Abstract
This study aimed to investigate the relationship between air pollutants (AP) and liver fibrosis in the older Chinese population with metabolic dysfunction-associated steatotic liver disease (MASLD). A total of 23,887 MASLD subjects aged ≥ 65 years were enrolled in this study. The annual average levels of various APs, including NO2, SO2, O3, CO, and PM, were collected. Logistic regression analysis was conducted to examine the associations between APs and the prevalence of liver fibrosis. The quantile g-computation model was employed to assess the combined effect of the AP mixture. After adjustment for possible confounders, the odds ratios of liver fibrosis at the highest quartile of O3, CO, SO2, PM2.5, and PM10 were 1.50 (1.30, 1.74), 1.26 (1.09, 1.46), 1.22 (1.06, 1.40), 1.49 (1.28, 1.74), and 1.83 (1.56, 2.13) compared to the lowest quartile, respectively. Stratified analysis showed that the effects of APs were more prominent among the males, participants who exercise inactively, those without obesity, and non-diabetic participants (Pfor interaction < 0.05). The overall AP mixture was positively associated with liver fibrosis, and PM10 mainly contributed to the positive association with liver fibrosis.
Similar content being viewed by others
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD)1 is one of the most widespread liver diseases, distinguished by excessive hepatic fat accumulation, affecting approximately 30% of the global population2. This condition may lead to liver fibrosis3 caused by persistent hepatic injury and the excess accumulation of extracellular matrix components such as fibronectin in liver cells. Liver fibrosis, in turn, can progress to various adverse outcomes, including liver cirrhosis, hepatocellular cancer4, and even death. Notably, several studies have demonstrated an increasing prevalence of liver fibrosis with advancing age5,6; thus, the current severe trend of population aging 7 may exacerbate morbidity and the healthcare utilization burden of MASLD-related fibrosis among the older population, necessitating urgent attention and intervention8.
In recent years, there has been increasing evidence that environmental pollutants, particularly air pollutants (AP), have effects on diseases of old age. Previous studies have demonstrated that in subjects aged ≥ 65 years, AP exposure was associated with increased risk of overweight and obesity9, dyslipidemia10,11, and disruptions in glucose homeostasis12. Animal studies have further revealed that APs can cause lipid metabolism disorder13,14 and induce liver fibrosis by activating oxidative stress and inflammatory pathways15, as well as impairing liver glucose metabolism16. As such, it is plausible to hypothesize that AP exposure may also play an important role in the development of liver fibrosis in MASLD. However, limited data exist concerning the association between AP and the severity of MASLD, especially in the elderly. Some studies have shown a positive association between ambient particulate matter (PM) exposure and advanced fibrosis in adults with MASLD17, whereas a negative association has been observed with increasing ozone (O3) concentration18; another study reported that exposure to higher concentrations of PM2.5, PM10, and nitrogen dioxide (NO2) was linked to an increased risk of incident cirrhosis19. These existing studies mainly focus on middle-aged adults, leaving uncertainty regarding whether AP levels later in life may still affect MASLD-related fibrosis outcomes. In addition, the above studies focus on a single AP; since individuals are exposed to a broad spectrum of APs simultaneously, the overall effects of multi-air pollutant exposures on liver fibrosis still need to be further clarified.
Therefore, in a large community-based cross-sectional study in Xiamen City, China, we aimed to investigate the association of various APs and the combined effect of the AP mixture with liver fibrosis in MASLD in subjects aged 65 years or older.
Materials and methods
Study population
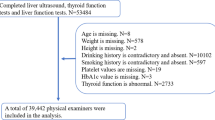
This study was a cross-sectional study based on a registered household population aged 65 years or older who underwent a physical examination from January 1, 2021, to December 31, 2021, in Xiamen, Fujian Province, China. Subjects aged ≥ 65 years in the community were informed whether to participate in the physical examination voluntarily. We included 228,664 older adults who completed the survey. Subjects with hepatic steatosis via abdominal ultrasonography (HD-15; Philips, Netherlands); presence of ≥ 1 metabolic risk factor: 1) Body mass index (BMI) ≥ 25 kg/m2 or waist circumference (WC) > 94 cm (males) and > 80 cm (females); 2) Fasting blood glucose ≥ 5.6 mmol/L or hemoglobin A1c ≥ 5.7% or a previous diagnosis of diabetes or treatment for diabetes; 3) Blood pressure ≥ 130/85 mmHg or treatment for hypertension; 4) Triglyceride (TG) ≥ 1.7 mmol/L or lipid-lowering therapy; 5) High-density lipoprotein cholesterol (HDL) ≤ 1 mmol/L (males) or ≤ 1.3 mmol/L (females) or lipid-lowering therapy; and without treatment for hepatitis B or without significant alcohol consumption (alcohol consumption of ≥ 30 g ethanol/day in men and ≥ 20 g ethanol/day in women20) were diagnosed as MASLD1.
To investigate the association between APs and liver fibrosis in MASLD, the following exclusion criteria were applied: (1) subjects without ultrasound examination (n = 94,276); (2) subjects with liver cirrhosis detected by ultrasound (n = 62); (3) subjects without valid APs exposure values due to incomplete address information (n = 46,005); (4) non-MASLD (n = 64,434). Finally, 23,887 MASLD subjects were enrolled.
We performed this study according to the tenets of the Declaration of Helsinki. This work was part of the routine procedure of Health Management Services for the elderly in the national basic public health services in China21. Written informed consent was obtained from all participants. The study protocol was approved by the Ethics Committee of Xiamen Center for Disease Control and Prevention [ID: XJK/LLSC (2024) 016]. All the data analyzed was anonymized.
Assessment of covariates
In all subjects, a standardized questionnaire survey was conducted to collect sociodemographic characteristics, lifestyle factors (smoking, alcohol intake, and physical activity), and medication history; medical examinations included height, weight, WC, blood pressure, blood biochemistry, hemoglobin A1c, and abdominal ultrasonography. The indicators of blood biochemistry, including fasting blood glucose, TG, total cholesterol (TC), low-density lipoprotein cholesterol (LDL), HDL, alanine transaminase (ALT), aspartate transaminase (AST), and platelet count of each subject, were evaluated using an auto-analyzer (AU5800; Beckman, California, USA).
Subjects who smoked at least 1 cigarette per day with continuous smoking for more than 6 months were defined as current smokers. Daily alcohol consumption was calculated as the volume of alcohol consumed per day (g) × alcohol concentration (%) × 0.822. Subjects with alcohol consumption > 0 g ethanol/day were defined as drinkers. The self-reported physical activity frequency was classified as inactive, occasional, often (> 1 time/week), and daily. BMI was calculated as weight divided by the square of height (kg/m2). WC was measured at the midpoint between the lower border of the rib cage and iliac crest, and central obesity was defined as a waist circumference ≥ 90 cm in men and ≥ 85 cm in women23. Hypertension was defined as systolic blood pressure of ≥ 140 mm Hg and/or diastolic blood pressure of ≥ 90 mm Hg and/or a history of antihypertensive medication and/or a self-reported history of hypertension. Diabetes was defined as a fasting blood glucose concentration ≥ 7.0 mmol/L, and/or a history of antidiabetic medication, and/or a self-reported history of diabetes.
Exposure assessment
The exposure data of O3, PM2.5, and PM10 (spatial resolution 1 km × 1 km) were obtained from China high air pollutants (CHAP) dataset. The concentration data of sulfur dioxide (SO2), carbon monoxide (CO), and NO2 (spatial resolution 15 km × 15 km) were sourced from a high-resolution air quality reanalysis dataset over China (CAQRA) dataset. Different sources of pollutants, such as traffic, industrial emissions, and domestic heating, were considered in these databases. Participants’ residential addresses were converted into geographic coordinates using the Baidu Map API (https://lbsyun.baidu.com/) and subsequently integrated into the CHAP and CAQRA datasets using the k-nearest neighbor algorithm for spatial analysis. Daily exposure levels of PM2.5, PM10, O3, SO2, CO, and NO2 were matched to each participant based on their geographic location. The average exposures over 1-year and 2-year periods were computed, with these periods being defined as exactly 1-year and 2-year preceding the date of the medical examinations, respectively. Two-year average levels of AP were considered as the exposure variable in the main analyses.
Diagnosis of liver fibrosis
Liver fibrosis in patients with MASLD was evaluated by metabolic dysfunction-associated fibrosis 5 (MAF-5)24 and Fibrosis-4 index (FIB-4)25. The MAF-5 score is a newly validated, age-independent referral tool to identify individuals at high risk of liver fibrosis with a relevant improvement of fibrosis case-finding in older populations. MAF-5 was calculated as follows: -11.3674 + WC (cm) × 0.0282 – BMI (kg/m2) × 0.1761 + WC (cm) × BMI (kg/m2) × 0.0019 + 2.0762 for diabetes (yes = 1, no = 0) + ln [AST (U/L)] × 2.9207 – platelet count (109/L) × 0.0059. A MAF-5 score of < 0, 0–1, and ≥ 1 was considered a strong predictor of low, medium, and high liver fibrosis risk, respectively. FIB-4 was calculated as follows: age (years) × AST (U/L)/ [platelet count (109/L) × ALT (U/L)1/2]. A low FIB-4 score of < 2 was considered a strong predictor of the absence of liver fibrosis in the elderly.
Statistical analyses
We described and compared the characteristics of MASLD subjects with low to medium liver fibrosis risk (MAF-5 < 1) and high liver fibrosis risk (MAF-5 ≥ 1). If following normal distribution, continuous variables were presented as mean ± standard deviation and compared by t-test. If not, they were presented as medians (interquartile range) and compared by the Mann–Whitney U test. Categorical variables were presented as numbers (%) and compared by the Pearson chi-square test or the Fisher exact probability test. We used restricted cubic splines with three knots to explore the dose–response relationship between APs and liver fibrosis in MASLD. Then, we divided all the participants into quartiles according to AP concentration. Using the lowest quartile group as the reference, we conducted unadjusted-, age-, sex-adjusted, and multivariate-adjusted (further adjusted for BMI, ALT, hypertension, diabetes, central obesity, physical activity, smoking, and alcohol drinking status) logistic regression models to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for liver fibrosis risk in the higher quartile groups.
To better understand the overall effect of the mixture of APs, we assessed the combined effect of the AP mixture on liver fibrosis using a quantile g-computation model based on the R qgcomp package26. This model was used to estimate the change in liver fibrosis risk for a synchronous one-quartile increase for all APs. The plot depicts APs and liver fibrosis prediction at the joint exposure levels via quantile g-computation and bootstrap variance with a bootstrap of up to 1000. The model was adjusted for age, sex, BMI, ALT, hypertension, diabetes, central obesity, physical activity, smoking, and alcohol drinking status.
We conducted a stratified analysis to test the potential interaction between sex, age, BMI, central obesity, diabetes, hypertension, alcohol consumption, smoking status, physical activity, and APs in liver fibrosis development in MASLD. To validate the robustness of the results, five sensitivity analyses were performed: (1) applying two-pollutant models to assess the confounding effects of co-occurring pollutants; (2) using additional noninvasive indices, i.e., FIB-4, as diagnosis criteria of liver fibrosis; (3) using a 1-year average of APs to assess long-term exposure; (4) excluding participants with medium risk of liver fibrosis (MAF-5 of 0–1); (5) calculating E-value to assess the robustness of our findings to unmeasured confounding27. The E-value quantifies the minimum strength of association that an unmeasured confounder would need to have with both the exposure and outcome to explain away the observed association.
All data were analyzed using SAS software, version 9.4 (SAS Institute, Cary, NC), and R software, version 4.4.0, with two-sided tests and P < 0.05 as statistically significant.
Results
Characteristics of the study participants
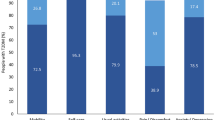
Characteristics of the study subjects according to the presence or absence of liver fibrosis, which was diagnosed by MAF-5, are shown in Table 1. The prevalence of high-risk liver fibrosis (MAF-5 ≥ 1) in the overall MASLD population was 15.5% (3,713/23,887). Patients with higher MAF-5 were significantly older, with a higher proportion of males, current smokers, and drinkers. They had a higher level of BMI, ALT, AST, TC, TG, systolic blood pressure, diastolic blood pressure, fasting blood glucose, fibrosis-4 score, and a higher prevalence of diabetes, central obesity, and hypertension (P < 0.001). The O3, CO, PM2.5, and PM10 concentrations were significantly increased in subjects with MAF-5 ≥ 1 as compared with those in subjects with MAF-5 < 1 (P < 0.05) (Fig. 1).
Association of single AP with liver fibrosis in MASLD
We utilized restricted cubic splines to assess the potential dose–response relationship of APs to liver fibrosis, as depicted in Fig. S1. We found that the liver fibrosis prevalence risk was nonlinear with the exposure–response curves for CO (Poverall = 0.01, Pnonlinear = 0.006), and linear with O3 (Poverall = 0.005, Pnonlinear = 0.14), PM2.5 (Poverall = 0.006, Pnonlinear = 0.10), PM10 (Poverall < 0.001, Pnonlinear = 0.22). Regarding NO2 and SO2, no significant association was observed (Poverall > 0.05, Pnonlinear > 0.05).
The associations between APs and liver fibrosis are shown in Table 2. After adjusting the covariates (model 3), participants exposed to the second, third, and highest quartiles of CO, SO2, and PM10 had elevated odds of liver fibrosis, compared with those exposed to the lowest quartile of AP levels. Similarly, individuals exposed to the third and highest quartiles of PM2.5 and O3 also demonstrated increased odds of liver fibrosis. When the association between each AP and liver fibrosis prevalence was considered linear, the OR per standard deviation increase in exposure to O3, PM2.5, and PM10 was 1.13 (95% CI 1.07–1.19), 1.09 (95% CI 1.04–1.15), and 1.18 (95% CI 1.12–1.24), respectively.
Association of AP mixture exposure with liver fibrosis in MASLD
The results of the quantile g-computation model showed that a quartile increase in the AP mixture was significantly associated with increased odds of liver fibrosis [OR = 1.15, 95%CI (1.07–1.24)]. As shown in Fig. 2, the positive effect of the AP mixture was attributed to PM10, accounting for 100.00% of all. On the other hand, the negative effect was driven by PM2.5 (66.34%) and SO2 (12.84%).
Combined association and weights of air pollutant mixture with liver fibrosis by quantile g-computation model. Model was adjusted for age, sex, body mass index, alanine transaminase, hypertension, diabetes, central obesity, physical activity, smoking, and alcohol drinking status. OR odds ratio, 95%CI 95% confidence interval, NO2 nitrogen dioxide, O3 ozone, SO2 sulfur dioxide, CO carbon monoxide, PM particulate matter.
Stratified analyses
Table S1-1–S1-6 show the stratified analysis of relationships of O3, CO, NO2, SO2, PM2.5, and PM10 with liver fibrosis by different characteristics. Sex, BMI, diabetes, and physical activity were observed to modify the association between specific AP exposure and liver fibrosis. Specifically, we observed a significantly stronger liver fibrosis risk in participants who exercised inactively and were exposed to CO (Pfor interaction = 0.041), SO2 (Pfor interaction < 0.001), and PM10 (Pfor interaction = 0.052) compared to participants who exercised actively. Furthermore, the relationship between O3 and liver fibrosis was observed to be modified by BMI (Pfor interaction = 0.014) and diabetes (Pfor interaction = 0.002). Males had a significantly higher fibrosis risk in the highest quartile of NO2 than the reference quartile; the adjusted OR (95% CI) was 1.33 (1.02, 1.73).
Sensitivity analyses
In the two-pollutant models (Table S2), the positive relationships between O3, PM2.5, and PM10 and liver fibrosis remained robust after adjusting for other APs, except for PM10. The effects estimated for O3 were not significant after adjustment for PM10, and the associations between PM2.5, CO, NO2, and SO2 and liver fibrosis shifted to negative after adjustment for PM10.
When using FIB-4 for the diagnosis of liver fibrosis to explore the association between AP and fibrosis, we observed consistent associations. The conclusion also remained unchanged after using a 1-year average AP exposure or excluding subjects with medium risk of liver fibrosis (Table S3).
The analysis yielded an OR of 1.83 (95% CI: 1.56–2.13) for the association between PM10 (the highest vs. lowest exposure group) and liver fibrosis. The E-value for this point estimate was calculated as 3.06, and for the lower confidence bound, it was 2.49. This suggested that unmeasured confounders would need to have substantial associations to nullify the observed effect.
Discussion
In this large community-based cross-sectional study in Xiamen City, China, we observed a positive association between higher levels of O3, CO, SO2, PM2.5, and PM10 and liver fibrosis in MASLD subjects aged ≥ 65 years, after adjusting for the potential confounders. Co-exposure to the AP mixture also increased liver fibrosis risk, and the positive effect was mainly driven by PM10. Additionally, factors such as sex, BMI, diabetes, and physical activity might serve as potential modifiers of these associations.
In our study, we observed that higher PM2.5 and PM10 levels had a significant risk effect against liver fibrosis in MASLD, aligning with previous research findings17,19. Additionally, our finding of the positive relationship between O3 concentration and liver fibrosis is similar to those of prior studies that focused on MASLD as the outcome28. However, a cross-sectional study conducted in Taiwan and Hong Kong reported a decrease in the prevalence of advanced fibrosis with increasing O3 concentration18. A plausible explanation for this discrepancy could be the lower O3 concentration in those study regions (mean [SD]: 52.0 ± 9.9 μg/m3) compared to our study (mean [SD]: 104.5 ± 2.6 μg/m3). Therefore, further studies on the health effects of a broader range of O3 concentrations on liver fibrosis are warranted. Positive relationships of SO2 and CO with liver fibrosis in MASLD were also found in our study. Currently, we have not identified any studies exploring the effects of these two APs on liver fibrosis in MASLD, but existing evidence does suggest that these exposures are strongly associated with MASLD28. While the health outcomes examined in that study differ from ours, it does provide consistent support for the impact of SO2 and CO on liver fibrosis. For NO2, we didn’t find significant relationships between NO2 and liver fibrosis, which was in contrast with findings that NO2 was linked to increased risks of incident cirrhosis using data from the UK Biobank cohort19 and the Rome administrative cohort29. The inconsistency may be attributable to differences in the study regions, study designs, etc. Justification still needs to be through more studies.
The association between APs and liver fibrosis in the elderly with MASLD may be related to oxidative stress, inflammation, and metabolic disruption30. Studies31 have demonstrated that APs exposure generates elevated levels of free radicals and oxidants, thereby inducing oxidative stress. Mechanistic research by Qiu et al.32 revealed that APs activate the reactive oxygen species-mediated PINK1/Parkin signaling pathway in hepatic stellate cells, leading to mitochondrial damage and mitophagy, which subsequently promotes liver fibrosis. Animal experiments further indicate that APs disrupt normal lipid metabolism33 and impair liver glucose metabolism16, evidenced by intrahepatic lipid accumulation, as well as interference with fatty acid oxidation and transport processes. Additionally, APs exposure induces inflammatory pathways15,34 and triggers the development of insulin or vascular endothelial growth factor resistance in the blood vessels, leading to increased insulin resistance and lipid synthesis, causing liver injury.
Recently, the importance of evaluating the health effects of multi-pollutant exposures has garnered increasing attention, as the various APs are highly correlated and often originate from the same emission sources. In the two-pollutant models, we observed that single-pollutant OR estimates for PM2.5 and O3 were initially positive for the outcome but shifted to negative and non-significant after adjusting for PM10. This shift may be due to the strong correlations observed between PM2.5 (Spearman’s r = 0.868), O3 (Spearman’s r = 0.685), and PM10 in our study and the stronger independent effect of PM10 on liver fibrosis compared to other pollutants. To further explore the association between joint exposure to various APs and MASLD-related liver fibrosis, the quantile g-computation method was used. We found the association of the AP mixture with liver fibrosis was positively significant, and the major contributing pollutant was PM10. Few studies have assessed the relationship between AP mixture and liver disease. For instance, Ye et al.35 found a positive correlation between AP score and severe liver disease. Another study indicated that exposure to higher concentrations of AP score was linked to increased risks of incident MASLD and cirrhosis19. Though the health outcome of these studies did not specifically focus on MASLD-related severe liver disease and only included PM2.5, PM2.5–10, PM10, NO2, and NOx in their study, the studies also supported our results that the importance of maintaining a low AP level, particularly PM10 to prevent the risk of MASLD-related liver fibrosis.
The odds of liver fibrosis were higher in subjects who exercise inactively compared to those who exercise actively when exposed to the same elevated CO, SO2, and PM10 levels. This finding aligns with previous research by Ji et al.28, which reported that the associations of APs with MASLD were stronger in participants who did not engage in leisure-time exercise than those who did. For other outcomes, such as death, previous studies have also found that36,37,38 the benefits of exercise outweigh the risks from pollutant inhalation during air pollution exposure. One possible explanation was that although physical activity increased the breathing rate and the amount of inhaled air, the proportion of additional pollutants inhaled in the total amount is relatively small and thus did not significantly affect the overall health benefits. Another possibility was that the long-term health benefits brought about by physical activity may offset the adverse effects of short-term exposure to a highly polluted environment36. Furthermore, our findings indicated that people with a high BMI and without diabetes may suffer greater effects of O3 on liver fibrosis risk. Obesity is well-recognized as a major risk factor in the development of liver fibrosis39, and it is plausible that obesity and O3 exposure may have an additive effect on the risk of liver fibrosis. Notably, the ORs were greater for people without diabetes, which was in accordance with findings from other studies40. The underlying reason for this observation requires further clarification. Additionally, we observed significant associations between NO2 exposure and liver fibrosis in males. This sex-specific association may be attributed to differences in physiological and behavioral factors between females and males, which can result in differential susceptibility to APs41.
Our study has several limitations. First, although MAF-5 is a valuable non-invasive tool for liver fibrosis screening, its accuracy is relatively lower compared to liver biopsy. However, a study conducted in Shenzhen, China, reported that in the MASLD population, MAF-5 demonstrated high diagnostic accuracy for liver fibrosis42. Further verification of the diagnostic accuracy of MAF-5 remains warranted. Second, the concentrations of APs were evaluated based on fixed addresses, without considering the patterns of participants’ daily activities. Although the variation in AP concentrations within a certain area is generally small, it still may lead to random exposure misclassification, which might attenuate the estimated associations. More advanced technology and methodologies are needed to account for individual mobility and exposure variability to improve exposure assessment. Third, the cross-sectional design of our study limits the ability to establish causal relationships between exposure to APs and liver fibrosis. Longitudinal studies are required to further investigate these associations. Fourth, other potential confounding factors, such as genetic factors, which may play a potential role in susceptibility to liver fibrosis, were not measured; however, our E-value of 3.06 suggests that the observed association was robust to unmeasured confounding, as it requires a confounder with substantial strength to explain it away. Given that common confounders43 typically had associations below this threshold, our findings may be reasonably reliable. Future research should aim to measure potential confounders directly to reduce uncertainty. Fifth, we did not distinguish between indoor and outdoor habitual exercise. However, previous studies have reported that outdoor exercises predominate among older adults36,37. Sixth, we did not distinguish between pollutant sources. Future studies should further subdivide pollution sources to better explain the impact of AP exposure on liver fibrosis. Although the current comprehensive exposure may integrate multiple sources, our results can still provide an overall risk estimate, laying the foundation for public health interventions. Finally, the participants in this study were recruited from southeastern China, which may limit the generalizability of our findings to other ethnic groups and geographical regions. Therefore, additional studies in diverse populations are necessary to confirm the associations observed in our study and to enhance the understanding of the impact of APs on liver fibrosis in MASLD subjects.
In conclusion, this study revealed significantly positive associations between exposure to O3, CO, SO2, PM2.5, and PM10 and liver fibrosis in elderly participants with MASLD. Meanwhile, mixed exposure analysis provided additional evidence that exposure to AP mixtures was significantly associated with liver fibrosis and was primarily driven by PM10. Additionally, our findings indicate that no exercise might amplify these adverse effects. These results highlight the importance of reducing exposure to APs in managing and potentially mitigating the progression of liver fibrosis in MASLD patients. Prospective cohort and mechanism research is needed to confirm the current findings and the role of APs in the pathogenesis of liver fibrosis in elderly MASLD patients.
Data availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- AP:
-
Air pollutant
- MASLD:
-
Metabolic dysfunction-associated steatotic liver disease
- NO2 :
-
Nitrogen dioxide
- O3 :
-
Ozone
- SO2 :
-
Sulfur dioxide
- CO:
-
Carbon monoxide
- PM:
-
Particulate matter
- SLD:
-
Steatotic liver disease
- OR:
-
Odds ratio
- 95%CI:
-
95% confidence interval
- FIB-4:
-
Fibrosis-4 score
- MAF-5:
-
Metabolic dysfunction-associated fibrosis 5
- BMI:
-
Body mass index
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- FBG:
-
Fasting blood glucose
References
Rinella, M. E., Lazarus, J. V., Ratziu, V., Francque, S. M., Sanyal, A. J., Kanwal, F. et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. (2023).
Miao, L., Targher, G., Byrne, C. D., Cao, Y. Y. & Zheng, M. H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 35(8), 697–707 (2024).
Parola, M. & Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Aspects Med. 65, 37–55 (2019).
Rinella, M. E. Nonalcoholic fatty liver disease: A systematic review. JAMA 313(22), 2263–2273 (2015).
Chen, T. P., Lai, M., Lin, W. Y., Huang, K. C. & Yang, K. C. Metabolic profiles and fibrosis of nonalcoholic fatty liver disease in the elderly: A community-based study. J. Gastroenterol. Hepatol. 35(9), 1636–1643 (2020).
Lan, Y., Guo, Z., Dai, J., Chen, K. & Chen, Y. Association between remnant cholesterol and metabolic dysfunction-associated steatotic liver disease in the elderly. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. (2024).
China Development Research Foundation. China Development Report 2020: Trends and policies for China’s aging population. https://www.cdrf.org.cn/jjh/pdf/2020zhongguofazhanbaogao1226.pdf.
Alqahtani, S. A. & Schattenberg, J. M. NAFLD in the elderly. Clin. Interv. Aging 16, 1633–1649 (2021).
Ye, L. et al. Associations of residential greenness and ambient air pollution with overweight and obesity in older adults. Obesity (Silver Spring, Md). 31(10), 2627–2637 (2023).
Li, A. et al. Investigating potential associations between O3 exposure and lipid profiles: A longitudinal study of older adults in Beijing. Environ. Int. 133(Pt A), 105135 (2019).
Wang, J. et al. Associations between individual exposure to fine particulate matter elemental constituent mixtures and blood lipid profiles: A panel study in Chinese people aged 60–69 years. Environ. Sci. Technol. 56(18), 13160–13168 (2022).
Li, A. et al. The effect of ambient ozone on glucose-homoeostasis: A prospective study of non-diabetic older adults in Beijing. Sci. Total Environ. 761, 143308 (2021).
Zhang, C. et al. PM(2.5) induced liver lipid metabolic disorders in C57BL/6J mice. Front. Endocrinol. 14, 1212291 (2023).
Xu, Z. et al. Real ambient particulate matter-induced lipid metabolism disorder: Roles of peroxisome proliferators-activated receptor alpha. Ecotoxicol. Environ. Saf. 231, 113173 (2022).
Zheng, Z. et al. Exposure to fine airborne particulate matters induces hepatic fibrosis in murine models. J. Hepatol. 63(6), 1397–1404 (2015).
Zheng, Z. et al. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J. Hepatol. 58(1), 148–154 (2013).
Xing, Y. et al. Associations between exposure to ambient particulate matter and advanced liver fibrosis in Chinese MAFLD patients. J. Hazard. Mater. 460, 132501 (2023).
Bo, Y. et al. Chronic exposure to ambient air pollution and the risk of non-alcoholic fatty liver disease: A cross-sectional study in Taiwan and Hong Kong. Ecotoxicol. Environ. Saf. 275, 116245 (2024).
Li, F. R. et al. Long-term exposure to air pollution and incident non-alcoholic fatty liver disease and cirrhosis: A cohort study. Liver Int. Off. J Int. Assoc. Study Liver 43(2), 299–307 (2023).
EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64(6), 1388–1402.
China NhafpcotPsRo. The national essential public health service specification (the 3rd version) (2017).
Gao, X. & Fan, J. G. Diagnosis and management of non-alcoholic fatty liver disease and related metabolic disorders: Consensus statement from the Study Group of Liver and Metabolism, Chinese Society of Endocrinology. J. Diabetes 5(4), 406–415 (2013).
Chinese Diabetes Society. Guideline for prevention and control of type 2 diabetes in China 2017. Chin. J. Diabetes Mellit. 10(1), 4–67 (2018).
van Kleef, L. A. et al. Metabolic dysfunction-associated fibrosis 5 (MAF-5) score predicts liver fibrosis risk and outcome in the general population with metabolic dysfunction. Gastroenterology 167(2), 357–67.e9 (2024).
Vilar-Gomez, E. & Chalasani, N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J. Hepatol. 68(2), 305–315 (2018).
Keil, A. P. et al. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ. Health Perspect. 128(4), 47004 (2020).
Haneuse, S., VanderWeele, T. J. & Arterburn, D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA 321(6), 602–603 (2019).
Ji, W. et al. Exposure to ambient air pollution and metabolic dysfunction-associated fatty liver disease: Findings from over 2.7 million adults in Northwestern China. Ecotoxicol. Environ. Saf. 272, 116109 (2024).
Orioli, R. et al. A cohort study on long-term exposure to air pollution and incidence of liver cirrhosis. Environ. Epidemiol. 4(4), e109 (2020).
He, X. et al. Air pollution exposure and prevalence of non-alcoholic fatty liver disease and related cirrhosis: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 289, 117469 (2025).
Chen, H. et al. Effects of air pollution on human health-mechanistic evidence suggested by in vitro and in vivo modelling. Environ. Res. 212(Pt C), 113378 (2022).
Qiu, Y. N. et al. PM2.5 induces liver fibrosis via triggering ROS-mediated mitophagy. Ecotoxicol. Environ. Saf. 167, 178–187 (2019).
Xu, M. X. et al. Prolonged PM2.5 exposure elevates risk of oxidative stress-driven nonalcoholic fatty liver disease by triggering increase of dyslipidemia. Free Radic. Biol. Med. 130, 542–556 (2019).
Hill, B. G., Rood, B., Ribble, A. & Haberzettl, P. Fine particulate matter (PM(2.5)) inhalation-induced alterations in the plasma lipidome as promoters of vascular inflammation and insulin resistance. Am. J. Physiol. Heart Circ. Physiol. 320(5), H1836-h50 (2021).
Ye, Z. et al. Various ambient air pollutants, residential green spaces, fibrosis 4 scores, genetic susceptibility, and risk of severe liver disease. Ecotoxicol. Environ. Saf. 263, 115246 (2023).
Sun, S. et al. Benefits of physical activity not affected by air pollution: A prospective cohort study. Int. J. Epidemiol. 49(1), 142–152 (2020).
Andersen, Z. J. et al. A study of the combined effects of physical activity and air pollution on mortality in elderly urban residents: The danish diet, cancer, and health cohort. Environ. Health Perspect. 123(6), 557–563 (2015).
Guo, C. et al. Effects of air pollution and habitual exercise on the risk of death: a longitudinal cohort study. CMAJ Can. Med. Assoc. J. 193(32), E1240-e9 (2021).
Chiang, D. J., Pritchard, M. T. & Nagy, L. E. Obesity, diabetes mellitus, and liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 300(5), G697-702 (2011).
Guo, B. et al. Exposure to air pollution is associated with an increased risk of metabolic dysfunction-associated fatty liver disease. J. Hepatol. 76(3), 518–525 (2022).
Wang, X. et al. Constituents of fine particulate matter and asthma in 6 low- and middle-income countries. J. Allergy Clin. Immunol. 150(1), 214–22.e5 (2022).
Li, M. et al. The fibrosis investigating navigator in diabetes (FIND): A tool to predict liver fibrosis risk in subjects with diabetes. Diabetes Obes. Metab. 27(3), 1184–1197 (2025).
Zhao, J. et al. Biological aging accelerates hepatic fibrosis: Insights from the NHANES 2017–2020 and genome-wide association study analysis. Ann. Hepatol. 30(2), 101579 (2024).
Acknowledgements
The authors are grateful to all participants in this study for their support.
Funding
This study was supported by the Xiamen health and wellness high-quality development science and technology plan project (2024GZL-GG75) and the Xiamen medical and health guiding project (3502Z20244ZD1364).
Author information
Authors and Affiliations
Contributions
YLC designed the study. HXZ, AHW, and JKH collected the data. HXZ and AHW processed the data. YQL performed statistical analysis. ZNG interpreted the data. YQL drafted the manuscript. ZNG and YLC revised the manuscript critically. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lan, Y., Zhang, H., Wu, A. et al. Long-term exposure to air pollution and liver fibrosis in the elderly with MASLD. Sci Rep 15, 29969 (2025). https://doi.org/10.1038/s41598-025-15114-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15114-5