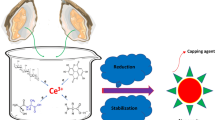
Abstract
The rising levels of environmental contamination and oxidative stress disorders have led to a growing demand for multifunctional nanomaterials that possess both biomedical and catalytic importance. CeO2 nanoparticles (NPs) were synthesized using a green solution combustion method involving Ficus carica F. extract, followed by an evaluation of their structural, biological, and photocatalytic properties. XRD confirmed a pure cubic fluorite phase (Fm-3m) with a crystallite size of 11.3 nm, which was also validated through Williamson–Hall and Rietveld refinements (χ2 = 1.9). FTIR spectroscopy revealed strong Ce–O vibrational modes at 955 cm−1, and a bandgap of 3.03 eV was determined through DRS. The SEM/TEM images displayed spherical, agglomerated particles with an average diameter of 13.5 nm. The BET analysis revealed a surface area of 30.081 m2/g, a pore radius of 1.374 nm, and a pore volume of 0.057 cm3/g, confirming the presence of microporosity. The analysis via XPS validated the existence of Ce, O, and C, revealing the coexistence of Ce3+/Ce4+ oxidation states alongside surface hydroxyl species. CeO2 NPs exhibited a dose-dependent redox activity that resulted in a reduction of cell viability to 48.82% at a concentration of 50 µM. H2O2 enhanced LDH release to 223.86%, whereas CeO2 NPs (1–7.5 µM) reduced it to 124.84%, demonstrating redox cycling antioxidant protection. Fluorescence imaging showed dose-dependent duality—antioxidant at low, pro-oxidant at high—consistent with mitochondrial damage and ATP depletion. Furthermore, CeO2 NPs demonstrated a remarkable 94.9% degradation of methylene blue under visible light, indicating their significant potential for both therapeutic and environmental applications.
Similar content being viewed by others
Introduction
Global challenges such as antibiotic resistance, environmental degradation, and advancements in medical technology have intensified the need for innovative biological solutions. Nanotechnology has emerged as a revolutionary solution through precise molecular control, leading to new applications in healthcare and environmental research1,2. Its ability to modulate biochemical interactions can improve medication delivery, targeted therapy, and regenerative medicine. Furthermore, nanoparticles play an essential role in antibacterial applications by compromising microbial structures3,4. As the previously discussed challenges grow more intense, it is crucial to develop innovative nanotechnology solutions that provide sustainable and effective answers in the fields of medicine, environmental remediation, and biological research.
Nanoparticle size, shape, and function depend on synthesis. Conventional physical and chemical procedures regulate these properties precisely, but they use dangerous chemicals, are energy-intensive, and produce harmful by-products5,6. The constraints have spurred interest in sustainable and eco-friendly synthesis methods. Plant extracts as stabilizing and reducing agents in green synthesis are popular. This green chemistry approach uses bioactive phytochemicals to improve nanoparticle biocompatibility and activity7,8. Numerous studies have examined metal oxide nanoparticles, including ZnO, TiO2, and CuO, for biological and catalytic uses. Their broad use is limited by photocorrosion, poor redox capacity, and high concentration toxicity. However, CeO2 nanoparticles have unique advantages such as a reversible redox shift from Ce3+ to Ce4+, increased oxygen storage, and chemical stability. These properties make CeO2 an attractive choice for several applications, especially in catalysis and biology. Plant-based approaches for synthesizing CeO2 nanoparticles are promising due to the inclusion of phytochemicals such as polyphenols, flavonoids, alkaloids, and terpenoids9. Their effects reduce metal ions and limit nanoparticle agglomeration10. Nanoparticles’ biomolecular surface coating boosts their antioxidant, anticancer, and antibacterial capabilities, making them useful for biomedical applications11.
The genus Ficus belongs to the family Moraceae, comprising many species rich in medicinal and nutritional values. Among these, Ficus carica is considered one of the oldest fruit trees in the world to be domesticated and highly valued for its pharmacological uses12. The fruits of Ficus carica are rich in phenolic compounds, flavonoids, and vitamins, with potent antioxidant, anti-inflammatory, and anticancer activity13. Details of all the possible phytochemicals present in Ficus carica fruit extract have been quoted in the Handbook of phytochemical constituents of GRAS herbs and other economic plants by James A. Duke (supporting information Table S1)14. Compared to other counterparts, these bioactive constituents establish that Ficus carica fruit extract is an apt candidate for synthesizing nanoparticles under the green chemistry route15. Apart from being within the sustainable practices of synthesis, it also inculcates the therapeutic prospects of the fruit in the generated nanoparticles.
Metal-Oxide like Cerium Oxide nanoparticles, synthesized with the aid of Ficus carica fruit extract, hold intrigue because of the dual functionality derived from the intrinsic properties of nano-metal and the bioactive molecules present in the extract16. The capacity of nanoceria to alternate between Ce3+ and Ce4+ oxidation states bestow it with exceptional antioxidant activity, closely resembling the actions of natural enzymes like superoxide dismutase and catalase17,18. This property equips CeO2 nanoparticles with the capability of scavenging ROS associated with a myriad of pathological conditions, such as cancer and aging. Bioactive compounds from the Ficus carica fruit extract would, on their part, improve the antioxidant and anticancer effects of nanoparticles, meaning it would reflect synergy in its potential as a therapy19,20.
Cancer is still one of the largest killers in the world, and therefore, it necessitates new, effective, and innovative therapeutic strategies21. Oxidative stress is implicated in the initiation, progression, and resistance of cancer because of the imbalance between the production of reactive oxygen species and antioxidant defenses22,23. Therefore, the antioxidant characteristic of CeO2 nanoparticles together with the anticancer property of Ficus carica fruit extract represent a novel and promising avenue to targeted cancer treatments that would obviate most of the typical side effects induced by the application of the former24. Moreover, the nanoparticles demonstrated their potential to modify oxidative stress in tumor cells, enhancing radio and chemotherapy efficacies25. Diseases associated with oxidative stress caused by free radicals, including neurodegenerative diseases, cardiovascular conditions, and diabetes, would be better addressed through the antioxidant properties of CeO2 nanoparticles26,27,28. With bioactive compounds extracted from Ficus carica fruit extract added to the mix, their therapeutic applications could be widened. Biocompatibility and low toxicity of green-synthesized CeO2 nanoparticles make them ideal for biomedical applications, thus countering the cytotoxicity problems that face chemically synthesized nanoparticles29,30.
The work here successfully demonstrated the environment-friendly synthesis of CeO2 nanoparticles by using the fruit extract of Ficus carica as the reducing and stabilizing agent. The structural, morphological, and optical properties of the as-synthesized nanoparticles have been ascertained with the help of various analytical techniques. Besides, we explored their biological activities in terms of their antioxidant and anticancer properties for evaluating further application in the biomedical field. This current study aims to provide a sustainable and effective method for synthesizing multifunctional nanomaterials intended for therapeutic applications by leveraging the potential bioactivity of Ficus carica fruit extract coupled with the unique properties of CeO2 nanoparticles.
Materials and methods
Fruit identification and chemicals procurement
Ficus carica fruits were identified with the help of a plant taxonomist (Dr. Murali M) and freshly plucked from the botanical garden at Manasagangotri campus situated at the University of Mysore (voucher number 12/1604; Repository: Herbarium lab, University of Mysore). The starting chemical cerium nitrate hexahydrate (Ce(NO3)3.6H2O) (99.999% trace metals basis) was procured from Sigma (USA) and utilized without further filtration.
Characterization tools
SHIMADZU UV 3600 Plus was utilized to record the sample’s solid-state reflectance spectrum. Rigaku SmartLab SE instrumentation was used to perform structural studies. The chemical composition of the prepared sample was identified using SHIMADZU, IRTRACER 100. Microscopic studies revealing the morphology and precursor elements were done using TESCAN BRONO MAIA3 XMH FESEM with EDS and JEOL Japan, JEM-2100 Plus HRTEM.
Preparation of Ficus carica F. extract
With the assistance of plant taxonomist (Dr. Murali, University of Mysore), fresh Ficus carica fruits were collected from the Boothal Farm (Mysuru) and deposited in the herbarium lab, University of Mysore, India (accession code: 2012/1604). The fruits were then washed, peeled, and blended into a pulp with distilled water. The mixture was heated at 60–80 °C for 30–60 min to extract bioactive compounds, followed by filtration using Whatman No. 1 filter paper to obtain a clear extract. The prepared extract, rich in reducing and stabilizing agents, was stored at 4 °C for immediate use in nanoparticle synthesis. This eco-friendly approach effectively utilizes natural phytochemicals, enhancing the green synthesis of nanoparticles for various applications.
Synthesis of CeO2 NPs
CeO2 NPs were synthesized using bio-mediated solution combustion synthesis (SCS). Initially, 3.26 g of Ce(NO3)3.6H2O was weighed accurately and placed in a combustion vessel (ceramic crucible). 5 mL of the obtained Ficus carica F. extract was added as a fuel, along with 15 mL double-distilled water, and stirred for 30 min to produce a homogenous mixture (20 mL). The mixture was then placed in a preheated muffle furnace set to 500 °C, where it underwent a typical combustion reaction, yielding pale-yellow amorphous results. Furthermore, the resulting product was calcined for 2 h at 750 °C to get the required CeO2 NPs. The sample was further characterized regarding phase identification, size, shape, and optical qualities. A schematic representing the synthesis process is shown in Fig. 1. Before performing in vitro evaluation, sterilization of the synthesized CeO2 NPs was done through UV light exposure for 30 min, aseptically.
Biological studies
The study investigates the concentration-dependent effects of CeO2 NPs synthesized from Ficus carica fruit extract on various biochemical parameters in cancer cell lines, including SH-SY5Y neuroblastoma cells. The studies emphasize the dual role of CeO2 NPs as both antioxidants and pro-oxidants depending on concentration and cellular context, particularly when it comes to oxidative stress modulation, mitochondrial function, and overall cell viability. CeO2 NPs display protective antioxidant properties at low concentrations (2.5–10 µM), while at higher concentrations (20 µM and above), they induce significant oxidative stress and cytotoxicity.
Cell culture and treatment
SH-SY5Y neuroblastoma cells were obtained from BioScienceClix labs, Mysuru (India), and utilized to investigate the antioxidant and cytotoxic properties of CeO2 NPs31. The cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin, incubated at 37 °C in a humidified setting with 5% CO2. Before treatment, the CeO2 NPs were dissolved in deionized water and sonicated. Cells were plated at a density of 1 × 104 cells per well in 96-well plates and left to incubate for 24 h before being exposed to various concentrations of CeO2 NPs (from 1 to 50 µM). To evaluate their protective or harmful impacts, the cells were also treated with hydrogen peroxide (H2O2) at a concentration of 100 µM concurrently.
MTT assay for cell viability
Cell viability was determined through an MTT assay using the methodology of32. During the administration of as-synthesized CeO₂ nanoparticles, 20 µL of 5 mg/mL MTT solution was transferred to the cells, which were seeded and then incubated at 37 °C for 4 h for formazan crystal generation. Thereupon, the media was aspirated, and the resultant formazan crystals were dissolved in DMSO. The absorbance was recorded using a microplate reader at 570 nm. Cell viability was calculated as the survival of cells relative to the control and expressed as a percentage. Further, the cell viability assay was performed to visually monitor and evaluate the CeO2 NPs cytotoxicity effect on SH-SY5Y cells. A total of 2,50,000 sub-cultured cells were separated and sown in each well of a 6-well microtiter plate, which was then incubated for 48 h. Following this, cells were treated with a 50 µg/µL dose of CeO2 NPs and incubated again for an additional 48 h. On the final day of the process, the cell staining was carried out and then incubated for 60 min in the dark. The cells were then washed with a PBS buffer solution. The fluorescence bioimaging of cells was then observed and recorded with an Invitrogen EVOS M5000 fluorescent inverted microscope.
Lactate dehydrogenase (LDH) assay
Membrane integrity and cytotoxicity were determined by observing lactate dehydrogenase (LDH) release into the culture medium with a commercial LDH assay kit (2(b), according to the procedures mentioned in33. After treatment with CeO₂ the following steps were performed on the cell-culture plates: (i) spin down plates for 5 min at 400 × g, to remove cells and obtain a supernatant; (ii) transfer 100 µL of cell-free supernatant from each well of the plate to a new 96-well plate; (iii) add 100 µL of the LDH reaction mixture to each well in the plate and mix gently. Once the plates were prepared, the LDH reaction was initiated, and the plates were incubated in the dark at 37 °C for 30 min to allow for the enzymatic conversion of lactate to pyruvate, reduction of NAD⁺, and development of colour; however, if applicable the stop solution (50 µL) was added. The plate was then read at 490 nm using a microplate reader with a reference wavelength of 620 nm to correct for any background. The cytotoxicity was calculated based on a positive (i.e., 1% Triton X-100 treated cells) and a negative (untreated) control using the following formula (Eq. 1):
Reactive oxygen species (ROS) and H₂O₂ assays
Reactive oxygen species (ROS) and hydrogen peroxide (H₂O₂) levels were obtained using the DCFH-DA and Amplex Red assays, respectively34. For total ROS measurements, cells were treated with 10 µM 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) in serum-free medium at 37 °C for 30 min in the dark. After incubation, cells were washed with phosphate-buffered saline (PBS) to remove excess probe and the non-fluorescent DCFH-DA was deacetylated and then oxidized intracellularly to create the fluorescent DCF compound. The intracellular fluorescence intensity was measured using a microplate reader with an excitation/emission wavelength of 485/535 nm.
For H₂O₂ quantification, culture supernatants were collected following treatment, and 50 µL of each sample was mixed with 50 µL of Amplex Red reagent (100 µM) in the presence of horseradish peroxidase (0.2 U/mL) for 30 min at 37 °C in the dark. The reaction of Amplex Red with H₂O₂ to produce resorufin, a red-fluorescent compound, was allowed to take place before measuring fluorescence with an excitation/emission wavelength of 530/590 nm. Fluorescence values were normalized to the untreated controls and expressed as relative fluorescence units (RFU). All experiments were completed in triplicate.
Oxidative stress marker assays
The effect that CeO₂ NPs had on oxidative stress was demonstrated by assessing the biochemical markers MDA, GSH, GSSG, and the enzyme activities of GPx, GR, GST, SOD, and CAT35. Lipid peroxidation was assessed by analyzing the MDA levels with the thiobarbituric acid reactive substances (TBARS) assay. In this assay, cell lysates were treated with thiobarbituric acid in acidic conditions and heated to produce a colored complex that could be measured at 532 nm. The levels of GSH and GSSG were assessed using Ellman’s reagent (DTNB). The GSH was quantified based on its reaction with DTNB to form a yellow chromophore that was measured at 412 nm, while the GSSG was determined after trapping free GSH and enzymatically recycled GSSG back to GSH. The enzyme activities of the antioxidants were analyzed spectrophotometrically by following reported methods: GPx was determined by following the oxidation of NADPH in the presence of glutathione and hydrogen peroxide at 340 nm; GR was determined by following the enzymatic reduction of GSSG by NADPH at 340 nm; GST was determined by following its catalysis of the conjugation of GSH to CDNB at 340 nm; SOD activity was assessed by the ability to inhibit the reduction of nitroblue tetrazolium (NBT) by superoxide radicals at 560 nm and CAT activity was evaluated on the ability to decompose hydrogen peroxide at 240 nm. All experiments were performed in triplicate, and the total protein content calculated by the Bradford or BCA assay was used for normalization.
Mitochondrial function and ATP/ADP measurement
Mitochondrial performance was measured by assessing the enzymatic activities of the electron transport chain (ETC) complexes I-IV and measuring the ATP/ADP ratio that represents the intracellular mitochondrial energy state36. Mitochondria were harvested from untreated control and treated cells using a previously standardized differential centrifugation protocol. In brief, cells were homogenized and resuspended in ice-cold isolation buffer (250 mM sucrose, 10 mM HEPES, 1 mM EDTA, pH 7.4), and all centrifugation steps were conducted on ice. The homogenate was spun at 800 ×g for 10 min to remove nuclei and debris, and the supernatant was spun again at 12,000 ×g for 15 min (4 °C) to pellet the mitochondria. Mitochondrial yield relied upon measuring the mitochondrial marker enzyme succinate dehydrogenase (SDH) to confirm that the mitochondria were of high purity and that lactate dehydrogenase (LDH) is absent to ensure no contamination with cytosol.
The enzymatic activities of each ETC Complex were measured spectrophotometrically according to standard kinetic assays using specific substrates for each complex. The activity of Complex I (NADH: ubiquinone oxidoreductase) was measured by the decrease in absorbance at 340 nm due to NADH oxidation in the presence of the coenzyme Q analogue (ubiquinone). Activity of Complex II (succinate dehydrogenase) was assessed by measuring 2,6-dichlorophenolindophenol (DCIP) reduction at 600 nm with succinate. Complex III (cytochrome bc₁ Complex) activity was performed by measuring cytochrome c reduction at 550 nm. Finally, Complex IV (cytochrome c oxidase) activity was measured based on the oxidation of reduced cytochrome c at 550 nm. All assays were performed at optimal pH and buffer, at 30 °C, and initiated upon the addition of substrates.
For analysis of cellular energy, ATP and ADP concentrations were assessed using a bioluminescence-based assay. Cells were lysed in the corresponding lysis buffer, and levels of nucleotides were assessed based on light emitted from the luciferase-mediated oxidation of luciferin; this is proportional to ATP concentrations. In the assay, ADP was enzymatically phosphorylated to ATP, and total luminescence was subsequently used to calculate the ATP/ADP ratio. All experiments were conducted in triplicate (n = 3), and data were presented as mean ± standard deviation to ensure a reasonable measure of statistical validity and reproducibility.
Statistical analysis
All the experiments were conducted in triplicate, and data are represented as mean ± standard deviation (SD). Statistical significance was calculated using a one-way analysis (ANOVA) with Tukey’s post hoc test. The statistical significance was set at p < 0.05. GraphPad Prism was used to analyse the data.
Degradation procedure of methylene blue (MB)
The visible light photocatalytic degradation of Methylene Blue (MB) dye was investigated using the synthesized CeO2 NPs. A 10 ppm MB solution was prepared and utilized in the experiments. For a typical run, 45 mg of CeO2 NPs catalyst was used with 100 mL of dye solution. To maintain a balance between dye adsorption and desorption on the catalyst’s surface, the solution was magnetically agitated in the dark for 30 min. A 300-W tungsten lamp was positioned constantly above the reaction unit to irradiate visible light. Samples were collected at predefined time intervals, centrifuged to remove the catalyst, and examined using a UV-Vis spectrophotometer spanning the 200–800 nm range. Photocatalytic activity was determined by measuring the MB dye’s absorbance intensity decrease, particularly at its characteristic peak at 664 nm. The degradation (%) was evaluated using the formula (Eq. 2)37,
where A0 and At denote the initial and time-dependent concentrations of MB, respectively.
Reusability and scavenging test
CeO2 nanoparticles were tested for reusability by repeated photocatalytic degradation cycles of MB dye under identical circumstances. After each cycle, the catalyst was centrifuged and washed with double-distilled water and ethanol to remove any remaining dye molecules. It was then dried in a hot air oven at 80 °C. The activated CeO2 catalyst was utilized to test its photocatalytic endurance and stability across 5 cycles. Further, Scavenger studies with selective quenching agents were conducted to investigate the involvement of various reactive species in the photocatalytic degradation process. Tert-butyl alcohol (TBA) (1 mM) was employed to scavenge hydroxyl radicals (•OH), disodium ethylenediaminetetraacetate (EDTA-2Na) (1 mM) for photogenerated holes (h⁺), and benzoquinone (BQ) (1 mM) for superoxide radicals (•O₂⁻). Scavengers were introduced separately to the MB-CeO2 solution before visible light irradiation. The degradation test was then carried out under similar circumstances as the control to identify the major reactive species responsible for the photocatalytic process.
Results and discussion
Reflectance studies
The as-shown Fig. 1 depicts the recorded optical reflectance spectra of as-synthesized CeO2 NPs in the range of 200–900 nm using a double-beamed UV-Vis spectrophotometer. The characteristic band 400–600 nm attributes for the effective charge transfer between Ce3+ and O2− ions38. The band-gap evaluation was done using the Kubelka-Munk relation (Eq. 3 and Eq. 4)39,
Where R∞ is referred to as the reflectance coefficient and F(R∞) is the Kubelka-Munk function. λ being the wavelength at which the spectrum is recorded, the evaluated band gap was found to be 3.03 eV (insight of Fig. 2).
FTIR studies
The functional groups associated with the surface of the as-synthesized CeO2 NPs were analysed using FTIR studies. FTIR spectrum of Ficus carica F. extract depicted the characteristic absorption bands due to the functional groups like O–H (broadband near 3300 cm⁻¹), C = O (sharp peak at 1630 cm⁻¹), and C–O (stretching bands near 1050 cm⁻¹) due to the presence of phenolics, flavonoids, and other bioactive compounds (Fig. 3)40. These functional groups are responsible for the reduction and stabilization of metal ions during the formation of nanoparticles. The FTIR spectra of as-synthesized CeO2 NPs depict a broad band in the 778–1243 cm−1 range, authenticating the metal-oxide phonon band (Ce-O)41. The 1464–1740 cm−1 and 3548–3934 cm−1 bands are attributed to the characteristic vibrational alcoholic group (OH). Further, a broad band between 1913 and 2409 cm−1 suggests the presence of methylene groups due to the aliphatic hydrocarbons42.
Structural studies
Structural assessments for the as-synthesized CeO2 NPs were done by recording PXRD spectra between the 2θ range of 20° and 90°. Figure 4 demonstrates the PXRD pattern of CeO2 NPs exhibiting a perfect match for JCPDS file #96-434-3162. The as-synthesized CeO2 NPs exhibited a perfect match with a cubic phase having phase group number 225 (F m-3 m), the lattice parameters evaluated as a = b = c = 5.41Å and α = β = γ = 90°. Major peaks detected at 2θ = 28.78°, 33.24°, 47.50°, 56.54°, 59.36°, 69.53°, 76.87°, 79.27°, and 88.73° were assigned with hkl values, (111), (200), (220), (311), (222), (400), (331), (420), and (422), respectively. The average crystallite sizes of the sample were evaluated by substituting the same obtained peak values in Scherrer’s relation (Eq. 5)43 given by,
Where 0.9 is the shape factor on assuming a circular shape, Dc, λ, and βhkl refer to crystallite size, wavelength of the X-rays used, and Full Width at Half Maximum (FWHM) of the selected peaks. The evaluated crystallite size results in 11.30 nm. Similarly, the empirical (Segal) method (Eq. 6) was interpreted in evaluating the %crystallinity of the synthesized CeO2 NPs sample,
Where Icryst and Iamp refer to the intensities of crystalline peaks and amorphous peaks, respectively. The value of %Crystallinity was estimated at 86.37%. Further, Specific Surface Area (SSA) was evaluated using the relation (Eq. 7),
Where Dc and ρ are the crystallite size and material density (CeO2 = 7.22 g/cm3), and found to be ~ 74.1 m2/g. The same result was utilized in evaluating the %porosity of 1.34%.
The same was affirmed in plotting the Williamson-Hall relation (W-H) (Eq. 8)44,45 for the as-synthesized CeO2 NPs as depicted in Fig. 5a.
Where ε refers to the lattice strain component, which was found to be 0.1 × 10−2. The results from the W-H plot suggest a well-agreed crystallite size of 12.62 nm with an R2 value of 0.976. Furthermore, for more quantitative phase information of the sample, Rietveld refinement was conducted using the FullProf suite software (version 5.20, December 2023, copyleft © 2023, JGP-JRC), utilizing the JCPDS file #96-434-3162. Figure 5b shows good agreement with the selected JCPDS file for the as-synthesized CeO2 NPs’ PXRD pattern with the refinement parameters Rp=42.6, Rwp=52.6, Rexp=11.88, and χ2 = 1.9. The Diamond-5 software (version 5.0.2, copyright ©, 1997–2024, Crystal Impact GbR, Boon, Germany) was utilized to extract the crystal structure (Fig. 5c) of the prepared CeO2 nanoparticles, matching with JCPDS card #96-434-3162, confirming the establishment of a cubic fluorite-type structure. Cerium ions occupy the corners and face-centered positions of the cubic unit cell, whereas oxygen ions dwell in the tetrahedral interstitial sites, which is typical of the standard crystallographic features of CeO2.
Morphology
The surface morphology of the synthesized CeO2 nanoparticles was examined using FESEM micrographs, as shown in Fig. 6. The images, captured at magnifications of 1 μm (Fig. 6a, b, c and d) and 100 nm (Fig. 6e, f and g), reveal notable agglomeration of the CeO2 NPs, a characteristic commonly observed in nanomaterials produced through solution combustion methods. The clustering of CeO2 NPs primarily results from the high-temperature solution combustion process, along with the influence of Ficus carica F. extract as a bio-reducing agent, which introduces organic residues that promote particle aggregation. Also, the recorded images visualize the spherical shape of the prepared CeO2 NPs. Additionally, Fig. 6h illustrates the distribution of particle sizes for the synthesized CeO2 nanoparticles.
The elemental composition and spatial arrangement of the synthesized CeO2 nanoparticles were analyzed using Energy Dispersive X-ray Spectroscopy (EDS) along with elemental mapping, as illustrated in Fig. 7. The EDS spectrum (Fig. 7a) demonstrates the successful formation of CeO2, showcasing prominent peaks attributed to cerium (Ce) around ~ 4.8–5.4 keV (Ce Lα and Lβ lines) and oxygen (O) at approximately ~ 0.5 keV. The presence of a small peak of carbon (C), typically observed at 0.28 keV, is linked to residual organic materials from the green fuel (Ficus carica F. extract) utilized in the bio-assisted solution combustion synthesis. The elemental mapping images (Fig. 7b and f) indicate a uniform distribution of Ce and O throughout the scanned area, thereby reinforcing the consistent synthesis of CeO2 nanoparticles.
Additionally, Fig. 7g presents a pie chart that depicts the distributions of atomic percentages of the identified elements. Cerium constitutes the primary weight component (46%), while oxygen exhibits a relatively elevated atomic percentage (45%), aligning with the stoichiometric composition of CeO2. The chart also reveals traces of carbon (9%), reinforcing the organic nature of the synthesis route. The results collectively confirm the effective synthesis of uniformly distributed, phase-pure CeO2 nanoparticles through an eco-friendly, plant-extract-mediated combustion method.
For deeper insights into the morphology of the as-synthesized CeO2 NPs, TEM analysis was conducted by capturing the sample’s morphology at 500 nm, 200 nm, and 50 nm resolution as depicted in Fig. 8 (a-e). The results obtained affirm the agglomeration along with the spherical morphology of the sample, with the evaluated average particle size of 13.5 nm46. This was well in agreement with the PXRD and W-H plot results. Figure 8(f), showcasing the SAED image, affirms the crystallinity of the sample, well in agreement with the recorded PXRD pattern. Furthermore, the lattice spacing was evaluated to be 0.312 nm by utilizing the recorded HRTEM image depicted in Figs. 8(g) and 8(h).
BET surface area analysis
Nitrogen adsorption-desorption isotherm analysis was used to analyze the surface area, pore volume, and pore size distribution of the produced sample after 2 hours of degassing at 160 °C. The isotherm pattern illustrated in Fig. 9(a) demonstrates a Type-II adsorption character, accompanied by an H3 hysteresis loop. This behavior is characteristic of non-rigid aggregates composed of plate-like particles that feature slit-shaped pores. The discovery aligns with the IUPAC classification and indicates the presence of a porous framework. The measured BET surface area of the sample (Fig. 9b) was determined to be 30.081 m2/g, accompanied by a pore diameter of 2.748 nm and a total pore volume of 0.057 cm3/g. According to IUPAC guidelines, porous materials are classified into three categories: microporous (with pore diameters less than 2 nm), mesoporous (ranging from 2 to 50 nm), and macroporous (with pore diameters exceeding 50 nm). The measurements (Fig. 9c) indicate that the pore radius categorizes the material within the microporous regime; however, the shape of the hysteresis loop suggests the existence of narrow mesopores alongside it, indicating a mesoporous structure. The extensive surface area and mesoporous nature of the resulting CeO2 NPs enhance the accessibility of active sites and facilitate mass transfer, thereby boosting their catalytic activity.
XPS analysis
X-ray Photoelectron Spectroscopy (XPS) was used to examine the surface chemical composition and oxidation states of the prepared sample, as shown in Fig. 10. The survey spectrum (Fig. 10a) demonstrates the presence of cerium (Ce), oxygen (O), and carbon (C) elements within the sample. The Ce 3 d high-resolution spectrum (Fig. 10b) reveals multiple peaks corresponding to Ce3+ and Ce4+ oxidation states, with prominent peaks observed at binding energies of 885.07 eV, 901.05 eV, and 916.83 eV, indicating the mixed-valence nature of cerium within the material. The O 1 s spectrum (Fig. 10c) reveals two distinct peaks at 529.13 eV and 532.22 eV, which are attributed to lattice oxygen (O2−) and surface-adsorbed hydroxyl or oxygen-containing species, respectively. The C 1 s spectrum (Fig. 10d) displays three distinct peaks at 284.69 eV, 288.40 eV, and 292.43 eV, which are associated with C–C/C = C, C = O, and π–π∗ shake-up satellite structures, respectively. This indicates the presence of adventitious carbon contamination on the surface. These findings confirm the effective integration of cerium and oxygen within the material, exhibiting a mixed Ce3+/Ce4+ valence state, alongside surface-bound functional groups that may influence catalytic or electronic properties.
MTT assay and cell viability
The data from Fig. 11 collectively demonstrate a dose-dependent cytotoxic effect of CeO2 NPs (referred to as CNP in Figures) on cell viability, with distinct variations depending on CeO2 NPs concentration and the presence of oxidative stress (H2O2). In Fig. 11(a), CeO2 NPs alone show minimal toxicity at 25 µM, maintaining high cell viability (96.98%). However, cell viability progressively declines as the concentration increases to 50 µM and beyond, dropping to 37.25% at 600 µM, indicating substantial cytotoxicity at elevated doses. Figure 11(b) further highlights the dual role of CeO2 NPs when co-treated with H2O2. While H2O2 alone reduces cell viability to 51.53%, adding low concentrations of CeO2 NPs (1–7.5 µM) provides a protective effect, increasing viability to a peak of 74.10% at 7.5 µM. However, at 50 µM, cell viability significantly drops to 31.47%, suggesting that high CeO2 NPs concentrations negate the protective effect and exacerbate cytotoxicity (Fig. 11c). Furthermore, it is clear from Fig. 11c that at lower concentrations (1–50 µM), CeO2 NPs alone can maintain high viability (94.73–98.58%) at 1–5 µM, but as concentrations increase to 20 µM and above, cell viability sharply declines to 48.82% at 50 µM, consistent with the cytotoxicity trends. Overall, these results demonstrate that CeO2 NPs are relatively safe at low concentrations (1–7.5 µM), particularly under oxidative stress conditions, but become increasingly cytotoxic at higher doses, irrespective of the presence of H2O2. In a study by Ming et al., 2016 assessed 5 nm and 25 nm CeO2 nanocrystals (NCs) were used to assess the in vitro biosafety and antioxidant capacity via INS-1 rat pancreatic β-cells. We assessed cell viability over the course of 72 h using the CCK-8 assay, revealing that both were nontoxic up to 200 µg/mL. Within the first 24 h, both + ve NCP and -ve NCP significantly increased cell viability, suggesting potential protective or proliferative effects at early time points47. A comparative study with different anticancer agents and different cell lines has been included in Table 1.
As indicated in Fig. 12(a), the findings of the LDH assay reveal a clear dose-dependent effect of CeO2 nanoparticles (CNPs) on membrane integrity with differences at normal and oxidative stress conditions. In Fig. 12(a), treatment with CeO2 NPs alone showed a trend toward an increase in LDH release, where the percentage of LDH release increased across increasing concentrations of CeO2 NPs (from 110.02% at 25 µM to 265.65% at 600 µM). The percent increase in LDH release indicates an increase in cellular membrane disruption, suggesting that CNPs at higher concentrations may have cytotoxic effects. The membrane damage may be due to a potential shift to the redox state of cerium ions with increased NP concentration, allowing for Ce4⁺ production rather than scavenging ROS, and increased NP production instead of scavenging ROS. Additionally, as NP concentrations increase, issues with sustained levels of intracellular DNP concentration, lysosomal destabilization, and undermining of the cellular cytoskeletal network may occur together, resulting in ruptured membranes and thus increased cytotoxicity. On the other hand, Fig. 12(b) displays the biphasic action of CeO2 NPs co-administered with hydrogen peroxide (H₂O₂), which is known to induce oxidative stress. H2O2 alone elevated LDH release to 223.86%, which confirms that inducing oxidative stress damaged cell membranes. Yet under co-treatment at lower concentrations of CeO2 NPs (1-7.5 µM), this effect was decreased to 124.84% LDH release at 7.5 µM CeO2 NPs. CeO2 NPs did show some protective (antioxidant) properties at lower doses, presumably through redox cycling between Ce³⁺ and Ce⁴⁺, mimicking the activity of the antioxidant enzymes superoxide dismutase (SOD) and catalase. The redox cycling may allow the CeO2 NPs to neutralize ROS on a cellular level, leading to the stabilization of chemical equilibrium or cellular redox balance and the protection of membrane integrity. The phenomenon becomes opposite to that of protection as concentrations of CeO2 NPs increased above 10 µM with H₂O₂, where the LDH release increased significantly again (285.41%) at 50 µM. The recovery of the protective effect can be explained by the redox paradox of CeO2 NPs, where high concentrations may themselves induce oxidative stress through either catalyzing ROS formation or disrupting the activity or formation of any normal endogenous antioxidant systems. Increased cytotoxicity at higher concentrations of CeO2 NPs may be attributed to redox cycling between Ce3+ and Ce4+ forms, leading to the excess generation of reactive oxygen species (ROS), which is explained in detail in the next section. Surface oxygen vacancies promote redox-mediated oxidative stress, disrupting cell redox equilibrium and playing a critical role in dose-dependent toxicity. The exogenous H2O2 as an oxidative agent may also have been at a point where, together with the high-dose CeO2 NPs, LDH release could have occurred with both agents working in synergy, which may have synergized the oxidative damage through lipid peroxidation and inflammatory processes, leading to worse cellular injury.
These results highlight the dual properties of CeO2 NPs as antioxidants at low doses and pro-oxidants at higher doses. The results, combined with previous studies, show that a proper dose is critical for future biomedical applications of CeO2 NPs. CeO2 NPs exhibit the potential for clinical intervention because of their unique redox-active properties, where low doses exhibit antioxidant properties and high doses exhibit pro-oxidant properties, but any potential benefits are easily lost, or negative side effects taken to the extreme by using improper doses. It is now a matter of establishing a safe and efficacious therapeutic window for use in future clinical translation of CeO2 NPs based interventions, especially for pathologies associated with oxidative stress. Further, the pro-oxidant nature of CeO2 NPs at a 50 µg/µL dose was validated by obtaining the fluorescence imaging of the control cells (Figure S1(a)) and the cells treated with CeO2 NPs (Figure S1(b)), as depicted in the supporting information.
ROS and H2O2 modulation
The results from the ROS and H2O2 assays, along with oxidative stress marker analyses, reveal a distinct concentration-dependent dual functionality of CeO2 nanoparticles (CeO2 NPs) in modulating oxidative stress. At lower concentrations (2.5–10 µM), CeO2 NPs exhibited antioxidant properties, as evidenced by reduced intracellular ROS and H2O2 levels, along with stable or elevated levels of key endogenous antioxidants such as glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione reductase (GR). This antioxidant effect was particularly pronounced under conditions of exogenous oxidative stress, such as H2O2 co-treatment, where CeO2 NPs effectively suppressed ROS accumulation and lipid peroxidation, as reflected by reduced MDA levels. The most potent protective response was observed at 7.5 µM (Fig. 13b). The underlying mechanism is largely attributed to the redox cycling ability of cerium atoms on the nanoparticle surface, which can reversibly shift between Ce3+ and Ce4+ oxidation states. This unique redox flexibility enables CeO2 NPs to mimic antioxidant enzymes—Ce3+ species act similarly to superoxide dismutase by neutralizing superoxide anions (O2•⁻), while Ce4+ species mimic catalase activity by decomposing hydrogen peroxide into water and molecular oxygen—thereby maintaining redox homeostasis and reducing oxidative stress.
However, at higher concentrations (≥ 20 µM), CeO2 NPs switch from being protective to acting as pro-oxidant agents, resulting in significant increases in ROS and H2O2 levels (Fig. 13a), indicative of elevated oxidative stress and potential cytotoxicity. This paradoxical behavior is attributed to several interrelated mechanisms. First, a redox imbalance arises at high concentrations as the nanoparticle surface becomes saturated with Ce4+ species, reducing the Ce3+/Ce4+ ratio and impairing regenerative redox cycling, thus diminishing the enzyme-mimetic antioxidant activity. Moreover, excess Ce4+ can catalyze the decomposition of H2O2 through Fenton-like reactions, generating hydroxyl radicals (•OH), which are highly reactive and damaging. Second, CeO2 NPs are known to exhibit photocatalytic activity under UV or ambient light exposure. Upon irradiation, they generate electron-hole pairs that interact with water and oxygen to form additional ROS such as O2•−, •OH, and 1O2, thereby amplifying oxidative stress even without external ROS inducers. Third, CeO2 NPs exhibit a dual role in modulating cellular redox balance, as evident from our data in Figs. 13(a) and 13(b). At higher concentrations (Fig. 13a), CeO2 NPs induce a dose-dependent increase in ROS levels, accompanied by a gradual decline in key antioxidant enzyme activities (GST, SOD, CAT) and a mild elevation in MDA, indicating enhanced lipid peroxidation and compromised antioxidant defence. Total GSH levels remain relatively stable, while GSSG levels rise slightly at the highest dose, suggesting moderate oxidative stress without a complete breakdown of redox homeostasis. In contrast, under H2O2-induced oxidative stress conditions (Fig. 13b), co-treatment with CeO2 NPs significantly reduces ROS levels in a concentration-dependent manner and partially restores antioxidant enzyme activities. This is coupled with decreased MDA and GSSG levels and improved total GSH, GPX, and GR activities, indicating a protective effect. These findings can be attributed to the redox-active nature of CeO2, where the Ce3+/Ce4+ valence switching allows the nanoparticles to mimic SOD and CAT activity, effectively scavenging ROS and supporting intracellular antioxidant mechanisms under stress conditions. Taken together, these findings underscore a concentration-dependent shift in CeO2 NP behavior—from cytoprotective at low doses to cytotoxic at higher doses—driven by both physicochemical parameters (Ce3+/Ce4+ ratio, surface characteristics) and biological interactions (redox cycling, photocatalysis, enzyme mimicry). This dual nature highlights the critical need for precise dose optimization and control over environmental factors, such as light exposure, to ensure safe and effective use of CeO2 NPs in therapeutic applications.
Mitochondrial function and ATP/ADP levels
The effects of CeO₂ nanoparticles (CeO2 NPs) on mitochondrial function were assessed by probing the activities of electron transport chain (ETC) complexes, levels of citrate synthase (CS), and ATP/ADP ratios, as shown in Fig. 14. Figure 14(a) shows that all treatment groups (5–40 µM CeO2 NPs) revealed similar levels of CS activity, implying that mitochondrial mass was intact and that the functional impairments observed were not due to a depletion or degradation of mitochondria. However, all the ETC Complexes I (CI), II (CII) and III (CIII) activities decreased in a concentration-dependent manner with increasing levels of CeO2 NPs, suggesting that several nanoparticles were interfering with the flow of electrons in the mitochondrial respiratory chain, likely due to oxidative damage or direct interaction with the active enzymatic centers of each complex. These interactions could impair NADH oxidation and transfer of electrons, leading to inefficient ATP generation and build-up of metabolites that ordinarily would not accumulate since it is downstream.
CIV activity appeared to only be modestly affected concerning the concentration range, indicating it could be reduced by CeO2 NPs, but unlike the previous complexes indicated less susceptibility to CeO2 NP-induced dysfunction. Similar observations regarding the relative insensitivity of CV to other oxidising agents of biological interest have been documented and may relate to differences in the subunit composition, differences in ROS sensitivity, as well as potential differences in membrane localization or accessibility. The alterations in the ETC’s function resulted in a reduction in bioenergetic reserves and capacity, evidenced by the decrease in ATP and concurrent increase in ADP (Fig. 14(b) and 14(c)). ATP decreased from 32.83 (control) to 24.45 at 40 µM CNP, while ADP increased from 5.69 to 7.32, indicative of less efficient oxidative phosphorylation. The increase in ADP alongside the decrease in ATP suggests that mitochondrial ATP synthase activity was unable to meet the cellular need for energy due to the upstream disruption of the ETC.
Comparative assays testing the low concentrations of CNPs (2.5–7.5 µM) with oxidative stress conditions (Fig. 14c), with H₂O₂, induced partial recovery of ATP levels and reduced ADP accumulation relative to H₂O₂ alone. This is further evidence that low concentrations of CNPs can act as mitochondrial protectants, possibly via scavenging ROS and stabilizing mitochondrial redox homeostatic control, but the protective mechanism expired at 7.5 µM with no additional benefit lost to the bioenergetic ceiling. In a study published by Fisichella et al. (2016), who explored the effect of pristine CeO2 NPs on mitochondrial activity in Caco-2 cells. The findings indicated that pristine CeO2 NPs resulted in under expression of 27 genes that encode for subunits of mitochondrial complexes I, III, IV, V. This under expression suggests that CeO2 NPs affect mitochondrial respiration as they alter the expression of key components of the ETC, leading to less ATP, and therefore less energy available to the cells48. In a study published by Rukhsana et al. (2023), the researchers examined the protective effects of nanoceria in H9c2 cardiomyoblasts exposed to angiotensin II-induced stress. Their results indicated that nanoceria pre-treatment dramatically decreased intracellular ROS formation, restored mitochondrial membrane potential, as well as increased the expression of mitochondrial biogenesis and fusion genes. Together, these findings suggest that CeO2 NPs could lessen mitochondrial dysfunction and oxidative stress at physiological doses, which could be therapeutically favourable49.
Mechanism prediction
Cerium oxide nanoparticles (CeO2 NPs) have a concentration-dependent bimodal role in different cellular applications, regulating cell viability, oxidative stress, and mitochondrial activity. In the MTT assay, CeO2 NPs affect mitochondrial dehydrogenase activity, where low concentrations (2.5–7.5 µM) increase cell viability by promoting redox cycling between Ce3+ and Ce4+, lowering reactive oxygen species (ROS). At elevated concentrations (> 10 µM), CeO2 NPs interfere with mitochondrial function, inhibiting electron transport chain (ETC) complexes and ATP synthesis, inducing apoptosis. In the LDH assay, low-dose CeO2 NPs stabilize membranes and reduce H2O2-induced damage, whereas high concentrations (> 10 µM) disrupt membranes, indicated by enhanced LDH leakage. ROS and H2O2 modulating studies confirm that low concentrations of CeO2 NPs function as antioxidants by eliminating ROS, reducing lipid peroxidation (MDA content), and increasing antioxidant enzymes (SOD, CAT, GPX). Conversely, > 20 µM concentrations trigger oxidative stress by exhausting glutathione (GSH) and increasing oxidized glutathione (GSSH) and thus producing redox imbalance. Mitochondrial function tests reveal that although low doses of CeO2 NPs guard against oxidative stress-induced dysfunction through the sustenance of ATP content and the conservation of CI–CIII activity, elevated doses induce mitochondrial depolarization, ATP loss, and uncontrolled ROS production, disrupting oxidative phosphorylation. The dose-dependent shift from an antioxidant to a pro-oxidant condition emphasizes the importance of careful dosing to capitalize on the therapeutic benefits of CeO2 NPs without suffering from cytotoxicity.
Photocatalytic dye degradation studies
Figure 15(a-d) shows a comprehensive investigation into the photocatalytic activity of CeO2 NPs in the degradation of MB dye under visible light. Figure 15(a) shows the UV-Vis absorption spectra of MB dye (starting concentration: 10 ppm) at different time intervals of visible light exposure in the presence of 45 mg CeO2 NPs. The MB absorption peak at 664 nm, caused by the π→π* electronic transition in the aromatic chromophore, decreases with increasing irradiation duration. This decline represents the gradual deterioration of the dye’s conjugated system as oxidative radicals destroy the MB molecules54. The constant reduction in absorbance ensures the effective production of reactive oxygen species (ROS), including hydroxyl radicals (•OH) and superoxide anions (\(\:{\text{O}}_{2}^{-}\)•) by photo-induced charge separation in CeO2. The absorption measurements provide direct evidence of MB dye mineralization or degradation into smaller, less colorful intermediates.
Figure 15(b) depicts the linear connection between deterioration (%) and irradiation time. There is a significant increase in degradation efficiency in the first 30–60 min, followed by a plateau, indicating a rapid accumulation of reactive species in the early stages and saturation of active sites with increasing time55. This trend is characteristic of Langmuir-Hinshelwood-type heterogeneous photocatalysis, in which degradation is surface-dependent and rate-limited by active site availability over extended periods.
Figure 15(c) depicts the kinetics of the degrading process determined from a linear fit of the data to the pseudo-first-order kinetic model (Eq. 9)56.
A straight-line graph indicates that CeO2 NPs-catalyzed MB degradation follows pseudo-first-order kinetics. The apparent rate constant (k1) obtained from the slope represents the photocatalytic system’s overall efficacy. The good linearity (R2 ~ 1) demonstrates that the reaction rate mostly relies on the concentration of MB in the solution under the given circumstances.
Figure 15(d) depicts the influence of starting solution pH on MB dye degradation efficiency. The degradation performance of CeO2 NPs varies significantly with pH, with alkaline conditions (pH ~ 7.19) showing the highest degradation. The increasing availability of OH⁻ ions at neutral pH may lead to increased formation of hydroxyl radicals, which react with photo-created holes. In acidic circumstances (pH < 5), breakdown efficiency drops, likely due to protonation of MB molecules and suppression of ROS production. Surface charge interactions between the dye and catalyst are pH-dependent, influencing adsorption and degradation. According to a review of the literature, if the pH of the solution is slightly higher than neutral pH (e.g., 7.19), but close to the point of zero charge (PZC), the surface will have a weak negative charge and little electrostatic interaction with nearby charged species57. A comparative study between the prepared catalyst and different catalysts against various dyes has been included in Table 1.
The photocatalytic performance of the synthesized catalyst was systematically evaluated under various conditions as depicted in Fig. 16. Catalyst dose is a critical factor in photocatalytic degradation since it directly influences the availability of active sites and light absorption effectiveness. A suitable concentration promotes deterioration, while excessive usage might cause light diffraction and catalyst aggregation, reducing efficiency. The effect of catalyst dosage revealed that degradation efficiency increased with rising catalyst concentration up to an optimum level of 45 mg (Fig. 16(a)). Similarly, experiments related to dye-dosage effect on degradation (%) indicate that an initial increment was observed from 5 ppm to 10 ppm, which later led to a drop in photocatalytic activity at 15 ppm (Fig. 16(b)), likely due to excessive dye molecules competing for active sites and hindering photon absorption. The non-linear pattern (15 ppm < 5 ppm < 10 ppm) suggests that the 10-ppm dye concentration resulted in the optimal surface contact with the photocatalyst. Lower concentrations reduce the use of active sites, while greater concentrations block light and cause surface sites to become saturated, reducing photocatalytic activity43. The recyclability test demonstrated that the catalyst retained substantial activity over four consecutive cycles, with only a slight decrease in efficiency in the 5th cycle (70.48%), confirming its stability and reusability. Quenching experiments were conducted using tetr-butyl alcohol (TBA), EDTA-2Na, and Benzoquinone (BQ) for •OH, h⁺, and •O₂⁻ scavengers (Fig. 16(c)). The results are indicated in Fig. 16(d), further indicating that superoxide radicals (•O₂⁻) played the dominant roles in the degradation process, while photogenerated holes (h⁺) had a comparatively lesser impact, thereby confirming a radical-mediated photocatalytic mechanism.
12. Plausible mechanism
Figure 17 indicates a series of photo-induced redox reactions that make up the proposed mechanism for the photocatalytic breakdown of Methylene Blue (MB) dye by CeO2 NPs. CeO2 NPs, when photosensitized to absorb light when exposed to UV or visible light, excite electrons (e⁻) in the valence band (VB) and create positive holes (h⁺) in the VB. When these charge carriers reach the surface, the holes oxidize hydroxide ions (OH⁻) or water (H₂O) to create hydroxyl radicals (•OH), while the electrons transform molecular oxygen (O₂) to create superoxide radicals (•O₂⁻). Both ROS are highly oxidative and play a key role in breaking down the intricate structure of MB dye into innocuous by-products, including CO2, H2O, and other inorganic compounds. To authenticate these results Electron Paramagnetic Resonance (EPR) spectrum was recorded for the prepared CeO2 NPs (Figure S2), which displays the typical asymmetric g value of 1.9452. The results ensure the prominent role of electron capture by oxygen vacancies detected in the EPR spectrum58. Light-generated electron-hole pairs and ROS work together to give CeO2 nanoparticles their high photocatalytic activity in breaking down dyes59,60. The predicted mechanism was also supported by the recombination dynamics studied using photoluminescence spectroscopy, as indicated in Text S1 and Figure S3 of the Supporting Information.
The Nyquist plot (Figure S4) of the synthesized CeO2 nanoparticles displays a distinct semi-circular arc in the high-frequency region, indicative of the charge transfer process occurring at the electrode–electrolyte interface. The impedance parameters obtained indicate a solution resistance (Rs) of 15.51 Ω and a charge transfer resistance (Rct) of 1.40 Ω. The related arc radius, derived by halving the Rct, measures approximately 0.70 Ω, a relatively small value.
The low arc radius value is directly linked to a lower charge transfer resistance, suggesting enhanced interfacial electron mobility and increased conductivity of CeO2 NPs61. The enhanced electron transfer properties are crucial for the effective photocatalytic separation and movement of photoinduced electron–hole pairs62. The enhanced conductivity minimizes electron–hole recombination, leading to a more effective generation of reactive oxygen species. The CeO2 nanoparticles produced in this study exhibit exceptional charge transport properties, making them highly promising for photocatalytic applications. The enhanced electron conductivity significantly contributes to the rapid degradation of organic contaminants such as Methylene Blue (MB), underscoring their potential in environmental remediation efforts.
Furthermore, to evaluate the energy bands’ position, the Mott-Schottky analysis was conducted on the prepared CeO2 NPs as depicted in Figure S5. The flat-band potential (Efb) was evaluated by extrapolating the Mott-Schottky plot to intercept the potential axis. The observed positive slope in Figure S5 indicates the material’s classification as an n-type semiconductor, in which the measured Efb approximates to conduction band (− 0.144 eV)63. This result was utilized in calculating the valence band position using the equation.(Eq.10)
Where ECB and Eg refer to the conduction band position (~−0.144 eV) and band-gap energy evaluated from the Kubelka-Munk relation (3.03 eV). The evaluated valence band position was found to be EVB=2.886 eV. The favourable alignment of the band edges suggests that the material possesses strong redox capabilities under visible light, making it a promising candidate for efficient photocatalytic applications.
Conclusion
In conclusion, CeO2 nanoparticles were effectively synthesized using a green solution combustion method, utilizing Ficus carica fruit extract as a reducing agent. This process yielded nanostructures exhibiting a pure cubic fluorite phase, with an average crystallite size of 11.3 nm, as confirmed by XRD, Williamson–Hall, and Rietveld analyses (χ2 = 1.9). Analysis using SEM and TEM revealed spherical agglomerated particles with an average diameter of 13.5 nm, consistent with nanoscale morphology. The BET analysis indicated a significant specific surface area of 30.081 m2/g, a pore radius of 1.374 nm, and a pore volume of 0.057 cm3/g, implying a highly microporous structure that is well-suited for catalysis and surface-based interactions. The XPS analysis revealed the presence of Ce, O, and adventitious C, providing clear evidence of mixed valence states (Ce3+/Ce4+) and surface hydroxyl functionalities, which are believed to contribute to the redox and catalytic properties of CeO2. The optical bandgap measured at 3.03 eV, along with a significant Ce–O vibrational band observed at 955 cm−1 through FTIR analysis, validates the material’s potential for UV-responsive applications. In biological tests, CeO2 NPs exhibited concentration-dependent dual redox activity: low doses (1–7.5 µM) demonstrated cytoprotective antioxidant activity, significantly reducing H2O2-induced LDH release from 223.86 to 124.84%, whereas high doses (50 µM) resulted in a decrease in cell viability to 48.82%, indicating cytotoxic and pro-oxidant effects. All these activities were once more validated through mitochondrial dysfunction, depletion of ATP levels, and disruption of electron transport chain activity, alongside fluorescence bioimaging. The dual behavior observed can be attributed to the redox cycling between Ce3+ and Ce4+, mimicking the enzymatic functions of superoxide dismutase (SOD) and catalase. Furthermore, CeO2 NPs demonstrated significant photocatalytic activity, successfully degrading 94.9% of methylene blue when exposed to visible light, thereby confirming their potential for environmental remediation. Collectively, these findings highlight the versatile nature of green-synthesized CeO2 nanoparticles and suggest their promising applications in biomedical therapeutics and environmental catalysis. However, they also emphasize the crucial importance of the synthesis method and the optimization of dosing in influencing their functional activities.
Data availability
All the data generated or analyzed during this study are included within the article and supplementary information.
References
Chang, Y. et al. Advances in postharvest nanotechnology: enhancing fresh produce shelf life and quality to reduce losses and waste. Postharvest Biol. Technol. 222, 113397. https://doi.org/10.1016/j.postharvbio.2025.113397 (2025).
Ghorbian, M., Ghobaei-Arani, M., Babaei, M. R. & Ghorbian, S. Nanotechnology and nanosensors in personalized healthcare: A comprehensive review. Sens. Bio-Sensing Res. 47, 100740. https://doi.org/10.1016/j.sbsr.2025.100740 (2025).
Kaur, N., Sahoo, J. & De, M. Development of Nanomaterials-Based agents for selective antibacterial activity. ChemBioChem 26 (1), e202400693. https://doi.org/10.1002/cbic.202400693 (2025).
Arbabi, L. S., Azarang, M., Sookhakian, M. & Basirun, W. J. The application of molybdenum disulfide nanosheets decorated with silver nanoparticles for antibacterial activity. J. Solgel Sci. Technol. https://doi.org/10.1007/s10971-025-06696-7 (2025).
Birhanu, M. Z., Shiferaw, R. G., Bachheti, R. K. & Ahmed, I. N. Challenges and Limitations of Chitin-Based Nanoparticles in Agriculture. Chitin-Based Nanoparticles for the Agriculture Sectors https://doi.org/10.1007/978-981-96-0920-8_10 (2025).
Awadalla, E., Bekheet, S. H. M., Gbr, S. A. & Ebrahim, Z. Toxicity of Silver Nanoparticles: Impacts on Human Health and Environmental Safety, and the Role of Green Synthesis as an Eco-Friendly Approach. Aswan Sci. Technol. Bull. https://doi.org/10.21608/astb.2025.334847.1011 (2025).
Reddy, S. G. Utilisation of Plant Extracts for Green Synthesis of Metallic Nanoparticles. In R. Malviya & S. Sundram (Eds.). Sustainable Green Biomaterials As Drug Delivery Systems https://doi.org/10.1007/978-3-031-79062-1_10 (2025).
Paiva-Santos, A. C. et al. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 597, 120311. https://doi.org/10.1016/j.ijpharm.2021.120311 (2021).
Kirubakaran, D. et al. A comprehensive review on the green synthesis of nanoparticles: advancements in biomedical and environmental applications. Biomedical Mater. Devices. https://doi.org/10.1007/s44174-025-00295-4 (2025).
Prasad, A. R., Kuruvilla, M. & Joseph, A. Synthesis and Fabrication of Green Nanoparticles. In Nanoformulations for Agricultural Applications https://doi.org/10.1002/9781119819127.ch2 (2025).
Yadav, A., Kumar, H., Kumar, P., Rani, G. & Maken, S. Syzygium cumini leaf extract mediated green synthesis of ZnO nanoparticles: A sustained release for anticancer, antimicrobial, antioxidant, and anti-corrosive applications. J. Mol. Struct. 1325, 141017. https://doi.org/10.1016/j.molstruc.2024.141017 (2025).
Falistocco, E. The Millenary History of the Fig Tree (Ficus carica L). Adv. Agric. Hortic. Entomol. https://doi.org/10.37722/AAHAE.202051 (2020).
Nigar, S. et al. Comparative analysis on phytonutrient properties of different Fig varieties (Ficus spp). Food Chem. Adv. 6, 100878. https://doi.org/10.1016/j.focha.2024.100878 (2025).
Duke, J. A. Handbook of Phytochemical Constituents of GRAS Herbs and Other Economic Plants (CRC, 1992).
Murugan, S., Senthilvelan, T., Govindasamy, M. & Thangavel, K. A comprehensive review on exploring the potential of phytochemicals and biogenic nanoparticles for the treatment of Antimicrobial-Resistant pathogenic bacteria. Curr. Microbiol. 82 (2), 90. https://doi.org/10.1007/s00284-025-04064-w (2025).
Foroutan, Z. et al. Plant-based synthesis of cerium oxide nanoparticles as a drug delivery system in improving the anticancer effects of free Temozolomide in glioblastoma (U87) cells. Ceram. Int. 48 (20), 30441–30450. https://doi.org/10.1016/j.ceramint.2022.06.322 (2022).
Sabouri, Z., Sabouri, M., Amiri, M. S., Khatami, M. & Darroudi, M. Plant-based synthesis of cerium oxide nanoparticles using rheum Turkestanicum extract and evaluation of their cytotoxicity and photocatalytic properties. Mater. Technol. 37 (8), 555–568. https://doi.org/10.1080/10667857.2020.1863573 (2022).
Sarani, M. et al. Facile synthesis of tl, pb, and Bi doped CeO2 nanoparticles and the evaluation of their in-vitro cytotoxicity and photocatalytic performance. J. Indian Chem. Soc. 101 (11), 101350. https://doi.org/10.1016/j.jics.2024.101350 (2024).
Foroutan, Z. et al. Green synthesis of cerium oxide nanoparticles via Linum usitatissimum seeds extract and assessment of its biological effects. Micro Nano Lett. 19 (6), e270003. https://doi.org/10.1049/mna2.70003 (2024).
Rahimi, P., Ghasemi, E., Esmaeili, D. & Hekmati, M. Synthesis of ficus carica sap-functionalized Nano-chitosan for biomedical applications: apoptosis induction and tumor cell line growth Inhibition. Results Chem. 14, 102115. https://doi.org/10.1016/j.rechem.2025.102115 (2025).
Crooks, S., Mitchell, G., Wynne, L. & Carter, G. Exploring the stigma experienced by people affected by parkinson’s disease: a systematic review. BMC Public. Health. 25 (1), 25. https://doi.org/10.1186/s12889-024-21236-8 (2025).
Astro, J., Clauss, G., Fontes, J. V., Oliveira, L. S. & Abbehausen, C. Oxidative stress mechanism by gold compounds: A close look at total ROS increase and the Inhibition of antioxidant enzymes. Chem. – Asian J. 20 (1), e202400792. https://doi.org/10.1002/asia.202400792 (2025).
Faderin, E. et al. Platinum nanoparticles in cancer therapy: chemotherapeutic enhancement and ROS generation. Med. Oncol. 42 (2), 42. https://doi.org/10.1007/s12032-024-02598-w (2025).
Dubey, S. et al. Utilizing plant phytoconstituents in metal oxide nanoparticle synthesis for cancer therapies. Curr. Pharm. Design. https://doi.org/10.2174/0113816128329342241120105041 (2025).
Nguyen, V. K. et al. Gold Nanoparticle-Enhanced Production of Reactive Oxygen Species for Radiotherapy and Phototherapy. Nanomaterials, 15(4), 317. (2025). https://www.mdpi.com/2079-4991/15/4/317
Dong, J. et al. Chiral Inorganic Nanomaterial-Based Diagnosis and Treatments for Neurodegenerative Diseases. Advanced Materials https://doi.org/10.1002/adma.202418723 (2025).
Bashandy, S. A. E. et al. Management of cardiovascular disease by cerium oxide nanoparticles via alleviating oxidative stress and adipokine abnormalities. Sci. Rep. 15 (1), 5709. https://doi.org/10.1038/s41598-025-85794-6 (2025).
Meng, L., Yang, M., Li, Y. & Zhang, K. Green synthesis of CeO2 nanoparticles supported over silica gel: its catalytic activity in the synthesis of pyrazolo[3,4-b]pyridines and its performance for the treatment of retinal damage in diabetic rat. J. Organomet. Chem. 1031, 123598. https://doi.org/10.1016/j.jorganchem.2025.123598 (2025).
Deshpande, N., Dwivedi, S., Revanasiddappa, B. C. & P. S. R., & Virtual screening, pharmacokinetics & MD simulation study of active phytoconstituents of ficus carica linn. Against PPAR-γ in diabetes mellitus. J. Biomol. Struct. Dynamics. 43 (1), 36–52. https://doi.org/10.1080/07391102.2023.2279286 (2025).
Ashagrie, Y. N. et al. Antidiabetic phytochemicals: an overview of medicinal plants and their bioactive compounds in diabetes mellitus treatment. Z. Für Naturforschung C. https://doi.org/10.1515/znc-2024-0192 (2025).
Guler, E. et al. In Vitro Neuroprotective Effect Evaluation of Donepezil-Loaded PLGA Nanoparticles-Embedded PVA/PEG Nanofibers on SH-SY5Y Cells and AP-APP Plasmid Related Alzheimer Cell Line Model.. Macromolecular Materials and Engineering https://doi.org/10.1002/mame.202400160 (2025).
Kumar, R. & Shikha, D. Thrombogenicity, DPPH assay, and MTT assay of sol–gel derived 3% silver-doped hydroxyapatite for hard tissue implants. Int. J. Appl. Ceram. Technol. 22 (1), e14884. https://doi.org/10.1111/ijac.14884 (2025).
Nazarkina, Z. K., Stepanova, A. O., Savostyanova, T. A. & Laktionov, P. P. Application of a modified lactate dehydrogenase assay to evaluate the viability of cells cultured on 3D scaffolds when commonly used assays fail. Cytotechnology 77 (1), 9. https://doi.org/10.1007/s10616-024-00670-x (2024).
Zhou, Y. et al. Antioxidant and Anti-Senescence Polyvinyl Alcohol-Gallic Acid Supramolecular Hydrogels for Stem Cell Culture. Advanced Healthcare Materials https://doi.org/10.1002/adhm.202402882 (2025).
Jin, Z. et al. TA-V nanozymes with acid resistance capabilities effectively target and alleviate ulcerative colitis lesions via oral delivery. ACS Appl. Mater. Interfaces. 17 (10), 14912–14923. https://doi.org/10.1021/acsami.4c20322 (2025).
Lin, H. et al. Structure and mechanism of the plastid/parasite ATP/ADP translocator. Nature https://doi.org/10.1038/s41586-025-08743-3 (2025).
Alhamdi, H. W. et al. Exploring the anticancer potency and photocatalytic efficiency of bio-derived CuO nanoparticles using Moringa Oleifera leaf extract. Results Chem. 7, 101430. https://doi.org/10.1016/j.rechem.2024.101430 (2024).
Wolski, L. et al. Enhanced adsorption and degradation of methylene blue over mixed niobium-cerium oxide – Unraveling the synergy between Nb and Ce in advanced oxidation processes. J. Hazard. Mater. 415, 125665. https://doi.org/10.1016/j.jhazmat.2021.125665 (2021).
Sooraj, M., Krishnapriya, M. J. & Manoj, E. Synthesis, characterization, band gap calculation, DFT study, and biological implication of two Nitrogen-rich proligands: polymorphic crystal structures and Hirshfeld surface analyses of a thiocarbohydrazone. J. Mol. Struct. 1321, 139857. https://doi.org/10.1016/j.molstruc.2024.139857 (2025).
Selvaraj, M., Mylsamy, N. P. P. T. R. & S, S. Extraction and Characterization of a New Natural Cellulosic Fiber from Bark of Ficus Carica Plant as Potential Reinforcement for Polymer Composites. J. Nat. Fibers https://doi.org/10.1080/15440478.2023.2194699 (2023).
Sarangi, S., Suryakanta, U., Nayak, N., Mandal, D. & Sahoo, T. R. Green synthesis and characterization of Gd-doped CeO2 NPs and their anticancer effects against colon cancer and breast cancer. J. Rare Earths. https://doi.org/10.1016/j.jre.2025.02.015 (2025).
Basumatary, R., Kalita, P., Bailung, H. & Brahma, R. Facile synthesis of CeO2 nanoparticles through plasma-liquid interaction. Materialia 39, 102367. https://doi.org/10.1016/j.mtla.2025.102367 (2025).
Majani, S. S. et al. Fe-doped SrZrO3 nanostructures as photocatalyst: Solid-state synthesis and UV-light driven eradication of thymol blue dye. Mater. Res. Bull. 183, 113184. https://doi.org/10.1016/j.materresbull.2024.113184 (2025).
Majani, S. S., Basavaraj, R. B., Iqbal, M., Venkatachalaiah, K. N. & Kollur, S. P. Dysprosium doped SrCeO3 nanophosphors for advanced photochemical applications: synthesis, characterization, photo-assisted dye degradation and latent fingerprint visualization. Mater. Sci. Semiconduct. Process. 182, 108674. https://doi.org/10.1016/j.mssp.2024.108674 (2024).
Majani, S. S. et al. Enhanced photocatalytic degradation activity of SrCeO3 nanophosphors: Aloe Vera gel-mediated synthesis and UV light-driven eradication of Titan yellow dye. Opt. Mater. 162, 116900. https://doi.org/10.1016/j.optmat.2025.116900 (2025).
Hkiri, K., Elsayed Ahmed Mohamed, H., Ghotekar, S. & Maaza, M. Green synthesis of cerium oxide nanoparticles using portulaca Oleracea extract: photocatalytic activities. Inorg. Chem. Commun. 162, 112243. https://doi.org/10.1016/j.inoche.2024.112243 (2024).
Lyu, G. M. et al. Hydrophilic ceo 2 nanocubes protect pancreatic β-cell line INS-1 from H 2 O 2-induced oxidative stress. Nanoscale 8 (15), 7923–7932. https://doi.org/10.1039/c6nr00826g (2016).
Fisichella, M. et al. Toxicity evaluation of manufactured ceo 2 nanoparticles before and after alteration: combined physicochemical and whole-genome expression analysis in Caco-2 cells. BMC Genom. 15, 1–15. https://doi.org/10.1186/1471-2164-15-700 (2014).
Gul, R., Dar, M. A., Nawaz, S. & Alfadda, A. A. Protective effects of nanoceria against mitochondrial dysfunction and angiotensin II-induced hypertrophy in H9c2 cardiomyoblasts. Antioxidants 12 (4), 877. https://doi.org/10.3390/antiox12040877 (2023).
Hadkar, V. M. & Selvaraj, C. I. Bio-inspired Ag3PO4-ZnO nanocomposites: investigation of its antioxidant, anticancer activity and photocatalytic degradation of methylene blue dye. J. Inorg. Organomet. Polym Mater. https://doi.org/10.1007/s10904-025-03895-1 (2025).
Al-darwesh, M. Y., Babakr, K. A., Qader, I. N. & Mohammed, M. A. Antimicrobial, anti-inflammatory, and anticancer potential of green synthesis TiO2 nanoparticles using sophora flavescens root extract. Chem. Pap. 79 (2), 1207–1221. https://doi.org/10.1007/s11696-024-03853-0 (2025).
Bharathi, A. et al. Microscopic characterization based synthesis of silver nanoparticles using merremia Quinquefolia (L.) hallier f. Leaf extract: their biological approaches and degradation of methylene blue dye. Microsc. Res. Tech. 88 (6), 1664–1680. https://doi.org/10.1002/jemt.24789 (2025).
Vasantharaj, S. et al. Biosynthesis of copper oxide nanoparticles using Tecoma stans flower extract and its antibacterial, anticancer, and photocatalytic activities. Biocatal. Agric. Biotechnol. 58, 103137. https://doi.org/10.1016/j.bcab.2024.103137 (2024).
Mamand, D. M. et al. Advanced spectroscopic approach for exploring the structural, optical, and electronic properties in dye-functionalized Chitosan biopolymers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 329, 125485. https://doi.org/10.1016/j.saa.2024.125485 (2025).
Shi, Y., Ma, C., Xing, Y., Sun, Y. & Ding, G. Manganese ferrite supported on activated alumina beads for activation of periodate towards efficient degradation of organic dye pollutants. Catal. Lett. 155 (2), 91. https://doi.org/10.1007/s10562-024-04919-0 (2025).
Mallik, T., Srabanti, G. & Ekka, D. Photocatalytic degradation of organic dyes by synthesised lanthanum oxide nanoparticle. Materials Res. Innovations https://doi.org/10.1080/14328917.2025.2462596 (2025).
Kamo, A., Ates Sonmezoglu, O. & Sonmezoglu, S. Unraveling the effects of Strain-Induced defect engineering on the Visible-Light-Driven photodynamic performance of Zn2SnO4 nanoparticles modified by larger barium cations. ACS Appl. Bio Mater. 7 (12), 8656–8670. https://doi.org/10.1021/acsabm.4c01447 (2024).
Bui, H. T. et al. Oxygen vacancy engineering of cerium oxide for the selective photocatalytic oxidation of aromatic pollutants. J. Hazard. Mater. 404, 123976. https://doi.org/10.1016/j.jhazmat.2020.123976 (2021).
Chen, L. et al. In-situ hydrothermal synthesis of MoS2 /TiO2 nanocomposites for enhanced and stable photocatalytic performance: methylene blue degradation pathway and mechanism. J. Taiwan Inst. Chem. Eng. 166, 105436. https://doi.org/10.1016/j.jtice.2024.105436 (2025).
Koli, M., Kanwar, B. & Singh, S. P. Impact of operating parameters on the electrooxidation of methylene blue and ciprofloxacin: a comprehensive analysis and degradation pathway. Environ. Sci. Pollut. Res. 32 (8), 4656–4669. https://doi.org/10.1007/s11356-025-35992-6 (2025).
Zhu, P., Hu, M., Duan, M., Xie, L. & Zhao, M. High visible light response Z-scheme Ag3PO4 / g-C3N4 / ZnO composite photocatalyst for efficient degradation of Tetracycline hydrochloride: preparation, properties and mechanism. J. Alloys Compd. 840, 155714. https://doi.org/10.1016/j.jallcom.2020.155714 (2020).
Wen, X. J. et al. Photocatalytic degradation of Ciprofloxacin by a novel Z-scheme CeO2–Ag/AgBr photocatalyst: influencing factors, possible degradation pathways, and mechanism insight. J. Catal. 358, 141–154. https://doi.org/10.1016/j.jcat.2017.11.029 (2018).
Kumar, A. et al. Enhanced visible light photocatalytic performance of CeO2@Acidified g-C3N4 nanoheterostructures for RhB degradation. Langmuir 41 (23), 14765–14777. https://doi.org/10.1021/acs.langmuir.5c00619 (2025).
Acknowledgements
The authors acknowledge the Director, Amrita Vishwa Vidyapeetham, Mysuru campus, for infrastructural facilities. The authors acknowledge the Nanotechnology Research Centre (NRC), SRMIST, for providing the research facilities. The authors acknowledge the DST and the SAIF, Mahatma Gandhi University, Kottayam, for characterization facilities. The authors extend their appreciation to the Ongoing Research Funding Program (ORF-2025-734), King Saud University, Riyadh, Saudi Arabia, for their support. Some of the research was carried out with the support of the Russian Science Foundation (Grant No. 23-65-10040).
Author information
Authors and Affiliations
Contributions
S.S.M., S.P.K.: Conceptualization, Methodology, Investigation, Formal analysis; S.S.M., P.S., P.K., C.S.S., C.S., P.B.S.S., V.S., E.S., S.P.K.: Data curation, Investigations, Formal analysis, Writing- Original draft preparation. K.S.A., M.I., R.G.A., V.S., E.S.: Visualization, Project administration, validation. S.P.K.: Resources, Supervision, Writing- Reviewing and Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
CRediT statement.
S.S.M., S.P.K.: Conceptualization, Methodology, Investigation, Formal analysis; S.S.M., P.S., P.K., C.S.S., C.S., P.B.S.S., V.S., E.S., S.P.K.: Data curation, Investigations, Formal analysis, Writing- Original draft preparation. K.S.A., M.I., R.G.A., V.S., E.S.: Visualization, Project administration, validation. S.P.K.: Resources, Supervision, Writing- Reviewing and Editing.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
41598_2025_13914_MOESM1_ESM.docx
Supplementary Material 1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Majani, S.S., Singh, P., Kumari, P. et al. Cerium oxide nanoparticles prepared through Bio-combustion using Ficus carica as effective antioxidant, anticancer and dye degrading agent. Sci Rep 15, 30285 (2025). https://doi.org/10.1038/s41598-025-13914-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13914-3