Abstract
There is increasing evidence that cerebrospinal fluid (CSF) circulation plays a key role in the pathophysiology of Alzheimer’s disease (AD), although the underlying mechanisms remain poorly understood. This study evaluated CSF circulation in patients with AD using magnetic resonance imaging (MRI)-derived measures, including choroid plexus (CP) volume, CSF volume, and diffusion tensor imaging along the perivascular space (DTI-ALPS) index. The study included 188 participants: 28 with AD, 40 with mild cognitive impairment (MCI), 66 with subjective cognitive decline (SCD), and 54 normal controls (NCs). Participants underwent structural T1-weighted MRI, diffusion tensor imaging, clinical assessment, and plasma biomarker analysis. After adjustments for age, sex, and education, group differences in the DTI-ALPS index, CP volume, and CSF volume were examined. Correlation and mediation analyses were conducted to assess the relationships of clinical parameters with DTI-ALPS index, CP volume, and CSF volume. The AD group exhibited a significantly lower DTI-ALPS index and higher CP and CSF volumes compared with the MCI, SCD, and NC groups. The DTI-ALPS index showed a strong negative correlation with both CP and CSF volumes, whereas CP volume was positively correlated with CSF volume. Mediation analysis revealed interactive relationships among the DTI-ALPS index, CP volume, and CSF volume. This study demonstrates that CSF circulation is disrupted in AD patients. Additionally, CP volume influences glymphatic drainage through its effects on CSF volume, indicating a potential role in AD pathophysiology.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by the accumulation of extracellular amyloid-β (Aβ) and intracellular tau proteins, ultimately resulting in cognitive decline, memory loss, and an inability to perform daily activities1. The prevalence of AD is projected to triple by 20502, but current treatments offer only symptomatic relief with modest efficacy2. There is increasing evidence that impaired clearance of protein aggregates is a key factor in AD development. Aβ pathology has been observed in the early stages of the disease3indicating that brain clearance mechanisms are already compromised at these initial stages. This clearance dysfunction is closely linked to cerebrospinal fluid (CSF) circulation3. Efforts to target the structures and systems involved in CSF circulation hold promise for preventing or treating AD.
CSF circulation plays a critical role in eliminating toxic metabolites and metabolic waste, thereby maintaining physiological homeostasis in the brain3. Research has shown that the choroid plexus (CP) and aquaporin-4 (AQP4) contribute to CSF production, the choroid plexus primarily generates CSF via AQP1, whereas AQP4 may facilitates CSF production through water filtration across parenchymal capillaries4. Furthermore, the presence of AQP4 along perivascular spaces of draining veins indicates that the glymphatic system can also contribute to CSF production through AQP4-dependent mechanisms5. Furthermore, AQP4-dependent glymphatic drainage and CP-driven CSF production are important aspects of CSF circulation5suggesting their involvement in clearance mechanisms. The localization of perivascular AQP4 has been strongly associated with AD status6and patients with AD exhibit substantial CP enlargement7indicating that CSF circulation is impaired in AD. Recent studies have defined increased CSF volume as the accumulation of CSF within the brain8,9. This parameter, included in the present study, serves as an indicator for evaluating CSF circulation. Thus far, CSF circulation has shown strong associations with aging and neurodegenerative pathologies, including the accumulation of Aβ3 and tau protein10. These associations emphasize the importance of studying CSF circulation mechanisms to develop strategies for preventing and treating neurodegenerative diseases.
Magnetic resonance imaging (MRI) studies involving intrathecal tracer injections have demonstrated the presence of a glymphatic system in both rodent and human brains5,11. However, the invasive nature of these methods has limited their application in human studies. Recently, non-invasive MRI surrogates have been developed to assess glymphatic drainage in AD. These surrogates include the diffusion tensor imaging along the perivascular space (DTI-ALPS) index and CP volume, an indicator of CSF production12,13. The DTI-ALPS index is derived from diffusivity measurements along the deep medullary vein at the level of the lateral ventricular body14. Although there is considerable debate regarding whether the DTI-ALPS index can accurately represent glymphatic drainage function—such as its inability to reflect specific brain regions or merely indicating glymphatic drainage in periventricular white matter areas surrounding the lateral ventricles15,16its reliability as a measure of glymphatic clearance has been supported by evidence of a significant correlation between the DTI-ALPS index and glymphatic clearance measured via intrathecal angiography17. The DTI-ALPS index has been investigated in various conditions, including neurodegenerative diseases18,19,20brain tumors8idiopathic intracranial hypertension21sleep disorders22fibromyalgia23and pediatric acute lymphoblastic leukemia9. Additionally, previous studies have demonstrated a significant positive correlation between CP volume and cognitive impairment severity in AD patients24. Both glymphatic drainage and CSF production are closely linked to sleep and circadian rhythms5,25,26. Notably, circadian variations in CSF volume suggest that this measure is sensitive to changes in CSF circulation26. Intrathecal injection studies in rodents have also demonstrated a correlation between CSF volume and glymphatic drainage27highlighting the potential utility of CSF volume as a metric for assessing both the glymphatic system and CSF circulation.
In this study, we evaluated CSF circulation in AD patients using three key metrics: CSF volume, CP volume, and the DTI-ALPS index, derived from two MRI sequences. We hypothesized that (1) CSF volume would remain significantly different across the four diagnostic groups, even after controlling for brain parenchymal volume; and (2) changes in CSF volume would be correlated with CP volume and the glymphatic system, based on their critical roles in CSF circulation.
Materials and methods
Demographical and clinical characteristics
This cross-sectional case-control study recruited 188 volunteers aged 44–89 years from the community. Participants were categorized into four groups: AD (n = 28), mild cognitive impairment (MCI, n = 40), subjective cognitive decline (SCD, n = 66), and normal control (NC, n = 54).
Inclusion criteria for the NC group were: (1) right-handedness; (2) absence of complaints regarding cognitive decline and no concerns about cognitive function; (3) scores within the normal range on standardized neuropsychological tests adjusted for age, sex, and education; (4) negative findings during physical examination; and (5)review medical history and family history(Including within three generations of the person’s paternal, maternal, direct and collateral lines) is negative, accessory examination don’t show disease could cause cognitive decline.
Inclusion criteria for the SCD group were based on previous definitions28: (1) self-perceived, sustained cognitive decline relative to a previously normal state, not attributable to an acute event; (2) memory-related concerns; and (3) failure to meet the criteria for MCI.
Inclusion criteria for the MCI group were based on previous definitions29: (1) a Clinical Dementia Rating (CDR) score of 0.5, without meeting criteria for dementia; (2) impairment in at least two measures within a single cognitive domain (e.g., memory, language, or processing speed/executive function); and (3) impairment across three sampled cognitive domains.
Inclusion criteria for the AD group30 were based on guidelines for the diagnosis of AD dementia, established by the National Institute on Aging-Alzheimer’s Association.
Exclusion criteria for all groups were: left-handedness, ambidexterity, and contraindications to MRI.
Neuropsychological assessments
Demographic and clinical data, including age, sex, and years of education, were collected. General cognitive performance was assessed using the Mini-Mental State Examination (MMSE)31Montreal Cognitive Assessment-Basic (MoCA-B)32and CDR33. To evaluate impairments in situational memory and executive function, additional neuropsychological tests were administered, including the Shape Trail Tests (STT-A and STT-B)34Pittsburgh Sleep Quality Index (PSQI)35Verbal Fluency Test (VFT)36Boston Naming Test (BNT)37Memory and Executive Screening (MES)38and Hamilton Depression Scale (HAMD)39.
Plasma biomarker measurement
Plasma biomarker concentrations were measured via the single-molecule array (Simoa) platform. The p-tau181 Advantage Kit was used to quantify p-tau181 levels, whereas the Simoa Human Neurology 4-Plex E (N4PE) assay (Quanterix) was utilized to measure Aβ40, Aβ42, neurofilament light chain (NfL), and glial fibrillary acidic protein (GFAP) concentrations. All analyte measurements were above the detection limit, and intra-assay variation coefficients were below 10%. Data were subsequently matched to phenotype information.
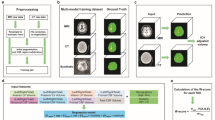
MRI acquisition.
MRI data were acquired using a 3-T MR scanner (Magnetom Prisma, Siemens Healthineers, Erlangen, Germany) equipped with a 64-channel head-neck coil. The imaging protocol included a three-dimensional (3D) magnetization-prepared rapid acquisition gradient-echo (MPRAGE) T1-weighted sequence for anatomical imaging and diffusion tensor imaging (DTI). The scanning parameters were as follows: (1) 3D T1-weighted imaging (MPRAGE sequence): echo time = 2.26 msec; repetition time = 2300 msec; inversion time = 900 msec; flip angle = 8°; slice thickness = 1 mm; field of view = 256 × 256 mm40; and voxel size = 1 × 1 × 1 mm2; (2) DTI (spin-echo echo planar imaging sequence): echo time = 68 msec; repetition time = 4500 msec; slice thickness = 2 mm; field of view = 245 × 245 mm40; voxel size = 2 × 2 × 2 mm2; number of slices = 74; 64 gradient directions for b = 1000 s/mm2; and 12 images for b = 0 s/mm2.
Image analysis
DTI-ALPS
The DTI-ALPS index was used to assess interstitial fluid movement41. Preprocessing of DTI data was performed using FSL software (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki), and quantitative diffusion maps were generated. Due to the symmetrical distribution of pathological features in AD patients and all subjects were right-handed, a 2.5-mm-thick mask was created specifically in the region of projection and association fibers at the level of the left lateral ventricular body42. Diffusion coefficient values were recorded along the x, y, and z axes as follows: projection fibers (Dxxproj, Dyyproj, Dzzproj) and association fibers (Dxxassoc, Dyyassoc, Dzzassoc). The DTI-ALPS index was calculated using the formula: DTI-ALPS index = [Mean (Dxxproj + Dxxassoc)]/[Mean (Dyyproj + Dzzassoc)].
CP and CSF volumes
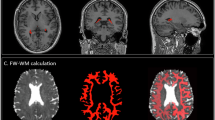
The lateral ventricular CP volume was manually segmented from 3D T1-weighted volumetric images using ITK-SNAP software (version 4.0)(see Fig. 1). CSF volume and total intracranial volume (TIV) were calculated using the CAT12 toolkit (http://www.neuro.uni-jena.de/cat/) within SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) in MATLAB R2022b (MathWorks). To adjust for inter-participant variability, CP and CSF volumes were normalized by expressing each as a ratio to TIV, in accordance with established methodology.
Bar graphs illustrate the distribution of choroid plexus (CP) volume, cerebrospinal fluid (CSF) volume, and diffusion tensor imaging (DTI) values along the perivascular space (ALPS) across all groups. Volumes are presented as ratios of local volume to total intracranial volume (TIV). Significance levels are indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, and NS = not significant.
Statistical analysis
All statistical analyses were performed using SPSS software (version 26.0, IBM), and two-tailed P-values < 0.05 were considered statistically significant. Figures were generated using GraphPad Prism (version 10.2.3, GraphPad Software). Categorical data were analyzed using the chi-square test. The Shapiro-Wilk test was utilized to assess normality, and Levene’s test was used to evaluate homogeneity of variance. For variables meeting normality and homogeneity requirements, one-way analysis of variance with Bonferroni post hoc adjustments was performed. For variables not meeting these criteria, the Kruskal-Wallis test (H-test) with Bonferroni corrections for multiple comparisons was used. Linear regression models were used to examine relationships between variables. Mediation analysis was conducted to explore relationships among CP volume, CSF volume, DTI-ALPS index, and MMSE scores; age was included as a covariate.
Ethics approval
This retrospective study was approved by the Ethics Committee at Hainan Affiliated Hospital of Hainan Medical University, and in accordance with the Declaration of Helsinki. Ethics approval No: Med-Eth-Re[2023] 290. Due to retrospective nature of the study Informed consent was waived by the Ethics Committee at Hainan Affiliated Hospital of Hainan Medical University.
Results
Participant characteristics
Demographic and clinical characteristics are summarized in Table 1. In total, 188 participants were included in the study. NCs were significantly younger than patients with AD (P < 0.0001), MCI (P = 0.0002), or SCD (P < 0.0001). SCD patients had a higher level of education compared with AD patients (P < 0.0001); NCs were more educated than AD patients (P = 0.029). According to neuropsychological assessments, AD patients performed worse than patients in the MCI, SCD, and NC groups regarding global cognition (MMSE, MoCA-B), executive function (STT-A, STT-B), memory (MES), and language ability (BNT, VFT). No significant differences in HAMD or PSQI were observed among the groups. In terms of plasma biomarkers, the Aβ42/40 ratio was lower among AD patients than among SCD patients (P = 0.011) and NCs (P = 0.039); NfL levels were higher in AD patients than in SCD patients (P = 0.0002) and NCs (P = 0.0002). Moreover, p-tau181 levels were higher in AD patients than in MCI patients (P = 0.018), SCD patients (P < 0.0001), and NCs (P < 0.0001). MCI patients also showed higher levels compared with SCD patients (P = 0.033) and NCs (P = 0.015). There were no significant differences among groups concerning GFAP and Aβ40 levels.
Group comparisons of DTI-ALPS, CP volume, and CSF volume
Data for the DTI-ALPS index, CP volume, and CSF volume across groups are summarized in Table 2. TIV showed no significant differences among the groups. AD patients exhibited significant differences in DTI-ALPS index, CP volume, and CSF volume compared with MCI patients (DTI-ALPS: P = 0.00116; CP volume: P < 0.0001; CSF volume: P < 0.0001), SCD patients (DTI-ALPS: P < 0.0001; CP volume: P < 0.0001; CSF volume: P < 0.0001), and NCs (DTI-ALPS: P = 0.00018; CP volume: P < 0.0001; CSF volume: P < 0.0001). Additionally, MCI patients exhibited significant differences from NCs in CP volume (P = 0.00987) and CSF volume (P = 0.03640) (Fig. 2). After adjusting for age, education, and sex as covariates, no significant differences were observed between MCI patients and NCs in CP volume or CSF volume. However, AD patients continued to show significant differences in DTI-ALPS index, CP volume, and CSF volume relative to MCI patients (DTI-ALPS: P = 0.00549; CP volume: P < 0.0001; CSF volume: P = 0.00020), SCD patients (DTI-ALPS: P = 0.01249; CP volume: P < 0.0001; CSF volume: P < 0.0001), and NCs (DTI-ALPS: P = 0.02282; CP volume: P < 0.0001; CSF volume: P < 0.0001). When education, sex, age, and gray/white matter volume were added as covariates, CSF volume remained significantly different in AD patients compared with MCI patients (P = 0.0029), SCD patients (P < 0.0001), and NCs (P = 0.00012).
Graphs show relationships among choroidal plexus (CP) volume, cerebrospinal fluid (CSF) volume, and diffusion tensor imaging (DTI) along the perivascular space (ALPS) value. (A–C) Scatter plots demonstrating the correlations between age and the ALPS index (A), choroid plexus volume (B), and CSF volume (C); (D–E) Regression analyses showing the associations between the ALPS index and choroid plexus volume (D), as well as CSF volume (E); (F) Scatter plot illustrating the relationship between CSF volume and choroid plexus volume. Linear regression trend lines and 95% confidence intervals (dashed lines) illustrate the association with age.
Correlations among DTI-ALPS, CP volume, and CSF volume and their relationships with clinical parameters
Table 3 summarizes correlations among DTI-ALPS, CP volume, and CSF volume, as well as their associations with age, neuropsychological assessments, and plasma biomarkers. The DTI-ALPS index decreased with age (P = 0.00534, R2 = 0.036), whereas CP volume (P < 0.0001, R2 = 0.160) and CSF volume (P < 0.0001, R2 = 0.251) increased with age (Fig. 3A-C). The DTI-ALPS index was negatively correlated with CP volume (P < 0.0001, R2 = 0.085) and CSF volume (P < 0.0001, R2 = 0.125) (Fig. 3D-E); CP volume was positively correlated with CSF volume (P < 0.0001, R2 = 0.322) (Fig. 3F). The DTI-ALPS index was positively correlated with MMSE (P = 0.00011, R2 = 0.072), MoCA-B (P = 0.00044, R2 = 0.059), BNT (P = 0.00346, R2 = 0.040), VFT (P = 0.01548, R2 = 0.026), and MES (P = 0.00686, R2 = 0.033) scores; it was negatively correlated with STT-A (P < 0.0001, R2 = 0.065) and STT-B (P < 0.0001, R2 = 0.322) scores. CP volume was negatively correlated with MMSE (P < 0.0001, R2 = 0.127), MoCA-B (P < 0.0001, R2 = 0.094), BNT (P = 0.00443, R2 = 0.038), VFT (P = 0.01491, R2 = 0.015), and MES (P < 0.0001, R2 = 0.111) scores; it was positively correlated with STT-A (P < 0.0001, R2 = 0.102) and STT-B (P < 0.0001, R2 = 0.134) scores. CSF volume was negatively correlated with MMSE (P < 0.0001, R2 = 0.199), MoCA-B (P < 0.0001, R2 = 0.173), BNT (P = 0.00334, R2 = 0.040), PSQI (P = 0.02196, R2 = 0.023), and MES (P < 0.0001, R2 = 0.166) scores; it was positively correlated with STT-A (P < 0.0001, R2 = 0.159) and STT-B (P < 0.0001, R2 = 0.142) scores. Concerning plasma biomarkers, the DTI-ALPS index was negatively correlated with p-tau181 (P = 0.00895, R2 = 0.041) and NfL (P = 0.00386, R2 = 0.057); CP volume was positively correlated with p-tau181 (P < 0.0001, R2 = 0.141) and NfL (P < 0.0001, R2 = 0.133) but negatively correlated with Aβ42/40 ratio (P = 0.00445, R2 = 0.056); and CSF volume was positively correlated with p-tau181 (P = 0.00043, R2 = 0.078), GFAP (P = 0.00653, R2 = 0.043), and NfL (P = 0.00033, R2 = 0.122) but negatively correlated with Aβ42/40 ratio (P < 0.0001, R2 = 0.157).
(A-C) Scatter plots demonstrating the correlations between Pittsburgh Sleep Quality Index (PSQI) and: (A) the DTI-ALPS index, (B) choroid plexus volume, and (C) cerebrospinal fluid (CSF) volume. Linear regression trend lines with 95% confidence intervals (dashed lines) illustrate the association with age.
Mediation analysis
Mediation analysis, with age included as a covariate, revealed several significant indirect effects. CP volume had a significant indirect effect on cognition, mediated by the DTI-ALPS index and CSF volume. These pathways explained 13.37% and 50.15% of the total effect, respectively (Fig. 3A-B). CSF volume had a significant indirect effect on cognition, mediated by CP volume; this pathway explained 17.60% of the total effect (Fig. 3C). The DTI-ALPS index had a significant indirect effect on cognition, mediated by CP volume; this pathway explained 27.78% of the total effect (Fig. 3D). CSF volume fully mediated the effect of changes in the DTI-ALPS index on cognitive impairment (Fig. 3E).
Mediation analysis results. (A) Mediation path diagram depicting diffusion tensor imaging (DTI) along the perivascular space (ALPS) as a potential mediator between choroid plexus (CP) volume (independent variable) and cognition (dependent variable). (B) Mediation path diagram showing that cerebrospinal fluid (CSF) volume fully mediates the relationship between DTI-ALPS (independent variable) and cognition (dependent variable). (C) Mediation path diagram showing CP volume as a potential mediator between DTI-ALPS (independent variable) and cognition (dependent variable). (D) Mediation path diagram showing CP volume as a potential mediator between CSF volume (independent variable) and cognition (dependent variable). (E) Mediation path diagram showing CSF volume as a potential mediator between CP volume (independent variable) and cognition (dependent variable). Abbreviation: MMSE = Mini-Mental State Examination. Significance levels are indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
In terms of relationships among CP volume, CSF volume, and DTI-ALPS index, CP volume had a significant indirect effect on CSF volume, mediated by the DTI-ALPS index, which explained 10.81% of the total effect (Fig. 4A). The DTI-ALPS index had a significant indirect effect on CSF volume, mediated by CP volume, explaining 32.81% of the total effect (Fig. 4B). Finally, CSF volume fully mediated the interaction between CP volume and the DTI-ALPS index (Fig. 4C-D).
Mediation analysis results. (A) Mediation path diagram showing that cerebrospinal fluid (CSF) volume fully mediates the relationship between choroidal plexus (CP) volume (independent variable) and diffusion tensor imaging (DTI) along the perivascular space (ALPS) (dependent variable. (B) Mediation path diagram showing that CSF volume fully mediates the relationship between DTI-ALPS (independent variable) and CP volume (dependent variable). (C) Mediation path diagram showing CP volume as a potential mediator between DTI-ALPS (independent variable) and CSF volume (dependent variable). (D) Mediation path diagram showing DTI-ALPS as a potential mediator between CP volume (independent variable) and CSF volume (dependent variable). Significance levels are indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Discussion
Unlike prior studies examining CP volume or DTI-ALPS in isolation, our work provides three novel insights: First evidence of CSF volume as a mediator linking CP enlargement to glymphatic dysfunction (Fig. 5).Tri-modal integration of MRI metrics (CP/CSF/DTI-ALPS) to model CSF circulation dynamics.Demonstration that CSF volume changes persist after correcting for brain atrophy (P < 0.0001), supporting its role as an independent biomarker.
CP volume for assessment of CSF circulation
Our findings regarding CP volume are consistent with previous studies7,43which showed that CP volume increases in AD patients and is positively correlated with cognitive impairment and the DTI-ALPS index (P < 0.0001, R2 = 0.085). Furthermore, CP volume exhibits an age-dependent increase (P < 0.0001, R2 = 0.160). Changes in CP volume and glymphatic impairment are closely associated with neuroinflammation44,45. We hypothesize that astrocytes, particularly their AQP4 channels, mediate interactions between the CP and glymphatic system. These interactions likely lead to a combined effect of CP volume and glymphatic function on cognitive performance. Reports of significant correlations between plasma GFAP levels, CP volume, and glymphatic function46,47 may support this hypothesis. However, in the present study, no significant correlation was observed between plasma GFAP levels and CP volume or the DTI-ALPS index. This lack of significance could be attributed to the relatively small sample size.
DTI-ALPS index as a glymphatic clearance pathway
The results for the DTI-ALPS index in this study align with those of prior studies, constituting a decrease in the DTI-ALPS index among AD patients and an age-dependent decline in this measure18,48,49. The DTI-ALPS index is derived from a specific periventricular region, but has been proposed as a surrogate marker reflecting global glymphatic function. However, it cannot evaluate clearance capacity in specific local brain regions or particular ROIs14and the DTI-ALPS index may only be affected by perivascular spaces in white matter15,16. As previously reported, a lower DTI-ALPS index was associated with more severe cognitive impairment9. There is increasing evidence of links among the glymphatic system, sleep, and astrocytic function5,45,49. However, in this study, the DTI-ALPS index did not show significant correlations with the PSQI or GFAP levels. This lack of significance may be related to the limited sample size. Recent studies have shown that glymphatic failure may precede amyloidosis18emphasizing the importance of identifying non-aging factors that initiate glymphatic system damage.
CSF volume is involved in CSF circulation changes CSF, the primary component of the central nervous system’s extracellular fluid, plays a critical role in the glymphatic system by facilitating the clearance of waste products from brain tissue3. Our findings revealed that CSF volume was increased in AD patients. Furthermore, CSF volume was positively correlated with sleep quality (P = 0.02196, R2 = 0.023) (Fig. 6) and negatively correlated with DTI-ALPS index (P < 0.0001, R2 = 0.125). These differences in CSF volume remained statistically significant when gray matter, white matter volume, and age were included as covariates. And previous studies primarily attributed CSF changes to age-related brain atrophy. There has been a lack of consistency across studies regarding the correlation between CSF volume and glymphatic drainage. Similar to our results, studies of brain tumors and acute lymphoblastic leukemia patients have demonstrated increased CSF volume associated with impaired glymphatic drainage. Previous researchers have defined this increase as CSF accumulation8,9. However, in the present study, the increase in CSF volume was primarily attributed to the effects of brain atrophy. In contrast, NCs and AQP4 knockout mice showed an inverse trend4,26,50. Intriguingly, the positive correlation observed between CSF volume and sleep quality, combined with the known relationship between the glymphatic system and sleep5suggests a positive relationship between CSF volume and glymphatic drainage. However, this relationship is complex in AD patients. It remains challenging to disentangle the effects of brain atrophy from aging and AD pathology on CSF volume. In this study, CSF volume was the only metric significantly correlated with sleep quality (P = 0.02196, R2 = 0.023), displaying a positive association. This finding is consistent with previous reports that CSF volume is sensitive to circadian rhythms and sleep status26,27. Although circadian rhythmicity has been demonstrated in the glymphatic system, the DTI-ALPS index does not exhibit circadian properties51suggesting that DTI-ALPS lacks sufficient sensitivity to detect circadian changes in the glymphatic system. Although one study showed an association between CP volume and sleep52there is no evidence to suggest that CP volume rapidly responds to changes in sleep status. The mechanisms underlying changes in CSF volume in AD patients remain unclear and warrant further investigation in subsequent studies.
CSF volume mediates the effect of CP to glymphatic drainage
The mechanism by which the CP affects glymphatic drainage has been speculated upon by some researchers. They propose that the CP supports glymphatic drainage by increasing CSF production. The mediation analysis in the present study supports this hypothesis, although the precise mechanism—whether through molecular signaling pathways or alterations in intracranial pressure—requires clarification in future research. The correlation between the glymphatic system and CSF volume may involve AQP4, which reportedly plays a key role in the formation of extrachoroidal CSF by facilitating water filtration within brain parenchymal capillaries4. This previous finding suggests that the movement of water mediated by the polarized state of AQP4 supports both CSF generation and waste clearance. Aquaporin-4 (AQP4) polarization is intrinsically linked to blood-brain barrier (BBB) integrity53. In AD, BBB dysfunction disrupts perivascular AQP4 localization, impairing glymphatic clearance and exacerbating amyloid-β accumulation54. This disruption may explain the observed correlations between glymphatic impairment and plasma biomarkers, reflecting neurovascular pathology. Intriguingly, both the CP and the glymphatic system contribute to CSF production and waste removal55. Additionally, these systems may influence intracranial pressure and ventricular volume through their respective aquaporins, AQP1 (associated with the CP) and AQP4 (associated with the glymphatic system)4,55,56. This interplay may explain the observed correlations among CP volume, the glymphatic system, and CSF volume. Mediation analyses offer valuable insights into the potential mechanisms through which interactions among the glymphatic system, CP volume, and CSF volume influence cognition.
Future directions
In addition to AQP1 and AQP4, two other aquaporins, AQP9 and AQP11, are abundantly expressed in the brain. AQP9, with a distribution similar to that of AQP4, exhibits pro-inflammatory effects57,58. Although less is known about AQP11, there is evidence that, despite its minimal expression—often below detection thresholds—it may be widely distributed in the brain. Its strong expression in hypothalamic neurons suggests a role in endocrine function57. Further experimental and clinical research is needed to elucidate the roles of individual aquaporins, as well as their specific contributions to CSF circulation and glymphatic function.
Limitations of this study
This study had some limitations. First, considering its cross-sectional nature and limited sample size, the findings should be interpreted with caution. Longitudinal studies are needed to better understand the mechanisms of CSF circulation, including changes in CP volume and permeability, along with their relationships with CSF volume and glymphatic function. Second, CSF volume does not fully represent CSF accumulation because we could not exclude the effect of brain atrophy on CSF volume. Third, the use of CP volume as a proxy for CSF generation is not entirely consistent with the change in CP permeability; it cannot be assumed that a larger CP volume corresponds to greater CSF production. Fourth, there was incomplete data regarding plasma biomarkers, which may have impacted our analysis. Further studies are needed to clarify the changes in CSF circulation in AD. Fifth, there was a lack of information about participants’ medication use, which could have had a significant impact on CSF production and circulation, thereby confounding the observed relationships.
Conclusion
This study assessed CSF circulation in AD using noninvasive MRI techniques. We found that in addition to the DTI-ALPS index and CP volume, CSF volume can serve as an indicator of AD progression and CSF circulation. Furthermore, we demonstrated that the CP influences glymphatic drainage through its effects on CSF volume, highlighting a complex interplay among the CP, CSF, and the glymphatic system. The exploration of changes in CP volume, CSF volume, and the DTI-ALPS index, as well as factors associated with CSF circulation, may provide new insights into early AD diagnosis and potential strategies to slow or reverse disease progression.
Data availability
The data supporting the findings of this study can be obtained from the corresponding author upon reasonable request.
References
Scheltens, P. et al. Alzheimer’s disease. Lancet (London England). 397, 1577–1590. https://doi.org/10.1016/s0140-6736(20)32205-4 (2021).
Vaz, M. & Silvestre, S. Alzheimer’s disease: Recent treatment strategies. Eur. J. Pharmacol. 887 https://doi.org/10.1016/j.ejphar.2020.173554 (2020).
Tarasoff-Conway, J. M. et al. Clearance systems in the brain-implications for alzheimer disease. Nat. Rev. Neurol. 11, 457–470. https://doi.org/10.1038/nrneurol.2015.119 (2015).
Trillo-Contreras, J. L., Toledo-Aral, J. J., Echevarría, M. & Villadiego, J. AQP1 and AQP4 contribution to cerebrospinal fluid homeostasis. Cells 8 https://doi.org/10.3390/cells8020197 (2019).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl Med. 4, 147ra111. https://doi.org/10.1126/scitranslmed.3003748 (2012).
Zeppenfeld, D. M. et al. Association of perivascular localization of Aquaporin-4 with cognition and alzheimer disease in aging brains. JAMA Neurol. 74, 91–99. https://doi.org/10.1001/jamaneurol.2016.4370 (2017).
Choi, J. D. et al. Choroid plexus volume and permeability at brain MRI within the alzheimer disease clinical spectrum. Radiology 304, 635–645. https://doi.org/10.1148/radiol.212400 (2022).
Gao, M. et al. A histopathologic correlation study evaluating glymphatic function in brain tumors by Multi-Parametric MRI. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.Ccr-24-0150 (2024).
Lin, L. P. et al. Glymphatic system dysfunction in pediatric acute lymphoblastic leukemia without clinically diagnosed central nervous system infiltration: a novel DTI-ALPS method. Eur. Radiol. 33, 3726–3734. https://doi.org/10.1007/s00330-023-09473-8 (2023).
Ishida, K. et al. Glymphatic system clears extracellular Tau and protects from Tau aggregation and neurodegeneration. J. Exp. Med. 219 https://doi.org/10.1084/jem.20211275 (2022).
Eide, P. K. & Ringstad, G. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol. Open. 4, 2058460115609635. https://doi.org/10.1177/2058460115609635 (2015).
Tadayon, E., Pascual-Leone, A., Press, D. & Santarnecchi, E. Choroid plexus volume is associated with levels of CSF proteins: relevance for alzheimer’s and parkinson’s disease. Neurobiol. Aging. 89, 108–117. https://doi.org/10.1016/j.neurobiolaging.2020.01.005 (2020).
Huang, S. Y. et al. Glymphatic system dysfunction predicts amyloid deposition, neurodegeneration, and clinical progression in alzheimer’s disease. Alzheimers Dement. 20, 3251–3269. https://doi.org/10.1002/alz.13789 (2024).
Steward, C. E. et al. Assessment of the DTI-ALPS parameter along the perivascular space in older adults at risk of dementia. J. Neuroimaging. 31, 569–578. https://doi.org/10.1111/jon.12837 (2021).
Taoka, T. et al. Diffusion tensor image analysis along the perivascular space (DTI-ALPS): revisiting the meaning and significance of the method. Magn. Reson. Med. Sci. 23, 268–290. https://doi.org/10.2463/mrms.rev.2023-0175 (2024).
Ringstad, G. Glymphatic imaging: a critical look at the DTI-ALPS index. Neuroradiology 66, 157–160. https://doi.org/10.1007/s00234-023-03270-2 (2024).
Zhang, W. et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage 238, 118257. https://doi.org/10.1016/j.neuroimage.2021.118257 (2021).
Huang, S. Y. et al. Glymphatic system dysfunction predicts amyloid deposition, neurodegeneration, and clinical progression in alzheimer’s disease. Alzheimers Dement. https://doi.org/10.1002/alz.13789 (2024).
Shen, T. et al. Diffusion along perivascular spaces as marker for impairment of glymphatic system in parkinson’s disease. NPJ Parkinson’s Disease. 8, 174. https://doi.org/10.1038/s41531-022-00437-1 (2022).
Chang, H. I. et al. Gray matter reserve determines glymphatic system function in young-onset alzheimer’s disease: evidenced by DTI-ALPS and compared with age-matched controls. J. Neuropsychiatry Clin. Neurosci. 77, 401–409. https://doi.org/10.1111/pcn.13557 (2023).
Schartz, D. et al. Diffusion-Weighted imaging reveals impaired glymphatic clearance in idiopathic intracranial hypertension. AJNR Am. J. Neuroradiol. 45, 149–154. https://doi.org/10.3174/ajnr.A8088 (2024).
Si, X. et al. Neuroimaging evidence of glymphatic system dysfunction in possible REM sleep behavior disorder and Parkinson’s disease. NPJ Parkinsons Dis. 8 https://doi.org/10.1038/s41531-022-00316-9 (2022).
Tu, Y., Li, Z., Xiong, F. & Gao, F. Decreased DTI-ALPS and choroid plexus enlargement in fibromyalgia: a preliminary multimodal MRI study. Neuroradiology 65, 1749–1755. https://doi.org/10.1007/s00234-023-03240-8 (2023).
Müller, J. et al. Choroid plexus volume in multiple sclerosis vs neuromyelitis Optica spectrum disorder: A retrospective, Cross-sectional analysis. Neurol. Neuroimmunol. Neuroinflamm. 9 https://doi.org/10.1212/nxi.0000000000001147 (2022).
Hablitz, L. M. et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 11, 4411. https://doi.org/10.1038/s41467-020-18115-2 (2020).
Nilsson, C. et al. Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am. J. Physiol. 262, R20–24. https://doi.org/10.1152/ajpregu.1992.262.1.R20 (1992).
Benveniste, H. et al. Anesthesia with Dexmedetomidine and Low-dose isoflurane increases solute transport via the glymphatic pathway in rat brain when compared with High-dose isoflurane. Anesthesiology 127, 976–988. https://doi.org/10.1097/aln.0000000000001888 (2017).
Jessen, F. et al. A conceptual framework for research on subjective cognitive decline in preclinical alzheimer’s disease. Alzheimers Dement. 10, 844–852. https://doi.org/10.1016/j.jalz.2014.01.001 (2014).
Bondi, M. W. et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J. Alzheimers Dis. 42, 275–289. https://doi.org/10.3233/jad-140276 (2014).
McKhann, G. M. et al. The diagnosis of dementia due to alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 7, 263–269. https://doi.org/10.1016/j.jalz.2011.03.005 (2011).
Li, H., Jia, J. & Yang, Z. Mini-Mental state examination in elderly chinese: A Population-Based normative study. J. Alzheimers Dis. 53, 487–496. https://doi.org/10.3233/jad-160119 (2016).
Chen, K. L. et al. Validation of the Chinese version of Montreal cognitive assessment basic for screening mild cognitive impairment. J. Am. Geriatr. Soc. 64, e285–e290. https://doi.org/10.1111/jgs.14530 (2016).
Morris, J. C. The clinical dementia rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. https://doi.org/10.1212/wnl.43.11.2412-a (1993).
Ding, Z. & Chan, A. S. The shape trail test is sensitive in differentiating older adults with mild cognitive impairment: A Culture-neutral Five-minute test. J. Prev. Alzheimers Dis. 11, 1166–1176. https://doi.org/10.14283/jpad.2024.80 (2024).
Buysse, D. J., Reynolds, C. F. 3, Monk, T. H., Berman, S. R., Kupfer, D. J. & rd, & The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. https://doi.org/10.1016/0165-1781(89)90047-4 (1989).
Mok, E. H., Lam, L. C. & Chiu, H. F. Category verbal fluency test performance in Chinese elderly with alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 18, 120–124. https://doi.org/10.1159/000079190 (2004).
Mack, W. J., Freed, D. M., Williams, B. W. & Henderson, V. W. Boston naming test: shortened versions for use in alzheimer’s disease. J. Gerontol. 47, P154–158. https://doi.org/10.1093/geronj/47.3.p154 (1992).
Guo, Q. H., Zhou, B., Zhao, Q. H., Wang, B. & Hong, Z. Memory and executive screening (MES): a brief cognitive test for detecting mild cognitive impairment. BMC Neurol. 12, 119. https://doi.org/10.1186/1471-2377-12-119 (2012).
Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 23, 56–62. https://doi.org/10.1136/jnnp.23.1.56 (1960).
Alzheimer’s disease facts and figures. Alzheimers Dement 17, 327–406, (2021). https://doi.org/10.1002/alz.12328 (2021).
Taoka, T. et al. Reproducibility of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating interstitial fluid diffusivity and glymphatic function: changes in alps index on multiple condition acquisition eXperiment (CHAMONIX) study. Jpn J. Radiol. 40, 147–158. https://doi.org/10.1007/s11604-021-01187-5 (2022).
Taoka, T. et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in alzheimer’s disease cases. Jpn J. Radiol. 35, 172–178. https://doi.org/10.1007/s11604-017-0617-z (2017).
Jiang, D. et al. Regional glymphatic abnormality in behavioral variant frontotemporal dementia. Ann. Neurol. 94, 442–456. https://doi.org/10.1002/ana.26710 (2023).
Ricigliano, V. A. G. & Stankoff, B. Choroid plexuses at the interface of peripheral immunity and tissue repair in multiple sclerosis. Curr. Opin. Neurol. 36, 214–221. https://doi.org/10.1097/wco.0000000000001160 (2023).
Feng, S. et al. High-intensity interval training ameliorates alzheimer’s disease-like pathology by regulating astrocyte phenotype-associated AQP4 polarization. Theranostics 13, 3434–3450. https://doi.org/10.7150/thno.81951 (2023).
Bouhrara, M. et al. Association of plasma markers of alzheimer’s disease, neurodegeneration, and neuroinflammation with the choroid plexus integrity in aging. Aging Dis. 15, 2230–2240. https://doi.org/10.14336/ad.2023.1226 (2024).
Eide, P. K. et al. Plasma neurodegeneration biomarker concentrations associate with glymphatic and meningeal lymphatic measures in neurological disorders. Nat Commun 14, (2084). https://doi.org/10.1038/s41467-023-37685-5 (2023).
Zhang, X. et al. Glymphatic system impairment in alzheimer’s disease: associations with perivascular space volume and cognitive function. Eur. Radiol. 34, 1314–1323. https://doi.org/10.1007/s00330-023-10122-3 (2024).
Siow, T. Y. et al. Association of sleep, neuropsychological performance, and Gray matter volume with glymphatic function in Community-Dwelling older adults. Neurology 98, e829–e838. https://doi.org/10.1212/wnl.0000000000013215 (2022).
Gomolka, R. S. et al. Loss of aquaporin-4 results in glymphatic system dysfunction via brain-wide interstitial fluid stagnation. Elife 12 https://doi.org/10.7554/eLife.82232 (2023).
Han, G. et al. Age- and time-of-day dependence of glymphatic function in the human brain measured via two diffusion MRI methods. Front. Aging Neurosci. 15, 1173221. https://doi.org/10.3389/fnagi.2023.1173221 (2023).
Kim, J., Lee, H. J., Lee, D. A. & Park, K. M. Choroid plexus enlargement in patients with obstructive sleep apnea. Sleep. Med. 121, 179–183. https://doi.org/10.1016/j.sleep.2024.07.005 (2024).
Cibelli, A. et al. Cx43 carboxyl terminal domain determines AQP4 and Cx30 endfoot organization and blood brain barrier permeability. Sci. Rep. 11, 24334. https://doi.org/10.1038/s41598-021-03694-x (2021).
Xie, A. et al. Highly BBB-permeable nanomedicine reverses neuroapoptosis and neuroinflammation to treat alzheimer’s disease. Biomaterials 312, 122749. https://doi.org/10.1016/j.biomaterials.2024.122749 (2025).
Rasmussen, M. K., Mestre, H. & Nedergaard, M. Fluid transport in the brain. Physiol. Rev. 102, 1025–1151. https://doi.org/10.1152/physrev.00031.2020 (2022).
Mehta, N. H. et al. Quantifying cerebrospinal fluid dynamics: A review of human neuroimaging contributions to CSF physiology and neurodegenerative disease. Neurobiol. Dis. 170, 105776. https://doi.org/10.1016/j.nbd.2022.105776 (2022).
Trillo-Contreras, J. L., Ramírez-Lorca, R., Villadiego, J. & Echevarría, M. Cellular Distribution of Brain Aquaporins and Their Contribution to Cerebrospinal Fluid Homeostasis and Hydrocephalus. Biomolecules 12, (2022). https://doi.org/10.3390/biom12040530
Zahl, S. et al. Aquaporin-9 in the brain inflammatory response: evidence from mice injected with the parkinsonogenic toxin MPP(). Biomolecules 13 https://doi.org/10.3390/biom13040588 (2023).
Acknowledgements
This work was supported in part by the Key Science and Technology Project of Hainan Province (ZDYF2024SHFZ058), the National Natural Science Foundation of China (82271977), the Hainan Academician Innovation Platform Fund, and the Hainan Province Clinical Medical Center.
Author information
Authors and Affiliations
Contributions
Guarantors of integrity of entire study, F.C.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, W.L., Y.G., H.Q.; approval of final version of submitted manuscript, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, all authors; experimental studies, W.L.; statistical analysis, W.L., H.C.; and manuscript editing, all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, W., Chen, H., Zhang, Z. et al. Multimodal MRI reveals impaired glymphatic function with choroid plexus enlargement and cerebrospinal fluid expansion in alzheimer’s disease. Sci Rep 15, 30409 (2025). https://doi.org/10.1038/s41598-025-15923-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15923-8