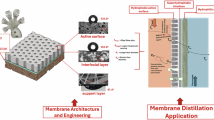
Abstract
Membrane distillation (MD) is an emerging membrane-based thermal desalination technology for desalination. However, designing highly efficient MD membranes faces complication between large pore size for high flux and reduced pore size for excellent anti-wetting property. In this work, a unique composite nanofibrous membrane was developed using a simultaneous electrospinning and electrospraying technique. The membrane consisted of electrospun PVDF nanofibers and electrosprayed fluorinated TiO2-PVDF microclusters. The simultaneous dual-nozzle fabrication created an intertwined network of nanofibers and microclusters, resulting in a highly porous membrane structure with enhanced flux. The microclusters contributed to increased surface roughness and reduced surface energy, providing excellent liquid entry pressure and wetting resistance. The membrane achieved a flux of 22.5 kg m-2 h-1 and a salt rejection factor (SRF) of 0.999 for 24 h of MD operation with 3.5% NaCl. Additionally, it demonstrated good performance against surfactant-contaminated saline feed, maintaining a flux of 14.8 kg m-2 h-1 and SRF of 0.999. Compared to a conventional nanofibrous membrane, the composite membrane exhibited up to 55% increase in flux, highlighting its performance advantage. This work introduces a simple, scalable, and time-efficient fabrication strategy for producing high-performance MD membranes and offers valuable insights into membrane design for water desalination applications.
Similar content being viewed by others
Introduction
Membrane technology for desalination and water purification processes has been widely used to obtain fresh water from contaminated saline water. Various membrane technologies, including pressure-assisted techniques like reverse osmosis and thermal desalination processes such as multistage flash distillation, have been globally established1. Alternatively, membrane distillation (MD) is a developing membrane-based and thermal desalination technique that attracts increasing attention from research and industrial sections due to its ability to utilize low-grade thermal energy, treat highly saline waters, and its potential integration in a zero-liquid discharge system. However, the technology readiness of the MD is still at a low level due mainly to the limitation of membrane materials such as wetting problems, low permeability, and weak membrane mechanical properties2,3,4. Membrane wetting is a main challenge to MD especially when complex saline feed is involved. Therefore, to realize the MD in a membrane market these issues must be properly addressed
The key strategies in improving the anti-wetting property of the membrane surface are to increase surface roughness and reduce surface energy. These principles are applied by incorporating nanoparticles and fluorinated compounds in the fabrication and modification of MD membranes5,6,7,8,9,10. Both approaches lead to remarkable enhancements in the anti-wetting properties producing membranes with superhydrophobic and omniphobic surfaces. However, the membranes suffer from the known trade-off between wetting resistance and vapor permeability. Superior wetting resistance against different low surface energy contaminants such as surfactants and organic solvents might not be totally advantageous in MD desalination, as it can compromise the vapor permeability11. Thus, a balance between wetting resistance and vapor flux is crucial in designing and developing MD membranes
Electrospinning is a well-established technique in creating nanofibrous membranes with high porosity and interconnected pore structures that provide vapor pathways with minimal tortuosity. The pores in electrospun nanofibrous membranes can be controlled by adjusting the fiber size. A trend has been identified, showing that the mean pore size is two to four times the mean fiber diameter and that the thin fibers produce small pores12. Moreover, electrospinning is versatile in producing fibers with different morphology and made of various materials, blends, and nanocomposites. Electrospinning feed solutions containing blends of hydrophobic polymers, nanoparticles, and fluorinated compounds are used to create nanofibrous membranes with improved wetting resistance13,14,15,16. Combining the advantages of tunable pore size and controlled surface property, electrospinning is an efficient technique to develop MD membranes with the desired balance between high porosity and excellent wetting resistance.
Similar to electrospinning, electrospraying also relies on the electrohydrodynamic phenomenon in which electrically charged fluid flows and deforms in the presence of an external electric field17. The combination of the two methods ensures the compatibility of techniques for a one-step membrane preparation. In addition, the difference in the shapes and structures of their resulting products is beneficial in developing membranes with unique internal and surface morphologies. Generally, electrospinning produces cylindrical fibrous morphology due to whipping and elongation of the charged jet before solidification. In contrast, electrospraying creates micro and nanoscale particles due to the polarization of the liquid drop at the nozzle tip causing the charged jet to be emitted. The addition of nanoparticles in the electrospraying solution further enhances the formation of hierarchical structures of micro- and nanospheres18,19.
The advantages of combining electrospinning and electrospraying methods in producing MD membranes are shown in recent studies. A two-step fabricated membrane with PVDF-HFP nanofibrous layer and PVDF-HFP/fluorinated TiO2 electrosprayed particles showed a slight improvement in the average flux but notable lower permeate conductivity20. However, considering the comparable salt rejection factor and the vapor flux, the modified membrane only showed equal performance with the single-layered PVDF-HFP nanofibrous membrane. In the study of Wu and coworkers, a subsequent electrospinning and electrospraying method was used to prepare a multi-layered membrane consisting of a layer of electrospun PVDF-HFP nanofibers and a layer of electrosprayed PVDF-HFP/fluorinated-SiO2 nanoparticles. The membrane has higher hydrophobicity, larger pores, and higher flux than the membrane with electrospun PVDF-HFP nanofibers only21. Likewise, electrospinning PVDF immediately followed by electrospraying of PVDF/Al2O3 nanoparticle composite resulted in a dual-layered membrane with a nanofibrous base layer and a bead-structured top layer22. A slight reduction in pore size and a large increase in hydrophobicity resulted in a minor increase in flux compared to that of the single-layered membrane. The studies exhibited how the electrosprayed particles primarily improved the surface roughness but adversely affected the pore size and porosity. They showed remarkable enhancement in the wetting resistance of the membranes but only minimal improvement and even negative effects in the flux. Thus, this work proposes the utilization of simultaneous actions of electrospinning and electrospraying in designing membranes with rough and highly hydrophobic surfaces and loose and porous structures.
Herein, a triple-layered membrane consisting of (1) an electrospun nanofibrous layer, (2) an intertwined nanofibers—fluorinated TiO2/PVDF bead layer, and (3) fluorinated TiO2/PVDF bead layer. Through the simultaneous action of electrospinning and electrospraying, a loose and porous layer was obtained. The short extension of the electrospraying action provided a moderate coating layer of particles for enhanced roughness and hydrophobicity. The increase in pore size is expected to cause the improvement in flux, while the enhancement in surface is expected to lead to better anti-wetting characteristics.
Methodology
Materials and chemicals
Poly (vinylidene fluoride) (PVDF, MW = 275 000 g/mol) was obtained from Sigma Aldrich. The solvents N, N-dimethylformamide (DMF, AR 99.8%), and acetone (AR 99.5%) were procured from RCI Labscan. Lithium chloride (LiCl, RPE) and sodium chloride (NaCl, RPE) were supplied by Carlo Erba reagents. Titanium dioxide powder (TiO2, > 95%) was obtained from Tokyo Chemical Industry Co., Ltd. 1H,1H,2H,2H-Perfluorodecyltriethoxysilane (FAS, 97%) and Sodium dodecyl sulfate (SDS, AR 99.0%) was purchased from Sigma-Aldrich. A commercial PVDF membrane (pore size: 0.45 μm) was obtained from CNW Technologies.
Preparation of electrospinning and electrospraying solutions
The electrospinning solution, hereafter referred to as solution 1, was prepared by dissolving 17.5 wt.% of PVDF in a 3:2 volume ratio of DMF: acetone. The mixture was heated at 75 °C with continuous stirring until dissolved. A trace amount (0.01 wt.%) of LiCl was added to improve the conductivity of the polymer solution. The solution was continuously stirred and heated at 75 °C for 12 h.
The electrospraying solution, hereafter referred to as solution 2, was prepared by dissolving 3 wt.% of PVDF in DMF under continuous stirring and heating at 75 °C. Then, TiO2 (0.3, 0.6, and 1.2 wt.%) was added to the mixture and was sonicated for 30 min. The PVDF-TiO2 suspension was continuously stirred at 75° for 12 h, followed by adding 3 wt.% of FAS.
Both solutions 1 and 2 were degassed by sonication for 15 min before loading into syringes.
Preparation of composite nanofibrous membrane
An electrospinning machine (Bionicia Fluidnatek LE-50) with two-nozzle spinneret capable of simultaneous electrospinning and electrospraying was used to fabricate a composite nanofibrous membrane. One horizontally positioned nozzle was used for electrospinning, while the other vertically positioned nozzle was used for electrospraying a PVDF/TiO2/FAS bead onto the fabric. Figure 1 provides a schematic diagram of the layer-by-layer fabrication process.
Solution 1 was loaded into a 10 mL Luer Lock tip plastic syringe with a 24G stainless steel needle positioned horizontally 15 cm from a grounded, aluminum foil-covered drum collector rotating at a speed of 200 rpm. The voltage and the flow rate were set to 25 kV and 1.0 mL/h, respectively. Solution 2 with different TiO2 loading was loaded into a 5 mL Luer Lock tip plastic syringe with a 21G stainless steel needle positioned vertically 8 cm above the same drum collector. The voltage and the flow rate were set to 18 kV and 1.5 mL/h, respectively. The membrane fabrication began with the electrospinning of Solution 1 for 4 h. Subsequently, electrospraying of Solution 2 was introduced simultaneously for 1.5 h. Finally, only Solution 2 was continuously electrosprayed for an additional 30 min. The temperature and humidity during the membrane fabrication were controlled 25 °C and 55%, respectively. All the composite electrospun nanofibrous membranes were fabricated using the same parameters and operating conditions. The control membrane was prepared using the parameters and operating conditions of Solution 1 electrospinning only. The prepared membranes were named as T03, T06, and T12 following the TiO2 loading of 0.3, 0.6, and 1.2 wt.%, respectively.
Membrane characterization
The membrane morphology was evaluated using scanning electron microscopy (Hitachi S-3400N). The images were analyzed using ImageJ software (version 1.53 k; https://imagej.nih.gov/ij/) to determine the mean fiber diameter, fiber size distribution, mean particle size, and particle size distribution. The surface roughness of the membranes was analyzed using a 3D measuring laser microscope (LEXT OLS5100). Elemental analysis was conducted using energy-dispersive X-ray analysis (Hitachi SU3500). The functional groups of the membranes were evaluated using ATR-FTIR (Bruker Alpha II). The transmittance spectra were obtained within 400–4000 cm−1 wavenumber range with 32 scans and a resolution of 2 cm−1. The crystallinity of TiO2 and the membranes were evaluated using X-ray diffractometer (Bruker D8 Advance), with Cu Kα radiation at wavelength 1.5418 Å, and operating under 40 kV and 40 mA. The diffraction angle, 2θ, was measured from 10° to 80°.
The liquid contact angles and the surface free energy of the prepared membranes were measured at room temperature using an optical contact angle system (Data Physics OCA-200) in sessile drop mode. For liquid contact angle measurement, the liquids used were deionized water, glycerol, diiodomethane, 3.5 wt.% NaCl, 0.5 mM sodium dodecyl sulfate (SDS), 0.5 mM cetyltrimethylammonium bromide (CTAB), and 0.5 mM Triton X. A 5 μL of liquid was dropped on the surface at a dosing rate of 1 μL/s. The contact angle was measured after 1 min when the liquid comes into contact with the surface. An average of five measurements was reported. For the evaluation of surface free energy using the double sessile drop mode, water and diiodomethane were used as the polar liquid and the non-dispersive liquid, respectively. The values were calculated using the Owens, Wendt, Rabel, and Kaelble model23.
The mean pore size and pore size distribution of the prepared membranes were measured using a capillary flow porometer (Porolux 1000). Porefil125 with a surface tension of 0.016 N/m was used as the wetting liquid agent. The liquid entry pressure (LEP) was estimated using Eq.1:
where γ is the surface tension of water (72 mN/m), θ is the water contact angle, and r is the pore size radius (μm).
The porosity of the membranes was determined by gravimetric analysis using butanol as the wetting liquid. Then, the porosity, ε, was calculated using Eq. 2. 5
where \({W}_{w}\) and \({W}_{D}\) are the weights of wet and dry membranes, respectively (gram); \({\rho }_{b}\) is the density of butanol (0.81 g/cm3) and \({\rho }_{PVDF}\) is the density of PVDF (1.78 g/cm3).
The mechanical properties of the membranes were assessed using a universal testing machine (Zwick Roell Z010) equipped with a 500 N load cell. Membrane samples were cut into strips measuring 20 mm in length and 5 mm in width. The extension rate was set at 20 mm/min, and the average values were calculated from five specimens. The membrane thickness was determined using a digital micrometer (Mitutoyo 293–340-30), with measurements taken at ten distinct locations across each membrane to calculate the average value.
Direct contact membrane distillation (DCMD)
The prepared membranes were used in a laboratory-scale DCMD process. The membrane was secured in a customized acrylic membrane cell with an effective area of 3.14 cm2. A 3.5 wt. % NaCl solution was used as the feed that was heated on a hotplate at a constant temperature of 65 °C. Deionized water was used as the permeate that was chilled at 20 °C using a chiller (Hailea HS-28A). The feed and permeate were circulated using a double-channel peristaltic pump (Longer BT100-3 J) set at 80.0 rpm or 75 mL/min. The increase in mass of the permeate was recorded and used to calculate water vapor flux, J (kg m−2 h−1), expressed in Eq. 3.
where Δm is the mass increase of permeate (kg), A is the effective membrane area (m2), and t is the time duration (h).
The conductivity of the permeate was measured using a calibrated conductivity meter (Mettler Toledo FiveEasy F30) and the NaCl concentration was determined using a predetermined calibration curve. The salt rejection factor, SRF, was calculated using Eq.4.
where \({C}_{p}\) and \({C}_{f}\) are the NaCl concentration of the permeate and the feed, respectively. Both J and SRF were monitored every 1 h of the operating test.
The DCMD performance of the membrane against surfactant-contaminated feed was evaluated using the same DCMD setup and with a feed solution containing 3.5 wt.% NaCl and 0.1 mM SDS.
Results and discussion
Morphology and porosity
The simultaneous electrospinning-electrospraying process was conducted using a two-nozzle spinneret, enabling the continuous fabrication of a multi-layer membrane. This membrane comprised a porous PVDF electrospun nanofiber (ENF) base layer, an intertwined fiber-bead layer, and a rough bead layer. Figure 2a1-d1 and 2 a2-d2 illustrate the surface morphology, while Figs. 2a3-d3 exhibit the fiber size distribution of the representative prepared membranes. All the membranes have comparable average fiber diameters of 166 ± 79 nm, as determined by ANOVA (p-value < 0.05). With the introduction of electrospraying, submicron-sized beads of PDVF/TiO2/FAS emerged, adhering to the nanofibers and filling the pores of the ENF. As the TiO2 concentration increased from 0.3% to 1.2%, the viscosity of the electrospraying solution rose from 10 cP to up to 12 cP (see Figure S1). This increase in viscosity resulted in larger droplet sizes at the needle tip and, subsequently, in the collected electrosprayed PVDF-TiO2 composite beads on the membrane surface. The formation of larger spherical clusters was observed as shown in Figs. 2a2-d2 and Figure S2. Additionally, the microbead density increased from 27 to 57 × 10⁻2 beads/μm2 with increasing TiO₂ content (Figure S3), highlighting the influence of TiO₂ concentration on bead formation. Membrane thickness measurements (Figure S4) showed an increase from 90 μm to 115 μm upon electrospraying the 0.3% TiO₂ PVDF/TiO₂/FAS solution. Further increases in TiO₂ concentration to 0.6% and 1.2% led to membrane thicknesses of approximately 140 μm. This trend in thickness correlates well with the observed increase in bead size and deposition.
The membrane pore size is a critical property, as it is directly related to the permeating flux and to the anti-wetting characteristics. Larger pore size is advantageous for achieving higher flux; however, excessively large pores can lower the liquid entry pressure, increasing the membrane’s susceptibility to wetting and liquid penetration. In the intertwined sections, the sprayed submicron particles can disrupt fiber packing, resulting in larger pores. During the concurrent processes of electrospinning and electrospraying, charge accumulation on the protruding spherical clusters enhances the repulsion between the nanofibers and microbeads, leading to a loosely packed arrangement of the nanofiber-composite particle network24. Later, when only spraying is applied, these microbeads fill in the pores. In the case of the spraying solution with 0.3 wt.% TiO2, the created bead size was smaller than under other conditions, leading to less pore filling compared to larger beads. As a result, the T03 membrane exhibits a much larger pore size compared to the T06 and T12 membranes (Figure 3). The increase in pore size in the composite membranes, particularly in T03, shows the advantage of simultaneous electrospinning and electrospraying compared to the pore size reduction in modified membranes fabricated through sequential electrospinning and electrospraying19,22. A similar observation was reported by Lee et al.16, where the composite membrane with nanoparticles has a larger pore size than the unmodified membrane.
High porosity in membranes is desirable to achieve larger surface area for evaporation leading to high flux and minimal heat loss25. The electrospraying of PVDF-fluorinated TiO2 composite particles is advantageous in creating ragged layer packing, providing a loose porous network. As shown in Fig. 3b, all membranes maintained high porosity levels, exceeding 80%, although the addition of microbeads did result in a slight reduction in porosity. The incorporation of micro spherical particles into the fibrous network does not block the pores; rather, it enhances the creation of additional porous channels for water vapor pathways26.
Surface roughness, hydrophobicity, and liquid wetting resistance
Hydrophobicity is a crucial property of membranes used in membrane distillation, as it primarily indicates the anti-wetting and anti-fouling characteristics. Enhancing the hydrophobicity of the membrane surface relies on two key factors: increasing surface roughness and decreasing surface energy27.
The 3D scanned images of the membrane surfaces (Figs. 4a–d) highlight the effect of electrosprayed microbeads in increasing surface roughness and promoting non-wetting behavior. The Cassie-Baxter model predicts that a highly rough surface can trap air pockets, reducing the liquid–solid interface and enhancing hydrophobicity28. Consistent with this model, increased surface roughness on hydrophobic materials leads to higher water contact angles. However, the results indicate that the relationship between roughness and hydrophobicity is not strictly linear, suggesting that simply increasing roughness is not sufficient. It is proposed that microbead density plays a crucial role in maximizing air entrapment and, consequently, hydrophobicity. For instance, at higher TiO2 loading (T12 membrane), larger microbeads deposited in dense areas occupied the open pores, potentially collapsing the air pockets. This resulted in a decreased surface roughness and a corresponding reduction in the water contact angle, demonstrating that a suitable microbead density is required to achieve maximum hydrophobicity and that the spatial arrangement of the roughness features is critical.
Figure. 4e illustrates the surface energies of the different membranes. The presence of fluoroalkyl silanes on the electrosprayed particles reduced the membrane’s surface energy, reinforcing the Cassie-Baxter state by further preventing liquid penetration5,29. The combined influence of increased surface roughness and reduced surface energy led to the overall increase in the hydrophobicity of the composite membrane surfaces, as illustrated in Fig. 4f. For the T12 membrane, which has a relatively smoother surface, the improvement in hydrophobicity was primarily attributed to the presence of fluoroalkyl silanes. As a result, the T12 membrane exhibited a significantly higher water contact angle compared to the unmodified nanofibrous membrane.
Liquid entry pressure (LEP), describing the minimum pressure for the liquid to enter the pores, was estimated by Eq. 1 and the values from Fig. 3a and4f. With the assumption of having cylindrical pore geometries (B = 1) in the membranes and using their mean pore sizes in the calculation, an overestimation of the LEP values could have been obtained. The highest LEP is found in the case of the T06 membrane, implying excellent anti-wetting properties, but high LEP could also denote a very small pore size, leading to reduced flux. The slight reduction in the mean pore size of T06 and T12 membranes led to the same extent of improvement in LEP. Meanwhile, the notable pore size enlargement in T03 consequently resulted in low LEP. Nevertheless, all the composite membranes are still considered to have good anti-wetting properties due to their comparable LEP values with other TiO2 composite nanofibrous membranes16,19,30 and are within the desired criteria for MD membranes. The key characteristics of the fabricated membranes are summarized in Table 1, together with the properties of the commercial PVDF membrane made from the phase inversion technique.
In addition to water resistance, MD membranes must exhibit resistance to other liquids. This broader liquid resistance is a crucial characteristic for ensuring robust performance when dealing with challenging saline feed solutions containing contaminants with low surface tensions. To further evaluate the liquid resistance of the membrane surface, various liquids with surface tension lower than that of water (γwater = 72 mN/m) were tested on the membrane surface and their contact angles were measured, as shown in Fig. 5. For the liquids with surface tension ranging from 50 to 72 mN/m such as diiodomethane (50.8 mN/m), glycerol (63.4 mN/m), and 3.5 wt.% NaC1 (72 mN/m), the composite nanofibrous membranes have higher liquid contact angles compared to the unmodified membrane. The improvement in the resistance against such liquids was attributed to the increased surface roughness and decreased surface energy created by the electrosprayed fluorinated TiO2 composite particles. Similar to the results of Chen and coworkers31, the enhancement in the resistance against both water and diiodomethane, makes the composite nanofibrous membrane as amphiphobic with contact angles above 120° for both polar and nonpolar liquids, respectively.
In addition, against the different surfactant solutions with much lower surface tensions (γ < 40 mN/m), the composite nanofibrous membranes have notable resistance compared to the unmodified membrane that was instantly wetted by such liquids. The considerable contact angles of T03 and T06 membranes against the low surface tension liquids indicated the advantage of the evenly distributed electrosprayed fluorinated TiO2 composite particles. In contrast, despite the presence of the same particles on T12 membrane, the agglomerated microbeads with minimal height variation led to negligible enhancement in surface roughness, and consequently to an insignificant resistance against low surface tension liquids, particularly to ionic surfactants (SDS and CTAB) with stronger interfacial activity than nonionic surfactants (Triton-X). The high bead density combined with low roughness creates numerous continuous contact points for surfactant molecules, allowing ionic surfactants (SDS and CTAB) to interact more extensively with the densely packed microbeads through electrostatic forces. Therefore, improved surface roughness and sufficient spacing of these roughness features have more influence than reduced surface energy in enhancing the wetting resistance against challenging liquids.
Mechanical property
Figure 6a-b presents the mechanical properties of unmodified PVDF and composite nanofibrous membranes. The incorporation of electrosprayed microbeads had a minimal effect on tensile stress, which remained relatively constant across all modified membranes. However, Young’s modulus initially decreased at 0.3% and 0.6% TiO₂ loading (T03 and T06), indicating material less stiffness due to the loose fiber-particle network formed by the simultaneous processes of electrospinning and electrospraying. At a higher TiO₂ content of 1.2% (T12), Young’s modulus increased again, likely as a result of denser bead accumulation and greater membrane thickness, which contributed to increased stiffness. Meanwhile, elongation at break showed a consistent downward trend with increasing TiO₂ concentration (Fig. 6b). This reduction in material’s ductility can be attributed to the formation of larger particle aggregates and microclusters especially at higher loadings, which introduced stress concentration points and weakened the membrane structure. As a result, the membranes became more brittle. These findings are in agreement with those reported by Shahabadi et al.32. Overall, the mechanical response reflects a trade-off between flexibility and structural rigidity, governed by both the bead distribution and membrane thickness. While moderate bead incorporation improves flexibility, excessive TiO₂ loading leads to agglomeration and reduced mechanical durability.
Chemical characteristics, elemental analysis, and crystallinity
Functional groups of the fabricated membranes were identified using ATR-FTIR as shown in Fig. 7. Pristine TiO2 exhibited sharp absorption bands between 400 to 500 cm−1 indicating the Ti–O and Ti–O-Ti bonds16,32. Pure FAS showed peaks between 1080 to 1280 cm−1 denoting the absorption bands for C-F2 and C-F3 vibrations, and peak at 962 cm−1 indicating the Si–O stretching vibration32. PVDF displayed characteristic C-F₂ absorption bands in the same region. The characteristic peaks at 842 and 1406 cm−1 of PVDF, showing the combination of the three phases (α-phase, β-phase and γ-phase), were identified5,33. The unmodified membrane showed sharp peaks at 874 and 1071 cm−1 representing the characteristic α-phase of PVDF. The peaks at 842, 1280, and 1435 cm−1 denote the β-phase, while the peak at 1238 cm−1 signifies the γ-phase of PVDF.
The spectra of the composite nanofibrous membrane also show these characteristic PVDF peaks. A small absorption peak at 760 cm⁻1 indicates the presence of TiO₂ and overlapping peaks between 1100 and 1300 cm⁻1 are associated with FAS. The major change in the modified membranes is observed as a broad peak around 1100 cm⁻1- 1250 cm⁻1, showing a jagged, non-smooth peak (see Fig. 7b). In addition, the peak at 1034 cm−1 could indicate the Ti–O–Si bond from the successful functionalization of TiO2 with FAS16. The peaks of the precursors were observed as overlapping peaks in the FTIR spectra confirming their presence in the composite material. However, these peaks from TiO2 overlap with those of PVDF, making it difficult for FTIR analysis to clearly differentiate the chemical functional groups of the microbeads. Therefore, elemental analysis was introduced for further characterization.
The EDX mapping images of both the unmodified and composite nanofibrous membranes are presented in Fig. 8 Fluorine, derived from both PVDF and FAS, was predominantly observed in the nanofibers and spherical clusters across all modified membranes. Given that both solution 1 and solution 2 contain PVDF, the composite membranes are primarily composed of PVDF, estimated to be around 97%. The accumulation of F and O in the spherical clusters correlates with the larger particle sizes illustrated in Fig. 8, suggesting encapsulation of TiO2 by the PVDF polymer chains. Furthermore, the small amount of Ti detected indicates a very low concentration (< 0.7%) of TiO2 within the overall membrane structure. It is important to note that minor traces of Si, O, and Ti were identified in the unmodified membrane, likely attributed to noise or interactions with the sample holder, as the electron beam penetrated the thin and porous film. The significantly higher intensities of Si, O, and Ti in the composite membranes confirm the presence of the added modifiers, thereby indicating the successful fabrication of the composite membrane.
The XRD patterns of the pristine TiO2 and the prepared membranes are illustrated in Fig. 9.The pristine TiO2 exhibits distinct sharp peaks at 2θ values of 25.4°, 37.9°, 48.1°, 54.0°, 68.4°, and 70.4°, which are indicative of the anatase phase20,34. Additionally, smaller sharp peaks at 2θ values of 55.1°, 68.4°, and 75.2° suggest the presence of the rutile phase. The unmodified membrane displays a peak at a 2θ value of 21°, representing the crystalline phase of PVDF13. The composite membranes reveal characteristic crystalline peaks corresponding to both PVDF and TiO2, confirming the retention of the chemical identities of the precursor materials within the composite structure. This evidence supports the successful incorporation of TiO2 into the PVDF nanofibrous membranes.
DCMD performance
The performance of the unmodified and the composite nanofibrous membranes was evaluated in the desalination of 3.5 wt.% NaCl solution for 24 h. The monitored flux of the membranes, in comparison with the commercial PVDF membrane, are shown in Fig. 10 a Notably, the composite nanofibrous membranes exhibited a significantly higher flux compared to the unmodified membrane, showing an increase in flux ranging from 15 to 55%. This enhancement highlights the positive impact of the electrospraying modification technique. Although the electrospraying process increased membrane thickness—which would typically raise resistance to vapor transport—this effect was effectively counterbalanced by an increase in pore size and reduced pore wetting, resulting in improved permeate flux35. As shown in Figure 6a, the average fluxes closely align with the trends in mean pore size (Fig. 3a) and microbead density (Figure S3), underscoring the strong influence of pore structure on membrane performance. Among the tested membranes, the T03 membrane exhibited the highest flux, attributable to its largest pore size and lowest microbead density, which together minimized resistance to vapor transport. Additionally, the enhanced surface roughness in the composite nanofibrous membranes promoted localized flow perturbations at the membrane-feed interface, which in turn reduced the temperature polarization effects, contributing to the observed flux enhancement36,37. Furthermore, consistent with the predicted lowest LEP values, the T03 membrane exhibited slight wetting, as indicated by a marginal decrease in salt rejection. However, this decrease was minimal, with salt rejection rates remaining close to unity, affirming the membrane’s effectiveness in resisting wetting by saline solutions. All tested membranes displayed excellent salt rejection, underscoring their robust anti-wetting properties. These findings affirm the efficacy of the modification techniques employed in this study to improve vapor flux while maintaining resistance to wetting. During the first 10 h of operation, all membranes underwent concentration polarization, which led to salt crystal formation. Among these, the commercial membrane showed the most significant reduction in flux with prolonged operation. The loose and porous structure of the developed ENF membranes, coupled with their reduced surface energy, minimized salt deposition, prevented pore blockage, and facilitated sustained high flux. Notably, the T03 membrane demonstrated superior flux stability compared to commercial PVDF membranes, indicating enhanced anti-wetting performance against saline water.
(a) 24-h DCMD performance monitoring and (b) The 24-h average fluxes of the unmodified and composite nanofibrous membranes in comparison to a commercial membrane against a 3.5 wt.% NaCl feed solution. (c) 24-h DCMD performance monitoring of the unmodified and T03 membranes against a 3.5 wt.% NaCl with 0.1 mM SDS feed solution. (d) Images of the membranes after 24-h DCMD operation using the 3.5 wt.% NaCl with 0.1 mM SDS feed solution.
The T03 composite membrane, which exhibited superior performance, was further tested with a surfactant-contaminated saline feed solution composed of 3.5 wt.% NaCl solution containing 0.1 mM SDS, assess its stability under challenging conditions known to promote membrane wetting. Surfactants reduce the surface tension of the feed solution, thereby lowering the membrane’s LEP and increasing the risk of pore intrusion by the liquid phase38. This leads to partial pore wetting, reducing the temperature gradient across the membrane and diminishing the driving force for vapor transport13,39. Fig. 10c displays the 24-h performance monitoring of T03 under these challenging conditions. A 53% reduction in flux was observed, compared to a 36% reduction for the commercial PVDF membrane. Although both membranes maintained excellent salt rejection, the T03 membrane exhibited greater susceptibility to surfactant-induced wetting. This difference in performance arises from key structural distinctions: the commercial membrane, with its smaller pores, rigid matrix, and thicker structure, offers inherent resistance to surfactant penetration and internal wetting. In contrast, the T03 nanofibrous membrane, with its interconnected pore network, broader pore size distribution, and thinner profile, offers less resistance to surfactant-induced wetting propagation. In electrospun membranes, surfactant-induced wetting typically begins at surface pores and can propagate through interconnected pathways deeper into the membrance40. This cascading wetting effect is especially pronounced in thinner membranes, where the interconnected pore structure spans the full thickness of the material, leading to greater thermal gradient disruption and, consequently, a more significant decline in flux. Despite this vulnerability under aggressive conditions, the T03 membrane maintained its structural integrity and high salt rejection, owing to its fluorinated surface chemistry and hierarchical roughness, which prevented complete pore wetting. As shown in Fig. 10d, the membrane surface exhibited some contamination after the 24-h run, but the overall structure remained intact, affirming the effectiveness of the surface modification strategy under standard operating conditions.
Table 2 presents a comparison of the 24-h average MD performance of the T03 composite membrane with other composite electrospun membranes reported in the literature. The T03 composite electrospun membrane from this study exhibited higher flux during 20 to 24 h of operation, attributed to its larger pores and loose porous structure. Some of the membranes in Table 2 that report significantly higher flux may result from various factors, including enhanced driving force conditions (i.e. greater temperature difference) and milder operating conditions (i.e. lower salt concentrations in the feed and shorter operating times). The composite electrospun membrane investigated in this study demonstrated its potential for achieving efficient and robust MD desalination at a moderate temperature difference and normal saline feed concentration. Furthermore, this study highlights the advantage of the facile membrane fabrication technique involving simultaneous electrospinning and electrospraying, leading to a highly porous nanofibrous membrane with enhanced anti-wetting characteristics.
When the MD performance is evaluated against SDS-contaminated saline feed (see Table 3), the composite T03 membrane developed in this study is comparable to most membranes reported in the literature. However, its performance is notably lower than that of two composite membranes—those incorporating reduced graphene oxide/FAS and the Teflon AF240031,41. This disparity may be attributed to the relatively lower water contact angle of the T03 membrane. To address this, future improvements could include extending the electrospraying duration to allow the fluorinated microclusters—which exhibit stronger water repellency—to form a more dominate surface. This would minimize exposure of the underlying fibers and increase the overall hydrophobicity of the membrane. Additionally, the superior membranes mentioned in the literature were post-modified using a dip-coating method, which provides more precise control over uniform coating compared to the one-step electrospinning and electrospraying technique used in this study. Despite this, the one-step fabrication method employed in this work offers significant advantages. It is simpler and less time-consuming than multi-step processes like dip-coating, making it a more efficient approach for producing composite nanofibrous membranes. This study highlights the excellent potential of this streamlined technique for creating high-performance membranes for MD applications. Nonetheless, to further enhance the performance of membranes fabricated via this one-step process, future work could explore multi-layered or gradient deposition using dual-nozzle control, in-situ functionalization or surface activation of the electrosprayed clusters to improve hydrophobicity and durability, and optimization of electrospraying parameters (e.g., voltage, flow rate, and duration) to fine-tune surface morphology and increase water contact angle uniformity.
Conclusion
The study presented a facile method of developing an electrospun nanofibrous membrane with enhanced anti-wetting properties and increased flux. Through the simultaneous electrospinning of PVDF nanofibers and electrospraying of fluorinated TiO2-PVDF spherical clusters, a loose network of particles and fibers was achieved, creating more passageways for the water vapor to pass through the membrane. The fluorinated TiO2-PVDF clusters increased the roughness and reduced the surface energy that are both needed in improving the surface resistance of the membranes against water and other liquids. The composite membranes with electrosprayed spherical clusters on the surface exhibited better resistance against NaCl, glycerol, diiodomethane, Triton X and SDS when compared to the unmodified nanofibrous membrane. The increase in pore size caused a notable improvement in the flux, while the increase in anti-wetting characteristics led to significant resistance against saline and surfactants.
The effect of increasing TiO2 concentration in the electrospraying solution was shown to have a major influence on the particle agglomeration that caused the minimal enhancements in the pore size and flux. Therefore, it was identified that a concentration of 0.3 wt.% of TiO2 in the electrospraying solution was enough to obtain the desired and remarkable improvement in the flux and anti-wettability of the composite nanofibrous membranes. The composite nanofibrous membrane achieved a 55% and 53% increase in flux when tested against a saline feed and a surfactant-containing saline feed, respectively. It has also maintained a salt rejection factor of 0.999 throughout the 24-h operation, indicating its excellent anti-wetting properties and high desalination performance.
The study has shown the efficiency of the simultaneous electrospinning and electrospraying techniques in producing electrospun nanofibrous membranes for MD application. The produced composite nanofibrous membrane has shown comparable performance with the membranes prepared using multi-step processes as reported in other works. This study exhibited the outstanding potential of a one-step assembly preparation of a composite nanofibrous membrane with unique fiber-and-particle arrangement for an effective MD process against saline water and challenging saline water.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Deshmukh, A. et al. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. R. Soc. Chem. https://doi.org/10.1039/c8ee00291f (2018).
Kim, K. C., Lin, X. & Li, C. Structural design of the electrospun nanofibrous membrane for membrane distillation application:. A Rev. https://doi.org/10.1007/s11356-022-23066-w (2022).
Zhong, L., Wang, Y., Liu, D., Zhu, Z. & Wang, W. Recent advances in membrane distillation using electrospun membranes: Advantages, challenges, and outlook. Environ. Sci. Water Res. Technol. 7, 1002–1019 (2021).
Hussain, A., Janson, A., Matar, J. M. & Adham, S. Membrane distillation: recent technological developments and advancements in membrane materials. Emergent Mater. 5, 347–367 (2022).
Yadav, P., Farnood, R. & Kumar, V. Superhydrophobic modification of electrospun nanofibrous Si@PVDF membranes for desalination application in vacuum membrane distillation. Chemosphere 287, 132092 (2022).
Lee, J., Boo, C., Ryu, W. H., Taylor, A. D. & Elimelech, M. Development of omniphobic desalination membranes using a charged electrospun nanofiber scaffold. ACS Appl. Mater. Interfaces 8, 11154–11161 (2016).
Dizge, N., Shaulsky, E. & Karanikola, V. Electrospun cellulose nanofibers for superhydrophobic and oleophobic membranes. J. Memb. Sci. 590, 117271 (2019).
Xu, Y. et al. In-situ silica nanoparticle assembly technique to develop an omniphobic membrane for durable membrane distillation. Desalination 499, 114832 (2021).
Pan, T. et al. ZnO Nanowires@PVDF nanofiber membrane with superhydrophobicity for enhanced anti-wetting and anti-scaling properties in membrane distillation. J. Memb. Sci. 621, 118877 (2021).
Ding, Z., Liu, Z. & Xiao, C. Excellent performance of novel superhydrophobic composite hollow membrane in the vacuum membrane distillation. Sep. Purif. Technol. 268, 118603 (2021).
Wang, W. et al. Trade-off in membrane distilllation with monolithic omniphobic membranes. Nat. Commun. 10, 1–9 (2019).
Ma, H., Burger, C., Hsiao, B. S. & Chu, B. Ultra-fine cellulose nanofibers: new nano-scale materials for water purification. J. Mater. Chem. 21, 7507–7510 (2011).
Li, J. et al. Fabrication of superhydrophobic PDTS-ZnO-PVDF membrane and its anti-wetting analysis in direct contact membrane distillation (DCMD) applications. J. Memb. Sci. 620, 118924 (2021).
Dong, Z.-Q.Z.Q., Ma, X.-H.X.H., Xu, Z.-L.Z.L. & Gu, Z.Y.Z.-Y. Superhydrophobic modification of PVDF-SiO2 electrospun nanofiber membranes for vacuum membrane distillation. RSC Adv. 5, 67962–67970 (2015).
Hou, D., Lin, D., Ding, C., Wang, D. & Wang, J. Fabrication and characterization of electrospun superhydrophobic PVDF-HFP/SiNPs hybrid membrane for membrane distillation. Sep. Purif. Technol. 189, 82–89 (2017).
Lee, E. J. et al. Advanced multi-nozzle electrospun functionalized titanium dioxide/polyvinylidene fluoride-co-hexafluoropropylene (TiO2/PVDF-HFP) composite membranes for direct contact membrane distillation. J. Memb. Sci. 524, 712–720 (2017).
Hosseini, S. S. & Valipouri, A. Electrospraying for membrane fabrication. Electrospun Nanofibrous Membr. Princ. Appl. https://doi.org/10.1016/B978-0-12-823032-9.00024-6 (2022).
Zhang, W. et al. Preparation of re-entrant and anti-fouling PVDF composite membrane with omniphobicity for membrane distillation. J. Memb. Sci. 595, 117563 (2020).
Zhang, W., Wang, Z. & Li, B. Omniphobic membrane with nest-like re-entrant structure via electrospraying strategy for robust membrane distillation. J. Memb. Sci. 640, 119824 (2021).
Guo, J., Deka, B. J., Kim, K. J. & An, A. K. Regeneration of superhydrophobic TiO2 electrospun membranes in seawater desalination by water flushing in membrane distillation. Desalination 468, 114054 (2019).
Wu, X.-Q. et al. Designing triple-layer superhydrophobic/hydrophobic/hydrophilic nanofibrous membrane via electrohydrodynamic technique for enhanced anti-fouling and anti-wetting in wastewater treatment by membrane distillation. J. Membr. Sci. Lett. 2, 1–7 (2022).
Attia, H., Johnson, D. J., Wright, C. J. & Hilal, N. Robust superhydrophobic electrospun membrane fabricated by combination of electrospinning and electrospraying techniques for air gap membrane distillation. Desalination 446, 70–82 (2018).
Deka, B. J., Guo, J. & An, A. K. Robust dual-layered omniphobic electrospun membrane with anti-wetting and anti-scaling functionalised for membrane distillation application. J. Memb. Sci. 624, 119089 (2021).
Ramakrishna S Fujihara K Teo W.-E Lim T.-C Ma Z (2005) An Introduction to Electrospinning and Nanofibers World Scientific
Zare, S. & Kargari, A. Membrane properties in membrane distillation. in Emerging Technologies for Sustainable Desalination Handbook 107–156 (Elsevier Inc., 2018). https://doi.org/10.1016/B978-0-12-815818-0.00004-7.
Jia, W., Kharraz, J. A., Guo, J. & An, A. K. Superhydrophobic (polyvinylidene fluoride-co-hexafluoropropylene)/ (polystyrene) composite membrane via a novel hybrid electrospin-electrospray process. J. Memb. Sci. 611, 118360 (2020).
Lobregas, M. O. S., Rangkupan, R., Riassetto, D. & Klaysom, C. Advancing surface-enhanced electrospun nanofiber membranes: Customizing properties for enhanced performance in membrane distillation. Macromol. Mater. Eng. 2300461, 1–20 (2024).
Li, X. et al. Omniphobic nanofibrous membrane with pine-needle-like hierarchical nanostructures: Toward enhanced performance for membrane distillation. ACS Appl. Mater. Interfaces 11, 47963–47971 (2019).
Chang, H. et al. A critical review of membrane wettability in membrane distillation from the perspective of interfacial interactions. Environ. Sci. Technol. 55, 1395–1418 (2021).
Ren, L.F.L.-F. et al. TiO2-FTCS modified superhydrophobic PVDF electrospun nanofibrous membrane for desalination by direct contact membrane distillation. Desalination 423, 1–11 (2017).
Chen, T., Ma, W., Lee, J., Jassby, D. & Rahaman, M. S. Development of robust and superamphiphobic membranes using reduced graphene oxide (rGO)/PVDF-HFP nanocomposite mats for membrane distillation. Environ. Sci. Nano 8, 2883–2893 (2021).
Seyed Shahabadi, S. M., Rabiee, H., Seyedi, S. M., Mokhtare, A. & Brant, J. A. Superhydrophobic dual layer functionalized titanium dioxide/polyvinylidene fluoride-co-hexafluoropropylene (TiO2/PH) nanofibrous membrane for high flux membrane distillation. J. Memb. Sci. 537, 140–150 (2017).
Kaspar, P. et al. Characterization of Polyvinylidene Fluoride (PVDF) electrospun fibers doped by carbon flakes. Polymers 12, 2766 (2020).
Wang, Y. et al. Beads-on-String Structured Nanofibers for Smart and Reversible Oil/Water Separation with Outstanding Antifouling Property. ACS Appl. Mater. Interfaces 8, 25612–25620 (2016).
An, A. K. et al. Enhanced vapor transport in membrane distillation via functionalized carbon nanotubes anchored into electrospun nanofibres. Sci. Rep. 7, 1–11 (2017).
Zhao, Q., Yu, S., Zhu, J., Gong, G. & Hu, Y. Beads-on-string structural nanofiber membrane with ultrahigh flux for membrane distillation. Sep. Purif. Technol. 334, 125999 (2024).
Guo, J., Deka, B. J., Wong, P. W., Sun, J. & An, A. K. Fabrication of robust green superhydrophobic hybrid nanofiber-nanosphere membrane for membrane distillation. Desalination 520, 115314 (2021).
Kharraz, J. A., Farid, M. U., Jassby, D. & An, A. K. A systematic study on the impact of feed composition and substrate wettability on wetting and fouling of omniphobic and janus membranes in membrane distillation. J. Memb. Sci. 641, 119873 (2022).
Zhao, L. et al. Theoretical guidance for fabricating higher flux hydrophobic/hydrophilic dual-layer membranes for direct contact membrane distillation. J. Memb. Sci. 596, 117608 (2020).
McGaughey, A. L. & Childress, A. E. Wetting indicators, modes, and trade-offs in membrane distillation. J. Memb. Sci. 642, 119947 (2022).
Chen, Y., Lu, K. J. & Chung, T.-S. An omniphobic slippery membrane with simultaneous anti-wetting and anti-scaling properties for robust membrane distillation 595 (J. Memb, 2020).
Tijing, L. D. et al. Superhydrophobic nanofiber membrane containing carbon nanotubes for high-performance direct contact membrane distillation. J. Memb. Sci. 502, 158–170 (2016).
Chew, N. G. P., Zhao, S., Malde, C. & Wang, R. Superoleophobic surface modification for robust membrane distillation performance. J. Memb. Sci. 541, 162–173 (2017).
An, X., Liu, Z. & Hu, Y. Amphiphobic surface modification of electrospun nanofibrous membranes for anti-wetting performance in membrane distillation. Desalination 432, 23–31 (2018).
Essalhi, M., Khayet, M., Tesfalidet, S., Alsultan, M. & Tavajohi, N. Desalination by direct contact membrane distillation using mixed matrix electrospun nanofibrous membranes with carbon-based nanofillers: A strategic improvement. Chem. Eng. J. 426, 131316 (2021).
Khayet, M., García-Payo, C. & Matsuura, T. Superhydrophobic nanofibers electrospun by surface segregating fluorinated amphiphilic additive for membrane distillation. J. Memb. Sci. 588, 117215 (2019).
Du, Y. et al. Electrospun Nanofibrous Polyphenylene Oxide Membranes for High-Salinity Water Desalination by Direct Contact Membrane Distillation. ACS Sustain. Chem. Eng. 7, 20060–20069 (2019).
Dong, Z.-Q., Wang, B.-J., Ma, X.-H., Wei, Y.-M. & Xu, Z.-L. FAS Grafted Electrospun Poly(vinyl alcohol) Nanofiber Membranes with Robust Superhydrophobicity for Membrane Distillation. ACS Appl. Mater. Interfaces 7, 22652–22659 (2015).
Hou, D. et al. Electrospun nanofibrous omniphobic membrane for anti-surfactant-wetting membrane distillation desalination. Desalination 468, 114068 (2019).
Xu, M. et al. Amphiphobic electrospun PTFE nanofibrous membranes for robust membrane distillation process. J. Memb. Sci. 641, 119876 (2022).
Wu, X.-Q. et al. Omniphobic surface modification of electrospun nanofiber membrane via vapor deposition for enhanced anti-wetting property in membrane distillation. J. Memb. Sci. 606, 1–11 (2020).
Qing, W. et al. Omniphobic PVDF nanofibrous membrane for superior anti-wetting performance in direct contact membrane distillation. J. Memb. Sci. 608, 1–7 (2020).
Acknowledgements
M.O.S.L acknowledges the scholarship and financial support from the Second Century Fund (C2F) of Chulalongkorn University. This research is supported by the 90th Anniversary of Chulalongkorn University Scholarship under the Ratchadapisek Somphot Endowment Fund (GCUGR1125671127D). It has received funding support from the NSRF via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation (grant number B16F640164) and from the Ratchadapiseksomphot Grant Chulalongkorn University (CU-GR-63-53-62-03).
Author information
Authors and Affiliations
Contributions
M.O.S.L: Conceptualization, Methodology, Data Curation, Write-Original draft, Writing-Review and Edit. R.R: Resources, Writing-Review and Editing, Funding acquisition. H.P.K: Writing-Review and Editing. C.K: Conceptualization, Resources, Supervision, Writing-Review and Edit, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lobregas, M.O.S., Rangkupan, R., Kuo, HP. et al. Integrated electrospinning and electrospraying for tailoring composite membranes of nanofibers and microbeads for membrane distillation. Sci Rep 15, 31005 (2025). https://doi.org/10.1038/s41598-025-14936-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14936-7