Abstract
The dose-dependent effects of omega-3 supplementation on cognitive function remain unclear. This study aimed to evaluate the relationship between omega-3 dosage and cognitive outcomes in adults. A systematic search was conducted in PubMed, Scopus, and ISI Web of Science up to December 2024. Only randomized controlled trials (RCTs) were included. For each trial, we estimated the change in cognitive function per 2000 mg/day increment in omega-3 supplementation. Standardized mean differences (SMDs) and 95% confidence intervals (CIs) were calculated using a random-effects model. Dose-dependent effects were assessed through a dose-response meta-analysis of mean differences. The certainty of the evidence was evaluated using the GRADE approach. In total, 58 studies met the inclusion criteria. Each 2000 mg/d omega-3 supplementation showed a significant improvement in attention (SMD: 0.98; 95%CI: 0.41,1.54; GRADE = low), perceptual speed (SMD: 0.50; 95%CI: 0.05,0.95; GRADE = moderate) language (SMD: 0.98; 95%CI: 0.41,1.54; GRADE = low), primary memory (SMD: 0.87; 95%CI: 0.17,1.56; GRADE = moderate), visuospatial functions (SMD: 0.86; 95%CI: 0.46,1.27; GRADE = moderate), global cognitive abilities (SMD: 1.08; 95%CI: 0.73,1.44; GRADE = low). Levels of episodic memory decreased with the increase in omega-3 dose and then appeared to increase with an upward curve (P for non-linearity = 0.01, P for dose-response = 0.005). Levels of global cognitive abilities increased with the increase in omega-3 dosage, and then appeared to decreased with a downward curve (P for non-linearity = 0.008, P for dose-response = 0.002). The existing evidence suggests that omega-3 supplementation may lead to a modest improvement in cognitive function among adults. However, well-designed randomized trials with long-term follow-up are necessary to confirm and strengthen these findings.
Similar content being viewed by others
Introduction
Cognitive function refers to the continuous process of learning throughout one’s life and encompasses various mental abilities, including working memory, attention, language comprehension and production, reasoning, problem-solving, and decision-making1. A decline in these abilities, known as cognitive decline, typically progresses with aging. Multiple etiological factors have been proposed for this decline, including inflammation, genetic abnormalities, and impaired neuronal and cerebrovascular functioning2. Cognitive impairment and neurocognitive disorders pose significant risks for older adults. Currently, over 55 million people live with dementia worldwide—a number projected to rise to 78 million by 2030. Globally, neurocognitive disorders, including dementias, are leading contributors to mortality, disability-adjusted life years (DALYs), and the burden on health and social care systems3. In 2020, the total medical and caregiving costs associated with Alzheimer’s dementia in the United States alone were estimated to exceed $500 billion, with projections reaching $1.6 trillion by 20504.
Aging is the primary risk factor for neurological diseases. However, modifiable lifestyle factors—such as poor diet, physical inactivity, smoking, and low cognitive engagement—have also been associated with increased dementia risk. Among these, diet has emerged as a particularly important modifiable factor. Prospective epidemiological studies have shown a reduced incidence of dementia associated with the consumption of foods rich in antioxidants, unsaturated fats, and especially fish and fish oil, which are high in omega-3 fatty acids, particularly docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)5,6,7,8.
Polyunsaturated fatty acids (PUFAs)—especially DHA—play a vital role in brain and nervous system function by supporting membrane integrity and neuronal activity. DHA may exert neuroprotective effects through anti-inflammatory mechanisms, in part by competing with pro-inflammatory omega-6 fatty acids. Based on these pathways, long-chain omega-3 fatty acids (LCn-3s) are hypothesized to offer protective effects against cognitive decline9. Epidemiological evidence supports a beneficial role of high concentrations of long-chain polyunsaturated fatty acids (LC-PUFAs) in preserving cognitive function10,11,12,13. Several clinical trials have further investigated the potential cognitive benefits of omega-3 supplementation in both cognitively healthy individuals and those with cognitive impairments14,15. While some meta-analyses have reported mixed or inconclusive findings regarding the effects of omega-3 on cognitive function16,17many of these studies either focused solely on healthy individuals or exclusively on those with cognitive impairments. Moreover, few have incorporated dose-response analyses, which are crucial for understanding the relationship between omega-3 intake levels and cognitive outcomes18,19.
This study aimed to investigate the effect of omega-3 on cognitive function in healthy individuals, as well as those with dementia, mild cognitive impairment, or risk factors for cognitive decline. A key and distinguishing feature of our work is the incorporation of dose-response analysis, which allowed us to quantitatively assess the relationship between omega-3 intake and cognitive outcomes. This approach provides a more nuanced understanding than prior studies, which often lacked detailed exploration of dosage effects. Subgroup analyses further enriched the findings by examining variability across different populations.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) standards were followed in this study20and the protocol was registered on PROSPERO (registration number: CRD42024568781).
Search strategy
We systematically searched the literature from database inception until December 2024 using online databases such as PubMed, Scopus, and Web of Science to find published English studies. No publication time limitations were imposed. Supplementary Table 1 outlines the search strategy and keywords used from the MeSH database. We manually searched the reference lists of the retrieved publications and relevant reviews. To facilitate referencing, all publications were stored in an EndNote library (version X9 for Windows, Thomson Reuters, Philadelphia, PA, USA), and duplicate citations were removed. The literature search was conducted independently by ZY and NA, and any discrepancies were resolved through consultation with the lead researcher (SS-B).
Eligibility criteria
Original trials were considered if they met the following criteria: (1) Randomized controlled trials (RCTs) with a parallel or crossover design in adults (≥ 18 years) with dementia, mild to moderate cognitive impairments, Alzheimer’s disease (AD) or healthy individuals; (2) Trials evaluating the effectiveness of omega-3 supplementation including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), alone or in combination, to a control group; (3) Publications that provided means and standard deviation (SD) of changes in outcomes of interest (Supplementary Table 2), or reported sufficient information to estimate those values; (4) trials that administered doses of omega-3 supplements.
Studies were excluded if they (1) Were non-randomized controlled trials, animal studies, and other experimental designs; (2) Were performed in pregnant women, children, and adolescents; and (3) letters, commentaries, conference presentations, reviews, meta-analyses and ecological studies.
Data extraction
Data extraction was conducted independently by two reviewers (ZY and NA). The following information was extracted from each trial: the last name of the first author, year of publication, study design (parallel or crossover), sample size, mean age, baseline body mass index (BMI), intervention duration, description of the intervention and control arms, dose of omega-3 supplementation, types of outcome measurements, and the means and SDs of changes in outcomes from baseline for each group. When necessary, results were standardized to identical units for meta-analysis by incorporating mean and SDs changes in cognitive function throughout the trial for both intervention and control groups. Numerical estimates presented in graphical format were extracted using Plot Digitizer (http://plotdigitizer.sourceforge.net/). All discrepancies were resolved through consultation with the principal investigator (SS-B).
Risk of bias assessment
Two independent investigators (ZY and NA) conducted the quality assessment to evaluate the risk of bias in the included studies. The Risk of Bias 2 (RoB 2) tool, designed for randomized trials, was used to assess potential sources of bias21.
Statistical analysis
Standardized mean difference (SMD) and its 95% confidence interval (CI) were used as the effect size in order to present the meta-analysis’s findings. This approach was adopted due to the diverse outcome measures employed by researchers in assessing cognitive function. The mean differences and their SDs in cognitive tests between the intervention and control groups were used to calculate the overall effect sizes. When mean changes were not available, they were calculated using changes in cognitive function during the intervention. Hozo et al. method was used for converting standard errors (SEs), 95% confidence intervals (CIs), and interquartile ranges (IQRs) to SDs22. We assessed the heterogeneity using I2 values. We also conducted sensitivity analyses by eliminating one study at a time to examine the influence of each study on the results. Publication bias was assessed using Egger’s test. Subgroup analyses were performed by using predetermined variables, such as types of outcome measurements, intervention duration, study’s location, participant’s age and sex, health status, baseline BMI, and co-intervention.
We used the method introduced by Crippa and Orsini23 to calculate the SMD and its corresponding SD of change in cognitive function domains for every 2000 mg/d increments in omega3 supplementation in the intervention group relative to the control group in each trial. This method required the dose (mg/d) of omega-3 supplementation, the standardized mean, and its corresponding SD of change in cognitive function domains, and the number of participants in each study arm. For comparisons including ≤ 5 studies, trial-specific results were pooled using a fixed-effects model24. Finally, a dose-response meta-analysis provided further insight into the shape of the effect of omega-3 supplementation on cognitive function domains24. Statistical analyses were conducted using STATA software version 16.1. A two-tailed P value of less than 0.05 was considered significant.
Certainty of evidence
The certainty in the evaluations was assessed by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach25. According to GRADE recommendations, evidence obtained from RCTs is initially considered to have a high level of assurance. This tool rates studies as high, moderate, low, or very low quality, with options to downgrade or upgrade based on pre-specified criteria. Downgrade criteria include study limitations, inconsistency, indirectness, imprecision, and publication bias, while a significant effect size, a dose-response gradient, and attenuation by plausible confounding can upgrade the quality.
Results
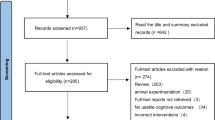
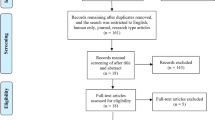
A total of 4,520 records were identified through the systematic search, of which 1,240 were duplicates and excluded. After screening titles and abstracts of the remaining 3,280 records, 3,191 were excluded for not meeting the eligibility criteria. Following full-text review of 89 publications, 31 additional studies were excluded Supplementary Table 3. The final meta-analysis included 58 studies. Among the excluded studies, 23 lacked sufficient data, 2 investigated unrelated interventions, and 1 assessed an irrelevant outcome. One was a study protocol, and full texts or supplementary data could not be retrieved for three studies. The detailed selection process is presented in the PRISMA flow chart Fig. 1.
Study characteristics
Key characteristics of the included studies are summarized in Table 1. Trials were published between 2006 and 2023 and conducted in USA and Canada (n = 14)12,26,27,28,29,30,31,32,33,34,35,36,37,38Europe (n = 20)15,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57and Asia /Oceania (n = 24)7,14,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79. Thirty two studies enrolled cognitively healthy participants15,26,28,29,30,31,32,33,34,35,36,41,42,43,44,46,47,48,50,51,53,54,57,60,62,64,65,70,71,73,76,77,78while 16 investigations included those with cognitive impairments7,12,14,27,37,40,55,58,61,63,66,68,69,72,79. An additional three studies focused specifically on Alzheimer’s disease (AD)38,45,56and three included participants with both AD and cognitive impairments52,59,67. Two trials involved participants with memory issues, and two assessed other mental disorders39,49,74,75. Nine studies used multi-component interventions combining omega-3 with compounds such as vitamins or antioxidants28,39,40,44,50,53,54,55,74,78. The daily dosage of omega-3 varied from 230 to 4950 mg/day, and the duration of the intervention ranged from 4 to 160 weeks. The most common cognitive assessment tools were the Mini-Mental State Examination (MMSE) for global cognition12,14,28,30,32,39,45,46,52,55,56,59,60,61,62,63,67,68, Trail Making A and B and Stroop Tests for attention and executive function15,26,28,30,33,35,36,37,39,44,47,49,50,55,69,76Block Design for visuospatial functions7,14,66,79and a variety of recall assessment tools for memory14,28,31,39,47.
Risk of bias assessment
Based on the RoB 2 tool, 16 trials were rated as good quality7,14,15,30,34,35,38,41,43,49,50,51,54,55,59,7919 as fair quality12,27,36,40,47,48,52,53,58,60,64,65,66,67,68,69,70,73,77and 23 trials were evaluated as poor quality26,29,31,32,33,37,39,42,44,45,46,56,57,61,62,63,71,72,74,75,76,78 (Supplementary Table 4). Quality ratings reflected differences in randomization methods, blinding, outcome reporting, and attrition rates.
Meta-analysis
Effects of omega-3 supplementation on cognitive outcomes
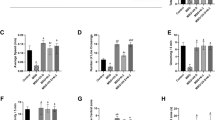
In the following sections, the reported effect sizes reflect the impact of daily supplementation with 2,000 mg of omega-3 fatty acids. SMDs with 95% confidence intervals (CIs) were calculated for each cognitive domain. Subgroup analyses, dose–response trends, and assessments of heterogeneity and publication bias are also reported where applicable. Across outcomes, substantial statistical heterogeneity (I2 > 95%) was consistently observed. This was expected due to the wide variation in study populations, intervention dosages and durations, baseline cognitive status, and the cognitive assessment tools used. Summary of main results have shown in Fig. 2.
Effects of omega-3 supplements on attention
A total of 32 study arms examined the impact of omega-3 supplementation on attention. The outcomes were not significantly affected (SMD: 0.12; 95%CI: -0.96,1.19, Supplementary Fig. 1) and the GRADE assessment rated the certainty of this evidence as low, suggesting limited confidence in this finding. Marked heterogeneity was detected among these studies (I2: 98.5%; P < 0.0001). Subgroup analyses (Table 2) were conducted based on participants’ age, baseline BMI, health status, geographical location, intervention duration, and the cognitive test used to assess attention. Notably, omega-3 supplementation was associated with improved attention in trials lasting more than 26 weeks and in participants with a baseline BMI > 25 (SMD: 0.45; 95%CI: 0.24 ,0.66 and SMD: 0.39; 95%CI: 0.19 ,0.59 respectively). Positive effects were also evident in studies conducted in Asia and Oceania, and those using Stroop tasks for attention assessment (SMD: 0.98; 95%CI: 0.72 ,1.24 and SMD: 0.72; 95%CI: 0.43,1.01 respectively). Significant reverse effects were seen in RCTs that did not have co-intervention (SMD: -0.19; 95%CI: -0.37, -0.02) (Table 2). Visual inspection of the funnel plot and Egger’s test did not suggest publication bias (P = 0.85) (Supplementary Fig. 2). The effect size remained non-significant after step-wise exclusion of each study from the main analysis (MD range: -1.16 to 1.41).
Dose-dependent effects of omega-3 on attention are indicated in Supplementary Fig. 3 and Table 3. Levels of attention increased proportionally with the increase in omega-3 dosage up to 1500 mg/d (SMD1500 mg/d: 0.58, 95%CI: -0.06, 1.22), followed by a modest decline at higher doses. However, the relationship was not statistically non-linear (P for non-linearity = 0.49), indicating that changes in effect were not significantly different across increasing doses. The overall dose-response trend did not reach statistical significance either (P for dose-response = 0.14).
Effects of omega-3 supplements on executive functions
Thirty-nine study arms assessed the effect of omega-3 supplementation on executive function. No statistically significant effect was observed (SMD: 0.53; 95% CI: -0.16, 1.21), and the low certainty of evidence suggests that future studies may alter this estimate. Considerable heterogeneity was detected across studies (I2 = 98.6%; P < 0.0001) Supplementary Fig. 4. Subgroup analyses (Table 2) revealed notable differences in effect based on study location and participant characteristics. A remarkable improvement in executive functions was found in trials conducted in European and Asian countries, as well as among participants with a baseline BMI greater than 25 (SMD: 0.28; 95%CI: 0.16,0.41 and SMD: 1.074; 95%CI: 0.87,1.26 and SMD: 0.39; 95%CI: 0.31,0.48 respectively). Conversely, a significant decline in executive function was observed in studies that included only women (SMD: -0.48; 95%CI: -0.91, -0.04), which highlights potential sex-specific differences in response. Significant positive effects were demonstrated in RCTs with having no co-intervention (SMD: 0.56; 95%CI: 0.46, 0.66) (Table 2). Funnel plot analysis and Egger’s test indicated no evidence of publication bias (P = 0.718) Supplementary Fig. 5. Sensitivity analyses demonstrated that the overall findings remained stable after the stepwise exclusion of individual studies (MD range: -0.35 to 1.31).
Dose-response modeling (Supplementary Fig. 6; Table 3) suggested no meaningful trend between omega-3 dosage and executive function outcomes. The effect size decreased slightly with increasing doses, but no statistically significant linear or non-linear relationship was observed (P for non-linearity = 0.92; P for dose-response = 0.97). This suggests that increasing omega-3 intake was not associated with a dose-dependent change in executive function performance.
Effects of omega-3 supplements on perceptual speed
A statistically significant improvement in perceptual speed was not observed (SMD: 0.44; 95%CI: -0.01,0.90), which was supported by moderate-certainty evidence, indicating a reasonably robust finding. Supplementary Fig. 7. However, substantial heterogeneity was detected among the studies (I2 = 96.4%, p < 0.0001). Based on subgroup analysis, studies conducted on both men and women revealed a significant enhancement in perceptual speed (SMD: 0.42; 95%CI: 0.34,0.49). Significant positive effects were illustrated in RCTs that did not have co-intervention (SMD: 0.59; 95%CI: 0.50, 0.68) (Table 2). No evidence of publication bias was detected according to funnel plot and Egger’s test (P = 0.51) Supplementary Fig. 8. Sensitivity analysis revealed that studies conducted by Philippa A. Jackson et al. (SMD: 0.58, 95%CI: 0.14, 1.03), Matthew P. Pase et al. (SMD: 0.47, 95%CI: 0.01, 0.93), Hisanori Tokuda et al. (SMD: 0.64, 95%CI: 0.21,1.07), Sandrine Andrieu et al. (SMD: 0.50, 95%CI: 0.04, 0.96), and Pinelopi S. Stavrinou et al. (SMD: 0.50, 95%CI: 0.03, 0.96) had a notable influence on the overall results.
Dose-response analysis illustrated in Supplementary Fig. 9 and Table 3 showed that perceptual speed declined with increasing omega-3 dosage up to 500 mg/d (SMD500mg/d: -0.41, 95%CI: -1.42, 0.60), and then appeared to increase with an upward curve (P for non-linearity = 0.18), and the overall dose-response relationship did not reach significance (P = 0.09).
Effects of omega-3 supplements on Language
Twenty-six arms of studies evaluated the effect of omega-3 supplementation on language abilities. While a substantial improvement in language was observed (SMD: 0.98; 95%CI: 0.41,1.54) Supplementary Fig. 10, the GRADE rating of low certainty limits the strength of this conclusion. Marked heterogeneity was detected across studies (I2 = 96.8%; P < 0.0001), likely reflecting variation in populations, intervention durations, and assessment methods prompting subgroup analysis which revealed statistically significant increase in language abilities after omega-3 supplementation when the duration was less than 48 weeks, the participants were younger than 60 years old, without any cognitive disorders, and when trials were carried out in American and European countries (SMD: 0.78; 95%CI: 0.65, 0.91 and SMD: 0.83; 95%CI: 0.68, 0.98 and SMD: 0.54; 95%CI: 0.41, 0.66 and SMD: 0.40; 95%CI: 0.29, 0.50 respectively) (Table 2). Additionally, the use of assessment tools other than verbal fluency exams significantly greater improvement language abilities (SMD: 0.35; 95%CI: 0.26,0.44) (Table 2). Significant positive effects were showed in RCTs with having no co-intervention (SMD: 0.359; 95%CI: 0.25, 0.46) (Table 2). Visual inspection of funnel plot and the results of Egger’s tests did not suggest any publication bias (P = 0.375) Supplementary Fig. 11. According to the sensitivity analysis, a study by Peter J. Rogers et al.54 had a notable impact on the overall findings (SMD: 0.34; 95%CI: -0.08,0.78).
Dose-dependent effects of omega-3 on language abilities are demonstrated in Supplementary Fig. 12 and Table 3. Language abilities increased with the increase in omega-3 dosage up to 1500 mg/d (SMD1500mg/d: 0.96, 95%CI: -0.55, 2.47), and then appeared to plateau. However, the non-significant P for non-linearity (P for non-linearity = 0.69) and overall dose-response trend (P for dose-response = 0.26) suggest that the relationship was approximately linear and not statistically dependent on dosage within the range studied.
Effects of omega-3 supplements on episodic memory
No statistically significant effect of omega-3 supplementation on episodic memory was observed (SMD: 0.27; 95%CI: -0.06, 0.59) (Supplementary Fig. 13), but the evidence was rated as low certainty, reflecting concerns about the consistency and precision of the results. Substantial heterogeneity was present across studies (I2: 92.9%; P < 0.0001). Subgroup analysis revealed a significant improvement in episodic memory among cognitively healthy individuals and in studies with intervention durations less than 48 weeks (SMD: 0.28; 95%CI: 0.20, 0.35 and SMD: 0.42; 95%CI: 0.33, 0.50 respectively). Significant positive effects were also demonstrated in RCTs that did not have co-intervention (SMD: 0.43; 95%CI: 0.34, 0.52) (Table 2). No evidence of publication bias was detected, based on funnel plot inspection and Egger’s test (P = 0.912) (Supplementary Fig. 14). Sensitivity analysis identified that individual studies by Karin Yurko-Mauro et al.12 (SMD: 0.32; 95%CI: 0.007, 0.64), Sandrine Andrieu et al.39 (SMD: 0.32; 95%CI: 0.03,0.61) and Rebecca Power et al.53 (SMD: 0.32; 95%CI: 0.005,0.64) had an influence on the overall results, each producing modest positive effects.
Dose-response analysis is presented in Supplementary Fig. 15 and Table 3. Levels of episodic memory decreased with the increase in omega-3 dose up to 1000 mg/d (SMD1000mg/d: -0.24, 95%CI: -0.98, 0.49), then appeared to increase with an upward curve. The test for non-linearity was statistically significant (P for non-linearity = 0.01), and the overall dose-response association reached significance (P for dose-response = 0.005), suggesting that benefits on episodic memory may only emerge at higher intake levels.
Effects of omega-3 supplements on primary memory
Our meta-analysis showed that omega-3 supplementation has a positive effect on primary memory (SMD: 0.77; 95% CI: 0.06, 1.48), and the high-certainty evidence strongly supports the reliability of this finding. (Supplementary Fig. 16). However, marked heterogeneity was detected across the included studies (I2 = 96.2%; P < 0.0001). We could explain the heterogeneity by doing subgroup analyses depending on the study’s location and memory assessment tools. Notably, significant improvements in primary memory were observed in studies conducted in America and those utilizing recall-based tests (SMD: 0.37; 95%CI: 0.11,0.63 and SMD: 0.61; 95%CI: 0.01,1.20 respectively) (Table 2). Significant positive effects were also demonstrated in RCTs that did not have co-intervention (SMD: 0.991; 95%CI: 0.852, 1.130) (Table 2). Funnel plot symmetry and Egger’s test results provided no indication of publication bias (P = 0.995) (Supplementary Fig. 17). Sensitivity analysis showed that individual studies by Lai Kuan Lee et al. (SMD: 0.67; 95%CI: -0.04, 1.40), Yan-Ping Zhang et al. (SMD: 0.55; 95%CI: -0.02, 1.14), Mengyue Li et al. (SMD: 0.71; 95%CI: -0.01, 1.45) had an influence on the overall results.
Dose-dependent effects of omega-3 on primary memory are revealed in Supplementary Fig. 18 and Table 3. Dose-response analysis (Supplementary Fig. 18 and Table 3) revealed a linear relationship between omega-3 dosage and primary memory performance, with greater benefits emerging at doses above 1000 mg/d. The absence of a significant non-linear trend (P for non-linearity = 0.48) alongside a significant dose-response association (P for dose-response = 0.02) supports a gradual, dose-related enhancement of primary memory.
Effects of omega-3 supplements on visuospatial functions
Omega-3 supplementation was linked to a significant improvement in visuospatial function (SMD: 0.89; 95% CI: 0.45, 1.33), with the GRADE assessment assigning a high certainty to this evidence, thereby reinforcing the strength of this result (Supplementary Fig. 19. Considerable heterogeneity was anticipated, and indeed, substantial variability was observed (I2 = 97.4%; P < 0.0001). To explore potential sources, subgroup analysis was performed based on intervention duration, location of the study, participants’ health status and baseline BMI, outcome assessment tools and co-intervention. However, none of them were able to explain the cause of heterogeneity except assessment tools (Table 2). No publication bias was found based on funnel plot and Egger’s test (P = 0.169) (Supplementary Fig. 20). Sensitivity analysis confirmed the stability of the findings, as no study influenced the overall results after individual study effects were removed (MD range: 0.20 to 1.44).
Dose-dependent effects of omega-3 on visuospatial functions are revealed in Supplementary Fig. 21 and Table 3. Visuospatial functions increased linearly, indicating a consistent effect across increasing doses of omega-3, particularly at doses above 500 mg (P for non-linearity = 0.37, P for dose-response = 0.004).
Effects of omega-3 supplements on global cognitive ability
Global cognitive abilities markedly improved by omega-3 supplementation (SMD: 1.00; 95%CI: 0.65,1.35), although the low certainty rating from GRADE suggests caution in interpreting the robustness of this finding. (Supplementary Fig. 22). To discover the potential source of the considerable heterogeneity (I2 = 97.1%; P < 0.0001), subgroup analysis was conducted. Studies with intervention duration less than 48 weeks, participants younger than 60 years old, cognitively healthy individuals, and participants with normal BMI accounted for the observed heterogeneity (Table 2). Significant reverse effects were seen in RCTs that did not have co-intervention (SMD: -0.07; 95%CI: -0.14, -0.01) (Table 2). Funnel plot asymmetry and Egger’s test indicated significant publication bias (P < 0.0001) (Supplementary Fig. 23). Despite this, sensitivity analysis confirmed the robustness of the overall effect estimate, as no single study significantly influenced the results (MD range: 0.50 to 1.41). Dose-dependent effects of omega-3 on language abilities are demonstrated in Supplementary Fig. 24 and Table 3. Enhancement of global cognitive abilities was observed with increasing omega-3 dosage up to 1500 mg/day. (SMD1500mg/d: 1.00, 95%CI: 0.31, 1.68), followed by downward trend at higher doses. The significant non-linearity (P for non-linearity = 0.008) suggests that the relationship between dose and cognitive effect was not consistent across the full dosage range, despite an overall significant dose-response trend (P for dose-response = 0.002).
GRADE assessment
The GRADE evidence profile and the certainty of omega-3 supplementation’s effects on cognitive function are shown in Supplementary Table 5. The certainty of evidence was low for attention, executive functioning, language, episodic memory, and global cognitive ability; moderate for perceptual speed, and high for primary memory and visuospatial functions.
Discussion
Globally, the role of omega-3 fatty acids in preserving and enhancing cognitive function has attracted increasing attention, particularly as populations age and the burden of cognitive decline grows. However, evidence remains inconclusive regarding the optimal dosage and the specific cognitive domains that benefit most. To the best of our knowledge, this is the first GRADE-assessed meta-analysis to evaluate the dose-response effects of omega-3 supplementation on adult cognitive function, including both cognitively healthy individuals and those with cognitive impairments. It represents a key methodological advancement over previous reviews, offering more detailed insights into the effects of different omega-3 dose levels which highlighting the novelty of our research.
Our primary analysis revealed that omega-3 supplementation is associated with a notable improvement in in perceptual speed, language, primary memory, visuospatial function, and global cognitive ability. The subgroup analysis indicates that greater cognitive benefits in the most of the cognitive domains are observed in participants who are cognitively healthy and in trials with intervention durations shorter than 48 weeks.
The dose-response analysis revealed valuable findings regarding omega-3 dosage and improvements in primary and episodic memory, visuospatial functions, and global cognitive ability. According to our analysis, the optimal dose of omega-3 supplementation—associated with the most consistent and pronounced effects—is between 1000 and 2500 mg per day. Moreover, no adverse effects were reported among participants consuming omega-3 supplements within this dosage range. This apparent optimal range may reflect both a biological plateauing of benefits at higher doses and the limited number of trials that evaluated doses beyond 2500 mg/day.
Sensitivity analysis indicated that several studies exerted a notable influence on the overall results. Their impacts might be linked to their methodological and population characteristics. For instance, studies conducted by Zhang was conducted on Chinese older adults with mild cognitive impairments, and assessed all cognitive domains comprehensively. Additionally, they prescribed high-dose DHA supplements (up to 2000 mg) which may have caused greater positive effect. In contrast, Marriott et al. included younger adults and used much more sensitive test, such as Stroop and reaction time task, which efficiently detect subtle changes. Rogers et al. conducted a 12-week study including healthy mid-age adult, which might be the reason of capturing early effects of omega-3 supplementation. Similarly, studies by Yurko-Mauro et al., Andrieu et al., and Power et al. targeted older adults (~ 69–75 years), but varied in terms of health conditions (e.g., subjective memory complaints vs. healthy aging) and intervention types, influencing effect sizes accordingly.
While these findings are promising, those should be interpreted with caution due to considerable heterogeneity and the low certainty of evidence, primarily driven by variability in study population, dosage and duration, outcome measures, baseline cognitive status, and the cognitive assessment tools used.
These findings seem to be in line with the results of A. Alex et al.80who showed a slight improvement in memory function. Although Mazereeuw et al.18 reported consistent findings for attention, immediate recall and processing speed, the effects of n-3 FAs were limited to CIND (cognitive impairment no dementia) populations. In contrast to our findings, several previous meta-analyses have reported divergent results. Ruth E Cooper et al.81 discovered no evidence of an effect of n-3 PUFA supplementation on cognitive performance in the general population, and Balachandar et al.2 found that DHA supplementation has an insignificant or no beneficial role in slowing or improving age-related decline in memory, executive functions, working memory, and attention. The differing results between our meta-analysis and those of Balachandar et al. (2020) and Cooper et al. (2015) likely stem from variations in study populations, cognitive domains assessed, and methodological approaches. While Balachandar et al. focused mainly on older adults with age-related cognitive decline and Cooper et al. included general populations without detailed subgroup analyses, our study encompassed both cognitively healthy and impaired individuals and examined multiple specific cognitive domains. Additionally, unlike the previous reviews, we conducted a dose-response analysis identifying an optimal omega-3 dose range (1000–2500 mg/day), which may clarify inconsistencies in prior findings due to heterogeneous dosing. Differences in intervention duration and updated inclusion criteria further contribute to the contrasting results observed.
In terms of underlying mechanisms, omega-3 poly-unsaturated fatty acids, particularly DHA and EPA, play crucial roles in brain structure and function. These fatty acids offer protective effects notably mitigating cognitive decline in aging population.
Perceptual speed declines have been closely associated with reduced white matter integrity, which disrupts neural connectivity and negatively impacts cognitive processing speed82. Notably, long-chain omega-3 fatty acids may help preserve white matter structure and support neural integrity, thereby enhancing perceptual speed83. Additionally, the hippocampus plays a central role in primary memory functions84and its atrophy has been strongly linked to memory impairments85. Omega-3 supplementation has demonstrated potential in attenuating hippocampal volume loss among older adults, offering a protective effect against age-related memory decline86. Furthermore, the parietal lobe is critically involved in visual-spatial processing, underscoring its importance in maintaining visuospatial cognitive functions87. Evidence indicates that long-chain omega-3 supplementation preserves brain structure—including the parietal lobe—by exerting beneficial effects on white matter integrity and gray matter volume57. Ultimately, omega-3 fatty acids serve as precursors for synthesis of neuroprotectin D1 (NPD1) and resolvins. These lipid mediators actively attenuate neuroinflammation, a major contributor to neurodegeneration and cognitive ability decline88,89.
The current meta-analysis has several strengths, including a large sample size that enabled a comprehensive investigation of the dose-response relationship between omega-3 supplementation and various cognitive domains. This dose-response analysis represents a key methodological advancement over previous reviews, offering more detailed insights into the effects of different omega-3 intake levels. Our meta-analysis is novel in combining dose-response modeling with a formal GRADE assessment of the certainty of evidence in the context of omega-3 supplementation and cognitive function. This integrated approach provides a more nuanced understanding of how different omega-3 doses relate to cognitive outcomes, while transparently evaluating the quality and strength of the evidence. Additionally, we performed subgroup analyses to explore variability across different populations, further enhancing the clinical relevance and interpretability of our findings.
This study also has a few limitations. First, several cognitive tests were used to reflect a single cognitive domain. Nonetheless, by giving the most popular cognitive test for analysis priority, an attempt was made to lessen this heterogeneity. Additionally, it was discovered that some cognitive tests lacked specificity for the assigned cognitive domain. Second, we couldn’t perform subgroup analysis based on cognitive assessment methods for all of the domains due to this variety of cognitive tests. Third, the majority of the studies were low quality based on risk of bias assessment. Finally, sensitivity analysis demonstrated that by removing five studies in perceptual speed7,36,54,79one study in language54and three studies in episodic memory12,39,53the overall effect size was significantly changed; therefore, the results for these outcomes should be interpreted with caution. To reduce heterogeneity and improve comparability, we recommend standardizing cognitive assessment tools across future studies. Moreover, higher-quality randomized controlled trials with rigorous methodology are needed to strengthen the certainty of evidence. Lastly, longer-term studies exploring optimal dosing strategies and their sustained impact on diverse cognitive domains are warranted to better inform clinical recommendations.
Conclusion
In conclusion, our meta-analyses indicate that omega-3 fatty acid supplementation may confer beneficial effects on several cognitive domains, including perceptual speed, language ability, primary memory, visuospatial functions, and overall cognitive performance. Furthermore, dose-response analysis suggests that memory, visuospatial functions, and global cognition may improve at specific dosage levels of omega-3 intake. However, based on the GRADE assessment, high certainty of evidence supports only the outcomes related to primary memory and visuospatial functions. The findings for other cognitive domains should be interpreted with caution.
Data availability
The data are available from the corresponding author on reasonable request.
References
Jiao, J. et al. Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: a systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 100, 1422–1436 (2014).
Balachandar, R., Soundararajan, S. & Bagepally, B. S. Docosahexaenoic acid supplementation in age-related cognitive decline: a systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 76, 639–648 (2020).
Gauthier, S. R. N. P., Morais, J. A. & Webster, C. World Alzheimer Report 2021: Journey Through the Diagnosis of Dementia (Alzheimer’s Disease International, 2021).
Aranda, M. P. et al. Impact of dementia: health disparities, population trends, care interventions, and economic costs. J. Am. Geriatr. Soc. 69, 1774–1783 (2021).
Kosti, R. I. et al. Fish intake, n-3 fatty acid body status, and risk of cognitive decline: a systematic review and a dose-response meta-analysis of observational and experimental studies. Nutr. Rev. 80, 1445–1458 (2022).
Cederholm, T., Salem, N. Jr. & Palmblad, J. ω-3 fatty acids in the prevention of cognitive decline in humans. Adv. Nutr. (Bethesda Md). 4, 672–676 (2013).
Bakre, A. T. et al. Association between fish consumption and risk of dementia: a new study from China and a systematic literature review and meta-analysis. Public Health. Nutr. 21, 1921–1932 (2018).
Morris, M. C. et al. Fish consumption and cognitive decline with age in a large community study. Arch. Neurol. 62, 1849–1853 (2005).
Devassy, J. G. et al. Omega-3 polyunsaturated fatty acids and Oxylipins in neuroinflammation and management of alzheimer disease. Adv. Nutr. 7, 905–916 (2016).
Dangour, A. D. et al. Fish consumption and cognitive function among older people in the UK: baseline data from the OPAL study. J. Nutr. Health Aging. 13, 198–202 (2009).
Schaefer, E. J. et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and alzheimer disease: the Framingham heart study. Arch. Neurol. 63, 1545–1550 (2006).
Yurko-Mauro, K. et al. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimer’s Dement. J. Alzheimer’s Assoc. 6, 456–464 (2010).
D’Ascoli, T. A. et al. Association between serum long-chain omega-3 polyunsaturated fatty acids and cognitive performance in elderly men and women: the Kuopio ischaemic heart disease risk factor study. Eur. J. Clin. Nutr. 70, 970–975 (2016).
Lee, L. K. et al. Docosahexaenoic acid-concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): a 12-month randomised, double-blind, placebo-controlled trial. Psychopharmacol. (Berl). 225, 605–612 (2013).
van de Rest, O. et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology 71, 430–438 (2008).
Brainard, J. S. et al. Omega-3, Omega-6, and polyunsaturated fat for cognition: systematic review and Meta-analysis of randomized trials. J. Am. Med. Dir. Assoc. 21, 1439–1450e1421 (2020).
Yang, L. et al. N-3 polyunsaturated fatty acids in elderly with mild cognitive impairment: A systemic review and Meta-Analysis. J. Alzheimers Dis. 99, S81–s95 (2024).
Mazereeuw, G. et al. Effects of ω-3 fatty acids on cognitive performance: a meta-analysis. Neurobiol. Aging. 33, 1482e1417–1482e1429 (2012).
Zhang, X. et al. Effect of n-3 long-chain polyunsaturated fatty acids on mild cognitive impairment: a meta-analysis of randomized clinical trials. Eur. J. Clin. Nutr. 74, 548–554 (2020).
Moher, D. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 (2009).
Sterne, J. A. C. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Res. ed). 366, l4898 (2019).
Hozo, S. P., Djulbegovic, B. & Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 5, 13 (2005).
Crippa, A. & Orsini, N. Dose-response meta-analysis of differences in means. BMC Med. Res. Methodol. 16, 91 (2016).
Crippa, A. & Orsini, N. Dose-response meta-analysis of differences in means. BMC Med. Res. Methodol. 16, 1–10 (2016).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj 336, 924–926 (2008).
Arellanes, I. C. et al. Brain delivery of supplemental docosahexaenoic acid (DHA): A randomized placebo-controlled clinical trial. EBioMedicine 59, 102883 (2020).
Boespflug, E. L. et al. Fish oil supplementation increases Event-Related posterior cingulate activation in older adults with subjective memory impairment. J. Nutr. Health Aging. 20, 161–169 (2016).
Carmichael, O. T. et al. A combination of essential fatty acids, Panax ginseng extract, and green tea catechins modifies brain fMRI signals in healthy older adults. J. Nutr. Health Aging. 22, 837–846 (2018).
Giles, G. E. et al. Omega-3 fatty acids and stress-induced changes to mood and cognition in healthy individuals. Pharmacol. Biochem. Behav. 132, 10–19 (2015).
Jackson, J. C. et al. Fish oil supplementation does not affect cognitive outcomes in cardiac surgery patients in the Omega-3 fatty acids for prevention of Post-Operative atrial fibrillation (OPERA) trial. J. Nutr. 148, 472–479 (2018).
Johnson, E. J. et al. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 11, 75–83 (2008).
Kang, J. H. et al. Marine n-3 fatty acids and cognitive change among older adults in the VITAL randomized trial. Alzheimers Dement. (N Y). 8, e12288 (2022).
Karr, J. E., Grindstaff, T. R. & Alexander, J. E. Omega-3 polyunsaturated fatty acids and cognition in a college-aged population. Exp. Clin. Psychopharmacol. 20, 236–242 (2012).
Leckie, R. L. et al. The effects of omega-3 fatty acids on neuropsychological functioning and brain morphology in mid-life adults: a randomized clinical trial. Psychol. Med. 50, 2425–2434 (2020).
Maltais, M. et al. Long-chain Omega-3 fatty acids supplementation and cognitive performance throughout adulthood: A 6-month randomized controlled trial. Prostaglandins Leukot. Essent. Fat. Acids. 178, 102415 (2022).
Marriott, B. P. et al. Impact of Fatty Acid Supplementation on Cognitive Performance among United States (US) Military Officers: The Ranger Resilience and Improved Performance on Phospholipid-Bound Omega-3’s (RRIPP-3) Study. Nutrients 13. (2021).
McNamara, R. K. et al. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol. Aging. 64, 147–156 (2018).
Quinn, J. F. et al. Docosahexaenoic acid supplementation and cognitive decline in alzheimer disease: a randomized trial. Jama 304, 1903–1911 (2010).
Andrieu, S. et al. Effect of long-term Omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 16, 377–389 (2017).
Baleztena, J. et al. Association between cognitive function and supplementation with omega-3 PUFAs and other nutrients in ≥ 75 years old patients: A randomized multicenter study. PLoS One. 13, e0193568 (2018).
Bischoff-Ferrari, H. A. et al. Effect of vitamin D supplementation, Omega-3 fatty acid supplementation, or a Strength-Training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. Jama 324, 1855–1868 (2020).
Daďová, K. et al. Calanus oil supplementation does not further improve Short-Term memory or Brain-Derived neurotrophic factor in older women who underwent exercise training. Clin. Interv Aging. 17, 1227–1236 (2022).
Dangour, A. D. et al. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am. J. Clin. Nutr. 91, 1725–1732 (2010).
Fairbairn, P. et al. Effects of a high-DHA multi-nutrient supplement and exercise on mobility and cognition in older women (MOBILE): a randomised semi-blinded placebo-controlled study. Br J. Nutr, 1–10. (2020).
Freund-Levi, Y. et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate alzheimer disease: OmegAD study: a randomized double-blind trial. Arch. Neurol. 63, 1402–1408 (2006).
Geleijnse, J. M., Giltay, E. J. & Kromhout, D. Effects of n-3 fatty acids on cognitive decline: a randomized, double-blind, placebo-controlled trial in stable myocardial infarction patients. Alzheimers Dement. 8, 278–287 (2012).
Jackson, P. A. et al. No effect of 12 weeks’ supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18–35 years. Br. J. Nutr. 107, 1232–1243 (2012).
Külzow, N. et al. Impact of Omega-3 fatty acid supplementation on memory functions in healthy older adults. J. Alzheimers Dis. 51, 713–725 (2016).
Lundbergh, B. et al. Fish oil supplementation May improve attention, working memory and attention-deficit/hyperactivity disorder symptoms in adults with autism spectrum disorder: a randomised crossover trial. Br J. Nutr, 1–11. (2022).
Moran, C. et al. Effects of a Six-Month Multi-Ingredient nutrition supplement intervention of Omega-3 polyunsaturated fatty acids, vitamin D, resveratrol, and Whey protein on cognitive function in older adults: A randomised, Double-Blind, controlled trial. J. Prev. Alzheimers Dis. 5, 175–183 (2018).
Patan, M. J. et al. Supplementation with oil rich in eicosapentaenoic acid, but not in docosahexaenoic acid, improves global cognitive function in healthy, young adults: results from randomized controlled trials. Am. J. Clin. Nutr. 114, 914–924 (2021).
Phillips, M. A. et al. No effect of Omega-3 fatty acid supplementation on cognition and mood in individuals with cognitive impairment and probable alzheimer’s disease: A randomised controlled trial. Int. J. Mol. Sci. 16, 24600–24613 (2015).
Power, R. et al. Omega-3 fatty acid, carotenoid and vitamin E supplementation improves working memory in older adults: A randomised clinical trial. Clin. Nutr. 41, 405–414 (2022).
Rogers, P. J. et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br. J. Nutr. 99, 421–431 (2008).
Stavrinou, P. S. et al. The Effects of a 6-Month High Dose Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins Supplementation on Cognitive Function and Functional Capacity in Older Adults with Mild Cognitive Impairment. Nutrients 12. (2020).
Tofiq, A. et al. Effects of peroral Omega-3 fatty acid supplementation on cerebrospinal fluid biomarkers in patients with alzheimer’s disease: A randomized controlled Trial-The OmegAD study. J. Alzheimers Dis. 83, 1291–1301 (2021).
Witte, A. V. et al. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cerebral cortex (New York, NY: 1991) 24, 3059–3068 (2014).
Bo, Y. et al. The n-3 Polyunsaturated fatty acids supplementation improved the cognitive function in the chinese elderly with mild cognitive impairment: A double-blind randomized controlled trial. Nutrients 9 (2017).
Chiu, C. C. et al. The effects of omega-3 fatty acids monotherapy in alzheimer’s disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog Neuropsychopharmacol. Biol. Psychiatry. 32, 1538–1544 (2008).
Danthiir, V. et al. An 18-mo randomized, double-blind, placebo-controlled trial of DHA-rich fish oil to prevent age-related cognitive decline in cognitively normal older adults. Am. J. Clin. Nutr. 107, 754–762 (2018).
Dong, R. et al. Effects of docosahexanoic acid on gut microbiota and fecal metabolites in HIV-Infected patients with neurocognitive impairment: A 6-Month randomized, Double-Blind, Placebo-Controlled trial. Front. Nutr. 8, 756720 (2021).
Hashimoto, M. et al. Perilla seed oil enhances cognitive function and mental health in healthy elderly Japanese individuals by enhancing the biological antioxidant potential. Foods 10 (2021).
Kamalashiran, C. et al. Outcomes of Perilla seed oil as an additional neuroprotective therapy in patients with mild to moderate dementia: A randomized control trial. Curr. Alzheimer Res. 16, 146–155 (2019).
Konagai, C. et al. Effects of Krill oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain function: a randomized controlled trial in healthy elderly volunteers. Clin. Interv Aging. 8, 1247–1257 (2013).
Kuszewski, J. C., Howe, P. R. C. & Wong, R. H. X. Evaluation of cognitive performance following Fish-Oil and Curcumin supplementation in Middle-Aged and older adults with overweight or obesity. J. Nutr. 150, 3190–3199 (2020).
Li, M. et al. Effect of folic acid combined with docosahexaenoic acid intervention on mild cognitive impairment in elderly: a randomized double-blind, placebo-controlled trial. Eur. J. Nutr. 60, 1795–1808 (2021).
Lin, P. Y. et al. Omega-3 fatty acids and blood-based biomarkers in alzheimer’s disease and mild cognitive impairment: A randomized placebo-controlled trial. Brain Behav. Immun. 99, 289–298 (2022).
Mahmoudi, M. J. et al. Effect of low dose ω-3 Poly unsaturated fatty acids on cognitive status among older people: a double-blind randomized placebo-controlled study. J. Diabetes Metab. Disord. 13, 34 (2014).
Mengelberg, A. et al. The effects of docosahexaenoic acid supplementation on cognition and well-being in mild cognitive impairment: A 12-month randomised controlled trial. Int J. Geriatr. Psychiatry 37 (2022).
Pase, M. P. et al. The effects of long-chain omega-3 fish oils and multivitamins on cognitive and cardiovascular function: a randomized, controlled clinical trial. J. Am. Coll. Nutr. 34, 21–31 (2015).
Salman, H. B., Salman, M. A. & Yildiz Akal, E. The effect of omega-3 fatty acid supplementation on weight loss and cognitive function in overweight or obese individuals on weight-loss diet. Nutr. Hosp. 39, 803–813 (2022).
Sinn, N. et al. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br. J. Nutr. 107, 1682–1693 (2012).
Stonehouse, W. et al. DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. Am. J. Clin. Nutr. 97, 1134–1143 (2013).
Sueyasu, T. et al. Effects of long-chain polyunsaturated fatty acids in combination with lutein and zeaxanthin on episodic memory in healthy older adults. Nutrients 15 (2023).
Tang, W. et al. Omega-3 fatty acids ameliorate cognitive dysfunction in schizophrenia patients with metabolic syndrome. Brain Behav. Immun. 88, 529–534 (2020).
Tokuda, H. et al. Effects of combining exercise with long-chain polyunsaturated fatty acid supplementation on cognitive function in the elderly: a randomised controlled trial. Sci. Rep. 10, 12906 (2020).
Tokuda, H. et al. Low doses of Long-chain polyunsaturated fatty acids affect cognitive function in elderly Japanese men: A randomized controlled trial. J. Oleo Sci. 64, 633–644 (2015).
Yasuno, F. et al. Combination of antioxidant supplements improved cognitive function in the elderly. J. Alzheimers Dis. 32, 895–903 (2012).
Zhang, Y. P. et al. Effects of DHA supplementation on hippocampal volume and cognitive function in older adults with mild cognitive impairment: A 12-Month randomized, Double-Blind, Placebo-Controlled trial. J. Alzheimers Dis. 55, 497–507 (2017).
Alex, A. et al. Long-chain omega-3 polyunsaturated fatty acids and cognitive decline in non-demented adults: a systematic review and meta-analysis. Nutr. Rev. 78, 563–578 (2020).
Cooper, R. E. et al. Omega-3 polyunsaturated fatty acid supplementation and cognition: A systematic review and meta-analysis. J. Psychopharmacol. 29, 753–763 (2015).
Madden, D. J., Bennett, I. J. & Song, A. W. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol. Rev. 19, 415–435 (2009).
Shinto, L. H. et al. ω-3 PUFA for secondary prevention of white matter lesions and neuronal integrity breakdown in older adults: A randomized clinical trial. JAMA Netw. Open. 7, e2426872 (2024).
Piekema, C. et al. The right hippocampus participates in short-term memory maintenance of object-location associations. NeuroImage 33, 374–382 (2006).
O’Shea, A. et al. Cognitive aging and the hippocampus in older adults. Front. Aging Neurosci. 8, 298 (2016).
Deshmukh, G. V. et al. The role of omega-3 fatty acid supplementation in slowing cognitive decline among elderly patients with alzheimer’s disease: A systematic review of randomized controlled trials. Cureus 16, e73390 (2024).
Seydell-Greenwald, A. et al. Bilateral parietal activations for complex visual-spatial functions: evidence from a visual-spatial construction task. Neuropsychologia 106, 194–206 (2017).
Bazan, N. G. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and alzheimer’s disease. J. Lipid Res. 50 Suppl, 400–405 (2009).
Serhan, C. N., Chiang, N. & Van Dyke, T. E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 (2008).
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
HSH and ZY were involved in the systematic search, screening, and extraction of data. HSH and KT contributed to the statistical analysis and interpreting the data. ZY and NA took part in drafting the manuscript. SS-B contributed to the interpretation of the results and critically revised the manuscript. All authors gave their approval for the final manuscript to be submitted. SS-B is the guarantor.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shahinfar, H., Yazdian, Z., Avini, N.A. et al. A systematic review and dose response meta analysis of Omega 3 supplementation on cognitive function. Sci Rep 15, 30610 (2025). https://doi.org/10.1038/s41598-025-16129-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16129-8