EC, and AL/US/EC processes, focusing on the % reduction of color and COD while considering consumption of power. Taking into consideration the results of the experiments, it is possible to draw the conclusion that particular processes, such as AL, US, and EC, perform less well when it comes to the treatment of wastewater derived from landfill leachate. On the other hand, when used, the hybrid model of airlift, US, and an EC process produces better results than the individual procedures. In comparison to the other processes, the combined AL/US/EC process demonstrates a high level of color reduction (100%) and COD reduction (100%) while using 6.5 kWhrm−3 of consumption of power. This is the conclusion that can be drawn from the experimental data. As a result of these discoveries, the combination of airlift and sonication with electrocoagulation provides an alternative to traditional physicochemical techniques for wastewater treatment.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Ramli, S. F. & Abdul Aziz, H. Use of ferric chloride and Chitosan as coagulant to remove turbidity and color from landfill leachate. Appl. Mech. Mater. 773–774, 1163–1167 (2015).
de Ballão, C. R., Martins, M. & de Freitas, A. M. K. G. Quality evaluation of landfill leachate after wetlands treatment: a Long-term case study. Water Air Soil. Pollut. 234, 765 (2023).
Faheem, K., Khan, S. U., Washeem, M. & Khan, S. U. Energy efficient removal of COD from landfill leachate wastewater using electrocoagulation: parametric optimization using RSM. Int. J. Environ. Sci. Technol. 19, 3625–3636 (2022).
Dolatabadi, M., Świergosz, T. & Ahmadzadeh, S. Electro-Fenton approach in oxidative degradation of dimethyl phthalate - The treatment of aqueous leachate from landfills. Sci. Total Environ. 772, 145323 (2021).
Hu, X., Wang, X., Ban, Y. & Ren, B. A comparative study of UV–Fenton, UV–H2O2 and Fenton reaction treatment of landfill leachate. Environ. Technol. 32, 945–951 (2011).
Wu, C., Chen, W., Gu, Z. & Li, Q. A review of the characteristics of Fenton and ozonation systems in landfill leachate treatment. Sci. Total Environ. 762, 143131 (2021).
Huang, Z. et al. Profile and removal of bisphenol analogues in hospital wastewater, landfill leachate, and municipal wastewater in South China. Sci. Total Environ. 790, 148269 (2021).
de Domingos, M. F. J. et al. Effect of the association of coagulation/flocculation, hydrodynamic cavitation, ozonation and activated carbon in landfill leachate treatment system. Sci. Rep. 13, 9502 (2023).
Cheng, S. Y. et al. Landfill leachate wastewater treatment to facilitate resource recovery by a coagulation-flocculation process via hydrogen bond. Chemosphere 262, 127829 (2021).
Abu Amr, S. S. & Aziz, H. A. New treatment of stabilized leachate by ozone/fenton in the advanced oxidation process. Waste Manage. 32, 1693–1698 (2012).
Nengzi, L. et al. Steel slags for enhanced removal of landfill leachate in a three-dimensional electrochemical oxidation system. Sci. Rep. 13, 12751 (2023).
Ricordel, C. & Djelal, H. Treatment of landfill leachate with high proportion of refractory materials by electrocoagulation: system performances and sludge settling characteristics. J. Environ. Chem. Eng. 2, 1551–1557 (2014).
Hamid, M. A. A., Aziz, H. A. & Yusoff, M. S. Electrocoagulation process in the treatment of landfill leachate. Sustainable Solutions Environ. Pollution. 257–304 https://doi.org/10.1002/9781119785439.ch7 (2021).
Rafiee, P., Hosseini, M. & Ebrahimi, S. The evolution patterns of temperature, pH, and voltage during the removal of chemical oxygen demand from a landfill leachate using electrocoagulation under different conditions. Reaction Kinetics Mech. Catal. 131, 319–334 (2020).
Jegadeesan, C., Somanathan, A. & Jeyakumar, R. B. Godvin sharmila, V. Combination of electrocoagulation with solar photo Fenton process for treatment of landfill leachate. Environ. Technol. 44, 4441–4459 (2023).
Sun, J., Li, X., Feng, J., Tian, X. & Oxone Co 2 D oxidation as an advanced oxidation process: comparison with traditional Fenton oxidation for treatment of landfill leachate. Water Res. 43, 4363–4369 (2009).
Torkashvand, J. et al. Application of ultrasound irradiation in landfill leachate treatment. Environ. Sci. Pollut. Res. 28, 47741–47751 (2021).
Ankoliya, D., Mudgal, A., Sinha, M. K., Patel, V. & Patel, J. Application of electrocoagulation process for the treatment of dairy wastewater: A mini review. Mater. Today Proc. 77, 117–124 (2023).
Boinpally, S., Kolla, A., Kainthola, J., Kodali, R. & Vemuri, J. A state-of-the-art review of the electrocoagulation technology for wastewater treatment. Water Cycle. 4, 26–36 (2023).
Bhagawati, P. B. et al. in Electrocoagulation Technology for Wastewater Treatment: Mechanism and Applications BT - Advanced Oxidation Processes in Dye-Containing Wastewater: Volume 1. 305–318 (eds Muthu, S. S. & Khadir, A.) (Springer Nature Singapore, 2022). https://doi.org/10.1007/978-981-19-0987-0_13
AlJaberi, F. Y. et al. Recent advances and applicable flexibility potential of electrochemical processes for wastewater treatment. Sci. Total Environ. 867, 161361 (2023).
Aljaberi, F. Y. Modelling current efficiency and ohmic potential drop in an innovateelectrocoagulation reactor. Desalin. Water Treat. 164, 102–110 (2019).
Jasim, M. A. & Aljaberi, F. Y. Treatment of oily wastewater by electrocoagulation technology: A general review (2018–2022). J. Electrochem. Sci. Eng. 13, 361–372 (2023).
Aljaberi, F. Y. Investigation of electrocoagulation reactor design effect on the value of total dissolved solids via the treatment of simulated wastewater. Desalin. Water Treat. 120, 141–149 (2018).
Abdollahi, J., Alavi Moghaddam, M. R. & Habibzadeh, S. The role of the current waveform in mitigating passivation and enhancing electrocoagulation performance: A critical review. Chemosphere 312, 137212 (2022).
Akansha, J., Nidheesh, P. V., Gopinath, A. & Anupama, K. V. Suresh kumar, M. Treatment of dairy industry wastewater by combined aerated electrocoagulation and phytoremediation process. Chemosphere 253, 126652 (2020).
Asaithambi, P., Sajjadi, B., Abdul Aziz, A. R. & Wan Daud, W. M. A. B. Performance evaluation of hybrid electrocoagulation process parameters for the treatment of distillery industrial effluent. Process Saf. Environ. Prot. 104, 406–412 (2016).
Farhadi, S., Aminzadeh, B., Torabian, A. & Khatibikamal, V. Alizadeh fard, M. Comparison of COD removal from pharmaceutical wastewater by electrocoagulation, photoelectrocoagulation, peroxi-electrocoagulation and peroxi-photoelectrocoagulation processes. J. Hazard. Mater. 219–220, 35–42 (2012).
Moradi, M. & Moussavi, G. Enhanced treatment of tannery wastewater using the electrocoagulation process combined with UVC/VUV photoreactor: parametric and mechanistic evaluation. Chem. Eng. J. 358, 1038–1046 (2019).
Emerick, T., Vieira, J. L., Silveira, M. H. L. & João, J. J. Ultrasound-assisted electrocoagulation process applied to the treatment and reuse of swine slaughterhouse wastewater. J. Environ. Chem. Eng. 8, 104308 (2020).
Moradi, M., Vasseghian, Y. & Arabzade, H. Mousavi khaneghah, A. Various wastewaters treatment by sono-electrocoagulation process: A comprehensive review of operational parameters and future outlook. Chemosphere 263, 128314 (2021).
Al-Qodah, Z. & Al-Shannag, M. On the Performance of Free Radicals Combined Electrocoagulation Treatment Processes. Separation and Purification Reviews vol. 48 143–158 Preprint at (2019). https://doi.org/10.1080/15422119.2018.1459700
Al-Qodah, Z., Tawalbeh, M., Al-Shannag, M., Al-Anber, Z. & Bani-Melhem, K. Combined electrocoagulation processes as a novel approach for enhanced pollutants removal: A state-of-the-art review. Sci. Total Environ. 744, 140806 (2020).
Alimohammadi, M., Mesdaghinia, A., Shayesteh, M. H., Mansoorian, H. J. & Khanjani, N. The efficiency of the electrocoagulation process in reducing fluoride: application of inductive alternating current and Polarity inverter. Int. J. Environ. Sci. Technol. 16, 8239–8254 (2019).
Payami Shabestar, M., Alavi Moghaddam, M. R. & Karamati-Niaragh, E. Evaluation of energy and electrode consumption of acid red 18 removal using electrocoagulation process through RSM: alternating and direct current. Environ. Sci. Pollut. Res. https://doi.org/10.1007/s11356-021-15345-9 (2021).
Sandhwar, V. K. & Prasad, B. Comparison of electrocoagulation, peroxi-electrocoagulation and peroxi-coagulation processes for treatment of simulated purified terephthalic acid wastewater: optimization, sludge and kinetic analysis. Korean J. Chem. Eng. 35, 909–921 (2018).
Das, P. P. et al. Integrated ozonation assisted electrocoagulation process for the removal of cyanide from steel industry wastewater. Chemosphere 263, 128370 (2021).
He, Z. Q. et al. Decolorization of C.I. Reactive yellow 84 in aqueous solution by electrocoagulation enhanced with ozone: influence of operating conditions. Environ. Technol. 28, 1257–1263 (2007).
Mehralian, M., Khashij, M. & Dalvand, A. Treatment of cardboard factory wastewater using ozone-assisted electrocoagulation process: optimization through response surface methodology. Environ. Sci. Pollut. Res. 28, 45041–45049 (2021).
Tavangar, T., Jalali, K., Alaei Shahmirzadi, M. A. & Karimi, M. Toward real textile wastewater treatment: membrane fouling control and effective fractionation of dyes/inorganic salts using a hybrid electrocoagulation – Nanofiltration process. Sep. Purif. Technol. 216, 115–125 (2019).
Ucevli, O. & Kaya, Y. A comparative study of membrane filtration, electrocoagulation, chemical coagulation and their hybrid processes for Greywater treatment. J. Environ. Chem. Eng. 9, 104946 (2021).
Gönder, Z. B., Balcıoğlu, G., Vergili, I. & Kaya, Y. An integrated electrocoagulation–nanofiltration process for carwash wastewater reuse. Chemosphere 253, 126713 (2020).
Al-Qodah, Z., Al-Qudah, Y. & Assirey, E. Combined biological wastewater treatment with electrocoagulation as a post-polishing process: A review. Sep. Sci. Technol. 55, 2334–2352 (2020).
Karimi, N., Mirbagheri, S. A., Nouri, R. & Bazargan, A. Sequential application of aerated electrocoagulation and γ-Fe2O3 nanoparticle adsorption for COD removal: consuming the least amount of energy and economic evaluation. Result. Eng. 17, 100770 (2023).
Khalifa, O., Banat, F., Srinivasakannan, C., Radjenovic, J. & Hasan, S. W. Performance tests and removal mechanisms of aerated electrocoagulation in the treatment of oily wastewater. J. Water Process. Eng. 36, 101290 (2020).
Nidheesh, P. V. & Gökkuş, Ö. Aerated iron electrocoagulation process as an emerging treatment method for natural water and wastewater. Sep. Sci. Technol. 58, 2041–2063 (2023).
Kumar, A., Nidheesh, P. V. & Suresh Kumar, M. Composite wastewater treatment by aerated electrocoagulation and modified peroxi-coagulation processes. Chemosphere 205, 587–593 (2018).
Priya, M. & Jeyanthi, J. Removal of COD, oil and grease from automobile wash water effluent using electrocoagulation technique. Microchem. J. 150, 104070 (2019).
Science, E. Study on ammonia and colour removal from leachate via aerated electrocoagulation (Ferum and aluminium Electrode) study on ammonia and colour removal from leachate via aerated electrocoagulation (Ferum and aluminium Electrode). https://doi.org/10.1088/1755-1315/1022/1/012067
Syakdani, A., Bow, Y., Dewi, T., Ja, A. & Arita, S. Combination of electrocogulation and aeration processes by addition NaCl for leachate treatment. Int. J. Adv. Sci. Eng. Inf. Technol. 10(1), 400–406 (2020).
Hassani, A., Malhotra, M., Karim, A. V., Krishnan, S. & Nidheesh, P. V. Recent progress on ultrasound-assisted electrochemical processes: A review on mechanism, reactor strategies, and applications for wastewater treatment. Environ. Res. 205, 112463 (2022).
Arka, A., Dawit, C., Befekadu, A., Debela, S. K. & Asaithambi, P. Wastewater treatment using sono-electrocoagulation process: optimization through response surface methodology. Sustain. Water Resour. Manag. 8, 61 (2022).
Raschitor, A., Fernandez, C. M., Cretescu, I., Rodrigo, M. A. & Cañizares, P. Sono-electrocoagulation of wastewater polluted with Rhodamine 6G. Sep. Purif. Technol. https://doi.org/10.1016/j.seppur.2014.08.003 (2014).
Özyonar, F., Gökkuş, Ö. & Sabuni, M. Removal of disperse and reactive dyes from aqueous solutions using ultrasound-assisted electrocoagulation. Chemosphere 258, 127325 (2020).
Prajapati, A. K. Sono-assisted electrocoagulation treatment of rice grain based distillery biodigester effluent: performance and cost analysis. Process Saf. Environ. Prot. 150, 314–322 (2021).
Oza, H., Singh, A., Sasikumar Jampa, S. & T. S. & Removal of arsenic from aqueous solution using combined ultrasonic and electrocoagulation process. Mater. Today Proc. 47, 728–732 (2021).
da Costa, P. R. F., de Costa, A., Castro, E. C. T. & Fajardo, S. S. L. Martínez-Huitle, C. A. A sequential process to treat a cashew-nut effluent: electrocoagulation plus electrochemical oxidation. J. Electroanal. Chem. 834, 79–85 (2019).
Valero, P. et al. Electrochemical advanced oxidation processes for Staphylococcus aureus disinfection in municipal WWTP effluents. J. Environ. Manage. 198, 256–265 (2017).
Mousset, E. et al. Cost comparison of advanced oxidation processes for wastewater treatment using accumulated oxygen-equivalent criteria. Water Res. 200, 117234 (2021).
GilPavas, E., Dobrosz-Gómez, I. & Gómez-García, M. Á. Optimization of solar-driven photo-electro-Fenton process for the treatment of textile industrial wastewater. J. Water Process. Eng. 24, 49–55 (2018).
Shaban, A., Basiouny, M. E. & AboSiada, O. A. Comparative study of the removal of Urea by electrocoagulation and electrocoagulation combined with chemical coagulation in aqueous effluents. Sci .Rep. 14, 30605 (2024).
Jean Claude, N. et al. Waste tea residue adsorption coupled with electrocoagulation for improvement of copper and nickel ions removal from simulated wastewater. Sci. Rep. 12, 3519 (2022).
El-Gawad, H. A. et al. Removal of chromium from tannery industry wastewater using iron-based electrocoagulation process: experimental; kinetics; isotherm and economical studies. Sci. Rep. 13, 19597 (2023).
Sadaf, S. et al. Electrocoagulation-based wastewater treatment process and significance of anode materials for the overall improvement of the process: A critical review. J. Water Process. Eng. 62, 105409 (2024).
Maha Lakshmi, P. & Sivashanmugam, P. Treatment of oil tanning effluent by electrocoagulation: influence of ultrasound and hybrid electrode on COD removal. Sep. Purif. Technol. 116, 378–384 (2013).
Borba, F. H. et al. Desirability function applied to the optimization of the Photoperoxi-Electrocoagulation process conditions in the treatment of tannery industrial wastewater. J. Water Process. Eng. 23, 207–216 (2018).
García-Morales, M. A., Roa-Morales, G., Barrera-Díaz, C., Bilyeu, B. & Rodrigo, M. A. Synergy of electrochemical oxidation using boron-doped diamond (BDD) electrodes and Ozone (O3) in industrial wastewater treatment. Electrochem. Commun. 27, 34–37 (2013).
Asaithambi, P. et al. Distillery industrial wastewater(DIW) treatment by the combination of sono(US), photo(UV) and electrocoagulation(EC) process. J. Environ. Manage. 320, 115926 (2022).
Somayajula, A., Asaithambi, P., Susree, M. & Matheswaran, M. Sonoelectrochemical oxidation for decolorization of reactive red 195. Ultrason. Sonochem. 19, 803–811 (2012).
Gu, X. et al. Efficient removal of Norfloxacin from water using batch airlift-electrocoagulation reactor: optimization and mechanisms analysis. RSC Adv. 13, 8944–8954 (2023).
Nidheesh, P. V. & Singh, T. S. A. Arsenic removal by electrocoagulation process: recent trends and removal mechanism. Chemosphere 181, 418–432 (2017).
Oturan, M. A. et al. Sonoelectro-Fenton process: A novel hybrid technique for the destruction of organic pollutants in water. J. Electroanal. Chem. 624, 329–332 (2008).
Menon, P., Singh, A., Pani, T. S., Nidheesh, P. V. & N. & Electro-Fenton assisted sonication for removal of ammoniacal nitrogen and organic matter from dye intermediate industrial wastewater. Chemosphere 269, 128739 (2021).
Dizge, N. et al. Sono-assisted electrocoagulation and cross-flow membrane processes for brewery wastewater treatment. J. Water Process. Eng. 21, 52–60 (2018).
He, C. C., Hu, C. Y. & Lo, S. L. Evaluation of sono-electrocoagulation for the removal of reactive blue 19 passive film removed by ultrasound. Sep. Purif. Technol. 165, 107–113 (2016).
Li, J. et al. A study on influential factors of high-phosphorus wastewater treated by electrocoagulation–ultrasound. Environ. Sci. Pollut. Res. 20, 5397–5404 (2013).
Asaithambi, P. et al. Sono assisted electrocoagulation process for the removal of pollutant from pulp and paper industry effluent. Environmental Sci. Pollution Research https://doi.org/10.1007/s11356-016-6909-5
Márquez, A. A., Coreño, O. & Nava, J. L. Abatement of a complex mixture of dyes in the presence of chlorides by electrocoagulation and active chlorine-based photoelectro-Fenton-like processes. Process Saf. Environ. Prot. https://doi.org/10.1016/j.psep.2022.11.050 (2022).
Sanni, I., Estahbanati, K., Carabin, M. R., Drogui, P. & A. & Coupling electrocoagulation with electro-oxidation for COD and phosphorus removal from industrial container wash water. Sep. Purif. Technol. 282, 119992 (2022).
Ahlawat, R., Srivastava, V. C., Mall, I. D. & Sinha, S. Investigation of the electrocoagulation treatment of cotton blue dye solution using aluminium electrodes. Clean. (Weinh). 36, 863–869 (2008).
Dalvand, A., Gholami, M., Joneidi, A. & Mahmoodi, N. M. Dye removal, energy consumption and operating cost of electrocoagulation of textile wastewater as a clean process. Clean. (Weinh). 39, 665–672 (2011).
Anotai, J., Singhadech, S., Su, C. C. & Lu, M. C. Comparison of o-toluidine degradation by fenton, electro-Fenton and photoelectro-Fenton processes. J. Hazard. Mater. 196, 395–401 (2011).
Irmak, S., Yavuz, H. I. & Erbatur, O. Degradation of 4-chloro-2-methylphenol in aqueous solution by electro-Fenton and photoelectro-Fenton processes. Appl. Catal. B. 63, 243–248 (2006).
Moreira, F. C., Garcia-Segura, S., Vilar, V. J. P., Boaventura, R. A. R. & Brillas, E. Decolorization and mineralization of sunset yellow FCF Azo dye by anodic oxidation, electro-Fenton, UVA photoelectro-Fenton and solar photoelectro-Fenton processes. Appl. Catal. B. 142–143, 877–890 (2013).
Campos, S. et al. Removal of contaminants of emerging concern by solar photo electro-Fenton process in a solar electrochemical raceway pond reactor. Process Saf. Environ. Prot. 169, 660–670 (2023).
Asaithambi, P., Govindarajan, R., Yesuf, M. B. & Alemayehu, E. Removal of color, COD and determination of power consumption from landfill leachate wastewater using an electrochemical advanced oxidation processes. Sep Purif. Technol 233, 115935 (2020).
Titchou, F. E. et al. Comparative study of the removal of direct red 23 by anodic oxidation, electro-Fenton, photo-anodic oxidation and photoelectro-Fenton in chloride and sulfate media. Environ. Res. 204, 112353 (2022).
Sridhar, R., Sivakumar, V., Immanuel, P., Prakash Maran, J. & V. & Treatment of pulp and paper industry bleaching effluent by electrocoagulant process. J. Hazard. Mater. 186, 1495–1502 (2011).
Zhong, Y. et al. A new magnetic adsorbent of eggshell-zeolitic imidazolate framework for highly efficient removal of Norfloxacin. Dalton Trans. 50, 18016–18026 (2021).
Zhao, L., Liu, J., Wang, H. & Dong, Y. hua. Sorption of copper and norfloxacin onto humic acid: effects of pH, ionic strength, and foreign ions. Environmental Science and Pollution Research 26, 10685–10694 (2019).
Wang, Y. et al. Effects of dissolved organic matter on the adsorption of Norfloxacin on a sandy soil (fraction) from the yellow river of Northern China. Sci. Total Environ. 848, 157495 (2022).
Lü, X. et al. Effect of iron ion configurations on Ni2 + removal in electrocoagulation. J. Environ. Sci. (China). 124, 823–834 (2023).
Ghanbari, F. et al. Insights into Paracetamol degradation in aqueous solutions by ultrasound-assisted heterogeneous electro-Fenton process: key operating parameters, mineralization and toxicity assessment. Sep. Purif. Technol. 266, 118533 (2021).
Dargahi, A. et al. Applications of advanced oxidation processes (electro-Fenton and sono-electro-Fenton) for degradation of Diazinon insecticide from aqueous solutions: optimization and modeling using RSM-CCD, influencing factors, evaluation of toxicity, and degradation Pat. Biomass Convers. Biorefin. https://doi.org/10.1007/s13399-021-01753-x (2021).
Şahinkaya, S. COD and color removal from synthetic textile wastewater by ultrasound assisted electro-Fenton oxidation process. J. Ind. Eng. Chem. 19, 601–605 (2013).
Amarzadeh, M. et al. Statistical modeling optimization for antibiotics decomposition by ultrasound/electro-Fenton integrated process: Non-carcinogenic risk assessment of drinking water. J. Environ. Manage. 324, 116333 (2022).
Abbasi, S., Zinatizadeh, A. A., Mirghorayshi, M., Zinadini, S. & McKay, T. Electrocoagulation technique for continuous industrial licorice processing wastewater treatment in a single reactor employing Fe-rod electrodes: process modeling and optimization and operating cost analysis. J. Environ. Chem. Eng. 10, 106686 (2022).
Patel, P., Gupta, S. & Mondal, P. Electrocoagulation process for Greywater treatment: statistical modeling, optimization, cost analysis and sludge management. Sep. Purif. Technol. 296, 121327 (2022).
Bayramoglu, M., Kobya, M., Can, O. T. & Sozbir, M. Operating cost analysis of electrocoagulation of textile dye wastewater. Sep. Purif. Technol. 37, 117–125 (2004).
Maurer, M. Specific net present value: an improved method for assessing modularisation costs in water services with growing demand. Water Res. 43, 2121–2130 (2009).
Simon, S., Suresh, B. K. & Anantha-Singh, T. S. A sequential aerated electrocoagulation and peroxicoagulation process for the treatment of municipal stabilized landfill leachate by iron and graphite electrodes. Chemosphere 339, 139692 (2023).
Kumari, S. & Kumar, R. N. How effective aerated continuous electrocoagulation can be for tetracycline removal from river water using aluminium electrodes? Chemosphere 305, (2022).
Jegadeesan, C., Somanathan, A., Jeyakumar, R. B., Sharmila, V. G. & Arafath, K. A. Y. Treatment of municipal solid waste landfill leachate by aeration assisted electrochemical peroxidation process using aluminium and iron electrodes. Desalin. Water Treat. 244, 131–146 (2021).
Alacahan, Ö. F. & Özyonar, F. Removal of Tetracycline using different treatment processes; electrocoagulation, ultrasound and ultrasound assisted electrocoagulation. Environ. Processes. 11, 58 (2024).
Dubey, S., Rekhate, C., Sharma, A. & Joshi, A. Kumar prajapati, A. Optimizing distillery effluent treatment through sono-electrocoagulation: A response surface methodology approach. Total Environ. Adv. 9, 200093 (2024).
Manikandan, S. & Saraswathi, R. Textile dye effluent treatment using advanced sono-electrocoagulation techniques: A Taguchi and particle swarm optimization modeling approach. Energy Sources Part. A: Recovery Utilization Environ. Eff. 45, 4501–4519 (2023).
Khoramipour, S., Mehralipour, J. & Hosseini, M. Optimisation of ultrasonic-electrocoagulation process efficiency in the landfill leachate treatment: a novel advanced oxidation process. Int. J. Environ. Anal. Chem. 103, 7587–7605 (2023).
Rai, P. K., Kant, V., Sharma, R. K. & Gupta, A. Process optimization for textile industry-based wastewater treatment via ultrasonic-assisted electrochemical processing. Eng. Appl. Artif. Intell. 122, 106162 (2023).
Posavcic, H., Halkijevic, I., Vouk, D. & Druskovic, M. Application of Box–Behnken design for Circulating flow sono-electrocoagulation for oily wastewater treatment. J. Environ. Sci. Health Tox Hazard. Subst. Environ. Eng. 57, 645–655 (2022).
Feng, H., Mao, W., Li, Y., Wang, X. & Chen, S. Characterization of dissolved organic matter during the O3-based advanced oxidation of mature landfill leachate with and without biological pre-treatment and operating cost analysis. Chemosphere 271, 129810 (2021).
Liew, L. W. et al. Microalgae cultivation in stabilized landfill leachate for simultaneous treatment and biomass production. J. Taiwan. Inst. Chem. Eng. 166, 105068 (2025).
Turan, A., Kobya, M., Iskurt, C., Gengec, E. & Khataee, A. A techno-economical assessment of treatment by coagulation-flocculation with aluminum and iron-bases coagulants of landfill leachate membrane concentrates. Chemosphere 314, 137750 (2023).
de Almeida, R., de Oroski, F., Campos, J. C. & A. & Techno-economic evaluation of landfill leachate treatment by hybrid lime application and nanofiltration process. Detritus 10, 170–181 (2020).
Li, J. et al. Treatment of landfill leachate nanofiltration concentrate by a three-dimensional electrochemical technology with waste aluminum scraps as particle electrodes: efficacy, mechanisms, and enhancement effect of subsequent electrocoagulation. Waste Manage. 173, 118–130 (2024).
Huang, C. et al. Efficient hydrogen peroxide synthesis with pluggable capsule cathode for Electro-Fenton treating actual landfill leachate. Chem. Eng. J. 498, 155318 (2024).
Li, M., Zhou, M. & Qin, X. A feasible electro-Fenton treatment of landfill leachate diluted by electro-Fenton effluent: evaluation of operational parameters, effect of Dilution ratio and assessment of treatment cost. J. Water Process. Eng. 47, 102754 (2022).
Kanchanapiya, P. & Tantisattayakul, T. Analysis of the additional cost of addressing per- and polyfluoroalkyl substance contamination from landfill leachate by reverse osmosis membranes in Thailand. J. Water Process. Eng. 45, 102520 (2022).
Ciftcioglu-Gozuacik, B. et al. Evaluation of volatile fatty acids and ammonia recovery approach from landfill leachate using pilot-scale mechanical vapor recompression. J, Environ. Manage 345, 118720 (2023).
Acknowledgements
Acknowledgments The authors acknowledge the financial support through the Researchers Supporting Project number (RSPD2025R768), King Saud University, Riyadh, Saudi Arabia and the authors are very thankful.
Funding
This research was funded by the Researchers Supporting Project number (RSPD2025R768), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Perumal Asaithambi: Investigation; Data curation; Resources; Writing - original draft. Abdelrahman O. Ezzat: Conceptualization; Methodology; Validation; Supervision.Omar H. Abd-Elkader: Conceptualization; Methodology; Validation; Supervision.Hamad A. Al-Lohedan: Conceptualization; Methodology; Validation; Supervision.Sivakumar Vigneshwaran: Conceptualization; Methodology; Validation; Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Asaithambi, P., Ezzat, A.O., Abd-Elkader, O.H. et al. Investigation on operating parameters for efficient reduction of contaminants from wastewater utilizing a combined airlift, sono, and electrocoagulation techniques. Sci Rep 15, 34045 (2025). https://doi.org/10.1038/s41598-025-13716-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-13716-7