Abstract
The aim of this study was to examine the pain-relieving effects of combining Carbamazepine (CBZ) and low-dose-Naltrexone (LDN) in trigeminal neuralgia (TN) to reduce the side effects of CBZ and the potential of synergistic antiallodynic interaction. Male Wistar rats were allocated randomly to 8 groups: Control, Sham, TN, TN (CBZ 100 mg), TN (CBZ 100 mg oral (p.o.) + LDN 0.5 mg, intraperitoneal (i.p.)), TN (CBZ 30 mg + LDN 0.5 mg), TN (CBZ 10 mg + LDN 0.5 mg), and TN (LDN 0.5 mg). TN was induced through chronic constriction injury of the infraorbital nerve (CCI-ION) . To evaluate hyperalgesia, we employed the von Frey, acetone, and air puff tests. Cognitive functions were assessed using the open field, elevated plus maze, tail suspension, and passive avoidance tests. Furthermore, total antioxidant capacity levels (TAC) were measured in the spinal trigeminal nucleus. Administering oral CBZ (100 mg) along with LDN for 7 days significantly reduced CCI-ION-induced mechanical allodynia and improved anxiety, depression, and passive avoidance learning and memory compared to the TN group. Co-administration of LDN and CBZ more effectively alleviated anxiety and depression than CBZ alone. The TN (LDN) group demonstrated significantly better avoidance memory compared to the TN (CBZ100) group. The TN group exhibited a lower TAC level compared to the sham group, and TAC levels were improved in the TN (CBZ 100 + LDN) and TN (LDN) groups. The data indicate that combining CBZ with LDN effectively reduces mechanical allodynia in TN rats, while LDN may also improve anxiety, depression, and cognitive impairment caused by chronic TN pain.
Similar content being viewed by others
Introduction
Trigeminal neuralgia (TN) is a clinical condition characterized by sudden, recurring pain in one or more divisions of the fifth cranial nerve, which substantially reduces the quality of life in those suffering from the condition. The pain attacks are brief, lasting from a fraction of a second to 2 min, and may be triggered by certain stimuli. TN can be classified as classical (without a known cause) or secondary (caused by a lesion or underlying condition). The condition occurs at an estimated annual rate of 12.6 per individual, with symptoms more commonly appearing on the right side than the left. It also affects women more frequently than men1,2,3. Chronic TN is associated with psychiatric morbidities and the presence of newly diagnosed psychiatric disorders in individuals with TN can predict poorer treatment outcomes4. TN increases the risk for diagnosis of depressive disorder, anxiety disorder, and sleep disorders5.
Although numerous causes have been proposed for trigeminal neuralgia (TN), its exact etiology remains uncertain. In 1932, Dandy and colleagues identified vascular compression of the trigeminal root as a potential cause of the disorder2,3,6,7. This suggested etiology has support as microvascular decompression surgery is effective. Other hypotheses suggest that development of TN may be facilitated by an increase in the expression of cytokines, neuropeptides, and neurotrophic factors following nerve injury or demyelination4,5,8.
Despite recent advancements, managing TN remains challenging. First-line therapies can alleviate pain in about 80% of patients. However, these treatments have limitations, such as poor tolerability, the need for careful dosage adjustments, and possible drug interactions, which can reduce their effectiveness over time9,10. The first-line medical treatments recommended for pain control in TN are Carbamazepine (CBZ) (400–1,200 mg/day) and Oxcarbazepine (600–1,800 mg/day), which are anticonvulsants that work by blocking Na+ and Ca2+ channels, leading to reductions in excitability of ectopic neuronal impulses. By blocking transmission at trigger sites, they decrease the intensity of pain, occurrence of pain paroxysms, and reduce triggering stimuli. Of the two, Oxcarbazepine can be preferential due to its lower risk of drug interactions and better tolerability; however, CBZ has a more established role in clinical management9,11,12. Other medical approaches include Lamotrigine, Gabapentin, Pregabalin, Baclofen, Phenytoin, and Botulinum toxin injections9,12. Surgical procedures such as microvascular decompression or gamma knife radiosurgery are considered for patients unresponsive to medical treatment. As not all patients respond to treatment, and the treatments utilized come with side effects, innovative therapeutic approaches for management of TN are warranted.
Low doses of Naltrexone (LDN), an opioid receptor antagonist, have been used clinically to manage inflammatory and painful disorders since the mid-1980s13. LDN is generally well-tolerated with a low incidence of adverse effects and no demonstrated potential for abuse13,14,15. In a rat model of orofacial neuropathic pain, LDN had an anti-nociceptive effect on facial mechanical allodynia, similar to the analgesic effect of CBZ, suggesting that LDN could a suitable alternative for treating TN15. However, additional investigation is necessary to determine LDN’s analgesic efficacy in the TN pain model as well as whether use of both compounds could alter cognitive-based behaviors.
Accordingly, our study was designed to investigate the effects of co-administration of LDN and CBZ on pain, anxiety and depression-like behaviors, and avoidance tendencies in a male rat model of TN. Additionally, we monitored LDN and CBZ-induced alterations in antioxidant activity using a total antioxidative capacity assay (TAC) in the spinal trigeminal nucleus to determine whether behavioral effects seen were associated with mitigation of oxidative stress.
Materials and methods
Subjects and housing
A total of 64 adult male Wistar rats aged 4 to 6 weeks (220–250 g) were obtained from the animal house of Kerman Medical University. The rats were habituated for 2 weeks under a 12 h light/dark cycle in a controlled condition with a temperature of 22 ± 2 °C and had ad libitum access to food and water. All experiments were done in accordance with the ARRIVE guidelines and National Institutes of Health Guide for the Care and Use of Laboratory Animals) NIH Publication No. 80–23, revised 1996). All procedures were approved by the Animal Research Ethics Committee of the Kerman Neuroscience Research Center (Ethics Code: IR.KMU.REC. 91000056). Euthanasia was carried out through cervical dislocation while the subject was under anesthesia with Ketamine-Xylazine.
Experimental design
The animals were allocated randomly to eight groups (n = 8 in each group). The groups were classified as Control (does not receive any treatment), Sham (received a skin incision closed with a polyester suture (4 − 0), TN (received CCI-ION operation), TN (CBZ 100 mg), TN (CBZ 100 mg + LDN 0.5 mg), TN (CBZ 30 mg + LDN 0.5 mg), TN (CBZ 10 mg + LDN 0.5 mg), and TN (LDN 0.5 mg). Our choice of concentration of Naltrexone was based on prior findings that this concentration worked synergistically with other drug classes to improve pain management15,16.
Chronic constriction of the infraorbital nerve (CCI-ION) caused TN on day 0. The nociceptive tests were conducted on the 7th, 14th, and 22nd days following surgery, as well as 1 h and 24 h after the first and last therapy doses. After 14 days CCI-ION, CBZ was administered orally using a gavage tube, and LDN was given intraperitoneally (i.p.) for 7 days. Final concentrations of 10, 30, and100 mg/kg/day were prepared by dissolving CBZ (Sigma-Aldrich) powder in a sterile saline solution (0.9% w/v sodium chloride) with dimethyl sulfoxide (DMSO), at a 1:9 (v/v) ratio17. Naltrexone powder (Naltrexone hydrochloride 50 mg, Sigma-Aldrich) was dissolved in saline (0.9% w/v sodium chloride) to achieve a final concentration of 0.5 mg/kg18.
All behavioral experiments were performed between 8:00 and 14:00 in order to avoid the influence of differences in circadian cycle. Animals were transferred to the laboratory one hour before the start of the experiment to acclimate to the laboratory conditions. All the groups underwent different behavioral studies, which were performed in the following order: pain behavior, depressive and anxiety-like behavior tests, and cognitive assessment (Behavioral procedures timeline; Fig. 1).
Surgical procedures
Surgical intervention for TN induction was executed under Ketamine-Xylazine (Ketamine 90 mg/kg + Xylazine 10 mg/kg, i.p.) anesthesia. The epilated skin area was thoroughly washed with iodine, followed by the application of 70% isopropyl alcohol to eliminate any residual iodine.
The modeling of TN was achieved through the use of CCI-ION, as described by Ding in 201719. The section of the rat’s facial surface located between the eye and the whisker pad was carefully shaved without causing damage to the whiskers. An incision measuring 3 cm in length and running parallel to the midline was made, starting from the caudal end of the third row of whisker lines and extending towards the ipsilateral orbit (Fig. 2).
The superficial fascia was carefully separated to expose the infraorbital nerve (ION) trunk, located outside the orbital cavity. Two chromic catgut ligatures (4 − 0) were loosely tied around the distal part of the ION, spaced 3 mm apart. To ensure proper constriction of the ION, a criterion proposed by Bennett and Xie was followed20. The ligatures were applied in such a way that the diameter of the ION was reduced by a just noticeable amount, and the circulation through the superficial vasculature was retarded, but not cut off. Finally, the skin incision was closed with a polyester suture (4 − 0). Rats in the Sham group underwent the same surgical procedure, including skin incision and ION nerve dissection, but did not experience actual nerve ligation21 (Fig. 3).
Following the surgical procedure, tramadol hydrochloride (20 mg/kg, i.p.) was administered at 12-hour intervals for the initial 72-hour period to alleviate pain and discomfort.
Behavioral tests
All of the behavioral experiments were conducted with the researchers blinded to treatment conditions. Before the baseline testing began, the animals were acclimated to the specific test environment for two consecutive days, involving 30-minute intervals per test. All operated animals were included in the treatment groups regardless of potential individual variability in the degree of development of allodynia, in order to reduce the number of animals used and adhere to ethical principles of animal research.
Pain behavior
Mechanical allodynia
Fourteen days post-surgery, the animal was placed in an individual plastic cage and allowed to acclimate to the environment for at least one hour. Using twenty von Frey filaments (NorthCoast, USA), the mechanical pain threshold was assessed on days 7, 14, and 22 after the operation. The filaments were capable of producing bending forces ranging from 0.02 g to 60.0 g (0.02, 0.04, 0.07, 0.16, 0.4, 0.6, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, 15.0, 26.0, 60). Each filament was administered to the area of bending adjacent to the center of the vibrissa pad. The pad was stimulated three times at thirty-second intervals on the nerve-injured side, with nine applications for each filament. The stimulation series began with the filament that produced the lowest force, and the procedure was repeated using filaments of increasing stiffness until a filament triggered one of the following nocifensive behaviors twice: brisk head withdrawal, escape or attack reactions, or short-lasting facial grooming. Based on previous research, individuals whose pain threshold measures below 2 g have been deemed a successful model for TN19,22.
Air-Puff test
The air-puff examination was executed using identical methods previously detailed. In summary, the rats were situated in a plexiglass enclosure and, after becoming acclimated to the laboratory surroundings, a constant surge of air was employed to the ipsilateral CCI-ION portion of the face. The air puffs were dispensed at a 90◦ angle with a distance of 1 cm. The bar level at which the rats demonstrated aggressive behaviors, such as averting their head or biting was documented23.
Facial cold allodynia score test
The animals were positioned within the identical plexiglass enclosure as previously outlined. To conduct this examination, a small quantity of acetone was administered onto the vibrissa pad located on the same side as the ION through a glass syringe, after which the quantity of scratching or rubbing actions executed within the ensuing 120 s was tallied. Body parts other than the facial region were not included for this assessment23.
Anxiety-like behavior
Open field test
The evaluation of anxiety and exploration behaviors in animals was conducted through a process whereby rats were permitted to freely explore an open field arena for 5 min. The testing apparatus employed was a traditional open field, comprising a Plexiglass square arena measuring 90 × 90 × 45 [H] cm. The apparatus was abstractly divided into central and peripheral areas. The activity of the rat was monitored by a video camera, which was connected to a computer. Parameters such as total time spent in the central zone, rearing (i.e., standing on hind legs), and grooming (such as licking, scratching, and face washing) were recorded for 5 min and assayed with offline analysis (Ethovision7.1, Noldus Information Technology, Netherland). Upon completion of the trial, the arena was cleaned with 70% ethyl alcohol24.
Elevated plus maze test
The maze used to evaluate anxiety-related behaviors in rodents consisted of two arms with open sides, and two enclosed arms, which were elevated above the ground. Each rat was positioned in the center of the maze, facing one of the open arms. The time spent in the open arms, as well as the number of entries into each arm, were recorded for a 5-minute period25,26.
Depressive-like behavior
Tail suspension test
The tail suspension test entails subjecting the test subject to hemodynamic stress by suspending it by the tail in a container, while the observation of efforts to escape is recorded for 6 min. Immobile behavior is the pertinent indicator of behavioral despair, with rats that display depressive-like symptoms exhibiting immobility within 2 to 4 min. The efficacy of anti-depressive interventions is gauged by their ability to reduce the duration of immobility of the rat27.
Passive avoidance test
The passive avoidance test is used to assess associative learning and memory in rodents. This fear-based test was conducted as the final behavioral assessment using a shuttle box apparatus. This apparatus is comprised of a light and dark compartment, which are connected by a guillotine door. In adaptation phase, animals were placed in the bright section of the apparatus. After 10 s, the door was opened, and the animals were allowed to freely move into the dark chamber without receiving any electric shocks for 30 s. One hour later, the avoidance learning phase began. The animals were placed in the bright chamber again, the door was opened, and they were allowed to enter the dark compartment. However, this time they received an electric shock (0.5 mA, 50 Hz, lasting 2 s) when they entered the dark chamber through the stainless-steel floor. This process was repeated up to five times with a 30-minute interval between each session to determine the animal’s ability to avoid entering the dark compartment and to record the number of shocks required for learning. The passive avoidance memory phase was evaluated 24 h after the learning phase. The rat was placed in the bright compartment with the door closed. After a 10-second interval, the door was opened, and the time taken for the rat to enter the dark chamber (known as the step-through latency), the time spent in the dark compartment (DCD) and the number of entries of the rat into the dark chamber (DCN) were recorded within a 300-second timeframe28.
Brain tissue separation
After completing the experiments, the animals were deeply anesthetized by CO2 gas. Their brains were extracted, and the pons were preserved in liquid nitrogen at − 80 °C for later assessment of total antioxidant capacity (TAC) levels.
Biochemical assay
Assay of TAC level in the spinal trigeminal nucleus
TAC levels were assayed in the spinal trigeminal nucleus as this structure is instrumental in the central sensitization mechanisms that are linked with the experience of facial pain29. The spinal trigeminal nucleus is responsible for the reception and processing of nociceptive signals emanating from the various organs and tissues of the craniofacial region, encompassing the meninges, via the trigeminal nerve.
To assay TAC levels, fixed samples were meticulously dissected, and spinal trigeminal nucleus were bilaterally extracted in accordance with the anatomical atlas established by Paxinos and Watson. The tissues were weighed prior to the homogenization procedure and then underwent a thorough rinsing process in ice-cold phosphate-buffered saline (PBS) (0.02 mol/L, pH 7.0-7.2) to eliminate any residual blood. The resultant suspension was subjected to ultrasonication for optimal homogenization. Subsequently, the homogenates were centrifuged for a duration of 15 min at a force of 1500×g (equivalent to 5000 rpm). A volume of 50 µl from the supernatant was removed, and an equal volume of standard solution was transferred utilizing a multichannel pipette to the enzyme-linked immunosorbent assay (ELISA) (Kiazist, Iran, rat KTAC) positioned within a 96-well tissue culture plate kept on ice, followed by the addition of 50 µl of conjugate solution. The mixture was allowed to incubate for 45 min at ambient room temperature, after which the plate was subjected to five wash cycles. A total of 100 µl of substrate was dispensed into each well, and the incubation continued at room temperature for an additional 10 min. Ultimately, 50 µl of stop solution was administered to terminate the reaction, yielding a yellow solution. The intensity of the resultant coloration was quantified spectrophotometrically at a wavelength of 450 nm using a microplate reader. The intensity of color exhibited an inverse relationship with the concentration TAC. A standard curve was constructed to correlate the intensity of color (optical density, O.D.) to the concentrations of the standards. The TAC concentration present in each sample was subsequently interpolated from this established standard curve.
Statistical analyses
Statistical analysis and figure production utilized GraphPad Prism version 8, developed by GraphPad Software (USA) . The Kolmogorov-Smirnov test was utilized to evaluate the normal distribution of the data. To compare the behavioral results of the Control and Sham groups, an unpaired Student’s t-test was conducted. To evaluate normally distributed data from behavioral tests (tail suspension test, open field test, and passive avoidance test) and assay of TAC level, one-way ANOVAs were performed, and the Tukey post hoc analysis was employed for multiple comparisons between TN and treatment groups. Repeated measures two-way ANOVA was employed to analyze the data of pain assessment tests (von Frey, acetone and air puff test). Data are expressed as mean ± SEM. Statistical significance between groups was determined when P < 0.05. Figures 1 and 2 were created using Bio Render, and Fig. 3 was prepared using Adobe Photoshop (version 26.4.1). Figures 4, 5, 6, 7, 8 and 9 were designed in GraphPad Prism.
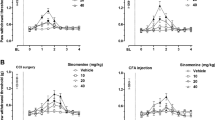
Mechanical (A), facial cold allodynia (B) and air puff test (C) of Control, Sham, nontreated-TN, and treated-TN rats. Repeated measure analysis showed a significant difference in pain threshold in all the groups at 7, 14, and 22 days after surgery in comparison to the Control and Sham (P < 0.05). The mechanical allodynia threshold of TN (CBZ100 + LDN) rats was significantly higher than the threshold of the TN group in day 22 (P < 0.05). Results are shown as mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 versus the Control, XP<0.05, XXP<0.01, XXXP<0.001 versus the Sham, and #P < 0.05, ##P < 0.01, ###P < 0.001 versus the TN rats.
Open field testing revealed that rearing time was significantly improved in treated TN rats with LDN (A). The TN rats showed increased face-grooming activities compared to Sham group. All treatment groups showed improvement in face-grooming activities (B). 7-day treatment with CBZ100 or CBZ100 + LDN increased the time spent in the central part of the open field compared to TN animals. Results are shown as mean ± SEM (n = 8). XXXP<0.001 versus the Sham operated group, and #P < 0.05, ##P < 0.01, ###P < 0.001 versus the TN rats and +P < 0.05, ++P < 0.01 versus CBZ 100 mg group.
Effects of CCI-ION surgery on depressive-like behaviors in the tail suspension test, represented as mobility time. Data is presented as mean ± SEM (n = 8), with significant differences determined in XXP<0.01, XXXP<0.001 versus Sham, and ##P < 0.01, ###P < 0.001 versus the TN rats, and +P < 0.05 versus CBZ 100 mg group.
Passive avoidance assessment in Sham, TN and TN treated rats. (A): The number of shocks required to reach learning in the passive avoidance test showed a significant difference between CBZ + LDN groups compared to TN group, (B): Step-through latency in passive avoidance learning was different between groups. The CBZ + LDN groups spent less time in the dark compartment compared to the TN group (C). LDN decreased the number of entries in the dark compartment compared to TN (D). Results are shown as mean ± SEM (n = 8). XP<0.05, XXP<0.01, XXXP<0.001 versus Sham, and #P < 0.05, ##P < 0.01, ###P < 0.001 versus nontreated TN rats, and +P < 0.05 versus CBZ 100 mg group.
Results
As expected, after the CCI-ION operation, the behavioral assessment scores of mechanical and cold allodynia, as well as results from the air-puff test indicated presence of persistent pain in the TN animals compared to Control and Sham.
Administration of CBZ in combination with LDN increases the tactile sensitivity threshold compared to CBZ alone
Tactile sensitivity elicited by mechanical allodynia was evaluated via the von Frey test. Initially, no significant differences were observed between the Sham groups in 7, 14 and 22 days after surgery (Fig. 4A-C), indicating that the Sham operation did not change the pain threshold. Reduction of pain threshold was observed in all TN groups on day 7, 14 and 22 after the operation groups compared to Control and Sham (P < 0.05). Mechanical allodynia responses were significantly higher in TN (CBZ100 + LDN) group compared to TN rats on day 22nd after the operation (P < 0.05) (Fig. 4A).
Thermal Allodynia was lower in TN rats, which were treated with the co-administration of LDN and 100 mg/ kg/day, in comparison to TN non-treated rats
Evaluation of the facial cold allodynia threshold in TN rats demonstrated a notable decrease in threshold levels (increasing grooming number) compared to Control and Sham rats on day 7 and 14 after operation (P < 0.001) (Fig. 4B), indicating the development of cold hypersensitivity in the nerve-injured side of the rats in all groups on days 7th and 14th after the CCI-ION operation. Untreated TN rats showed an increase in grooming 22 days post-surgery compared to the Control and Sham group (P < 0.001). TN rats treated with (CBZ 10 mg + LDN), (CBZ 30 mg + LDN) and (LDN) (P < 0.01) exhibited higher grooming numbers than those seen in the Control and Sham groups. Seven-day administration of CBZ decreased the facial cold allodynia threshold in the TN (CBZ) group compared to the TN group (P < 0.05), while in groups co-administrated with LDN, there was a significant difference when compared to TN rats (P < 0.001).
Air pressure
Pain sensitivity to air pressure increased in TN rats, but it was lower in the group receiving a combination of CBZ and LDN. As expected, a significant difference was found between the Control and the Sham rats when compared to all TN groups on the 7th and 14th days after the operation (P < 0.05). Rats treated with the co-administration of any concentration of CBZ + LDN showed a significant increase in Pain sensitivity in comparison when compared to the TN groups (Fig. 4C).
A combination of CBZ and LDN could reverse the neuropathic pain-induced anxiety
Due to the lack of difference between the Control and Sham groups in all evaluated parameters, the Control group was excluded from subsequent graphical representations. A significant decrease in rearing number was revealed in TN rats in comparison to the Sham group (P < 0.001), while rearing was significantly improved in treated TN groups compared to nontreated TN rats (P < 0.001) (Fig. 5A). Furthermore, the concomitant administration of LDN and CBZ at a dosage of 100 mg significantly diminished the anxiety index in comparison to the administration of CBZ100 mg alone (P < 0.05).
Treatment with varying concentrations of CBZ and LDN resulted in improvements in anxious behavior as evidenced by differences in grooming activity in all groups versus the TN group (P < 0.001), suggesting that both medications decrease anxiety behavior. However, TN (CBZ100 + LDN) and TN (CBZ30 + LDN) showed a significant reduction compared to TN (CBZ100) (P < 0.01) (Fig. 5B).
The time spent in the central part of the open field was used as an indicator of anxiety and TN rats showed a significant difference in this parameter compared to the Sham group (P < 0.001). A 7-day intervention utilizing CBZ100 or CBZ100 in conjunction with LDN significantly enhanced the duration of time spent in the central zone of the open field when contrasted with the time spent by TN subjects (P < 0.05) and (P < 0.01), respectively (Fig. 5C).
Combination of CBZ and LDN has demonstrated efficacy in ameliorating anxiety-related behaviors in the rat model of TN
As shown in Fig. 6, the frequency and amount of time spent within the enclosed arms of the elevated plus maze, which was used as a metric of anxiety-like behavior exhibited a statistically significant difference when the time spent by the TN rats was compared to the time spent by the Sham group at 22 days after CCI-ION operation (P < 0.01). The combination of CBZ and LDN exhibited anti-anxiety-like behavior effects (Fig. 6A-C, P < 0.05).
Neuropathic pain-induced depressive-like behavior was reversed significantly by LDN
The tail suspension test is a preclinical test with good predictive validity of anti- depressant-effects27. TN (P < 0.001), and TN rats treated with CBZ100 mg (P < 0.01) showed a significant decrease in mobility time compared to that seen in the Sham rats. The CBZ + LDN treatment group exhibited a significantly greater amount of time struggling to get rid of adverse conditions, which indicates the improvement of depressive-like behaviors in these groups compared to that seen in the TN group (P < 0.001) (Fig. 7). Indeed, significant differences were noted in TN (CBZ 100 + LDN) and TN (CBZ 30 + LDN) groups (P < 0.05), indicating a reduction in depressive-like behavior associated with neuropathic pain compared to the TN (CBZ 100) group.
Neuropathic pain-induced impairment of performance in the passive avoidance test was reversed by LDN
Passive avoidance learning was significantly different between the CBZ + LDN groups when compared to the TN group as evaluated by comparing the number of shocks received (Fig. 8A). In the memory phase of the passive avoidance test that occurred 24 h after the learning phase, there was a significant difference between the step-through latency in TN (P < 0.001) and CBZ 100 mg (P < 0.05) groups compared to Sham (Fig. 8B). All the groups treated with LDN showed a significant amelioration in passive avoidance learning and memory in comparison to nontreated TN rats (P < 0.01) (Fig. 8A-D). Additionally, the recovery of impaired passive avoidance memory in the TN group treated with LDN was significantly higher than that seen in the group treated with CBZ 100 mg (P < 0.05) (Fig. 8B), which suggests that CBZ could not reverse the effect of TN on cognitive abilities.
LDN significantly increases TAC level in the spinal trigeminal nucleus
TN group showed a significantly lower TAC in the spinal trigeminal nucleus than the Sham operated group (P < 0.05). Further, the CBZ 100 + LDN and LDN groups exhibited a significantly higher TAC (P < 0.05), when compared to TN rats, suggesting a positive effect of LDN on TAC (Fig. 9).
Discussion
Chronic TN heightens the risk of mental health conditions and can lead to reductions in the quality of life. CBZ is the primary medication prescribed for managing TN9,30,31. However, many patients encounter difficulties tolerating this drug due to its associated side effects. These adverse effects can include drowsiness, visual accommodation disorders, liver inflammation, changes in liver enzyme levels, kidney impairment, heart failure, delayed multi-organ failure, leukopenia, thrombocytopenia, and more27,30,32. In instances where analgesic relief for TN is inadequate with CBZ or if it induces adverse effects, alternative anticonvulsant medications can be utilized including Lamotrigine, Baclofen, Phenytoin, Gabapentin, Clonazepam, Valproate, Mexiletine, and Topiramate33. The pain-relieving properties of LDN, demonstrated in a rat model of orofacial neuropathic pain have suggested its potential as an alternative treatment for TN15. The primary objective of the current research was to examine the effects of Co-administration of LDN and CBZ on pain levels, anxious and depressive-like behaviors, avoidance tendencies and antioxidant activity in a male rat model of TN. This study aimed to explore whether incorporating LDN with CBZ could effectively manage TN symptoms at a reduced CBZ dosage, thereby minimizing side effects, without re-evaluating CBZ’s well-documented side effects34,35,36.
In the present study, using a rat model of TN induced by CCI-ION surgery, we found that treating rats with LDN for 7 days, similar to CBZ, alleviated facial mechanical and thermal allodynia. Importantly, combining LDN with different concentrations of CBZ had significant impact on managing chronic TN. Interestingly, using lower doses of CBZ (30 mg/kg/day and 10 mg/kg/day) along with LDN showed a similar effect to using CBZ alone at 100 mg/kg/day in reducing thermal and mechanical allodynia. Our findings support previous clinical and preclinical results that have shown the analgesic effect of LDN in various chronic pain conditions31,37.
Administering LDN intrathecally has been shown to effectively reverse tactile allodynia in rats with spinal and infraorbital nerve ligation in a dose-dependent manner15,38. Ultra-low doses of Naltrexone enhanced cannabinoid-induced analgesia and morphine antinociception, while also attenuating the development of morphine tolerance. Naltrexone was observed to enhance morphine’s analgesic effects and prevent tolerance by blocking excitatory opioid activity, amplifying morphine’s inhibitory actions39. Furthermore, in a study on cannabinoid-opioid interactions, it was suggested that the activation of opioid receptors coupled to Gs-proteins might diminish cannabinoid-induced antinociception and/or impair motor functioning, providing a potential mechanism for this interaction37,40.
Classical TN represents a severe neuropathic facial pain disorder, often accompanied by heightened risks of anxiety and depression41. In our TM rat model, anxious and depressive-like behaviors were noted in addition to the facial sensory changes after the CCI-ION injury. CBZ has been used to treat different psychological disorders such as anxiety and post-traumatic stress disorder. Its effects on the neurotransmitter systems, particularly the serotonergic, GABAergic, and noradrenergic pathways, as well as its influence on G-protein modulation, may contribute to its effectiveness. Furthermore, the anxiolytic effects of CBZ may stem from its capacity to inhibit the release of excitatory neurotransmitters through the inhibition of voltage-gated Na + channels, alongside its modulation of glutamatergic neurotransmission. Indeed, CBZ plays a critical role in addressing organic functional disorders within the limbic system by mitigating kindling phenomena42. Our results demonstrated that the anxious and depressive-like behavior in the TN group was significantly improved when treated with both CBZ and LDN, suggesting a synergistic interaction between these two agents in managing TN related psychiatric symptoms. In the tail suspension assay, noteworthy distinctions were observed among the TN (CBZ 100 + LDN) and TN (CBZ 30 + LDN) groups, signifying a diminution in depressive-like behaviors correlated with neuropathic pain when CBZ and LDN were co-administered in contrast to the group receiving CBZ 100 monotherapy.
CBZ selectively enhances the levels of acetylcholine in the central nervous system while concurrently diminishing choline levels, both of which are crucial for cognitive processes such as learning and memory43. However, CBZ has not been reported to enhance learning and memory processes. On the other hand, in rats and mice, Naltrexone has been shown to improve working memory performance44. These effects were confirmed in our study by use of the passive avoidance test, which revealed significant differences between CBZ + LDN groups compared to the TN group. In the memory phase of the passive avoidance test, there was a significant difference in the step-through latency in the TN and CBZ 100 mg groups when compared to the Sham. All of the groups treated with LDN showed a significant improvement in passive avoidance learning and memory in comparison to untreated TN rats. Additionally, the recovery of impaired passive avoidance memory in the TN group treated with LDN was significantly higher than that of the group treated with CBZ 100 mg, which could indicate that CBZ could not reverse the effect of TN in cognitive abilities.
The molecular effects of co-administering CBZ and LDN in the spinal trigeminal nucleus were also studied, which showed a significant increase in oxidative stress markers in the TN group. However, these markers decreased notably after 7 days of treatment with both CBZ and LDN. This finding suggests a synergistic interaction between these two agents in managing TN.
CBZ is well-established in TN management for its ability to inhibit voltage-gated Na + channels, thereby reducing neuronal hyperexcitability and preventing repetitive firing in the trigeminal nerve45. By stabilizing overactive neurons, CBZ alleviates pain associated with TN. However, its efficacy is often limited by dose-related side effects and incomplete control of pain, particularly in refractory cases. Our findings suggest that coadministration of LDN may enhance CBZ’s therapeutic potential by targeting complementary molecular pathways, especially those related to oxidative stress and neuroinflammation. At standard therapeutic dosages, Naltrexone markedly inhibits activity at mu- and delta-opioid receptors, while exerting a comparatively diminished effect on kappa-opioid receptors. Since activity of endogenous beta-endorphins at mu-opioid receptors is linked to endogenous analgesic mechanisms, it may appear paradoxical to prescribe Naltrexone to patients suffering from chronic pain. One would expect that this medication would diminish the analgesic effects derived from beneficial endogenous opioid activity. Furthermore, Naltrexone concurrently exerts an antagonistic influence on non-opioid receptors, specifically Toll-like receptor 4 (TLR4), which is located on macrophages, including microglia. Microglia are responsible for the production of inflammatory and excitatory mediators that can induce negative behaviors, including heightened pain sensitivity, fatigue, cognitive impairment, sleep disturbances, mood disorders, and overall malaise. By inhibiting the activation of microglia, Naltrexone attenuates the synthesis of reactive oxygen species and other potentially neuroexcitatory and neurotoxic substances, a mechanism that has been shown to confer neuroprotective and analgesic actions46. These effects are particularly relevant to the pathophysiology of TN, where neuroinflammatory processes play a critical role in sensitizing the nervous system and amplifying pain signaling. Previous studies have demonstrated that LDN can reduce pro-inflammatory cytokines, such as TNF-α and IL-1β while promoting the release of anti-inflammatory cytokines like IL-1047. However, no significant changes in TNF-α levels in the brainstem and spinal cord were seen following LDN administration15. Oxidative stress is known to worsen neuropathic pain by damaging neurons and increasing sensitivity to pain stimuli. The ability of LDN to reduce oxidative factors suggests that its benefits in TN may extend beyond its anti-inflammatory properties. This is consistent with earlier research showing that LDN can modulate glial activity and neuroinflammatory pathways by targeting TLR4 and µ-opioid receptors. Although our study did not directly evaluate TLR4, previous studies have shown increased TLR4 expression in peripheral nerve structures, such as the trigeminal ganglion, in animal models of TN48. Therefore, LDN’s effects on oxidative stress in TN could also be related to its pain relief. Additionally, LDN’s impact on neurotrophic factors like brain-derived neurotrophic factor (BDNF) has been shown to play a role in neuropathic and inflammatory pain49. BDNF is involved in maladaptive neuroplasticity, contributing to the persistence of chronic pain. LDN has been found to help reduce pain sensitivity by affecting brain chemicals like brain-derived neurotrophic factor (BDNF) and interleukin-10 (IL-10), which are involved neuropathic pain processes15. Although we did not assess BDNF in our study, prior research suggests that LDN’s influence on BDNF expression may depend on the baseline pain status of the animal, potentially reducing BDNF levels in the presence of chronic pain. This modulation of BDNF, combined with CBZ’s Na + channel inhibition, may contribute to the observed improvements in TN symptoms15.
The results we obtained are consistent with findings from other studies that investigated the potential for combination therapies in managing TN and other neuropathic pain conditions38,50. The combination of CBZ and LDN appears to provide a broader therapeutic effect by simultaneously targeting neuronal hyperactivity, oxidative stress, and neuroinflammation. The mechanistic actions of both drugs appear complementary and could explain why our study showed a greater reduction in oxidative markers when the drugs were combined, as CBZ reduces excitatory firing while LDN can reduce glial activation and oxidative damage.
The combination of Naltrexone and CBZ has been shown clinically to produce a synergistic interaction as the intrathecal administration of Naltrexone with oral CBZ15 showed heightened management of pain. However, clinically, this combination is still underexplored. While there isn’t yet strong evidence showing a direct synergy between LDN and CBZ clinically, there’s reason to believe they could complement each other. Naltrexone’s ability to modulate the immune system, paired with Carbamazepine’s effect on nerve signaling, could mean that together, they might offer synergistic pain relief, similar to that seen with LDN and Gabapentin and Pregabalin, especially for conditions like trigeminal neuralgia or chronic neuropathic pain38. More research is needed to explore how they might work together more effectively and determine the best ways to use them in clinical practice. Further, future studies should evaluate concentration-dependent effects as while we chose to use 0.5 mg of Naltrexone as that concentration previously was found to be synergistic with other drugs to enhance pain relief (Pineda-Farias et al., 2017), other concentrations could result in differential effects. As TN is difficult to manage effectively, the potential for a combined treatment strategy that helps ease nerve pain is worth further investigations51.
Besides the synergistic effects of coadministration of CBZ and LDN on ameliorating pain, the combination also improved anxiety-like behavior, depressive-like behavior, and cognitive function, which were significantly affected by CCI-ION. . These improvements were observed with CBZ at concentrations of 10 or 30 mg/kg/day, as well as with CBZ at a concentration of 100 mg/kg/day, either alone or in combination with LDN. This indicates that the dosage of CBZ can be lowered by 70% while still achieving the same therapeutic effect. This could potentially result in fewer side effects associated with CBZ and delay the development of drug resistance.
One notable limitation of our study is the exclusive use of a male rat model, which restricts our ability to evaluate sex differences and may not accurately reflect the processes occurring in humans. Additionally, our research focused solely on the effects of i.p. administration of LDN, leaving unexplored the potential impacts of other administration routes. Crucially, the study did not assess the long-term effects of LDN treatment, nor did it investigate the other underlying molecular mechanisms involved in these processes. In this study, ION-CCI surgery was used to induce TN symptoms, a common approach in TN research. Future studies should explore alternative administration methods, such as oral or intravenous routes, to validate current findings. Investigating different TN induction techniques could also enhance the understanding of these results. Moreover, including female rats in future research is essential to assess potential sex-specific effects of LDN, given the known higher prevalence of TN in females. Finally, our study was designed to evaluate the effectiveness of the adjunct of LDN to CBZ under the principle that reducing levels of CBZ would mitigate against CBZ-associated side effects. However, while we did not note any difference in adverse events between the treated and untreated TN groups, we did not directly evaluate this point in a control group of animals due to animal welfare issues. Accordingly, while we showed in our TN model that LDN and CBZ were effective, follow up studies should determine whether the combination is associated with adverse events (i.e. liver toxicity). Future research should also explore the long-term effects of this co-administration in both TN and other chronic neuropathic pain conditions to better understand its potential as a comprehensive pain management strategy.
Conclusion
Our study found that combining CBZ and LDN reduces nerve injury-induced tactile allodynia in rats. The combination also exerted positive effects on anxiety and depression, potentially minimizing side effects. Co-administration of LDN and CBZ improved passive avoidance impairment following chronic nerve injury. Our findings suggest that the co-administration of these medications may result in a significant reduction in TN symptoms. If confirmed in human studies, these combinations could offer valuable therapeutic options for managing neuropathic pain, as well as cognitive impairments associated with TN.
Data availability
Data availability: The datasets analysed during the current study are available in the uploaded supplementary file [Raw data Sci Rep].
References
Maarbjerg, S., Gozalov, A., Olesen, J. & Bendtsen, L. Concomitant persistent pain in classical trigeminal neuralgia–evidence for different subtypes. Headache: J. Head Face Pain. 54 (7), 1173–1183 (2014).
Møller, A. R. & Jannetta, P. J. Microvascular decompression in hemifacial spasm: intraoperative electrophysiological observations. Neurosurgery 16 (5), 612–618 (1985).
Zhong, J. et al. Microvascular decompression surgery: surgical principles and technical nuances based on 4000 cases. Neurol. Res. 36 (10), 882–893 (2014).
Rudich, Z., Lerman, S. F., Gurevich, B. & Shahar, G. Pain specialists’ evaluation of patient’s prognosis during the first visit predicts subsequent depression and the affective dimension of pain. Pain Med. 11 (3), 446–452 (2010).
Wu, T-H. et al. Risk of psychiatric disorders following trigeminal neuralgia: a nationwide population-based retrospective cohort study. J. Headache Pain. 16, 1–8 (2015).
Dandy, W. E. The treatment of trigeminal neuralgia by the cerebellar route. Ann. Surg. 96 (4), 787 (1932).
Dandy, W. E. Concerning the cause of trigeminal neuralgia. Am. J. Surg. 24 (2), 447–455 (1934).
Love, S. & Coakham, H. B. Trigeminal neuralgia: pathology and pathogenesis. Brain 124 (12), 2347–2360 (2001).
Cruccu, G. et al. AAN-EFNS guidelines on trigeminal neuralgia management. Eur. J. Neurol. 15 (10), 1013–1028 (2008).
Besi, E., Boniface, D., Cregg, R. & Zakrzewska, J. Comparison of tolerability and adverse symptoms in oxcarbazepine and carbamazepine in the treatment of trigeminal neuralgia and neuralgiform headaches using the Liverpool adverse events profile (AEP). J. Headache Pain. 16 (1), 1–7 (2015).
Besi, E., Boniface, D., Cregg, R. & Zakrzewska, J. Comparison of tolerability and adverse symptoms in oxcarbazepine and carbamazepine in the treatment of trigeminal neuralgia and neuralgiform headaches using the Liverpool adverse events profile (AEP). J. Headache Pain. 16, 1–7 (2015).
Di Stefano, G., La Cesa, S., Truini, A. & Cruccu, G. Natural history and outcome of 200 outpatients with classical trigeminal neuralgia treated with carbamazepine or oxcarbazepine in a tertiary centre for neuropathic pain. J. Headache Pain. 15, 1–5 (2014).
Younger, J., Parkitny, L. & McLain, D. The use of low-dose Naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin. Rheumatol. 33, 451–459 (2014).
Trofimovitch, D. & Baumrucker, S. J. Pharmacology update: low-dose Naltrexone as a possible nonopioid modality for some chronic, nonmalignant pain syndromes. Am. J. Hospice Palliat. Medicine®. 36 (10), 907–912 (2019).
de Oliveira, C. L. et al. Low-dose Naltrexone reverses facial mechanical allodynia in a rat model of trigeminal neuralgia. Neurosci. Lett. 736, 135248 (2020).
Pineda-Farias, J. B., Caram-Salas, N. L., Salinas-Abarca, A. B., Ocampo, J. & Granados-Soto, V. Ultra-Low doses of Naltrexone enhance the antiallodynic effect of Pregabalin or Gabapentin in neuropathic rats. Drug Dev. Res. 78 (8), 371–380. https://doi.org/10.1002/ddr.21409 (2017).
Grabenstatter, H. L. & Dudek, F. E. Effect of carbamazepine on spontaneous recurrent seizures recorded from the dentate gyrus in rats with kainate-induced epilepsy. Epilepsia 60 (4), 636–647 (2019).
Tawfik, D. I. et al. Evaluation of therapeutic effect of low dose Naltrexone in experimentally-induced crohn’s disease in rats. Neuropeptides 59, 39–45 (2016).
Ding, W. et al. An improved rodent model of trigeminal neuropathic pain by unilateral chronic constriction injury of distal infraorbital nerve. J. Pain. 18 (8), 899–907 (2017).
Bennett, G. J. & Xie, Y-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33 (1), 87–107 (1988).
Liu, M., Zhong, J., Xia, L., Dou, N. & Li, S. The expression of voltage-gated sodium channels in trigeminal nerve following chronic constriction injury in rats. Int. J. Neurosci. 129 (10), 955–962 (2019).
Deseure, K. & Hans, G. H. Chronic constriction injury of the rat’s infraorbital nerve (IoN-CCI) to study trigeminal neuropathic pain. JoVE (Journal Visualized Experiments). 103, e53167 (2015).
Islam, J. et al. Optogenetic stimulation of the motor cortex alleviates neuropathic pain in rats of infraorbital nerve injury with/without CGRP knock-down. J. Headache Pain. 21, 1–13 (2020).
Mohammadi, F., Esfahlani, M. A. & Shabani, M. Erythropoietin ameliorates harmaline-induced essential tremor and cognition disturbances. Neurosci. Lett. 704, 153–158 (2019).
Mansoori, M. et al. Chronic migraine caused a higher rate of tendency to cannabinoid agonist compared to morphine. Acta Bio Medica: Atenei Parmensis 91(4), e2020185 (2020).
Shabani, M., Ilaghi, M., Naderi, R. & Razavinasab, M. The hyperexcitability of laterodorsal tegmentum cholinergic neurons accompanies adverse behavioral and cognitive outcomes of prenatal stress. Sci. Rep. 13 (1), 6011 (2023).
Cryan, J. F., Mombereau, C. & Vassout, A. The tail suspension test as a model for assessing antidepressant activity: review of Pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev.. 29 (4–5), 571–625 (2005).
Rajaei, Z., Hosseini, M. & Alaei, H. Effects of Crocin on brain oxidative damage and aversive memory in a 6-OHDA model of parkinson’s disease. Arq. Neuropsiquiatr. 74, 723–729 (2016).
Patel, N. M., Jozsa, F. & Das, J. M. Neuroanatomy, Spinal Trigeminal Nucleus (StatPearls Publishing, 2022).
Sindrup, S. H. & Jensen, T. S. Pharmacotherapy of trigeminal neuralgia. Clin. J. Pain. 18 (1), 22–27 (2002).
Smith, J. P. et al. Therapy with the opioid antagonist Naltrexone promotes mucosal healing in active crohn’s disease: a randomized placebo-controlled trial. Dig. Dis. Sci. 56, 2088–2097 (2011).
Reisner, L. & Pettengill, C. A. The use of anticonvulsants in orofacial pain. oral surgery, oral medicine, oral pathology. Oral Radiol. Endodontology. 91 (1), 2–7 (2001).
Bennetto, L., Patel, N. K. & Fuller, G. Trigeminal neuralgia and its management. Bmj 334 (7586), 201–205 (2007).
Besi, E., Boniface, D. R., Cregg, R. & Zakrzewska, J. M. Comparison of tolerability and adverse symptoms in oxcarbazepine and carbamazepine in the treatment of trigeminal neuralgia and neuralgiform headaches using the Liverpool adverse events profile (AEP). J. Headache Pain. https://doi.org/10.1186/s10194-015-0563-z (2015).
Killian, J. M. & Fromm, G. H. Carbamazepine in the treatment of neuralgia. Use of side effects. Arch. Neurol. 19 (2), 129–136. https://doi.org/10.1001/archneur.1968.00480020015001 (1968).
Taylor, J. C., Brauer, S. & Espir, M. L. Long-term treatment of trigeminal neuralgia with carbamazepine. Postgrad. Med. J. 57 (663), 16–18. https://doi.org/10.1136/pgmj.57.663.16 (1981).
Terner, J. M., Barrett, A. C., Lomas, L. M., Negus, S. S. & Picker, M. J. Influence of low doses of Naltrexone on morphine antinociception and morphine tolerance in male and female rats of four strains. PAIN® 122 (1–2), 90–101 (2006).
Pineda-Farias, J. B., Caram‐Salas, N. L., Salinas‐Abarca, A. B., Ocampo, J. & Granados‐Soto, V. Ultra‐low doses of Naltrexone enhance the antiallodynic effect of Pregabalin or Gabapentin in neuropathic rats. Drug Dev. Res. 78 (8), 371–380 (2017).
Powell, K. J. et al. Paradoxical effects of the opioid antagonist Naltrexone on morphine analgesia, tolerance, and reward in rats. J. Pharmacol. Exp. Ther. 300 (2), 588–596. https://doi.org/10.1124/jpet.300.2.588 (2002).
Paquette, J. & Olmstead, M. Ultra-low dose Naltrexone enhances cannabinoid-induced antinociception. Behav. Pharmacol. 16 (8), 597–603 (2005).
Zhang, Y. et al. Dysregulation of pain-and emotion-related networks in trigeminal neuralgia. Front. Hum. Neurosci. 12, 107 (2018).
Rezvanfard, M., Zarrindast, M-R. & Bina, P. Role of ventral hippocampal GABAA and NMDA receptors in the anxiolytic effect of carbamazepine in rats using the elevated plus maze test. Pharmacology 84 (6), 356–366 (2009).
Sudha, S., Lakshmana, M. K. & Pradhan, N. Changes in learning and memory, acetylcholinesterase activity and monoamines in brain after chronic carbamazepine administration in rats. Epilepsia 36 (4), 416–422 (1995).
Canli, T., Cook, R. G. & Miczek, K. A. Opiate antagonists enhance the working memory of rats in the radial maze. Pharmacol Biochem. Behav 36(3), 521–525 https://doi.org/10.1016/0091-3057(90)90250-l (1990).
Gambeta, E., Chichorro, J. G. & Zamponi, G. W. Trigeminal neuralgia: an overview from pathophysiology to Pharmacological treatments. Mol. Pain. 16, 1744806920901890 (2020).
Watkins, L. R. et al. Glia as the bad guys: implications for improving clinical pain control and the clinical utility of opioids. Brain. Behav. Immun. 21 (2), 131–146 (2007).
Largent-Milnes, T. M., Guo, W., Wang, H-Y., Burns, L. H. & Vanderah, T. W. Oxycodone plus ultra-low-dose Naltrexone attenuates neuropathic pain and associated µ-opioid receptor–Gs coupling. J. Pain. 9 (8), 700–713 (2008).
Toljan, K. & Vrooman, B. Low-dose Naltrexone (LDN)—review of therapeutic utilization. Med. Sci. 6 (4), 82 (2018).
Zhang, Z., Wang, X., Wang, W., Lu, Y-G. & Pan, Z. Z. Brain-Derived neurotrophic Factor–Mediated downregulation of brainstem K+–Cl–Cotransporter and Cell-Type–Specific GABA impairment for activation of descending pain facilitation. Mol. Pharmacol. 84 (4), 511–520 (2013).
Eide, P. K. Familial occurrence of classical and idiopathic trigeminal neuralgia. J. Neurol. Sci. 434, 120101 (2022).
Hamann, S. & Sloan, P. Oral Naltrexone to enhance analgesia in patients receiving continuous intrathecal morphine for chronic pain: a randomized, double-blind, prospective pilot study. J. Opioid Manag. 3 (3), 137–144 (2007).
Acknowledgements
Funding for this study was provided by Kerman University of Medical Sciences as a grant (91000056) conducted by Yeganeh Naderi.
Author information
Authors and Affiliations
Contributions
Yeganeh Naderi and Monavareh Soti contributed to the study conception and design, acquisition of animal data, analyzing data, interpretation of the findings, and writing the manuscript. Masoud Soltani, Yasaman Naderi and Kristi A. Kohlmeier assisted with study conception and design, data analysis, interpretation of the findings, and critical revision of the manuscript. Mohammad Shabani contributed to the study’s conception and design, analyzed the data, interpreted the findings, and wrote the manuscript. He also provided a critical revision for important intellectual content. All authors critically reviewed the content and approved final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All experiments were done in accordance with the ARRIVE guidelines and National Institutes of Health Guide for the Care and Use of Laboratory Animals) NIH Publication No. 80–23, revised 1996). All procedures were approved (91000056) by the Research and Ethics Committee of Kerman Universities of Medical Sciences, Kerman, Iran. All experiments were performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Naderi, Y., Soti, M., Soltani, M. et al. Co-administration of low-dose-Naltrexone and Carbamazepine remarkedly ameliorate allodynia and cognitive deficit in a rat model of trigeminal neuralgia. Sci Rep 15, 31335 (2025). https://doi.org/10.1038/s41598-025-14820-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-14820-4