Abstract
Niemann-Pick disease type C (NPC) is a life-threatening neurodegenerative disease caused by impaired intracellular cholesterol trafficking. A cyclic heptasaccharide, 2-hydroxypropyl-β-cyclodextrin (HP-β-CD), is currently under clinical investigation for treating NPC, but its ototoxicity remains problematic. We previously reported that β- and γ-forms of cyclodextrin (CD) derivatives with various substituents capable of accommodating/solubilizing unesterified cholesterol (UC) exerted more pronounced therapeutic and ototoxic effects when administered intracerebroventricularly than subcutaneously for NPC treatment. However, the impact of substituent variations on these effects is unconfirmed for cyclic hexasaccharides, α-CD derivatives. Here, we investigated these effects for α-CD derivatives with various replaced substituents from the perspective of their interaction with UC. Even when administered intracerebroventricularly, a representative α-CD derivative had no impact on survival in NPC model mice. The normalization of intracellular cholesterol trafficking in NPC model cells and the auditory dysfunction in wild-type mice, both caused by HP-β-CD, were not seen for the α-CD derivatives, regardless of their substituent variations. Solubility and molecular simulation analyses showed negligible UC-solubilizing ability of α-CD derivatives because of their insufficient capacity to accommodate the UC molecule, rather than their substituent variations. These results underscore the importance of UC accommodation by CD for both efficacy and ototoxicity in NPC treatment.
Similar content being viewed by others
Introduction
Niemann-Pick disease type C (NPC) is a lysosomal storage disorder caused by mutations in the NPC1 or NPC2 genes, which encode lysosome-resident proteins, transmembrane NPC1 and luminal NPC2, responsible for the trafficking of endocytosed cholesterol from lysosomes to other cellular organelles1,2. After reaching the endoplasmic reticulum, cholesterol is esterified for storage by acyl-coenzyme A: cholesterol acyltransferase, a resident enzyme in the organelle3. Hereditary loss of function of these proteins disrupts intracellular cholesterol trafficking, leading to the excessive accumulation of unesterified cholesterol (UC) in lysosomes and shortages of esterified cholesterol (EC) in other subcellular compartments. The common clinical manifestations of NPC include neurodegeneration and hepatosplenomegaly, ultimately resulting in premature death4.
Cyclodextrins (CDs) are cyclic oligosaccharides composed of six, seven, or eight D-glucopyranose units, termed α-, β-, and γ-CDs, respectively, and are considered the most promising therapeutic candidates for NPC5. Their cyclic structure, with a hydrophilic exterior surface and hydrophobic interior cavity, allows CDs to form water-soluble inclusion complexes with appropriately sized, less polar guest molecules, such as UC6,7. Among CDs, a cyclic heptasaccharide with substitution of the 2-hydroxypropyl groups, 2-hydroxypropyl-β-CD (HP-β-CD), which has the ability to solubilize lipophilic compounds, including UC, is currently being administered on a compassionate basis and is also under clinical investigation for the treatment of NPC8,9,10 based on the discovery of its therapeutic effects and subsequent evidence accumulation in preclinical studies5. Although clinical studies have demonstrated that peripheral or central administration of HP-β-CD attenuates NPC-related manifestations, its ototoxicity remains an obstacle to its approval8,11.
We previously reported that several β- and γ-CD derivatives with various substituents capable of accommodating/solubilizing UC exerted more pronounced therapeutic and ototoxic effects when administered for NPC treatment intracerebroventricularly (ICV) compared with subcutaneously (SC)12,13,14,15,16,17,18,19. We also showed that substituent variations of β- and γ-CD affect the mode of UC inclusion and the stability of the complex, which is highly relevant to the degree of its therapeutic and toxic effects in the treatment of NPC. In contrast, 2-hydroxypropyl-α-CD (HP-α-CD), an α-CD derivative substituted with 2-hydroxypropyl groups, exerted negligible ototoxicity when administered to mice via either route19. HP-α-CD also failed to ameliorate lipid accumulation in NPC model mice after SC administration20. Note that CDs hardly penetrate the blood–brain barrier and therefore require direct administration into the central nervous system to ameliorate the neurological features of NPC21. In addition, substituents of CDs potentially affect not only physicochemical properties, such as their own hydrophilicity, but also the extension and/or asymmetrization of their hydrophobic cavity, thereby possibly altering their ability to accommodate UC or the mode by which this is achieved22,23. However, the impact of substituent variations in α-CD derivatives has not been investigated in the context of therapeutic and ototoxic effects, particularly in the ICV treatment of NPC with respect to evaluation of their interactions with UC.
In this study, we therefore investigated the therapeutic and ototoxic potentials of several α-CD derivatives with various substituents in experimental models of NPC after ICV administration and compared them with those of HP-β-CD based on evaluation of their in vitro interactions with UC. Unlike HP-β-CD, α-CD derivatives failed to normalize impaired intracellular cholesterol trafficking in NPC cellular models. In animal studies, α-CD derivatives had no apparent effect on either premature death of NPC model mice or auditory function in healthy wild-type (WT) mice, even when administered ICV. Solubility analysis and molecular simulation demonstrated that α-CD derivatives showed negligible capacity to solubilize UC, likely because their cavity size is insufficient to accommodate the steroid skeleton of the UC molecule, regardless of the substituent type. These results collectively indicate that the diameter of the CD ring, which is responsible for the ability to accommodate UC, rather than its substituents, plays an important role in both the therapeutic effect on NPC and the development of ototoxicity.
Materials and methods
Reagents
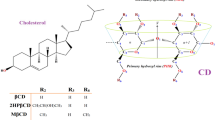
The 2-hydroxypropylated CD derivatives, HP-β-CD (average degrees of substitution, DS: 4.6) and HP-α-CD (average DS: 3.3–5.0) were kindly donated by Nihon Shokuhin Kako Co., Ltd. (Tokyo, Japan). The branched CD derivatives, mono-6-O-α-glucosyl-α-CD (G1-α-CD, DS: 1), mono-6-O-α-maltosyl-α-CD (G2-α-CD, DS: 1), di-6,6-O-α-glucosyl-α-CD (G1G1-α-CD, DS: 2), and di-6,6-O-α-maltosyl-α-CD (G2G2-α-CD, DS: 2) were generously supplied by Ensuiko Sugar Refining Co., Ltd. (Tokyo, Japan). Their molecular structures are shown in Fig. 1. The three possible positional isomers of G1G1-α-CD and G2G2-α-CD reagents were not distinguished in this study. These α-CD derivatives were investigated because both hydroxypropylation, a typical modification that increases the hydrophilicity of native CD and is suggested to extend its hydrophobic cavity, and branching, which similarly enhances hydrophilicity and lowers cytotoxicity, are expected to change the interactions of CD with target molecules19,22. All other reagents and solvents were of reagent grade. Deionized and distilled water was used throughout the study.
Chemical structures of CD derivatives. Chemical structures of HP-β-CD (A), HP-α-CD (B), G1-α-CD (C), G2-α-CD (D), and the three possible positional isomers of G1G1-α-CD (E) and G2G2-α-CD (F). (A, B) The 2-hydroxypropyl groups are randomly substituted onto the 2-, 3-, and 6-hydroxyl groups of β- and α-CD. (C, D) The α-glucosyl and α-maltosyl groups are solely substituted onto the 6-hydroxyl group of α-CD. (E, F) The two α-glucosyl or α-maltosyl groups are substituted onto the two 6-hydroxyl groups of α-CD (left, middle, and right represent 61,62-, 61,63-, and 61,64-isomers, respectively).
Mice and treatment
Animal studies are reported in compliance with the ARRIVE guidelines. Male and female Npc1 homozygous mutant (BALB/cNctr-Npc1m1N, Npc1−/−) mice24 kindly donated by Prof. Kousaku Ohno and Dr. Katsumi Higaki, were used as a murine model of NPC. Age-matched WT mice were used as controls. The mice were bred and housed under specific pathogen-free conditions in the Center for Animal Resources and Development (CARD), Kumamoto University. The mice were housed in cages in a controlled temperature (24 °C) room with a 12 h light-dark cycle and free access to food and water. The animal experiments were performed at the Department of Clinical Chemistry and Informatics, Graduate School of Pharmaceutical Sciences, Kumamoto University. CDs were dissolved in distilled water, and the osmotic pressure was adjusted with sodium chloride to near physiological osmolality. The pH was adjusted to 7.4 using sodium hydroxide, and the solution was filtered through an Advantec DISMIC-13CP 0.45-µm filter (Toyo Roshi Kaisha, Ltd., Tokyo, Japan). ICV administration was performed using stereotaxic instruments (IMPACT-1000 C and KDS 310 Plus; Muromachi Kikai Co., Ltd., Tokyo, Japan) under anesthesia (medetomidine: midazolam: butorphanol, 0.3:4.0:5.0 mg/kg, intraperitoneally). The injection volume was 1 µL/15 g for ICV treatment. All experimental procedures conformed to the animal use guidelines of the Committee for Ethics on Animal Experiments of Kumamoto University (approval numbers A2021-106 and A2023-122).
Measurement of cellular cholesterol in cultured cells
WT and Npc1-null Chinese hamster ovary (Npc1-null) cells developed previously were used25. The cells were grown in a culture medium consisting of a 1:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM) and F-12 (Life Technologies, Tokyo, Japan) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells were maintained at 37 °C in a saturated humidity atmosphere of 95% air and 5% CO2. The cholesterol levels in cultured cells and in aqueous solution were measured in accordance with a previously developed method using a Determiner L FC kit (Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan) and a microplate reader (Tecan Group, Ltd., Männedorf, Switzerland)26. Intracellular cholesterol content was normalized against total protein concentration, as determined with a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.).
Auditory brainstem response (ABR)
Auditory thresholds were measured using ABR System 3 (Tucker-Davis Technologies, FL, USA). The animals were anesthetized by intraperitoneal administration of xylazine and ketamine-HCl in saline. Electrodes were placed beneath the pinna of the treated ear and at the vertex just below the surface of the skin, and the ground electrode was then placed under the contralateral ear. An average of 512 sweeps was calculated at 4, 8, 12, 20, and 32 kHz. The stimulus levels near the threshold were varied in 5-dB steps, and the threshold was defined as the lowest level at which waves in the ABR could be clearly detected by visual inspection. The mice anesthetized with xylazine and ketamine-HCl in saline were euthanized by cervical dislocation method.
Solubilization curve analysis
UC solubility with CD derivatives was measured according to our previous study19. An excess of UC (10 mg) was added to each concentration of CDs in distilled water and shaken at 37 °C for 3 h at 180 rpm. Then, an aliquot was filtered through a Millex-HP PES 0.45-µm filter (Merck Millipore Ltd., Cork, Ireland). The filtrate was mixed and shaken for 10 min with an appropriate volume of chloroform/methanol (2:1, v/v). After centrifugation (1500 × g, 10 min, 4 °C), the chloroform phase was recovered and evaporated. The residue was dissolved in a solvent consisting of 2-propanol, polyoxyethylene alkyl ether, and polyoxyethylene lauryl ether (87:10:3), and the UC concentration was measured using a Determiner L FC kit and a microplate reader (Tecan Group, Ltd.). Three replicate samples were analyzed.
Molecular docking simulation
The 3D structure file for UC was obtained from PubChem (CID 5997). The structure of each CD derivative was constructed using Avogadro software (ver. 1.2.0)27 on the basis of the 3D structures of native β- and α-CDs that were retrieved from the Protein Data Bank (PDB ID: 3CGT and 4FEM, respectively)28. Based on a previous report29, the structures of HP-β-CD and HP-α-CD were prepared by assuming that four 2-hydroxyl groups of glucopyranose units were substituted by hydroxypropyl groups as equally spaced as possible, in accordance with the DS of these CD reagents. In G1-α-CD and G2-α-CD, a single 6-hydroxyl group of the glucopyranose unit was substituted by α-glucosyl and α-maltosyl groups, respectively. In G1G1-α-CD and G2G2-α-CD, considering three possible positional isomers, 61,62-, 61,63- and 61,64-hydroxyl groups of glucopyranose units were substituted by an α-glucosyl or α-maltosyl group, respectively, in accordance with a previous study30. The molecular geometry of UC, HP-β-CD, and α-CD derivatives were fed into GAMESS (ver. 2023 R1)31 for geometry optimization using the semiempirical quantum chemistry method, PM3, and no imaginary frequencies were obtained. Treating the geometry optimized structures of UC and CDs as a flexible ligand and rigid receptor, respectively, molecular docking simulation to obtain complexes was then performed with 100 runs using the Lamarckian genetic algorithm in AutoDock 4.232.
Statistics
Statistical analyses were performed using GraphPad Prism ver. 9.5.1 (GraphPad Software, San Diego, CA, USA). Multiple comparisons were performed to assess statistical significance. When uniform variance of the results was identified by Bartlett’s analysis (P < 0.05), one-way ANOVA was used to test for statistically significant differences. When significant differences (P < 0.05) were identified, the results were further analyzed by Dunnett’s or the Tukey–Kramer multiple range test to determine the significance of differences between the groups. Where uniform variance of the results was not identified, non-parametric multiple comparisons were performed. After confirming significant differences (P < 0.05) using Kruskal–Wallis analysis, the differences were then examined by applying Dunn’s multiple comparison test.
Results
Effect of ICV injection of a representative α-CD derivative in NPC model mice
HP-β-CD and γ-CD derivatives, which form 1:1 inclusion complexes with UC, significantly extend the lifespan of NPC model mice, particularly when administered ICV rather than SC. In contrast, HP-α-CD, a representative α-CD derivative, which does not solubilize UC, had no obvious effect when administered SC17,19,20. To investigate the therapeutic effect of the ICV application of HP-α-CD, we administered 21.4 and 42.8 µmol/kg (a frequently used dose of 30 mg/kg HP-β-CD and double that dose) to Npc1−/− mice once at 4 weeks of age. The model mice showed progressive body weight loss after 8 weeks of age, and this trend was not mitigated by either dose of HP-α-CD with no statistically significant differences between groups at any time point (Fig. 2A). HP-α-CD at both doses also had negligible effects on the shortened lifespan and mean survival time of Npc1−/− mice (Fig. 2B and C).
Effect of ICV administration of representative α-CD derivative on body weight and lifespan in NPC model mice. (A) Weekly changes in body weight (percentage over baseline at 4 weeks of age) of Npc1−/− mice. Kaplan–Meier survival curve (B) and mean lifetime (C) of untreated and HP-α-CD treated Npc1−/− mice. The experimental groups were untreated Npc1−/− mice, 21.4 µmol/kg HP-α-CD-treated Npc1−/− mice, and 42.8 µmol/kg HP-α-CD-treated Npc1−/− mice. Data represent the mean ± SEM, n = 4 for each group. ns; not significant vs. untreated group.
Effect of α-CD derivatives on disrupted intracellular cholesterol trafficking in NPC model cells
We previously demonstrated that HP-α-CD, unlike β- and γ-CD derivatives, exhibited no obvious effects on abnormal intracellular cholesterol trafficking in NPC model cells19. To test the impact of substituents of α-CD derivatives on this cellular NPC phenotype and their therapeutic potentials for NPC, the levels of intracellular cholesterol were measured in Npc1-null cells after exposure to various α-CD derivatives and compared with those for HP-β-CD, at 0.1, 1, and 10 mM. Compared with the findings for WT cells, the intracellular total cholesterol (TC) and UC levels were approximately doubled (Fig. 3A and B) and the molar EC/TC ratio was approximately halved (Fig. 3C) in Npc1-null cells. These changes were significantly normalized by HP-β-CD in a concentration-dependent manner, indicating increased delivery of lysosomal accumulated UC to the endoplasmic reticulum and conversion to EC, but were largely unchanged for all tested α-CD derivatives. Note that these evaluations were conducted separately for every CD derivative; consequently, the cholesterol levels in vehicle-treated Npc1-null cells were different among the tests for CD derivatives particularly in HP-α-CD. In simultaneous evaluations of HP-β-CD and HP-α-CD effects, the latter still showed no obvious advantage over vehicle-treated Npc1-null cells (data not shown).
Limited effect of α-CD derivatives on the perturbed intracellular cholesterol trafficking in NPC model cells. Concentration-dependent effects of HP-β-CD and α-CD derivatives on intracellular TC (A) and UC (B) levels, and on the EC/TC molar ratio (C) in Npc1-null cells. Cholesterol levels were measured 24 h after the exposure to 0.1, 1, and 10 mM CD. Data represent the mean ± SEM of three independent experiments performed separately for each CD derivative. *P < 0.05, **P < 0.01 vs. vehicle-treated WT cells; #P < 0.05, ##P < 0.01 vs. vehicle-treated Npc1-null cells.
Effect of ICV injection of α-CD derivatives on auditory function in mice
Previous studies have suggested that the central administration of CDs tends to induce more significant auditory dysfunction than their peripheral administration at each therapeutic dose19,33. To evaluate the effects of α-CD and its substituents on auditory function, we measured the ABR thresholds in WT mice after a single ICV dose of 21.4 µmol/kg of its derivatives (Fig. 4). Compared with saline treatment, HP-β-CD significantly increased the ABR threshold at every measured frequency, while all α-CD derivatives tested were associated with negligible signs of hearing loss.
Ototoxic effect of ICV administration of α-CD derivatives. ABR hearing thresholds expressed as decibels of sound pressure level (SPL) for five frequencies (4, 8, 12, 20, and 32 kHz) were measured 1 week after a single ICV (21.4 µmol/kg) administration of CD derivatives into WT mice at 8 weeks of age. Data represent the mean ± SEM, n = 4 for each treatment group. *P < 0.05, **P < 0.01 vs. saline-treated group.
Solubility curve analysis of UC with α-CD derivatives
We have previously shown that HP-α-CD, unlike β- and γ-CD derivatives, exhibited no ability to solubilize UC because of differences in the inner diameter of the CD ring19. To verify that the inability to solubilize UC is inherent to the α-CD derivatives and independent of their variety of substituent, the solubility of UC was measured with the α-CD derivatives and compared with that of HP-β-CD. At the concentration range of 0–10 mM, the enhancement of UC solubility exhibited an upward deviation from linearity with increasing HP-β-CD concentration, while UC solubilization was negligible in all α-CD derivatives tested (Fig. 5A). Even in magnified diagram views (0–5 µM of UC), no obvious UC solubilization with α-CD derivatives was observed (Fig. 5B). For further strict verification of the inability of α-CD derivatives to solubilize UC, we examined higher concentrations of 50 and 100 mM (Fig. 5C). Enhanced UC solubility was observed with HP-β-CD in a concentration-dependent manner, but not with α-CD derivatives. These results indicate that the variety of substituents on α-CD derivatives has no influence on their inability to solubilize UC.
Ability of α-CD derivatives to solubilize UC. (A) Solubilization curves of UC with HP-β-CD and α-CD derivatives at concentrations of 0–10 mM. (B) Magnified view of the area enclosed by the dashed line in A for each α-CD derivative. n = 12 for UC solubility without CDs, n = 5 with HP-β-CD, n = 3 with α-CD derivatives. (C) UC solubilization of HP-β-CD (n = 3) and α-CD derivatives (n = 4) at extremely high concentrations of 50 and 100 mM. Data represent the mean ± SEM.
Molecular docking simulation of α-CD derivatives with UC
To confirm that α-CD derivatives do not form inclusion complexes with UC, regardless of their substituents, we predicted probable docking conformations of UC with α-CD derivatives and compared them with that of HP-β-CD. Through 100 docking runs, we obtained two dominant binding modes of the 1:1 HP-β-CD: UC complex: in Type I, the hydroxyl terminus of the UC molecule is directed toward the secondary face of the CD molecule and in Type II, the hydroxyl terminus is directed toward the primary face of the CD molecule (Fig. 6A). These conformations showed that the HP-β-CD ring tended to surround the steroid skeleton of the UC molecule, indicating the formation of soluble inclusion complexes. In contrast, Type I and/or Type II conformations were also obtained for α-CD derivatives, but the lowest binding energy conformations indicated an inability to form a soluble inclusion complex with UC (Fig. 6B–J). The UC hydrophobic tail/hydroxyl terminus embedded within the cavity of α-CD derivatives and most of the steroid skeleton/hydrophobic tail protruded outside the cavity, as shown in the “overflowing” clusters. In “floating” clusters of HP-α-CD and the G2G2-α-CD, 61,64-isomer, UC remained unbound and floating in the vicinity of the larger opening of the α-CD derivatives (Fig. 6B and J). As shown in Fig. 6K, the lowest energies for the dominant binding conformations of HP-β-CD were lower than those of the α-CD derivatives, and the energies tended to be lower in Type I than in Type II or floating clusters in all CDs. Taken together, these data indicate that the ring diameter of the α-CD derivatives, regardless of the variety of their substituents, have insufficient capacity to accommodate the steroid skeleton of the UC molecule.
Probable binding conformation of the predominant UC inclusion modes of CD derivatives. (A−J) The lowest UC binding energy conformations (upper) and the lowest binding energy clusters are depicted with different colors (lower) for HP-β-CD and α-CD derivatives (A, HP-β-CD; B, HP-α-CD; C, G1-α-CD; D, G2-α-CD; E−G, three different isomers of G1G1-α-CD; H−J, three different isomers of G2G2-α-CD). The oxygen atom in the UC molecule is colored red. The structures of HP-β-CD and α-CD derivatives are colored orange and violet, respectively. The hydroxyl terminus of the UC molecule is directed toward the 2- and 3-hydroxyl groups of the CD molecule in Type I (left) and is directed towards the 6-hydroxyl group of the CD molecule in Type II (right). (B−J) In “overflowing” clusters, the UC hydrophobic tail/hydroxyl terminus is embedded within the cavity of α-CD derivatives and most of the steroid skeleton/hydrophobic tail protrudes outside the cavity. (B) and (J) In “floating” clusters, UC remained unbound and floating in the vicinity of the larger opening of HP-α-CD and the G2G2-α-CD, 61,64-isomer. (K) The lowest binding free energies (kcal/mol) of docking conformations for each CD derivative with UC.
Discussion
One of the most problematic adverse events in the clinical development of CD therapy for NPC is auditory dysfunction. In a recent report we showed that none of the tested β- and γ-CD derivatives with various substituents that are effective in NPC model mice avoided auditory toxicity, similar to HP-β-CD19. Therefore, there is an urgent need to identify the characteristics of CD derivatives that cause no ototoxicity. Here, we report no obvious effects of α-CD derivatives with various substituents on either early mortality of NPC model mice or on auditory function in WT mice, regardless of their substituent variations. The ability of β- and γ-CD derivatives to include/solubilize UC was absent in α-CD derivatives, indicating that this ability plays a critical function both in exerting a therapeutic effect on NPC and in the development of ototoxicity.
We have noted that the ability of CDs to solubilize UC varies depending on the diameter of their rings and on their substituents. When comparing hydroxypropylated CDs with different ring diameters, the UC-solubilizing capacity was significantly higher for the β-form, followed by the γ-form and then the α-form34. In a comparison of β-CD derivatives, the shape of the phase-solubility diagrams for UC differed depending on the presence or absence and the types of substituents35. For γ-CD derivatives, the solubility of UC increased linearly as a function of the CD concentration, with the gradient varying depending on the substituent and the DS19. Our recent examination of two different α-CD derivatives indicated that they have a negligible effect on enhancing UC solubility and inducing efflux from cells19,36. The present study further demonstrated that all tested α-CD derivatives lack the ability to accommodate and solubilize the UC molecule, indicating that these characteristics are inherent to α-CD having insufficient ring diameter/capacity, irrespective of the substituents and DS. Although the hydroxypropylation or branching of CD potentially affect not only physicochemical properties, such as their own hydrophilicity22 but also the extension and/or asymmetrization of their hydrophobic cavity23, our results suggest that these physicochemical and structural alterations have no impact on interactions with UC in terms of α-CD derivatives.
In NPC treatment, the CD–UC interaction is considered to be inextricably linked to the onset of both therapeutic and ototoxic effects. We recently demonstrated that the abilities of CD derivatives to form a 1:1 complex with UC, rather than their excessive UC-solubilizing capacity caused by 2:1 CD: UC complexation, were significantly correlated with their ability to normalize intracellular cholesterol trafficking and to prolong lifespan in cellular and murine models of NPC, respectively17,19. We highlighted that these results were obtained because the 1:1 complex may serve as a UC shuttle that catalytically enhances the cellular cholesterol flux, while the 2:1 complex formed by β-CD derivatives, which becomes predominant with increasing CD concentration, may function as a strong sink to excessively solubilize UC, thereby contributing to cytotoxicity. Given that cellular endocytosis of CD is responsible for UC reduction in NPC3, their 1:1 complexation at sites where UC accumulates, such as lysosomes, should be necessary for the onset of therapeutic effects. Previous studies showed a partial correlation between the abilities of CD derivatives to solubilize UC and to induce ototoxicity in mice19,20. A plausible mechanism of CD-induced ototoxicity is loss of cochlear outer hair cells resulting from the removal of UC from their lateral walls37; therefore, the CD–UC complexation at that site may be critical for the development of hearing loss. In addition, the route of CD administration is considered to be associated with the intensity of both the therapeutic and the ototoxic effects in the treatment of NPC. CDs hardly cross the blood–brain barrier; therefore, direct administration into the central nervous system is required to improve the neurological features of NPC19,21,38. However, the transfer of circulating CDs into the cochlear lymphatic fluid is considered to be restricted by the blood labyrinth barrier, so administration into the cerebrospinal fluid, which is connected to the cochlear lymph fluid through features such as the cochlear aqueduct, appears to confer a risk of rapid and severe ototoxicity5,39. Considering that the tested α-CD derivatives have negligible ability to include/solubilize UC and exhibit neither therapeutic nor ototoxic effects, even when administered ICV, the above results collectively support the concept that the complexations of CD with UC in NPC neuronal lysosomes and on cochlear outer hair cell membranes are responsible for these effects, respectively.
The function of CDs in NPC treatment suggests that, besides interacting with UC, they also regulate cellular signaling mediated by relevant proteins. Methyl-β-CD improves defective autophagy flux in NPC model cells derived from patients through activation of AMP-activated protein kinase (AMPK) via direct binding40. AMPK signaling may also be relevant to CD ototoxicity because noise exposure-induced AMPK activation is potentially linked to outer hair cell injury and hearing loss in mice41. Singhal et al. proposed that the increases in lysosome-associated membrane protein-1 resulting from HP-β-CD and HP-γ-CD treatments contributed to improved UC accumulation in NPC model cells42. These pharmacological activities of CD, particularly its direct interaction with relevant proteins, have been considered to be due to the inclusion of hydrophobic residues of the proteins into the CD ring43. It remains unclear whether these pharmacological mechanisms are primarily responsible for the therapeutic and ototoxic effects of CD; however, our results showing that the effects for UC accumulation were absent for α-CD derivatives suggest that their narrower cavity is insufficient for direct modulation of this cellular signaling.
In summary, the ability of β- and γ-CD derivatives to include/solubilize UC was notably absent in α-CD derivatives. This highlights that this ability is critical for exerting both therapeutic and ototoxic effects in the treatment of NPC. Native α-CD has been used as a pharmaceutical excipient or functional dietary supplement based on its high biocompatibility in oral and parenteral use, while its derivatives are rarely put to practical application44,45,46. Although the tested α-CD derivatives had no therapeutic effects on NPC, the finding that they caused no auditory dysfunction will contribute to their development for clinical and non-clinical use. The presented findings indicate the safety of α-CD derivatives in terms of maintaining auditory function and the importance of the interaction of CD with UC in the treatment of NPC.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Vanier, M. T. Niemann-Pick disease type C. Orphanet J. Rare Dis. 5, 16 (2010).
Kwon, H. J. et al. Structure of N-Terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 137, 1213–1224 (2009).
Rosenbaum, A. I., Zhang, G., Warren, J. D. & Maxfield, F. R. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc. Natl. Acad. Sci. U S A. 107, 5477–5482 (2010).
Bianconi, S. E. et al. Evaluation of age of death in Niemann-Pick disease, type C: utility of disease support group websites to understand natural history. Mol. Genet. Metab. 126, 466–469 (2019).
Ishitsuka, Y., Irie, T. & Matsuo, M. Cyclodextrins applied to the treatment of lysosomal storage disorders. Adv. Drug Deliv. Rev. 191, 114617 (2022).
Uekama, K., Hirayama, F. & Irie, T. Cyclodextrin drug carrier systems. Chem. Rev. 98, 2045–2076 (1998).
Saokham, P., Muankaew, C., Jansook, P. & Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 23, 1161 (2018).
Matsuo, M. et al. Effects of cyclodextrin in two patients with Niemann-Pick type C disease. Mol. Genet. Metab. 108, 76–81 (2013).
Matsuo, M. et al. Effects of intracerebroventricular administration of 2-hydroxypropyl-β-cyclodextrin in a patient with Niemann-Pick type C disease. Mol. Genet. Metab. Rep. 1, 391–400 (2014).
Sharma, R. et al. Long-term administration of intravenous Trappsol® Cyclo™ (HP-β-CD) results in clinical benefits and stabilization or slowing of disease progression in patients with Niemann-Pick disease type C1: Results of an international 48-week Phase I/II trial. Mol. Genet. Metab. Rep. 36, 100988 (2023).
Ory, D. S. et al. Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1–2 trial. Lancet 390, 1758–1768 (2017).
Tanaka, Y. et al. Efficacy of 2-hydroxypropyl-β-cyclodextrin in Niemann-Pick disease type C model mice and its pharmacokinetic analysis in a patient with the disease. Biol. Pharm. Bull. 38, 844–851 (2015).
Soga, M. et al. HPGCD outperforms HPBCD as a potential treatment for Niemann-Pick disease type C during disease modeling with iPS cells. Stem Cells. 33, 1075–1088 (2015).
Hoque, S. et al. Differential effects of 2-hydroxypropyl-cyclodextrins on lipid accumulation in Npc1-null cells. Int. J. Mol. Sci. 21, 898 (2020).
Yasmin, N. et al. In vitro and in vivo evaluation of 6-O-α-maltosyl-β-cyclodextrin as a potential therapeutic agent against Niemann-Pick disease type C. Int. J. Mol. Sci. 20, 1152 (2019).
Fukaura, M. et al. Intracerebroventricular treatment with 2-hydroxypropyl-β-cyclodextrin decreased cerebellar and hepatic glycoprotein nonmetastatic melanoma protein B (GPNMB) expression in Niemann–Pick disease type C model mice. Int. J. Mol. Sci. 22, 452 (2021).
Yamada, Y. et al. Differential mode of cholesterol inclusion with 2-hydroxypropyl-cyclodextrins increases safety margin in treatment of Niemann-Pick disease type C. Br. J. Pharmacol. 178, 2727–2746 (2021).
Yamada, Y. et al. Fine-tuned cholesterol solubilizer, mono-6-O-α-D-maltosyl-γ-cyclodextrin, ameliorates experimental Niemann–Pick disease type C without hearing loss. Biomed. Pharmacother. 155, 113698 (2022).
Yamada, Y. et al. Different solubilizing ability of cyclodextrin derivatives for cholesterol in Niemann–Pick disease type C treatment. Clin. Transl. Med. 13, e1350 (2023).
Davidson, C. D. et al. Efficacy and ototoxicity of different cyclodextrins in Niemann-Pick C disease. Ann. Clin. Transl. Neurol. 3, 366–380 (2016).
Vecsernyés, M. et al. Cyclodextrins, blood–brain barrier, and treatment of neurological diseases. Arch. Med. Res. 45, 711–729 (2014).
Koizumi, K., Okada, Y., Kubota, Y. & Utamura, T. Inclusion complexes of poorly water-soluble drugs with glucosyl-cyclodextrins. Chem. Pharm. Bull. 35, 3413–3418 (1987).
Aree, T. Effect of the ring size and asymmetry of cyclodextrins on their inclusion ability: a theoretical study. J. Incl. Phenom. Macrocycl. Chem. 77, 439–445 (2013).
Loftus, S. K. et al. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science 277, 232–235 (1997).
Higaki, K. et al. Isolation of NPC1-deficient Chinese hamster ovary cell mutants by gene trap mutagenesis. J. Biochem. 129, 875–880 (2001).
Kondo, Y. et al. In vitro evaluation of 2-hydroxyalkylated β-cyclodextrins as potential therapeutic agents for Niemann-Pick type C disease. Mol. Genet. Metab. 118, 214–219 (2016).
Hanwell, M. D. et al. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 4, 17 (2012).
Berman, H. M. et al. The protein data bank. Nucleic Acids Res. 28, 235–242 (2000).
Mischnick, P. Determination of the patterns of substitution of hydroxyethyl- and hydroxypropyl-cyclomaltoheptaoses. Carbohydr. Res. 192, 233–241 (1989).
Koizumi, K., Tanimoto, T., Okada, Y. & Matsuo, M. Isolation and characterization of three positional isomers of diglucosylcyclomaltoheptaose. Carbohydr. Res. 201, 125–134 (1990).
Barca, G. M. J. et al. Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys. 152, 154102 (2020).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791 (2009).
Cronin, S., Lin, A., Thompson, K., Hoenerhoff, M. & Duncan, R. K. Hearing loss and otopathology following systemic and intracerebroventricular delivery of 2-hydroxypropyl-beta-cyclodextrin. J. Assoc. Res. Otolaryngol. 16, 599–611 (2015).
Irie, T., Fukunaga, K. & Pitha, J. Hydroxypropylcyclodextrins in parenteral use. I: Lipid dissolution and effects on lipid transfers in vitro. J. Pharm. Sci. 81, 521–523 (1992).
Shiotani, K. et al. Differential effects of sulfate and sulfobutyl ether of beta-cyclodextrin on erythrocyte membranes in vitro. Pharm. Res. 12, 78–84 (1995).
Nakao, S. et al. Dimethyl-α-cyclodextrin induces capacitation by removing phospholipids from the plasma membrane of mouse sperm. Biol. Reprod. 108, 671–681 (2023).
Crumling, M. A., King, K. A. & Duncan, R. K. Cyclodextrins and iatrogenic hearing loss: new drugs with significant risk. Front. Cell. Neurosci. 11, 355 (2017).
Yamada, Y. et al. Intracerebroventricular 2-hydroxypropyl-γ-cyclodextrin alleviates hepatic manifestations without distributing to the liver in a murine model of Niemann–Pick disease type C. Life Sci. 350, 122776 (2024).
Salt, A. N. & Hirose, K. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear. Res. 362, 25–37 (2018).
Dai, S. et al. Methyl-β-cyclodextrin restores impaired autophagy flux in Niemann-Pick C1-deficient cells through activation of AMPK. Autophagy 13, 1435–1451 (2017).
Hill, K., Yuan, H., Wang, X. & Sha, S. H. Noise-induced loss of hair cells and cochlear synaptopathy are mediated by the activation of AMPK. J. Neurosci. 36, 7497–7510 (2016).
Singhal, A., Szente, L., Hildreth, J. E. K. & Song, B. Hydroxypropyl-beta and -gamma cyclodextrins rescue cholesterol accumulation in Niemann–Pick C1 mutant cell via lysosome-associated membrane protein 1. Cell. Death Dis. 9, 1019 (2018).
Serno, T., Geidobler, R. & Winter, G. Protein stabilization by cyclodextrins in the liquid and dried state. Adv. Drug Deliv. Rev. 63, 1086–1106 (2011).
Frank, D. W., Gray, J. E. & Weaver, R. N. Cyclodextrin nephrosis in the rat. Am. J. Pathol. 83, 367–382 (1976).
Irie, T. & Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 86, 147–162 (1997).
Puskás, I., Szente, L., Szőcs, L. & Fenyvesi, É. Recent list of cyclodextrin-containing drug products. Period Polytech. Chem. Eng. 67, 11–17 (2023).
Acknowledgements
We thank Jeremy Allen, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
Y.Y.: Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Conceptualization, Funding acquisition. M.T.: Writing – original draft, Methodology, Investigation. Y.I.: Investigation. Y.K.: Writing – review & editing, Methodology. T.T. and N.N.: Resources. T.M., H.T., and Y.O.: Methodology. K.M., T.H., and H.A.: Supervision. T.S., Y.K., and H.K.: Methodology. K.H.: Resources, Methodology. K.M., K.M., and N.Y.: Writing – review & editing. R.I., and M.M.: Supervision. T.I.: Writing – review & editing, Supervision, Funding acquisition. Y.I.: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yamada, Y., Tanaka, M., Ikeda, Y. et al. Inability of α-cyclodextrins to accommodate cholesterol potentially underlies their lack of efficacy and ototoxicity in Niemann-Pick disease type C treatment. Sci Rep 15, 30857 (2025). https://doi.org/10.1038/s41598-025-15599-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15599-0