Abstract
Colorectal cancer is the third most commonly diagnosed cancer in the world that affects both men and women. Approximately 40% of colorectal cancer patients exhibit cognitive impairment in executive functions including verbal learning, verbal memory, and information processing that is independent of chemotherapy. However, little information is currently available regarding the neural mechanisms underlying colorectal cancer-related cognitive decline (CRCD). In this study, we utilized highly sensitive 7 Tesla magnetic resonance imaging methods combined with standardized cognitive assessments to investigate the changes in brain local functional connectivity in early-stage colorectal cancer survivors compared to healthy controls. We observed that early-stage colorectal cancer survivors exhibited increased regional homogeneity (ReHo) in the left hippocampus, parahippocampal gyrus, and inferior temporal gyrus, along with decreased ReHo in the left inferior frontal gyrus, which were associated with reduced verbal memory performance compared to healthy controls. Furthermore, survivors exhibited significantly weaker inter-regional functional connectivity, suggesting a potential disruption in coordination among regions critical for verbal memory. Collectively, these findings indicate a maladaptive mechanism in the medial temporal lobes that is associated with declines in verbal memory processes among colorectal cancer survivors. ReHo analysis was found to be a valuable tool for characterizing the neurophysiological basis of colorectal CRCD and presents the medial temporal lobe as a promising target for therapeutic interventions.
Similar content being viewed by others
Introduction
Colorectal cancer ranks as the third most commonly diagnosed cancer worldwide and the second leading cause of cancer-related deaths1. In the United States, approximately 153,000 new colorectal cancer cases were diagnosed in 2023 with over one-third of cases being localized at diagnosis (stage I and II)2. By 2040, projections estimate global cases could reach 3.2 million per year due to progressively aging populations and lifestyle factors such as diet, smoking, alcohol, and others1. Shortly after diagnosis (but prior to receiving adjuvant therapy), over 40% of colorectal cancer patients exhibit objective cognitive impairment in areas including working memory, verbal learning, verbal memory, and information processing as assessed by a battery of neuropsychological tests3. These impairments have no significant added effects from adjuvant chemotherapy and can persist for at least two years while negatively impacting the survivors’ quality-of-life4. Previous studies have suggested that the colorectal cancer itself may be the primary contributor to cognitive decline5,6. However, little information exists for understanding the neural mechanisms of colorectal cancer-related cognitive decline (CRCD), which hinders the development of treatment for colorectal CRCD and has left the cognitive and psychological needs of colorectal cancer patients and survivors largely unmet.
Despite exhibiting significant objective cognitive impairment as assessed by neuropsychological testing, colorectal cancer patients often subjectively self-report as cognitively normal when evaluated by standardized questionnaires3,4,5,6. This is counterintuitive and it makes their cognitive decline less likely to be detected by themselves or clinicians, and can also accrue an invaluable impact on productivity and quality-of-life that is often underestimated among cancer patients and survivors7,8. Evidence-based therapies that have proven effective in other neurological and psychiatric conditions are inaccessible due to the lack of clearly defined biological targets among colorectal cancer survivors. For these reasons, a part of colorectal cancer survivorship care needs to focus on investigating the neural basis of colorectal CRCD with the goal of identifying potential biological targets for the development or adaptation of pharmacological or neuro-cognitive interventions for mitigating the adverse effects of CRCD.
Magnetic resonance imaging (MRI) is one of the most widely used non-invasive imaging tools for studying brain structure and function. Within the realm of functional MRI (fMRI), resting-state regional homogeneity (ReHo) is a neuroimaging marker used to measure the local functional connectivity (FC) and synchronization of fMRI signals within specific regions of the brain without the need of any task-based requirements from the subjects9. Because it is a data-driven whole-brain approach, ReHo does not require a priori assumptions regarding the brain regions being investigated. For each imaging voxel of the brain, ReHo can be utilized to quantify the similarities between it and its nearest neighboring voxels over time, thus characterizing the degree of FC within that local area. High ReHo values suggest strong local synchronization, indicating that voxels within a region are consistently active or inactive together, while low ReHo values suggest desynchronization10. A number of brain regions are associated with specific aspects of cognition and are known to have distinct FC properties within and amongst themselves. Alterations in ReHo and their correlations with cognition have been reported in various neurological and psychiatric disorders, including Alzheimer’s disease, mild cognitive impairment, depression, and attention-deficit/hyperactivity disorder, highlighting its potential as a biomarker for understanding these conditions11,12,13. In this study, we compared the cognitive performance and ReHo between early-stage colorectal cancer survivors and age/sex/education matched healthy controls on a whole-brain level using state-of-the-art 7 T MRI. By correlating their MRI results with cognitive assessment scores, we were able to identify brain regions with altered local FC associated with colorectal CRCD. Furthermore, we conducted an inter-regional FC analysis to determine if the regional hyperconnectivity within the medial temporal lobes represents a potential compensatory or maladaptive mechanism in colorectal cancer survivors that may compromise cognitive functioning.
Methods
The study was approved by the Carle Foundation Hospital Internal Review Board (IRB) #22CCC3706. All research was performed in accordance with relevant guidelines and regulations. All research was performed in accordance with the Declaration of Helsinki. All participants were provided written informed consent prior to enrollment in this study.
Participants
Thirty-seven total subjects were recruited including 19 colorectal cancer survivors (stage I or II, age 60.6 ± 8.1 years) who had surgery without neo-adjuvant or adjuvant therapy within twelve months, and 18 age/sex/education matched healthy controls (age 57.6 ± 9.0 years). Exclusion criteria included history of cancer, history of any neurologic or psychiatric condition, brain injury, alcoholism, substance abuse, and any condition precluding the use of 7 T MRI. Detailed inclusion and exclusion criteria are listed in Table 1.
Cognitive assessment
All participants were evaluated using objective neuropsychological tests and subjective self-reported questionnaires as described in Table 2. Cognitive assessments and MRI scans were completed on the same day. The primary endpoint for subjective cognitive assessments is perceived cognitive impairment as measured by the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) subscale14.
MRI acquisition
Each participant underwent an MRI scan on a Siemens MAGNETOM Terra 7 T scanner (Siemens Healthineers, Erlangen, Germany) with an 8Tx/32Rx head coil. MRI acquisition protocol consisted of (1) a T1-weighted MP2RAGE (Magnetization Prepared 2 Rapid Gradient Echoes) sequence with the following parameters: TR = 4530 ms, TE = 2.32 ms, TI 1 = 750 ms, TI 2 = 2950 ms, flip angle 1 = 4°, flip angle 2 = 5°, field of view (FOV) = 224 × 224 mm2, matrix size = 300 × 300, slice thickness = 0.75 mm, and acquisition time = 9 min 28 s; (2) a T2*-weighted resting state BOLD (blood-oxygenation level dependent) fMRI sequence with the following parameters: TR = 1180 ms, TE = 25 ms, flip angle = 40°, FOV = 240 × 240 mm2, matrix size = 150 × 150, slice thickness = 1.60 mm, and acquisition time = 8 min 22 s; and (3) two single-shot spin echo B1 mapping sequences (one acquired in the anterior-to-posterior (AP) direction and the other in the posterior-to-anterior (PA) direction) were utilized to correct for B1 field inhomogeneities in our resting state fMRI acquisitions with the following parameters: TR = 8780 ms, TE = 56 ms, flip angle = 90°, refocus flip angle = 180°, FOV = 240 × 240 mm2matrix size = 150 × 150, slice thickness = 1.60 mm, and acquisition time = 44 s.
MRI processing
MRI preprocessing was accomplished using SPM12 (statistical parametric mapping software; https://www.fil.ion.ucl.ac.uk/spm/software/spm12/), FSL (FMRIB Software Library), DPABI V8.2 (Data Processing & Analysis for Brain Imaging), and MATLAB (version R2023a; The MathWorks Inc., Natick, MA, USA). T1 MP2RAGE anatomical images were denoised, bias field corrected, and segmented into white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF) using SPM12’s new segment algorithm24,25. Standard preprocessing of resting state fMRI signals included the following steps26: (1) Discard the first five volumes to ensure steady-state longitudinal magnetization; (2) Distortion correction using FSL’s topup and B1 field maps; (3) Motion correction using DPABI’s 24-parameter model including the six motion parameters from rigid body translations and rotations derived from realignment as well as their temporal derivatives. Additionally, head motion scrubbing was applied, with a Jenkinson framewise displacement (FD) threshold of 0.2 mm27. Time points exceeding this threshold, along with one preceding and two succeeding time points, were regressed out from the signal via nuisance regression. Two participants (one survivor and one healthy control) with maximum translation > 3 mm or maximum rotation > 3o were excluded from further analysis; (4) Removal of nuisance signals including mean WM and CSF signals; (5) Temporal filtering (0.01–0.1 Hz); (6) Spatial normalization to the Montreal Neurological Institute (MNI) template MNI152 at a resolution of 3 × 3 × 3 mm3 using SPM12’s DARTEL algorithm.
Voxel-wise ReHo analysis
DPABI was utilized to analyze 3D ReHo using a 27-voxel neighborhood approach in which the resting state BOLD time series for each voxel was compared to the time series of its 26 neighboring voxels in 3D space. Kendall’s coefficient of concordance (KCC) was calculated to quantify the degree of rank correlation among these 27 time series which reflects the level of synchrony in their low-frequency fluctuations. This KCC value was then assigned as the ReHo value for the central voxel and represents the local functional homogeneity within that 27-voxel region. This process was repeated for every voxel in the brain. Spatial smoothing was then applied using a 6 mm full width at half maximum (FWHM) Gaussian kernel to reduce subject variability and to facilitate group-level statistical analysis. Non-parametric statistical analyses were performed using FSL’s PALM (Permutation Analysis of Linear Models) package. For group comparisons (e.g., survivors vs. controls), two-sample independent t-tests were performed. 5000 permutations were applied to generate the null distribution for each statistical map. Given our cross-sectional design with independent groups, no explicit exchangeability blocks were defined. Analysis was constrained to GM voxels by utilizing an AAL3 atlas mask28. Age, sex, years of education, BDI score, and STAI score were included as co-variates. Significance levels were controlled for multiple comparisons using family-wise error (FWE) correction based on threshold-free cluster enhancement (TFCE), with a corrected significance threshold of p< 0.0529.
Correlation analysis of ReHo and cognitive assessment
To evaluate the relationship between ReHo and cognitive performance in colorectal cancer survivors and healthy controls, Spearman’s partial correlation analysis was utilized with age, sex, years of education, BDI score, and STAI score being included as co-variates. To reduce the number of cognitive assessments included in this analysis, we selected only the tests that showed significant differences between the groups. The statistical threshold was set to a corrected p-value < 0.05 based on |Z| > 3.05 and cluster size > 1,350 mm3 (within the AAL3 atlas GM mask; search volume: 1,483,326 mm328,30.
Inter-regional functional connectivity analysis
To help interpret the ReHo results and determine whether they reflect a compensatory or maladaptive mechanism, we conducted an inter-regional FC analysis within cortical areas involved in verbal memory. Preprocessed fMRI data were spatially smoothed using a 6 mm FWHM Gaussian kernel from which a total of 26 regions-of-interest (ROIs) associated with verbal memory were selected for further analysis. These encompassed areas within the prefrontal cortex, medial temporal lobes, and posterior parietal lobes31,32,33,34 including the following ROIs: the bilateral superior frontal gyrus (SFG), middle frontal gyrus (MFG), the pars triangularis of the inferior frontal gyrus (IFGtri), medial SFG, medial orbital SFG, hippocampus (HIP), parahippocampal gyrus (PHG), fusiform gyrus (FFG), inferior parietal gyrus (IPG), superior parietal gyrus (SPG), precuneus, middle temporal gyrus (MTG), and inferior temporal gyrus (ITG)28. For each ROI, the average time series was calculated across all voxels within the region. FC was then computed as the Pearson’s correlation coefficient between the time series of each pair of ROIs. Individual FC matrices (26 × 26) were generated for each participant. A two-sample t-test was conducted to compare FC differences between survivors and controls while controlling for age, sex, years of education, BDI score, and STAI score. To correct for multiple comparisons, the Network-Based Statistic (NBS) method was utilized35. A primary threshold (p < 0.001) was applied to individual connections to form suprathreshold networks from which permutation testing (N = 1000) was then used to generate a null distribution of network sizes. The significance of observed subnetworks was determined by comparing them to this distribution.
Results
A total of 37 participants were initially recruited for the study, however, two participants (one healthy control and one colorectal cancer survivor) were excluded from analysis due to excessive motion artifacts (maximum translation > 3 mm or maximum rotation > 3o).
Participant demographic and clinical data
Participant information including demographics is summarized in Table 3 from which it can be seen that colorectal cancer survivors did not differ from the healthy controls in terms of age, years of education, depression, and anxiety scores. Cognitive assessment scores are summarized in Table 4 from which it can be seen that early-stage colorectal cancer survivors exhibited lower scores in the Recognition Discrimination Index (RDI) of the HVLT-R compared to healthy controls (p = 0.03) as well as slower information processing speed as assessed by TMT-A (p = 0.02). No statistically significant differences between both groups were found in other tests or questionnaires.
Group differences in ReHo
shows the ReHo differences between colorectal cancer survivors (n = 18) and healthy controls (n = 17). Survivors exhibited significantly increased ReHo in the left HIP, PHG, and ITG, along with significantly decreased ReHo in the left IFGtri and left postcentral gyrus. Detailed information regarding these regions is provided in Table 5 (corrected p < 0.05).
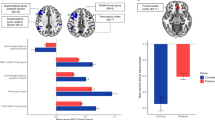
Figure 1 Brain map of Z statistics differences between colorectal cancer survivors and controls (corrected p < 0.05). Survivors showed significantly increased ReHo (orange) in the left hippocampus (HIP), parahippocampal gyrus (PHG), and inferior temporal gyrus (ITG), as well as significantly decreased ReHo (blue) in the left pars triangularis of the inferior frontal gyrus (IFGtri) and left postcentral gyrus. The color bar represents Z statistic values. The corresponding coordinates for regions labeled with numbers are listed in Table 5.
Correlations between ReHo and cognitive scores in survivors
As seen in Fig. 2, the correlation maps of RDI scores and ReHo for survivors (n = 18) revealed significant negative correlations in the left HIP, left PHG, and right FFG, along with significant positive correlations in the left IPG and bilateral IFG. Further details regarding these regions are provided in Table 6 (corrected p < 0.05).
Correlation maps of RDI scores and ReHo for survivors (n = 18). Significant negative correlations were observed in the left hippocampus (HIP), left parahippocampal gyrus (PHG), and right fusiform gyrus (FFG), while significant positive correlations were found in the left inferior parietal gyrus (IPG) and bilateral inferior frontal gyrus (IFG) including the pars triangularis of the IFG (IFGtri), the opercular part of the IFG (IFGoperc), and the IFG pars orbitalis (IFGorb). The color bar represents Z-statistic values. The corresponding coordinates for regions labeled with numbers are listed in Table 6.
Additionally, as seen in Fig. 3, correlation maps of TMT-A scores and ReHo for survivors revealed significant positive correlations in the bilateral postcentral gyrus. Further details regarding these regions are provided in Table 7 (corrected p < 0.05).
Correlations between ReHo and cognitive scores in controls
Figure 4 illustrates the correlation maps of RDI scores and ReHo for controls (n = 17) which showed only one significant positive correlation between ReHo within the right medial orbitofrontal cortex (OFCmed)/rectus gyrus and the RDI (corrected p < 0.05). Details are provided in Table 8 (corrected p < 0.05).
FC group differences within regions associated with verbal memory
Figure 5 highlights the significant differences in inter-regional FC of areas associated with verbal memory between healthy controls and colorectal cancer survivors (corrected p < 0.05). Compared to controls, the survivors exhibited significantly reduced FC between the medial temporal lobes (e.g., left MTG and right ITG) and prefrontal lobes (e.g., left SFG and bilateral medial SFG).36. The full anatomical labels are listed in the Methods section “Inter-Regional Functional Connectivity Analysis”.
(A) Circos plot illustrating significant differences in inter-regional FC among colorectal cancer survivors (n = 18) and healthy controls (n = 17) in regions associated with verbal memory (corrected p < 0.05). Brain region names and their corresponding lobes are annotated as such: FRN (frontal lobe, yellow), TL (temporal lobe, green), and PPC (posterior parietal cortex, purple). The color bar represents t-statistic values. (B) Axial view showing brain regions with significant differences between groups, primarily in the left temporal gyrus and the lateral and medial superior frontal gyrus. (C) Sagittal view of the medial left hemisphere showing brain regions with significant between-group differences including connections between the left middle temporal gyrus (MTG) and left superior frontal gyrus (SFG), as well as the left MTG and left medial SFG. These figures were generated using BrainNet Viewer 1.7 [https://www.nitrc.org/projects/bnv]36. The full anatomical labels are listed in the Methods section “Inter-Regional Functional Connectivity Analysis”
Discussion
The goal of this study was to investigate colorectal cancer-related changes in intrinsic local brain connectivity by analyzing ReHo values from resting state fMRI signals in early-stage colorectal cancer survivors. The results revealed a significant increase in local FC within the medial temporal regions in survivors compared to healthy controls. These neural changes were associated with reduced cognitive performance, particularly in verbal memory. Furthermore, FC analysis showed significantly weaker inter-regional connections among verbal memory-related regions in survivors, which may suggest a potential disruption in brain network coordination between the medial temporal and prefrontal lobes. Taken together, these findings highlight the potential of ReHo as a valuable tool for characterizing the neurophysiological basis of colorectal CRCD while also providing evidence that support the medial temporal lobe as a promising target for therapeutic interventions.
Cognitive performance
The HVLT-R is a neuropsychological assessment tool that evaluates verbal learning and memory15,37. It is commonly used to assess cognitive function in various clinical populations including individuals with brain injuries, neurodegenerative disorders, and cancer-related cognitive impairment38. In this study, early-stage colorectal cancer survivors exhibited significantly lower RDI scores compared to healthy controls (p = 0.03), although no statistically significant differences were observed in total recall, delayed recall, or retention. A lower RDI score may indicate memory retrieval difficulties or poor recognition accuracy. The TMT-A is another widely used neuropsychological test that primarily assesses information processing speed, visual attention, and motor function (hand-eye coordination)17. Here, the survivors exhibited significantly slower performance as indicated by increased completion time and decreased T scores compared to controls (p = 0.02). However, no significant differences were found in TMT-B performance, which prioritizes executive functioning compared to TMT-A. It is worth noting that the reduced cognitive performance amongst survivors does not meet the criteria of being cognitively impaired by the ICCTF recommendation of two or more test scores at or below -1.5 standard-deviations from the normative mean or a single test score at or below -2.0 standard-deviations from the mean38. For our study, cognitive impairment would be defined as a T score < 35 on two recall trials or < 30 on one recall trial for the HVLT-R, and a scaled score < 6 on two tests or < 4 on one test for the TMT and COWAT, respectively.
Increased ReHo in colorectal cancer survivors
ReHo reflects the synchronization of neural activity within localized brain regions and offers insights into the functional coherence of brain networks9,10,11,12,13. Our findings revealed that early-stage colorectal cancer survivors exhibited significantly increased ReHo in the medial temporal lobe including the left HIP, PHG, and ITG when compared to healthy controls. Notably, ReHo values within these regions were negatively correlated with the RDI of the HVLT-R.
The HIP plays a central role in memory processes including encoding, consolidation, and retrieval, all of which form the basis of the ability to differentiate between previously learned words and distractor words as assessed by the RDI39,40. The PHG complements the HIP by facilitating communication between the neocortex and the HIP which contributes to the encoding of verbal information into short-term memory41,42. The left ITG is known to be involved in visual word recognition, semantic processing, and memory retrieval, all of which are essential for performance on the RDI43. Additionally, the ITG works in conjunction with the HIP and PHG to integrate visual and memory-related information, directly influencing recognition memory performance44.
Interestingly, despite the observed local hyperconnectivity in these regions, the negative correlation between RDI and ReHo in colorectal cancer survivors suggests that increased local neural synchrony may not be effective at recovering recognition memory. There are two possible explanations for these results: (1) Compensatory mechanism: Since the survivors’ memory performance still remained clinically normal, the increased ReHo may represent a compensatory response to cancer-related changes, such as neuroinflammation, and reflects the brain’s attempt to preserve cognitive function despite underlying disruptions. (2) Maladaptive mechanism: Alternatively, hyperconnectivity in the medial temporal lobe may interfere with functional coordination among regions critical for memory and recognition, such as the prefrontal and parietal lobes. This disruption could impair the integration of signals necessary for optimal cognitive performance. To distinguish between these two possibilities, we conducted an inter-regional FC analysis from which our findings support the maladaptive mechanism. This is because the resting state FC results revealed significantly weaker inter-regional connectivity among key regions involved in verbal memory and learning, particularly between the temporal lobes and prefrontal regions (Fig. 5), which may increase the likelihood of a breakdown in functional coordination. This in turn, could disrupt verbal memory function in early-stage colorectal cancer survivors.
Similar regional hyperexcitability and hyperconnectivity in the medial temporal lobes, particularly in the hippocampus, have been observed in various neurological disorders including epilepsy, mild cognitive impairment, and early-stage Alzheimer’s disease45,46,47,48. These regions serve as critical hubs for cognitive functioning and are especially vulnerable to damage due to their high metabolic demands, elevated glucose consumption, and inherently high neuronal firing rates. Such hyperactivity and hyperconnectivity are often considered manifestations of an imbalance between excitation and inhibition, potentially contributing to network dysfunction and cognitive impairment48. This phenomenon can be transitory and have potential to be restored to normalcy, however, if the severity of the damage and its impact on network traffic redistribution are excessive or prolonged, structural damage to the hub and its connections is likely to occur. This can lead to significant disruptions in network organization, ultimately resulting in widespread cognitive impairment47. Given these parallels, a longitudinal investigation into the progression of colorectal CRCD is of great interest and may offer valuable insights into disease mechanisms and the development of targeted interventions towards improving survivorship and cognitive outcomes.
Decreased ReHo in colorectal cancer survivors
Our findings also revealed significantly decreased ReHo in the left IFGtri and left postcentral gyrus among early-stage colorectal cancer survivors compared to healthy controls with ReHo in the IFG being positively correlated with the RDI scores in survivors. The IFG plays a crucial role in the RDI by facilitating word retrieval, cognitive control, and the inhibition of irrelevant or competing information during recognition memory tasks49,50. For the colorectal cancer survivors, lower ReHo in the IFG along with its correlation with poorer RDI performance likely contributes to decline in verbal memory (both retrieval and interreference resolution) in colorectal cancer survivors.
Additionally, we also observed an association between reduced ReHo in the postcentral gyrus with slower TMT-A performance. As part of the primary somatosensory cortex, the postcentral gyrus is important for integrating sensory input and supporting motor planning, both of which are fundamental for tasks requiring rapid responses and precise coordination such as TMT-A51,52. Reduced ReHo in this region reflects diminished neural synchronization which may contribute to slower processing speeds on TMT-A due to the compromised integration of sensory and motor signals.
Correlations between ReHo and cognitive assessments in healthy controls
To strengthen the interpretation and specificity of our findings regarding colorectal cancer survivors, we conducted parallel voxel-wise ReHo-cognition correlation analysis within the control group. This revealed only one significant positive ReHo correlation between the right OFCmed/rectus gyrus and the RDI (Fig. 4; Table 8). While the OFCmed and gyrus rectus are not primarily considered core verbal memory regions, they are integral to broader cognitive functions that support memory. The OFCmed, as part of the ventromedial prefrontal cortex, is critically involved in decision-making, the evaluation and monitoring of retrieved information, and temporal context memory53,54. In healthy controls, greater local synchronization in this region potentially reflects more efficient integration of evaluative or contextual information, thereby contributing to better verbal memory discrimination55. This association likely represents a normative and beneficial brain-behavior relationship.
This finding demonstrates a clear specificity in both anatomical location and nature of the brain-behavior relationship observed in the survivor group. For instance, in survivors, significant inverse correlations between ReHo and RDI were found in medial temporal lobe regions (the right fusiform gyrus, left hippocampus, and left parahippocampal gyrus). In contrast, significant positive correlations in survivors were seen in the bilateral inferior frontal gyrus and left inferior parietal gyrus. These diverse locations and correlation directions are anatomically distinct from the single OFCmed/rectus gyrus association identified in controls. This pattern helps to emphasize that the neural alterations affecting verbal memory in survivors involve unique regional changes and altered functional-cognitive architecture compared to the healthy brain. This in turn supports the existence of specific colorectal cancer related neurological alterations as opposed to general brain-behavior relationships.
Further considerations
While the current pilot study offers significantly new insights into colorectal CRCI, several limitations warrant consideration for future research. Firstly, the limited sample size (18 survivors, 17 controls) may affect the robustness and reproducibility of our fMRI findings. Despite observing statistically significant results, we acknowledge the risk of uncontrollable Type II errors due to this sample size. Future studies will need to incorporate larger cohorts to enhance statistical power and ensure the stability and generalizability of the findings, including the exploration of potential sex differences. Secondly, the cross-sectional design only provides a snapshot of a survivor’s cognitive and neural health. A longitudinal approach is needed to effectively explore how both local and whole-brain functional connectivity, particularly within the temporal lobes, evolve over time and to delineate the progression trajectory of CRCI. Previous studies have shown that hippocampal hyperactivity is evident during the early stages of neurodegeneration but later transition into hypoactivity as the disease progresses46,56. Tracking these dynamic changes in brain patterns among colorectal cancer survivors could yield valuable insights into the neural mechanisms underlying cognitive alterations, which in turn, can inform targeted interventions. For example, future research could investigate whether this hyperconnectivity in the medial temporal lobe diminishes over time as other brain networks adapt or compensate during the early phases of recovery. It is worth noting that while our results support a maladaptive mechanism, these findings remain preliminary. As our current cross-sectional evidence cannot definitively determine whether the observed local hyperconnectivity reflects an early manifestation of overcompensation or a direct functional failure, comprehensive validation through larger scale, longitudinal studies is required. Another recommendation is to include additional neuropsychological tests to assess cognitive function. While the current neuropsychological tests and questionnaires align with the recommendation of the International Cancer Cognition Task Force38, incorporating additional assessments, such as the Wechsler Adult Intelligence Scale-Digit Span and Coding tests, could provide a more comprehensive understanding of the cognitive effects in colorectal cancer survivors.
Conclusions
Understanding the neural mechanisms underlying CRCD is crucial for developing interventions and improving survivorship care. Our study identified a potential maladaptive process in the medial temporal lobe associated with colorectal CRCD.
Data availability
Data and analysis for this study is available upon request. Please contact Dr. Zhaoyue Shi at zhaoyue.shi@carle.com.
References
Morgan, E. et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 72 (2), 338–344 (2023).
Menon, G. & Cagir, B. Colon cancer. In StatPearls. (StatPearls Publishing, 2024).
Vardy, J. et al. Cognitive function and fatigue after diagnosis of colorectal cancer. Ann. Oncol. 25 (12), 2404–2412 (2014).
Vardy, J. L. et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: a prospective, longitudinal, controlled study. J. Clin. Oncol. 33 (34), 4085 (2015).
Cruzado, J. A. et al. Longitudinal study of cognitive dysfunctions induced by adjuvant chemotherapy in colon cancer patients. Support. Care Cancer. 22 (7), 1815–1823 (2014).
Visovatti, M. A., Reuter-Lorenz, P. A., Chang, A. E., Northouse, L. & Cimprich, B. Assessment of cognitive impairment and complaints in individuals with colorectal cancer. Oncol. Nurs. Forum Vol. 43 (2), 169 (2016).
Wieldraaijer, T. et al. Follow-up of colon cancer patients; causes of distress and need for supportive care: results from the ICARE cohort study. Eur. J. Surg. Oncol. (EJSO). 43 (1), 118–125 (2017).
Han, C. J., Yang, G. S. & Syrjala, K. Symptom experiences in colorectal cancer survivors after cancer treatments: a systematic review and meta-analysis. Cancer Nurs. 43 (3), E132–E158 (2020).
Zang, Y., Jiang, T., Lu, Y., He, Y. & Tian, L. Regional homogeneity approach to fMRI data analysis. Neuroimage 22 (1), 394–400 (2004).
Jiang, L. & Zuo, X. N. Regional homogeneity: a multimodal, multiscale neuroimaging marker of the human connectome. Neuroscientist 22 (5), 486–505 (2016).
Zhang, Z. et al. Altered spontaneous activity in alzheimer’s disease and mild cognitive impairment revealed by regional homogeneity. Neuroimage 59 (2), 1429–1440 (2012).
Yao, Z., Wang, L., Lu, Q., Liu, H. & Teng, G. Regional homogeneity in depression and its relationship with separate depressive symptom clusters: a resting-state fMRI study. J. Affect. Disord. 115 (3), 430–438 (2009).
Uddin, L. Q. et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J. Neurosci. Methods. 169 (1), 249–254 (2008).
Henneghan, A. M. et al. Measuring self-reported cancer-related cognitive impairment: recommendations from the cancer neuroscience initiative working group. JNCI: J. Natl. Cancer Inst. 113 (12), 1625–1633 (2021).
Benedict, R. H., Schretlen, D., Groninger, L. & Brandt, J. Hopkins verbal learning Test–Revised: normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol. 12 (1), 43–55 (1998).
Ruff, R. M., Light, R. H., Parker, S. B. & Levin, H. S. Benton controlled oral word association test: reliability and updated norms. Arch. Clin. Neuropsychol. 11 (4), 329–338 (1996).
Reitan, R. M. The Halstead-Reitan neuropsychological test battery: therapy and clinical interpretation (1985).
Tombaugh, T. N. Trail making test A and B: normative data stratified by age and education. Arch. Clin. Neuropsychol. 19 (2), 203–214 (2004).
Wagner, L. I., Sweet, J., Butt, Z., Lai, J. S. & Cella, D. Measuring patient self-reported cognitive function: development of the functional assessment of cancer therapy-cognitive function instrument. J. Support Oncol. 7 (6), W32–W39 (2009).
Broadbent, D. E., Cooper, P. F., FitzGerald, P. & Parkes, K. R. The cognitive failures questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 21 (1), 1–16 (1982).
Goldberg, D. P. & Williams, P. A user’s guide to the General Health Questionnaire. (1988).
Beck, A. T., Steer, R. A. & Carbin, M. G. Psychometric properties of the Beck depression inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 8 (1), 77–100 (1988).
Spielberger, C. D. Assessment of state and trait anxiety: Conceptual and methodological issues. Southern Psychol. (1985).
Marques, J. P. et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 49 (2), 1271–1281 (2010).
O’Brien, K. R. et al. Robust T1-weighted structural brain imaging and morphometry at 7T using MP2RAGE. PloS One, 9(6), e99676. (2014).
Yan, C. G., Wang, X. D., Zuo, X. N. & Zang, Y. F. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics 14, 339–351 (2016).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17 (2), 825–841 (2002).
Rolls, E. T., Huang, C. C., Lin, C. P., Feng, J. & Joliot, M. Automated anatomical labelling atlas 3. Neuroimage 206, 116189 (2020).
Smith, S. M. & Nichols, T. E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44 (1), 83–98 (2009).
Forman, S. D. et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 33 (5), 636–647 (1995).
Shallice, T. et al. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature 368 (6472), 633–635 (1994).
Jonides, J. et al. The role of parietal cortex in verbal working memory. J. Neurosci. 18 (13), 5026–5034 (1998).
Narayanan, N. S. et al. The role of the prefrontal cortex in the maintenance of verbal working memory: an event-related FMRI analysis. Neuropsychology 19 (2), 223 (2005).
Emch, M., Von Bastian, C. C. & Koch, K. Neural correlates of verbal working memory: an fMRI meta-analysis. Front. Hum. Neurosci. 13, 180 (2019).
Zalesky, A., Fornito, A. & Bullmore, E. T. Network-based statistic: identifying differences in brain networks. Neuroimage 53 (4), 1197–1207 (2010).
Xia, M., Wang, J. & He, Y. BrainNet viewer: a network visualization tool for human brain connectomics. PloS One 8(7), e68910 (2013).
Shapiro, A. M., Benedict, R. H., Schretlen, D. & Brandt, J. Construct and concurrent validity of the Hopkins verbal learning Test–revised. Clin. Neuropsychol. 13 (3), 348–358 (1999).
Wefel, J. S., Vardy, J., Ahles, T. & Schagen, S. B. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 12 (7), 703–708 (2011).
Burgess, N., Maguire, E. A. & O’Keefe, J. The human hippocampus and Spatial and episodic memory. Neuron 35 (4), 625–641 (2002).
Sweatt, J. D. Hippocampal function in cognition. Psychopharmacology 174, 99–110 (2004).
Van Strien, N. M., Cappaert, N. L. M. & Witter, M. P. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nat. Rev. Neurosci. 10 (4), 272–282 (2009).
Aminoff, E. M., Kveraga, K. & Bar, M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 17 (8), 379–390 (2013).
Frackowiak, R. S. Functional mapping of verbal memory and Language. Trends Neurosci. 17 (3), 109–115 (1994).
Lin, Y. H. et al. Anatomy and white matter connections of the inferior Temporal gyrus. World Neurosurg. 143, e656–e666 (2020).
Ranasinghe, K. G. et al. Neuronal synchrony abnormalities associated with subclinical epileptiform activity in early-onset alzheimer’s disease. Brain 145 (2), 744–753 (2022).
Targa Dias Anastacio, H., Matosin, N. & Ooi, L. Neuronal hyperexcitability in alzheimer’s disease: what are the drivers behind this aberrant phenotype? Translational Psychiatry. 12 (1), 257 (2022).
Stam, C. J. Hub overload and failure as a final common pathway in neurological brain network disorders. Netw. Neurosci. 8 (1), 1–23 (2024).
Bullmore, E. & Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 13 (5), 336–349 (2012).
Burton, M. W. The role of inferior frontal cortex in phonological processing. Cogn. Sci. 25 (5), 695–709 (2001).
Hirshorn, E. A. & Thompson-Schill, S. L. Role of the left inferior frontal gyrus in Covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia 44 (12), 2547–2557 (2006).
Hanakawa, T., Dimyan, M. A. & Hallett, M. Motor planning, imagery, and execution in the distributed motor network: a time-course study with functional MRI. Cereb. Cortex. 18 (12), 2775–2788 (2008).
Tao, L. et al. Impairment of the executive function in breast cancer patients receiving chemotherapy treatment: a functional MRI study. Eur. J. Cancer Care 26(6), e12553. (2017).
Rolls, E. T., Cheng, W. & Feng, J. The orbitofrontal cortex: reward, emotion and depression. Brain Commun. 2 (2), fcaa196 (2020).
Duarte, A., Henson, R. N., Knight, R. T., Emery, T. & Graham, K. S. Orbito-frontal cortex is necessary for Temporal context memory. J. Cogn. Neurosci. 22 (8), 1819–1831 (2010).
Mızrak, E., Bouffard, N. R., Libby, L. A., Boorman, E. D. & Ranganath, C. The hippocampus and orbitofrontal cortex jointly represent task structure during memory-guided decision making. Cell Rep. 37(9). (2021).
O’brien, J. L. et al. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology 74 (24), 1969–1976 (2010).
Acknowledgements
The authors gratefully thank coordinators Ali Fadl-Alla, Hannah Parks, and Rebecca Sinkes for subject recruitment and neurocognitive test administration, MRI technologists Trenton Lyons, Todd Keller, and Rachel Quaid for 7 T MRI scanning support, Prof. Bruce Damon for advice on the study design, and Prof. Brad Sutton for providing the 7 T MRI scanning protocols.
Funding
This work was supported by the Stephens Family Clinical Research Institute in collaboration with Carle Health Center for Philanthropy through the Innovations in Medical Research Award and the Pete Elmer Family Digestive Health Research Funds.
Author information
Authors and Affiliations
Contributions
The study was designed by JQN and ZS with clinical input from TR, AH, RY, and KR. IRB documentation was performed by DD. Data collection was performed by JQN while data analysis was performed by JQN, NN, SC, and ZS. JQN and ZS wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nguyen, J.Q., Riddle, T., Nguyen, N. et al. Regional hyperconnectivity in the medial temporal lobes as a maladaptive mechanism for colorectal cancer-related cognitive decline. Sci Rep 15, 29954 (2025). https://doi.org/10.1038/s41598-025-15547-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15547-y
Keywords
This article is cited by
-
Altered spatial patterns of intrinsic brain activity and cognitive decline in colorectal cancer survivors
Journal of Cancer Survivorship (2026)