Abstract
Atrial fibrillation (AF) and heart failure (HF) often coexist, posing increased risks of HF events, rehospitalization, mortality, and stroke. While antiarrhythmic drugs have limitations, catheter ablation (CA) has emerged as a preferred rhythm control treatment for AF, yet its clinical outcomes remain inconclusive. Previous meta-analyses have predominantly included randomized controlled trials (RCTs) or cohort studies with limited sample sizes and outcome measures, which do not comprehensively and accurately reflect the clinical prognosis of patients with AF and HF following CA. However, the high prevalence of AF and HF comorbidity and the significant economic burden it imposes underscore the importance of focusing on the clinical prognosis of these patients. This meta-analysis systematically includes high-quality RCTs and cohort studies in evidence-based medicine, comprising a total of 34 studies and 777,668 patients.Meta-analysis revealed that CA significantly reduced the risk of HF events (RR, 0.63; 95% CI, 0.51–0.77), cardiovascular (CV) mortality (RR, 0.54; 95% CI, 0.45–0.66), CV hospitalization (RR, 0.81; 95% CI, 0.71–0.93), all-cause mortality (RR, 0.57; 95% CI, 0.46–0.70), all-cause rehospitalization (RR, 0.87; 95% CI, 0.76–0.996),AF recurrence (RR, 0.45; 95% CI, 0.36–0.57), and stroke (RR, 0.69; 95% CI, 0.57–0.83) when compared to NCA. CA demonstrated superior benefits in improving outcomes for patients with AF and HF, including HF events, mortality, rehospitalization, AF recurrence and incidence of stroke. Additionally, CA shows similar therapeutic effects in improving cardiac structure, cardiopulmonary function, and quality of life.These findings support the efficacy of CA in managing AF and HF.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) and heart failure (HF) exhibit a bidirectional epidemiological relationship, with over one-third of AF patients having concurrent HF1 and 50% of HF patients developing AF1. AF quintuples the risk of new-onset HF in the general population2, while 13.7% of AF patients die from HF within one year3. AF prevalence escalates with HF severity, rising from 5% in NYHA Class I to 50% in Class IV4. Their coexistence amplifies hospitalizations, cardiovascular mortality5,6, and stroke risk (tripled in AF-HF patients)7, alongside substantial economic burdens: annual U.S. healthcare costs reach $18,000(HF) and $13,000 (AF) per patient, with cardiovascular expenditures projected to consume 4.6% of GDP by 20508. Underdiagnosis of HFpEF further obscures cost estimates9., while aging populations drive rising prevalence10,11,12.
Shared risk factors (aging, hypertension, diabetes, structural heart disease) and reciprocal pathophysiological mechanisms underpin AF-HF synergy13. AF-induced rapid ventricular rates impair ventricular filling, reducing left ventricular ejection fraction (LVEF) and precipitating arrhythmogenic cardiomyopathy14,15. Conversely, HF-driven atrial remodeling via elevated left atrial pressure and pulmonary vein ectopy promotes AF12,16.
Maintenance of sinus rhythm correlates with improved prognosis17, yet antiarrhythmic drugs face tolerability limitations. Catheter ablation (CA) emerges as the preferred rhythm control strategy, slowing AF progression5,18,19. However, clinical outcomes remain contested: while CA reduced HF events in Di Biase4 and Li Fangchao20, Bunch21, Kuck22, and Parkash23 reported no difference. Similarly, CA lowered mortality in Sohns24 and Marrouche25 but not in Hunter26 or Kuck22. CA consistently reduced AF recurrence4,26, though some studies observed no difference16,27. LVEF improvements post-CA were significant in select cohorts21,26 but absent in others7,22, paralleling inconsistent cardiopulmonary function4,7,23,25 and quality-of-life outcomes7,22,26,28,29.
These discrepancies highlight unresolved questions regarding CA’s efficacy across HF subtypes, follow-up durations, and geographic regions. This systematic review and meta-analysis evaluate CA versus non-ablation therapies in AF-HF patients, stratifying outcomes by HF phenotype, study design, follow-up period, region, and AF type to clarify clinical benefits.
Materials and methods
Protocol and registration
The protocol for this systematic review and meta-analysis was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42024561102). This study was conducted and reported in accordance with the PRISMA reporting guideline.
Search strategy
Using the PICOS principle, searches were performed using the following electronic databases: Cochrane library, PubMed, Embase, WOS, CNKI, CSTJ, CBMdisc, COJ (last search conducted in June 2024), There are no language restrictions, The key search string was (Atrial Fibrillation) AND (Heart Failure) AND (Catheter Ablation) AND (Randomized controlled trial) OR (Cohort Study). The search strategies across databases were consistent (Figure S1). Pairs of independent reviewers (X.T.Z. and P.J.X.) screened titles and abstracts of identified studies after deduplication. Full texts of potentially eligible studies were reviewed in duplicate, and disagreements were resolved through discussion with a third reviewer (B.P.T.).
PICOS principle
Patients: patients with AF combined with HF
Intervention
The CA group included patients in whom pulmonary vein isolation (PVI) was the primary ablation strategy, with or without left atrial posterior wall isolation and additional linear ablation.
Comparison
The NCA group included patients treated with rate control (i.e., rate-lowering drugs or pacemaker implantation after atrioventricular junction ablation) or rhythm control (i.e., antiarrhythmic drugs, electrical cardioversion, or a combination of both) strategies.
Outcomes
The primary outcome was HF events, defined as unplanned hospitalizations due to worsening HF; Secondary outcomes included CV mortality, all-cause mortality, CV hospitalization, all-cause hospitalization, AF recurrence, SR maintenance, incidence of stroke/TIA. Other outcomes of interest were change in LVEF, left atrial diameter (LAD), Brain Natriuretic Peptide (BNP), N-terminal pro-brain natriuretic peptide (NT-proBNP), improvement in NYHA functional classification, as well as assessment of cardiopulmonary function through the 6-Minute walking test (6MWT) and Peak VO2, evaluation of quality of life through the Minnesota Living With Heart Failure Questionnaire (MLHFQ), with a total score range from 0 to 105, where lower scores indicate better quality of life.
Study design
RCT and cohort study.
Eligibility criteria
The eligibility criteria for including articles in the meta-analysis were as follows: articles reporting on patients with AF combined with HF treated with CA were included. If the same study was reported at different follow-up times, all reports were included for analysis.
Exclusion criteria
The exclusion criteria were as follows: (1) Duplicate articles; (2) Different study designs and objectives; (3) Irrelevant conference proceedings; (4) Studies that did not report the required outcomes; (5) Inability to access the full text; (6) Reviews.
Data extraction and management
Pairs of reviewers (X.T.Z. and P.J.X.) independently abstracted study data in duplicate using standardized data extraction sheets. Disagreements were resolved through discussion with a third reviewer (B.P.T.). Data extraction included: (1) Basic information of the articles: study title, first author, publication date, source of study, study region, study type, journal/magazine title. (2) Basic characteristics of the study subjects: age, gender, sample size, HF subtypes, follow-up time, baseline data for LVEF, LAD, BNP, NT-proBNP, NYHA classification, 6MWT, Peak VO2, presented using mean and standard deviation (SD) for quantitative data. (3) Treatment strategies adopted by the CA and NCA groups. (4) Key elements of bias risk assessment; (5) Outcome indicators of interest. Relevant information was obtained through tables, figures, and supplementary materials in the studies. For data presented only in graphics without direct access to numerical values, measurements were taken thrice using Engauge Digitizer 11.1 (Mark Mitchell, Torrance California), and the average was recorded. For quantitative data where mean and SD were not reported directly in the articles, data transformation was conducted using online Mean Variance Estimation (hkbu.edu.hk). For studies with follow-up times within 24 months, the outcome indicators from the last follow-up were included for analysis. For studies with follow-up times exceeding 24 months, outcome indicators from both the 24-month mark and the last follow-up were included for analysis. When the same study reported both raw data and multiple imputations for outcomes, the raw data were selected for analysis.
Data analysis
We conducted a meta-analysis of outcome indicators from 34 articles using Stata 17.0 (Stata Corp, College Station, Texas, TX, USA) software. Count data were expressed as risk ratio (RR) and 95% confidence interval(CI) to represent the combined effect size. For quantitative data, weighted mean differences (WMD) and their 95% CI were used to present the combined effect size compared to baseline changes. Heterogeneity was assessed using χ² test and I² statistic; If P ≥ 0.05 and I²≤50%, low heterogeneity was considered, and a fixed-effect model was used for combining effect sizes. If P<0.05 and I²>50%, high heterogeneity was considered, and a random-effects model was used. The same effect model was used to combine effect sizes for subgroup analysis of the same study outcomes. Sensitivity analysis was conducted to verify the stability of the results for overall outcomes. For binary results with high heterogeneity, Galbraith plots and L′Abbe plots were created, and meta-regression was used to further explore the sources of heterogeneity. To further explore differences in clinical prognosis among subgroups, this meta-analysis conducted subgroup analyses based on study type(RCT or cohort study), follow-up time (≤ 24 months or > 24 months, based on the median follow-up time), HF subtype (Heart Failure with reduced Ejection Fraction, HFrEF, or HFpEF), study region (Asia, Europe and America, Global, or Oceania), and treatment methods in the NCA group (medication or pacemaker implantation after atrioventricular junction ablation).
Due to the limited original studies on HFpEF, in studies by Yao Xu30and Packer31, over 85% of patients had HFpEF; therefore, all participants in the studies were classified as HFpEF. In cases where some studies included both types of HF but subgroup data for both groups were not clear and unavailable, these studies were excluded from subgroup analysis. Funnel plots and Egger’s tests were used to assess publication bias, and if significant publication bias was detected, trim-and-fill methods were applied. Forest plots of combined subgroup results were created using WPS Office 11.1.0.15320 (Beijing Kingsoft Office Software Co., Ltd. Beijing. China). All reported P values were two-tailed, with statistical significance set at P<0.05.
Study risk of bias assessment and certainty of evidence
Pairs of reviewers (X.T.Z. and P.J.X.) independently conducted bias assessment and cross-checked the evaluations. Disagreements were resolved through discussion with a third reviewer (B.P.T.). The risk of bias in RCTs was evaluated using the Cochrane Collaboration Risk of Bias tool version 2. An outcome-specific assessment was performed for each trial in the following domains: randomization process, deviations from intended interventions, missing outcome data, outcome measurements, and selection of reported results. The Newcastle-Ottawa Scale (NOS) was used to evaluate cohort studies, with a NOS score of ≥ 7 stars indicating high-quality articles.The Grading of Recommendations Assessment, Development, and Evaluation framework was used to assess the certainty of the evidence.
Results
Study characteristics
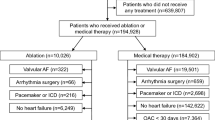
Using the search strategy outlined in Part 2, 2780 relevant studies were gathered, of which 34 were selected for meta-analysis based on inclusion criteria. Among these, there were 18 RCTs and 16 cohort studies, with a total of 4 Chinese articles and 30 English articles, including 777,668 patients. The included articles all reported clinical outcomes of patients with AF combined with HF treated by CA. The CA group underwent CA primarily focused on PVI, with additional left atrial posterior wall isolation, cavotricuspid isthmus ablation, or mitral isthmus ablation as determined by the operator based on the patient’s condition. The NCA group received rhythm or rate control. The HF subtypes varied across studies, categorised as HFrEF (n = 23) or HFpEF (n = 7), or both (n = 4). The study types also varied, being classified as RCTs (n = 18) or cohort studies (n = 16). Follow-up times varied in studies, categorised as ≤ 24 months (n = 21) or > 24 months (n = 13). The study regions varied, categorised as Asia (n = 6), Europe and America (n = 23), Global (n = 2), or Oceania (n = 3). The treatment methods in the NCA group varied across studies, with medication treatment (n = 32) or pacemaker implantation after atrioventricular node ablation (n = 2).The article selection process is shown in Fig. 1 and the basic information of the articles is presented in Table 1.
Summary outcomes
HF events
Nineteen studies evaluated the incidence of HF events in patients with AF combined with HF. The meta-analysis showed that the risk of HF events in the CA group was 0.63 times that of the NCA group (95% CI, 0.51–0.77; P < 0.0001), with high heterogeneity (P < 0.0001, I²=80.5%). Evaluation using the GRADE Evidence Profile system indicated a moderate recommendation for RCTs and very low recommendation for cohort studies.
CV mortality
Ten studies assessed the CV mortality rate in patients with AF combined with HF. The meta-analysis showed that the risk of CV mortality in the CA group was 0.54 times that of the NCA group (95% CI, 0.45–0.66; P < 0.0001), with no significant heterogeneity (P = 0.13, I²=32.3%). Evaluation using the GRADE Evidence Profile system indicated a moderate recommendation for RCTs and very low recommendation for cohort studies.
All-cause mortality
Twenty-two studies evaluated the all-cause mortality rate in patients with AF combined with HF. The meta-analysis showed that the risk of all-cause mortality in the CA group was 0.57 times that of the NCA group (95% CI, 0.46–0.70; P < 0.0001), with significant heterogeneity (P < 0.0001, I²=74.3%). Evaluation using the GRADE Evidence Profile system indicated a moderate recommendation for RCTs and very low recommendation for cohort studies.
CV hospitalization
Three studies evaluated the CV hospitalization rate in patients with AF combined with HF. The meta-analysis revealed that the CA group had a 0.81 times lower risk of CV rehospitalization compared to the NCA group (95% CI, 0.71–0.93; P < 0.01), with no significant heterogeneity (P = 0.18, I²=36.3%). Evaluation using the GRADE Evidence Profile system indicated a moderate recommendation for RCTs and very low recommendation for cohort studies.
All-cause hospitalization
Six studies assessed the all-cause hospitalization rate in patients with AF combined with HF. The meta-analysis showed that the CA group had a 0.87 times lower risk of all-cause rehospitalization compared to the NCA group (95% CI, 0.76–0.996; P = 0.04), with no significant heterogeneity (P = 0.001, I²=68.4%). Evaluation using the GRADE Evidence Profile system indicated a low recommendation for RCTs and very low recommendation for cohort studies.
AF recurrence
Fifteen studies evaluated the AF recurrence rate in patients with AF combined with HF. The meta-analysis demonstrated that the risk of AF recurrence in the CA group was 0.45 times lower than that in the NCA group (95% CI, 0.36–0.57; P < 0.0001), with high heterogeneity (P < 0.0001, I²=89%). Evaluation using the GRADE Evidence Profile system indicated a low recommendation for RCTs and very low recommendation for cohort studies.
SR maintenance
Thirteen studies assessed the SR maintenance rate in patients with AF combined with HF. The meta-analysis showed that the CA group had a 3.08 times higher SR maintenance rate compared to the NCA group (95% CI, 2.19–4.34; P < 0.0001), with high heterogeneity (P < 0.0001, I²=85.7%). Evaluation using the GRADE Evidence Profile system indicated a low recommendation for RCTs and very low recommendation for cohort studies.
Stroke/TIA events
Ten studies evaluated the incidence of stroke/TIA in patients with AF combined with HF. The meta-analysis revealed that the CA group had a 0.69 times lower risk of stroke/TIA compared to the NCA group (95% CI, 0.57–0.83; P < 0.0001), with no significant heterogeneity (P = 0.14, I²=31%). Evaluation using the GRADE Evidence Profile system indicated a moderate recommendation for RCTs and very low recommendation for cohort studies.
Change in LVEF
Twenty-one studies evaluated the change in LVEF in patients with AF combined with HF. The meta-analysis showed that the increase in LVEF in the CA group was 6.36% higher than that in the NCA group (95% CI, 4.63–8.09; P < 0.0001), with high heterogeneity (P < 0.0001, I²=92.5%). Evaluation using the GRADE Evidence Profile system indicated a low recommendation for RCTs and very low recommendation for cohort studies.
Change in LAD
Six studies assessed the change in LAD in patients with AF combined with HF. The meta-analysis revealed that the decrease in LAD in the CA group was 5.11 mm greater than that in the NCA group (95% CI, −7.52, −2.69; P < 0.0001), with high heterogeneity (P < 0.0001, I²=92.1%). Evaluation using the GRADE Evidence Profile system indicated a low recommendation for RCTs and very low recommendation for cohort studies.
Change in BNP
Five studies evaluated the change in BNP in patients with AF combined with HF. The meta-analysis demonstrated that the decrease in BNP in the CA group was 120.54 pg/ml greater than that in the NCA group (95% CI, −160.85, −80.22, P < 0.0001), with no significant heterogeneity (P = 0.73, I²=0%). Evaluation using the GRARE Evidence Profile system indicated a moderate recommendation for RCTs and low recommendation for cohort studies.
Change in NT-proBNP
Four studies evaluated the change in NT-proBNP in patients with AF combined with HF. The meta-analysis showed that the decrease in NT-proBNP in the CA group was 731.70 ng/L greater than that in the NCA group (95% CI, −816.49, −646.90, P < 0.0001), with no significant heterogeneity (P = 0.37, I²=5.7%). Evaluation using the GRADE Evidence Profile system indicated a low recommendation for RCTs and very low recommendation for cohort studies.
Change in 6MWT
Nine studies evaluated the change in 6MWT in patients with AF combined with HF. The meta-analysis revealed that the increase in 6MWT in the CA group was 21.79 m greater than that in the NCA group (95% CI, 2.45–41.12, P = 0.03), with high heterogeneity (P < 0.0001, I²=77%). Evaluation using the GRADE Evidence Profile system indicated a low recommendation for RCTs.
Change in NYHA
Eight studies assessed the change in NYHA functional class in patients with AF combined with HF. The meta-analysis showed that the decrease in NYHA class in the CA group was 0.69 grade greater than that in the NCA group (95% CI, −0.92, −0.45, P < 0.0001), with high heterogeneity (P < 0.0001, I²=78.5%). Evaluation using the GRADE Evidence Profile system indicated a low recommendation for RCTs and very low recommendation for cohort studies.
Change in peak VO2
Three studies evaluated the change in peak VO2 in patients with AF combined with HF. The meta-analysis demonstrated that the increase in peak VO2 in the CA group was 3.43 ml/kg/min greater than that in the NCA group (95%CI, 1.53–5.34, P < 0.0001), with no significant heterogeneity (P = 0.73, I²=0%). Evaluation using the GRADE Evidence Profile system indicated a moderate recommendation for RCTs.
Change in MLHFQ
Eleven studies assessed the change in MLHFQ in patients with AF combined with HF. The meta-analysis showed that the decrease in MLHFQ score in the CA group was 10.21 points greater than that in the NCA group (95%CI, −15.08, −5.34, P < 0.0001), with high heterogeneity (P < 0.0001, I²=81.4%). Evaluation using the GRADE Evidence Profile system indicated a low recommendation for RCTs and very low recommendation for cohort studies.All results are shown in Table 2, GRADE Evidence Profile in Supplementary Table S1 online, and the summary forest plot in Supplementary Fig. S2 online. L′Abbe plots and Galbraith radial plots are shown in Supplementary Fig. S22 online.
Subgroup analysis
HF events
(1) Subgroup Analysis by HF Subtype: Thirteen studies investigated the incidence of HF events in patients with AF combined with HFrEF. The meta-analysis showed that the risk of HF events in the CA group was 0.68 times that of the NCA group (95% CI, 0.6–0.78; P < 0.0001), with no significant heterogeneity (P = 0.47, I²=0%). Five studies explored the incidence of HF events in patients with AF combined with HFpEF. The meta-analysis showed that the risk of HF events in the CA group was 0.58 times lower than that of the NCA group (95% CI, 0.36–0.95; P = 0.03), with high heterogeneity (P < 0.0001, I²=82%). Meta-regression indicated P < 0.0001.
(2) Subgroup Analysis by Study Type: Eight RCTs investigated the incidence of HF events in patients with AF combined with HF. The meta-analysis showed that the risk of HF events in the CA group was 0.65 times lower than that of the NCA group (95% CI, 0.55–0.76; P < 0.0001), with no significant heterogeneity (P = 0.43, I²=0.1%). Eleven cohort studies explored the incidence of HF events in patients with AF combined with HF. The meta-analysis showed that the risk of HF events in the CA group was 0.61 times lower than that of the NCA group (95% CI, 0.45–0.81; P < 0.0001), with high heterogeneity (P < 0.0001, I²=85.3%). Meta-regression indicated P = 0.593.
(3) Subgroup Analysis by NCA Group Treatment: Eighteen studies involving NCA therapy with medication explored the incidence of HF events in patients with AF combined with HF. The meta-analysis showed that the risk of HF events in the CA group was 0.63 times lower than that of the NCA group (95% CI, 0.51–0.78; P < 0.0001), with high heterogeneity (P < 0.0001, I²=81.4%). One study involving NCA therapy with atrioventricular junction ablation and pacemaker implantation investigated the incidence of HF events in patients with AF combined with HF. The meta-analysis showed that the risk of HF events in the CA group was 0.50 times lower than that of the NCA group (95% CI, 0.05–5.14; P = 0.56). Meta-regression indicated P = 0.856.Subgroup analysis and meta-regression results are shown in Table 3, and the combined forest plot is depicted in Supplementary Fig. S3 online.
CV mortality
(1) Subgroup Analysis by HF Subtype: Eight studies investigated the CV mortality rate in patients with AF combined with HFrEF. The meta-analysis revealed that the risk of CV mortality in the CA group was 0.53 times lower than that in the NCA group (95% CI, 0.44–0.65; P < 0.0001), with no significant heterogeneity (P = 0.18, I²=29.1%). Three studies explored the CV mortality rate in patients with AF combined with HFpEF. The meta-analysis showed that the risk of CV mortality in the CA group was 0.68 times that of the NCA group (95% CI, 0.37–1.27; P = 0.23), with no significant heterogeneity (P = 0.24, I²=30.9%).
(2) Subgroup Analysis by Study Type: Six RCTs investigated the CV mortality rate in patients with AF combined with HF. The meta-analysis revealed that the risk of CV mortality in the CA group was 0.59 times lower than that in the NCA group (95% CI, 0.43–0.80; P < 0.01), with no significant heterogeneity (P = 0.38, I²=5.8%). Four cohort studies examined the CV mortality rate in patients with AF combined with HF. The meta-analysis showed that the risk of CV mortality in the CA group was 0.52 times lower than that of the NCA group (95% CI, 0.41–0.66; P < 0.0001), with high heterogeneity (P = 0.05, I²=59%). Subgroup analysis is presented in Table 4, and the combined forest plot is illustrated in Supplementary Fig. S4 online.
All-cause mortality
(1)Subgroup Analysis by HF Subtype: Fifteen studies examined the all-cause mortality rate in patients with AF combined with HFrEF. The meta-analysis revealed that the risk of all-cause mortality in the CA group was 0.71 times lower than that in the NCA group (95% CI, 0.58–0.87; P < 0.01), with no significant heterogeneity (P = 0.07, I²=36%). Six studies investigated the all-cause mortality rate in patients with AF combined with HFpEF. The meta-analysis showed that the risk of all-cause mortality in the CA group was 0.66 times lower than that in the NCA group (95% CI, 0.42–1.05; P = 0.08), with high heterogeneity (P = 0.02, I²=59.2%). Meta-regression indicated P = 0.308.
(2)Subgroup Analysis by Study Type: Ten RCTs explored the all-cause mortality rate in patients with AF combined with HF. The meta-analysis showed that the risk of all-cause mortality in the CA group was 0.69 times lower than that in the NCA group (95% CI, 0.53–0.90; P < 0.01), with no significant heterogeneity (P = 0.26, I²=19.3%). Twelve cohort studies investigated the all-cause mortality rate in patients with AF combined with HF. The meta-analysis revealed that the risk of all-cause mortality in the CA group was 0.50 times lower than that in the NCA group (95% CI, 0.38–0.66; P < 0.0001), with high heterogeneity (P < 0.0001, I²=81.1%). Meta-regression indicated P = 0.263.
(3)Subgroup Analysis by NCA Group Treatment Method: Twenty-one studies involving NCA therapy with medication examined the all-cause mortality rate in patients with AF combined with HF. The meta-analysis showed that the risk of all-cause mortality in the CA group was 0.57 times lower than that in the NCA group (95% CI, 0.46–0.71; P < 0.0001), with high heterogeneity (P < 0.0001, I²=75.3%). One study involving NCA therapy with atrioventricular node ablation and pacemaker implantation studied the all-cause mortality rate in patients with AF combined with HF. The meta-analysis showed that the risk of all-cause mortality in the CA group was 0.20 times lower than that in the NCA group (95% CI, 0.01–3.95, P = 0.29). Meta-regression indicated P = 0.779. Subgroup analysis and meta-regression are presented in Table 5, and the combined forest plot is depicted in Supplementary Fig. S5 online.
CV hospitalization
(1) Subgroup Analysis by HF Subtype: 2 studies investigated the CV hospitalization rate in patients with AF combined with HFrEF. The meta-analysis showed that the risk of CV rehospitalization in the CA group was 0.84 times lower than that in the NCA group (95% CI, 0.73–0.96; P = 0.01), with no significant heterogeneity (P = 0.63, I²=0%). 1 study explored the CV hospitalization rate in patients with AF combined with HFpEF, showing that the risk of CV rehospitalization in the CA group was 0.40 times lower than that in the NCA group (95% CI, 0.20–0.79; P < 0.01).
(2) Subgroup Analysis by Study Type: 1 RCT examined the CV hospitalization rate in patients with AF combined with HF, revealing that the risk of CV rehospitalization in the CA group was 0.77 times lower than that in the NCA group (95% CI, 0.63–0.96; P = 0.02), with no significant heterogeneity (P = 0.55, I²=0%). 2 cohort studies investigated the CV hospitalization rate in patients with AF combined with HF, indicating that the risk of CV rehospitalization in the CA group was 0.84 times lower than that in the NCA group (95% CI, 0.71-1.00; P = 0.05), with high heterogeneity (P = 0.07, I²=62.1%). The subgroup analysis results are presented in Table 6, and the combined forest plot is shown in Supplementary Fig. S6 online.
All-cause hospitalization
(1) Subgroup Analysis by HF Subtype: 3 studies explored the all-cause hospitalization rate in patients with AF combined with HFrEF. The meta-analysis indicated that the risk of all-cause rehospitalization in the CA group was 0.91 times lower than that in the NCA group (95% CI, 0.77–1.08; P = 0.28), with substantial heterogeneity (P = 0.02, I²=66.4%). 2 studies investigated the all-cause hospitalization rate in patients with AF combined with HFpEF, showing that the risk of all-cause rehospitalization in the CA group was 0.72 times lower than that in the NCA group (95% CI, 0.42–1.25; P = 0.24), with significant heterogeneity (P < 0.01, I²=80%). Meta-regression yielded P = 0.666.
(2) Subgroup Analysis by Study Type: 2 RCTs examined the all-cause hospitalization rate in patients with AF combined with HF. The meta-analysis revealed that the risk of all-cause rehospitalization in the CA group was 0.85 times lower than that in the NCA group (95% CI, 0.61–1.19; P = 0.34), with substantial heterogeneity (P < 0.01, I²=82.5%). 4 cohort studies explored the all-cause hospitalization rate in patients with AF combined with HF, indicating that the risk of all-cause rehospitalization in the CA group was 0.88 times lower than that in the NCA group (95% CI, 0.75–1.03; P = 0.10), with significant heterogeneity (P = 0.02, I²=62.2%). Meta-regression yielded P = 0.954. The subgroup analysis and meta-regression results are provided in Table 7, and the combined forest plot is depicted in Supplementary Fig. S7 online.
AF recurrence
(1) Subgroup Analysis by HF Subtype: 10 studies examined the rate of AF recurrence in patients with AF combined with HFrEF. The meta-analysis indicated that the risk of AF recurrence in the CA group was 0.50 times lower than that in the NCA group (95% CI, 0.42–0.60; P < 0.0001), with substantial heterogeneity (P < 0.01, I²=66.7%). 4 studies investigated the AF recurrence rate in patients with AF combined with HFpEF, revealing that the risk of AF recurrence in the CA group was 0.52 times lower than that in the NCA group (95% CI, 0.33–0.84; P < 0.01), with substantial heterogeneity (P < 0.0001, I²=83.3%). Meta-regression yielded P = 0.195.
(2) Subgroup Analysis by Study Type: 8 RCTs explored the AF recurrence rate in patients with AF combined with HF. The meta-analysis showed that the risk of AF recurrence in the CA group was 0.45 times lower than that in the NCA group (95% CI, 0.32–0.64; P < 0.0001), with substantial heterogeneity (P < 0.0001, I²=93.7%). 7 cohort studies examined the AF recurrence rate in patients with AF combined with HF, indicating that the risk of AF recurrence in the CA group was 0.45 times lower than that in the NCA group (95% CI, 0.33–0.63; P < 0.0001), with substantial heterogeneity (P < 0.01, I²=69.5%). Meta-regression yielded P = 0.821. The subgroup analysis and meta-regression results are provided in Supplementary Table S2 online, and the combined forest plot is depicted in Supplementary Fig. S8 online.
SR maintenance
(1) Subgroup Analysis by HF Subtype: 11 studies investigated the SR maintenance rate in patients with AF combined with HFrEF. The meta-analysis revealed that the SR maintenance rate in the CA group was 2.81 times higher than that in the NCA group (95% CI, 2.03–3.89; P < 0.0001), with substantial heterogeneity (P < 0.0001, I²=79.8%). 1 study explored the SR maintenance rate in patients with AF combined with HFpEF, showing that the CA group had a SR maintenance rate 2.38 times higher than the NCA group (95% CI, 1.45–3.91; P < 0.01). Meta-regression yielded P = 0.682.
(2) Subgroup Analysis by Study Type: 9 RCTs examined the SR maintenance rate in patients with AF combined with HF. The meta-analysis indicated that the SR maintenance rate in the CA group was 3.26 times higher than that in the NCA group (95% CI, 2.03–5.23; P < 0.0001), with substantial heterogeneity (P < 0.0001, I²=90.1%). 4 cohort studies investigated the SR maintenance rate in patients with AF combined with HF, revealing that the CA group had a SR maintenance rate 2.82 times higher than the NCA group (95% CI, 1.82–4.35; P < 0.0001), with substantial heterogeneity (P = 0.03, I²=61.5%). Meta-regression yielded P = 0.429. The subgroup analysis and meta-regression results are provided in Supplementary Table S3 online, and the combined forest plot is depicted in Supplementary Fig. S9 online.
Stroke/TIA events
(1) Subgroup Analysis by HF Subtype: 5 studies investigated the incidence of stroke/TIA in patients with AF combined with HFrEF. The meta-analysis showed that the CA group had a 0.69 times lower risk of stroke/TIA compared to the NCA group (95% CI, 0.43–1.11; P = 0.13), with no significant heterogeneity (P = 0.95, I²=0%). 2 studies explored the stroke/TIA incidence in patients with AF combined with HFpEF, indicating that the CA group had a 0.76 times lower risk of stroke/TIA compared to the NCA group (95% CI, 0.61–0.94; P = 0.01), with significant heterogeneity (P < 0.01, I²=80.6%). Meta-regression yielded P = 0.195.
(2) Subgroup Analysis by Study Type: 4 RCTs investigated the stroke/TIA incidence in patients with AF combined with HF. The meta-analysis revealed that the CA group had a 0.69 times lower risk of stroke/TIA compared to the NCA group (95% CI, 0.36–1.30; P = 0.25), with no significant heterogeneity (P = 0.75, I²=0%). 6 cohort studies explored the stroke/TIA incidence in patients with AF combined with HF, showing that the CA group had a 0.69 times lower risk of stroke/TIA compared to the NCA group (95% CI, 0.57–0.83; P < 0.0001), with significant heterogeneity (P = 0.04, I²=52.4%). Meta-regression yielded P = 0.429. The subgroup analysis results are presented in Supplementary Table S4 online, and the combined forest plot is shown in Supplementary Fig. S10 online.
Change in LVEF
(1) Subgroup analysis by HF Subtype: 19 studies explored the change in LVEF in patients with AF combined with HFrEF. The meta-analysis showed that the CA group had an increase in LVEF 6.37% higher than the NCA group (95% CI, 3.95–8.79; P < 0.0001), with substantial heterogeneity (P < 0.0001, I²=92.6%). 4 studies investigated the change in LVEF in patients with AF combined with HFpEF, demonstrating that the CA group had an increase in LVEF 7.20% higher than the NCA group (95% CI, −0.09–14.5, P = 0.05), with significant heterogeneity (P < 0.0001, I²=90.6%). Meta-regression yielded P = 0.869.
(2) Subgroup analysis by Study Type: 14 RCTs examined the change in LVEF in patients with AF combined with HF. The meta-analysis indicated that the CA group had an increase in LVEF 4.82% higher than the NCA group (95% CI, 3.45–6.19, P < 0.0001), with substantial heterogeneity (P < 0.0001, I²=81.2%). 7 cohort studies explored the change in LVEF in patients with AF combined with HF, revealing that the CA group had an increase in LVEF 9.63% higher than the NCA group (95% CI, 4.65–14.62, P < 0.0001), with substantial heterogeneity (P < 0.0001, I²=92.7%). Meta-regression yielded P = 0.019.
(3) Subgroup Analysis by Non-Ablation Group Treatment Method: 19 studies investigating medication treatment in the NCA group explored the change in LVEF in patients with AF combined with HF, showing that the CA group had an increase in LVEF 6.25% higher than the NCA group (95% CI, 4.41–8.10, P < 0.0001), with substantial heterogeneity (P < 0.0001, I²=93.1%). 2 studies involving atrioventricular node ablation and pacemaker implantation in the NCA group studied the change in LVEF in patients with AF combined with HF, demonstrating that the CA group had an increase in LVEF 7.29% higher than the NCA group (95% CI, 4.75–9.83, P < 0.0001), with no significant heterogeneity (P = 0.59, I²=0.0%). Meta-regression yielded P = 0.666. The subgroup analysis and meta-regression results are presented in Supplementary Table S5 online, and the combined forest plot is depicted in Supplementary Fig. S11 online.
Change in LAD
(1) Subgroup Analysis by HF Subtype: In an analysis of 5 studies on patients with AF combined with HFrEF, the meta-analysis indicated that the reduction in LAD in the CA group was 3.18 mm greater than that in the NCA group (95% CI, −5.18, −1.18; P < 0.01), with a substantial heterogeneity (P < 0.0001, I²=87.2%). One study explored the improvement in LAD in patients with AF combined with HFpEF, showing that the CA group had a reduction in LAD 11.69 mm greater than the NCA group (95% CI, −16.09, −7.3; P < 0.0001), with significant heterogeneity (P = 0.06, I²=72%). The meta-regression yielded P = 0.008.
(2) Subgroup Analysis by Study Type: A meta-analysis of 3 RCTs investigating the improvement in LAD in patients with AF combined with HF showed that the CA group had a reduction in LAD 2.34 mm greater than the NCA group (95% CI, −5.25, 0.56; P = 0.11), with significant heterogeneity (P < 0.0001, I²=83.4%). The analysis of 3 cohort studies exploring the improvement in LAD in patients with AF combined with HF revealed that the CA group had a reduction in LAD 7.77 mm greater than the NCA group (95% CI, −11.02, −4.51; P < 0.0001), with significant heterogeneity (P < 0.0001, I²=91%). The meta-regression yielded P = 0.083.
(3) Subgroup Analysis by NCA Group Treatment: In an examination of 5 studies focusing on medication treatment in the NCA group for patients with AF combined with HF, the meta-analysis revealed that the CA group had a reduction in LAD 4.99 mm greater than the NCA group (95% CI, −7.67, −2.31; P < 0.0001), with substantial heterogeneity (P < 0.0001, I²=92.9%). For 1 study involving AV node ablation and pacemaker implantation in the NCA group, the analysis showed that the CA group had a reduction in LAD 6.0 mm greater than the NCA group (95% CI, −8.33, −3.67; P < 0.0001). The meta-regression yielded P = 0.865. The results of the subgroup analysis and meta-regression are presented in Supplementary Table S6 online, and the combined forest plot can be found in Supplementary Fig. S12 online.
Change in BNP
(1) Subgroup Analysis by HF Subtype: In an analysis of 4 studies on patients with AF combined with HFrEF, the meta-analysis showed that the reduction in BNP in the CA group was 131.07 pg/ml greater than that in the NCA group (95% CI, −184.25, −77.90, P < 0.0001), with no significant heterogeneity (P = 0.64, I²=0%). One study examining the improvement in BNP in patients with AF combined with HFpEF revealed that the CA group had a reduction in BNP 106.30 pg/ml greater than the NCA group (95% CI, −168.10, −44.50, P < 0.01).
(2) Subgroup Analysis by Study Type: In an analysis of 4 RCTs investigating the improvement in BNP in patients with AF combined with HF, the meta-analysis indicated that the reduction in BNP in the CA group was 116.56 pg/ml greater than that in the NCA group (95% CI, −161.18, −71.95, P < 0.0001), with no significant heterogeneity (P = 0.60, I²=0%). One cohort study exploring the improvement in BNP in patients with AF combined with HF showed that the reduction in BNP in the CA group was 138.20 pg/ml greater than that in the NCA group (95% CI, −232.29, −44.11, P < 0.01). The subgroup analysis results are presented in Supplementary Table S7 online, and the combined forest plot can be found in Supplementary Fig. S13 online.
Change in NT-proBNP
(1) Subgroup Analysis by HF Subtype: Three studies investigated the change in NT-proBNP in patients with AF combined with HFrEF. The meta-analysis revealed that the reduction in NT-proBNP in the CA group was 731.23 ng/L greater than that in the NCA group (95% CI, −817.69, −644.77; P < 0.0001), with no significant heterogeneity (P = 0.20, I² = 37.1%). One study examined the change in NT-proBNP in patients with AF combined with HFpEF, showing that the CA group had a reduction in NT-proBNP 743.40 ng/L greater than the NCA group (95% CI, −1176.87, −309.93; P < 0.01).
(2) Subgroup Analysis by Study Type: Three RCTs studied the change in NT-proBNP in patients with AF combined with HF. The meta-analysis indicated that the reduction in NT-proBNP in the CA group was 741.39 ng/L greater than that in the NCA group (95% CI, −1069.15, −413.63; P < 0.0001), with no significant heterogeneity (P = 0.20, I² = 37.1%). One cohort study exploring the change in NT-proBNP in patients with AF combined with HF showed that the CA group had a reduction in NT-proBNP 731.00 ng/L greater than the NCA group (95% CI, −818.78, −643.22; P < 0.0001). The subgroup analysis results can be found in Supplementary Table S8 online, and the combined forest plot is in Supplementary Fig. S14 online.
Change in 6MWT
(1) Subgroup Analysis by HF Subtype: Eight studies investigated the improvement in 6MWT in patients with AF combined with HFrEF. The meta-analysis revealed that the increase in 6MWT in the CA group was 20.34 m greater than that in the NCA group (95% CI, −0.56-41.23, P = 0.06), with substantial heterogeneity (P < 0.0001, I² = 79.5%). One study examined the improvement in 6MWT in patients with AF combined with HFpEF, showing that the CA group had an increase in 6MWT 35.20 m greater than the NCA group (95% CI, −6.70-77.10, P = 0.10). The meta-regression yielded P = 0.674.
(2) Subgroup Analysis by Follow-Up Time Subgroup: Seven studies with follow-up time ≤ 24 months explored the improvement in 6MWT in patients with AF combined with HF. The meta-analysis revealed that the increase in 6MWT in the CA group was 22.28 m greater than that in the NCA group (95% CI, −2.14-46.70, P = 0.07), with substantial heterogeneity (P < 0.0001, I² = 83.9%). Two studies with follow-up time > 24 months investigating the improvement in 6MWT in patients with AF combined with HF demonstrated that the CA group had an increase in 6MWT 17.50 m greater than the NCA group (95% CI, −6.48-41.47, P = 0.15), with no significant heterogeneity (P = 0.54, I² = 0%). The meta-regression yielded P = 0.886.
(3) Subgroup Analysis by NCA Group Treatment Subgroup: Seven studies focusing on medication treatment in the NCA group explored the improvement in 6MWT in patients with AF combined with HF. The meta-analysis revealed that the increase in 6MWT in the CA group was 15.32 m greater than that in the NCA group (95% CI, 0.21–30.44, P = 0.05), with no significant heterogeneity (P = 0.11, I² = 38.7%). One study involving atrioventricular node ablation and pacemaker implantation in the NCA group examining the improvement in 6MWT in patients with AF combined with HF showed that the CA group had an increase in 6MWT 55.00 m greater than the NCA group (95% CI, 41.98–68.02, P < 0.0001). The meta-regression yielded P = 0.067. The results of the subgroup analysis can be found in Supplementary Table S9 online, and the combined forest plot is in Supplementary Fig. S15 online.
Change in NYHA
(1) Subgroup Analysis by HF Subtype: Seven studies investigated the improvement in NYHA classification in patients with AF combined with HFrEF. The meta-analysis showed that the reduction in NYHA classification in the CA group was 0.69 grade greater than that in the NCA group (95% CI, −0.95, −0.44, P < 0.0001), with significant heterogeneity (P < 0.0001, I² = 81.2%). One study examined the improvement in NYHA classification in patients with AF combined with HFpEF, revealing that the reduction in NYHA classification in the CA group was 0.56 grade greater than that in the NCA group (95% CI, −1.17, 0.05, P = 0.07). The meta-regression yielded P = 0.780.
(2) Subgroup Analysis by Study Type: In an analysis of five RCTs exploring the improvement in NYHA classification in patients with AF combined with HF, the meta-analysis indicated that the reduction in NYHA classification in the CA group was 0.65 grade greater than that in the NCA group (95% CI, −0.89, −0.41, P < 0.0001), with significant heterogeneity (P = 0.04, I² = 60.2%). Three cohort studies investigating the improvement in NYHA classification in patients with AF combined with HF showed that the reduction in NYHA classification in the CA group was 0.73 grade greater than that in the NCA group (95% CI, −1.22, −0.24, P < 0.01), with significant heterogeneity (P < 0.0001, I² = 88.7%). The meta-regression yielded P = 0.693. The results of the subgroup analysis and meta-regression are presented in Supplementary Table S10 online, and the combined forest plot is depicted in Supplementary Fig. S16 online.
Change in peak VO2
(1) Subgroup Analysis by HF Subtype: Two studies investigated the improvement in peak VO2 in patients with AF combined with HFrEF. The meta-analysis revealed that the increase in peak VO2 in the CA group was 3.37 (ml/kg/min) greater than that in the NCA group (95% CI, 1.14–5.60, P < 0.01), with no significant heterogeneity (P = 0.43, I² = 0%). One study examined the improvement in peak VO2 in patients with AF combined with HFpEF, showing that the increase in peak VO2 in the CA group was 3.60 (ml/kg/min) greater than that in the NCA group (95% CI, −0.05-7.25, P = 0.05). Subgroup analysis results can be found in Supplementary Table S11 online, and the combined forest plot is shown in Supplementary Fig. S17 online.
Change in MLHFQ
(1) Subgroup Analysis by HF Subtype: Ten studies explored the improvement in MLHFQ in patients with AF combined with HFrEF. The meta-analysis indicated that the reduction in MLHFQ in the CA group was 9.16 points greater than that in the NCA group (95% CI, −14.03, −4.28, P < 0.0001), with substantial heterogeneity (P < 0.0001, I² = 80.2%). Two studies investigated the improvement in MLHFQ in patients with AF combined with HFpEF, showing that the reduction in MLHFQ in the CA group was 18.55 points greater than that in the NCA group (95% CI, −47.94, 10.84, P = 0.22), with significant heterogeneity (P < 0.0001, I² = 92.2%). The meta-regression yielded P = 0.333.
(2) Subgroup Analysis by Study Type: In an analysis of ten RCTs examining the improvement in MLHFQ in patients with AF combined with HF, the meta-analysis revealed that the reduction in MLHFQ in the CA group was 9.96 points greater than that in the NCA group (95% CI, −15.27, −4.66, P < 0.0001), with substantial heterogeneity (P < 0.0001, I² = 82.8%). One cohort study investigated the improvement in MLHFQ in patients with AF combined with HF, demonstrating that the reduction in MLHFQ in the CA group was 13.10 points greater than that in the NCA group (95% CI, −20.34, −5.86, P < 0.0001). The meta-regression yielded P = 0.757.
(3) Subgroup Analysis by NCA Group Treatment: Ten studies focusing on medication treatment in the NCA group explored the improvement in MLHFQ in patients with AF combined with HF. The meta-analysis revealed that the reduction in MLHFQ in the CA group was 8.46 points greater than that in the NCA group (95% CI, −12.41, −4.52, P < 0.0001), with substantial heterogeneity (P < 0.01, I² = 64.6%). One study involving Atrioventricular node ablation and pacemaker implantation in the NCA group examined the improvement in MLHFQ in patients with AF combined with HF, showing that the reduction in MLHFQ in the CA group was 22 points greater than that in the NCA group (95% CI, −26.66, −17.34, P < 0.0001). The meta-regression yielded P = 0.112. Results of the subgroup analysis and meta-regression can be found in Supplementary Table S12 online, and the combined forest plot is in Supplementary Fig. S18 online. Detailed subgroup analyses stratified by geographic region and follow-up duration for all prespecified outcomes are presented in the Supplementary Appendix.
Risk of bias assessment
Detailed assessment results of risk bias can be found in the Supplementary Fig. S19 online. Due to the open-label design of most included RCTs, both patients and researchers were aware of group allocation and treatment, leading to a significant risk of bias in deviations from expected interventions, particularly in the trials by Marrouch25 and Packer31, where a large number of participants dropped out or were lost to follow-up. The open-label design also introduced bias in outcome measurements, particularly in the assessment of subjective outcomes. Among the cohort studies, 13 articles scored ≥ 7 points, with 3 articles scoring 5 points. Some articles did not provide detailed information on the source of the CA cohort and baseline data, possibly due to limitations in publication space.
Sensitivity analyses
Sensitivity analysis using the same model as the main effect and obtaining consistent conclusions yielded stable results. The sensitivity analysis diagram can be found in the supplementary material. For detailed results, please refer to Supplementary Table S13 online and Supplementary Fig. S20 online.
Reporting biases
The funnel plot of outcome reporting bias and Egger’s test results revealed publication bias in HF events, AF recurrence, and change in NYHA with P < 0.05. Publication bias was addressed using the trim-and-fill method, resulting in statistically significant and consistent results with the main effect, demonstrating stability.For detailed results, please refer to Supplementary Fig. S21 online and Supplementary Table S13 online.
Discussion
AF combined with HF increases the risk of HF events, mortality, hospital readmission, and stroke. Previous studies have suggested that CA may reduce adverse clinical outcomes compared to NCA, but current clinical research results remain inconsistent. A previous meta-analysis51 evaluated the clinical outcomes of patients with AF combined with HF undergoing CA, showing improvements in HF hospitalization rate, cardiovascular death rate, and all-cause mortality rate. However, it only included RCTs of patients with AF combined with HFrEF, with limited study and patient numbers. The present meta-analysis is the first comprehensive analysis and discussion of clinical outcomes in this population, incorporating RCTs and cohort studies, and exploring the broad applicability of CA in this patient population through subgroup analysis. The results of this meta-analysis indicate that, compared to NCA, CA can reduce HF event rates, mortality rates, hospital readmission rates, AF recurrence, and stroke occurrence in patients with AF combined with HF. CA can also improve cardiac structure, cardiopulmonary function, and quality of life, with most outcomes showing no significant differences in subgroup analyses. Consistency was observed in most subgroup and sensitivity analyses, suggesting that patients with AF combined with HF may benefit from early intervention with CA.
The global prevalence of HF ranges from 1–3%52, with the prevalence of HFrEF remaining stable or even decreasing over the years, while the prevalence of HFpEF is on the rise and may become the predominant form of HF in the future53. AF is often coexisting with HFpEF, leading to a 30–40% increase in HF hospitalization and mortality rates54. Conventional treatments in the form of pharmacotherapy for HFrEF show limited effectiveness or contraindications in HFpEF patients. Currently, CA is recommended as a Class I treatment for AF in HFrEF patients18, reducing HF event rates in HFrEF patients but showing no or limited efficacy in HFpEF patients55. This meta-analysis, based on a larger sample size, indicates that CA treatment can reduce HF events in HFpEF patients, while also reducing CV hospitalizations, AF recurrences, and stroke/TIA rates, but no significant benefits have been observed in cardiovascular mortality rates. Additionally, CA can improve LAD, BNP, NT-proBNP, and NYHA classification in HFpEF patients, but no significant improvements have been observed in LVEF,6MWT, and peak VO2 improvement. The lack of significant clinical prognosis improvement may stem from limitations in HFpEF research: the inclusion criteria of the CABANA trial did not definitively diagnose HF but relied solely on NYHA functional assessment to define HF. Many symptoms overlap between AF and HF, which is particularly challenging in HFpEF patients. Moreover, the CABANA trial used the composite endpoint of all-cause mortality, stroke, severe bleeding, or cardiac arrest as the primary endpoint, with the rest being secondary endpoints31. The inclusion criteria in the study by Parkash et al. combined NYHA classification and elevated NT-proBNP, providing a more comprehensive definition for HFpEF patients, and was the first study to compare HFrEF and HFpEF patients in a subgroup analysis23. The study by Chieng et al. used inclusion criteria that included symptoms, BNP, cardiac evidence, and even invasive hemodynamic parameters, increasing the accuracy of patient inclusion but limiting its applicability28. The non-significant improvements in LVEF, 6MWT, and peak VO2 among HFpEF patients may be attributed to the disease’s distinct pathophysiology: first, HFpEF is primarily characterised by impaired left ventricular diastolic function, whereas catheter ablation mainly enhances atrial contractility with limited effects on ventricular diastolic performance, potentially explaining the modest clinical benefits; second, HFpEF patients typically exhibit sympathetic overactivation and reduced vagal tone, leading to myocardial β-adrenergic receptor desensitisation and calcium dysregulation - while ablation reduces atrial arrhythmia triggers, its capacity to correct autonomic imbalance remains limited compared with HFrEF, resulting in less pronounced improvements in LVEF, 6MWT, and peak VO2. Ongoing RCTs, such as the comparison of CA for AF in HFpEF patients with conventional treatment (CABA-HFPEF; NCT05508256) and CA for AF in HFpEF patients (STABLE-SR IV; NCT06125925), may provide more definitive evidence for the benefits of CA treatment in HFpEF patients. Upon revisiting published meta-analyses per your suggestion, we identified important comparisons: Chen56 found no significant differences in HFpEF patients between CA and medical therapy (particularly AAD-based rhythm control) in HF hospitalisations, all-cause admissions, or mortality - aligning with our findings except for HF hospitalisation benefits. Methodological differences exist as Chen56 included case-control studies. Androulakis57 reported improved sinus rhythm maintenance and reduced HF admissions (but not all-cause mortality) post-CA, consistent with our results. Notably, Androulakis’ study57 had limited sample size and heterogeneous HFpEF definitions (LVEF > 45% or > 50%) versus our stricter criteria.
Following short-term follow-up of patients with AF combined with HF receiving CA treatment revealed a decrease in HF event rates4. However, long-term follow-up did not show significant benefits23, prompting a subgroup analysis to investigate the impact of follow-up duration on clinical outcomes. This meta-analysis further demonstrates that CA can reduce both short-term and long-term HF event rates, mortality rates, AF recurrence rates, LVEF, BNP levels, functional status, and MLHFQ scores in patients with AF and HF. CA did not improve short-term hospitalization rates and stroke occurrences in AF combined with HF patients, but benefits were observed during long-term follow-up. The lack of significant improvement in short-term clinical prognosis may stem from study limitations: the study by Marrouche et al. had more patients crossing over from the CA group to the control group compared to the drug control group, potentially resulting in no difference in hospitalization rates between the two groups in the short term25. The study by Olshausen et al. was a cohort study, where unmatched confounding factors may have impacted the outcomes41. Nevertheless, it is still recommended that all patients with AF combined with HF should undergo early CA treatment to improve both short-term and long-term clinical outcomes. CA is associated with improvements in HF event rates, mortality rates, AF recurrence, NYHA classification, and MLHFQ scores in Asian and Western patients with AF combined with HF. However, there were no significant improvements in HF event rates, cardiovascular mortality rates, and stroke in global studies. This meta-analysis suggests that CA is highly applicable to Asian and Western patients, improving cardiac structure and cardiopulmonary function, thereby enhancing quality of life and prognosis. This indicates the suitability of CA for patients with AF combined with HF in Asian and Western regions; Atrioventricular node ablation with pacemaker implantation as an alternative ablation strategy is also used in patients with AF combined with HF. Subgroup analysis of the NCA group found that in the subgroup analysis of pharmacotherapy in the NCA group, CA was associated with improvements in LVEF, LAD, 6MWT, and MLHFQ. In the subgroup analysis of atrioventricular node ablation with pacemaker implantation in the NCA group, no significant benefits were observed in HF event rates and all-cause mortality rates in the CA group. However, studies on atrioventricular node ablation with pacemaker implantation in the NCA group are limited, and previous studies used ventricular pacing. Future research is necessary to assess the comparison between CA and atrioventricular node ablation with pacemaker implantation systems to further investigate the clinical outcomes of these treatments.
Therefore, CA can improve the clinical prognosis of patients with AF combined with HF. We hypothesize that this is achieved through the following mechanisms: firstly, CA can restore SR, making the rhythm more regular to improve diastolic and systolic function, thereby improving LVEF and alleviating symptoms such as palpitations, fatigue, shortness of breath, and exercise intolerance. Secondly, CA can reduce AF recurrence, recurrent AF can contribute to the progression of HF by causing further damage to the heart muscle and promoting electrical remodelling. CA targets the areas in the heart responsible for triggering AF, interrupting the abnormal electrical signals and reducing the likelihood of AF recurrence. By maintaining sinus rhythm for longer periods, CA can prevent the negative impact of AF on HF progression. Moreover, CA has been associated with improvements in heart function and structure. AF combined with HF can result in structural changes to the heart over time, such as atrial dilation and ventricular dysfunction. By addressing the underlying arrhythmia through CA, it can help preserve cardiac function, prevent further adverse remodelling, and potentially reverse some of the structural changes that have occurred.
CA can significantly improve echocardiographic measurements in patients with AF combined with HF, including LVEF, LAD, and cardiopulmonary function markers such as BNP, NT-proBNP, NYHA classification, 6MWT, and peak VO2. Rhythm control and the maintenance of sinus rhythm are crucial in the clinical management of patients with AF combined with HF. In conclusion, we speculate that CA, by improving cardiac structural remodeling and maintaining SR, can reduce HF events, mortality rates, hospital readmission rates, stroke occurrence, AF recurrence rates, and also enhance the quality of life in this patient population. So we recommend early initiation of CA treatment for patients in this category.
Strengths and limitations
The key strength of this study lies in the comprehensive inclusion of cohort studies and RCTs on patients with AF combined with HF, with a large number of articles and patient samples, making the conclusions more reliable. The results remained consistent in sensitivity analysis, indicating stability. In the meantime our comparative analysis with the two referenced meta-analyses55,56 revealed three key distinctions: methodologically, we enhanced robustness through sensitivity analyses, Galbraith/L’Abbe plots for dichotomous outcomes with high heterogeneity (I²>50%), and meta-regression - exceeding comparator studies’ approaches; demographically, our inclusion of both HFrEF/HFpEF patients (incorporating RCTs/cohort studies) with prespecified subgroup analyses provided broader representation (n = 777,668) than prior works55,58; analytically, we evaluated 16 endpoints with subgroup stratification (study design/follow-up duration/HF phenotype/region/NCA strategies), assessed publication bias via funnel plots/Egger’s test/trim-and-fill method, and implemented GRADE scoring - collectively offering superior clinical applicability. The limitations of the study as follows: most of the included RCTs in this meta-analysis were open-label in design, where both patients and researchers were aware of the group allocation and treatment details. This awareness could lead to patient dropout or withdrawal, as well as introduce bias in subjective outcome measurements, causing substantial errors. However, several included studies implemented methodologies to mitigate open-label bias: (i) the CASTLE-AF and CAMERA-MRI trials employed independent endpoint committees for blinded adjudication of hard endpoints (heart failure hospitalisations/deaths); (ii) certain studies utilised cardiac MRI-derived LVEF/LAD measurements, BNP levels, or 6-minute walk distance as primary endpoints to reduce subjective assessment bias; (iii) in the RAFT-AF trial, MLHFQ questionnaire data were automatically collected via third-party electronic platforms to minimise investigator intervention risks. Additionally, there was significant heterogeneity in this meta-analysis. Combining subgroup analyses, funnel plots, and shape of the distribution, and meta-regression assessment, the heterogeneity may originate from differences in the inclusion criteria of study patients: Hunter26 and Zakeri35 studies included patients with persistent AF, while Packer31 and Marrouche38 studies simultaneously included patients with persistent and paroxysmal AF. Studies by Jones7 and Wong36 included patients with HFrEF, while Chieng28and Fukui27 studies included patients with HFpEF. The studies included also used varying criteria to define HFpEF, leading to further heterogeneity. Rahman45study defined HFpEF as HF patients with LVEF ≥ 50%, Fukui27 defined HFpEF as HF patients with LVEF ≥ 50% and left ventricular diastolic dysfunction, and Chieng28 defined HFpEF as HF patients with elevated BNP, PCWP, and LVEF ≥ 50%. Treatment methods also varied: the ablation group included primarily pulmonary vein isolation as the ablation strategy, possibly with additional linear ablation; control group treatments varied with AATAC4 using amiodarone, RAFT-AF23requiring only rate control medication, and Rauber44 study employing atrioventricular node ablation with pacemaker implantation as the treatment method. Studies by Rauber44 Chieng28 had small sample sizes, leading to wider confidence intervals in outcomes, increasing the risk of false positive and false negative results. Heterogeneity was particularly pronounced for quantitative outcomes including LVEF, LAD and NYHA classification. Beyond previously identified factors, we attribute this variability to the inherent subjectivity of these endpoints - particularly in open-label studies where assessors’ knowledge of treatment allocation may influence measurements, compounded by inter-study methodological differences in measurement protocols, operator techniques and centre-specific practices, all contributing to elevated heterogeneity. Additionally, the combination of cohort studies with RCTs in meta-analyses may substantially increase observed heterogeneity. This effect is particularly evident in studies that fail to employ propensity score matching, where significant selection bias and residual confounding are likely to persist. When conflicting results emerge from subgroup analyses by study design, we strongly recommend prioritising conclusions derived from RCT evidence, given its superior protection against confounding through randomisation.
Conclusions
This systematic review and meta-analysis found that catheter ablation is superior to NCAin reducing HF event rates, mortality rates, hospital readmission rates, AF recurrence, and stroke occurrence in patients with atrial fibrillation combined with HF. It also has similar therapeutic effects in improving cardiopulmonary function and quality of life. However, there is a limited number of studies in the subgroup analysis of HFpEF and atrioventricular node ablation with pacemaker implantation. Further evidence from large-scale RCTs, both ongoing and in the future, is needed to draw definitive conclusions.
Data availability
All data generated or analysed during this study are included in this manuscript and its supplementary information files.
References
Pabel, S. & Sossalla, S. Atrial fibrillation and heart failure: novel insights into the chicken and egg dilemma. Eur. Heart J. 43, 3376–3378 (2022).
Odutayo, A. et al. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ (Clin Res. ed,) 354, i4482 (2016).
Joglar, J. A. et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation 149, e1–e156 (2024).
Di Biase, L. et al. Ablation versus Amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 133, 1637–1644 (2016).
Becher, N., Metzner, A., Toennis, T., Kirchhof, P. & Schnabel, R. B. Atrial fibrillation burden: a new outcome predictor and therapeutic target. Eur. Heart J. 45, 2824–2838 (2024).
Mulder, B. A., Rienstra, M., Van Gelder, I. C. & Blaauw, Y. Update on management of atrial fibrillation in heart failure: a focus on ablation. Heart 108, 422–428 (2022).
Jones, D. G. et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J. Am. Coll. Cardiol. 61, 1894–1903 (2013).
Kazi, D. S. et al. Forecasting the economic burden of cardiovascular disease and stroke in the united States through 2050: a presidential advisory from the American heart association. Circulation 150, e89–e101 (2024).
Hashemi, D. et al. Economic impact of heart failure with preserved ejection fraction: insights from the ALDO-DHF trial. ESC Heart Fail. 7, 786–793 (2020).
Newman, J. D. et al. Implications of atrial fibrillation for guideline-directed therapy in patients with heart failure: JACC state-of-the-art review. J. Am. Coll. Cardiol. 83, 932–950 (2024).
Tsao, C. W. et al. Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation 145, e153–e639 (2022).
McDonagh, T. A. et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 42, 3599–3726 (2021).
Arbelo, E. et al. [2023 ESC Guidelines for the management of cardiomyopathies]. G. Ital. Cardiol. () 24, e1–e127 (2023).) 24, e1–e127 (2023). (2006).
Pabel, S. et al. Effects of atrial fibrillation on the human ventricle. Circ. Res. 130, 994–1010 (2022).
Paulus, M. G. et al. Tachycardiomyopathy entails a dysfunctional pattern of interrelated mitochondrial functions. Basic. Res. Cardiol. 117, 45 (2022).
Choi, A. D., Hematpour, K., Kukin, M., Mittal, S. & Steinberg, J. S. Ablation vs medical therapy in the setting of symptomatic atrial fibrillation and left ventricular dysfunction. Congestive Heart Fail. 16, 10–14 (2010).
Nguyen, B. O. et al. Optimal treatment of underlying conditions improves rhythm control outcome in atrial fibrillation - data from RACE 3. Am. Heart J. 226, 235–239 (2020).
Hindricks, G. et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur. Heart J. 42, 373–498 (2021).
Andrade, J. G. et al. Healthcare utilization and quality of life for atrial fibrillation burden: the CIRCA-DOSE study. Eur. Heart J. 44, 765–776 (2023).
Li, F. C. Observation of the therapeutic efficacy of radiofrequency ablation for persistent atrial fibrillation combined with heart failure in rhythm control. Pract. Clin. Pract. Integr. Med. 22, 16–18 (2022).
Bunch, T. J. et al. Five-year outcomes of catheter ablation in patients with atrial fibrillation and left ventricular systolic dysfunction. J. Cardiovasc. Electrophysiol. 26, 363–370 (2015).
Kuck, K. H. et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA trial. Circ. Arrhythm. Electrophysiol. 12, e007731 (2019).
R, P. et al. Randomized ablation-based rhythm-control versus rate-control trial in patients with heart failure and atrial fibrillation: results from the RAFT-AF trial. Circulation 145, 1693–1704 (2022).
Sohns, C. et al. Catheter ablation in end-stage heart failure with atrial fibrillation. N. Engl. J. Med. 389, 1380–1389 (2023).
Marrouche, N. F. et al. Catheter ablation for atrial fibrillation with heart failure. N Engl. J. Med. 378, 417–427 (2018).
Hunter, R. J. et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ. Arrhythm. Electrophysiol. 7, 31–38 (2014).
Fukui, A. et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J. Cardiovasc. Electrophysiol. 31, 682–688 (2020).
Chieng, D. et al. Atrial fibrillation ablation for heart failure with preserved ejection fraction: a randomized controlled trial. JACC: Heart Fail. 11, 646–658 (2023).
MacDonald, M. R. et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart 97, 740–747 (2011).
Xu, Y., Cai, H. & Li, H. S. Comparison of efficacy of radiofrequency ablation and drug therapy in patients with atrial fibrillation complicated with heart failure. Adv. Cardiovasc. Dis. 43, 274–281 (2022).
Packer, D. L. et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation 143, 1377–1390 (2021).
Zhu, X. Y. & Huang, C. X. The impact of catheter ablation on left ventricular function in atrial fibrillation patients with reduced left ventricular ejection fraction. Chin. J. Cardiovasc. Med. 24, 310–313 (2019).
Sun, J. et al. Comparison of catheter ablation therapy and medication treatment on prognosis in atrial fibrillation patients with heart failure. Liaoning Med. J. 32, 3–6 (2018).
Hunter, R. J. et al. A randomised controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the Camtaf trial). PACE - Pacing Clin. Electrophysiol. 34, 1367 (2011).
Zakeri, R. et al. Long-term outcomes following catheter ablation versus medical therapy in patients with persistent atrial fibrillation and heart failure with reduced ejection fraction. Eur J Heart Fail. 25, 77–86 (2023).
Wong, C. et al. Long-term outcomes following catheter ablation in patients with atrial fibrillation and heart failure: 7-year follow-up of the ARC-HF trial. Circulation 138, 171–179 (2018).
Mohanty, S. et al. Long-term outcome of pulmonary vein isolation versus Amiodarone therapy in patients with coexistent persistent atrial fibrillation and congestive heart failure. Card Fail Rev. 39, 52–53 (2018).
Marrouche, N. F., Kheirkhahan, M. & Brachmann, J. Association between improvement in left ventricular function, mortality and hospitalization post ablation of atrial fibrillation in patients with heart failure -the castle-af trial. Heart Rhythm. 16, 368 (2019).
Sugumar, H. et al. Catheter ablation versus medication in atrial fibrillation and systolic dysfunction late outcomes of CAMERA-MRI study. JACC: Clin. Electrophysiol. 6, 1721–1731 (2020).
Prabhu, S. et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J. Am. Coll. Cardiol. 70, 1949–1961 (2017).
Von Olshausen, G. et al. Catheter ablation for patients with atrial fibrillation and heart failure: insights from the Swedish heart failure registry. Europace 24, i346 (2022).
Samuel, M., Abrahamowicz, M., Joza, J., Essebag, V. & Pilote, L. Catheter ablation is associated with reduced all-cause mortality in a real-world cohort of patients with atrial fibrillation and heart failure. Canadian J. Cardiology 35, 1006–1009 (2019).
Geng, J. et al. Catheter ablation versus rate control in patients with atrial fibrillation and heart failure: A multicenter study. Med. (Baltim). 96, e9179 (2017).
Rauber, M. et al. Conduction system pacing with AV node ablation versus catheter ablation for treatment of persistent atrial fibrillation in patients with heart failure with reduced ejection fraction. Europace 24, i261 (2022).
Rahman, A. et al. Efficacy of catheter ablation of atrial fibrillation in heart failure with preserved ejection fraction. J. Card. Fail. 25, S84–S85 (2019).
Lima, F. et al. Hospital readmissions after catheter ablation for atrial fibrillation among patients with heart failure in the united States. Eur. Heart J. 41, 443 (2020).
Samuel, M. et al. Long-term effectiveness of catheter ablation in patients with atrial fibrillation and heart failure. Europace 22, 739–747 (2020).
Patel, H. et al. MP-483497-005 impact of catheter ablation for atrial fibrillation in patients with heart failure with preserved ejection fraction. Heart Rhythm. 21, S101–S102 (2024).
Zolotarova, T., Brynza, M. & Bilchenko, O. Outcomes of heart failure with preserved ejection fraction after radiofrequency catheter ablation for atrial fibrillation. Europace 23, iii134 (2021).
Khan, M. N. et al. 34.Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 359, 1778–1785 (2008).
Pasqualotto, E. et al. Catheter ablation for atrial fibrillation in heart failure with reduced ejection fraction patients: A meta-analysis. Heart Rhythm. 21, 1604–1612 (2024).
Savarese, G. et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc. Res. 118, 3272–3287 (2023).
Becher, P. M., Lund, L. H., Coats, A. J. S. & Savarese, G. An update on global epidemiology in heart failure. Eur. Heart J. 43, 3005–3007 (2022).
Kotecha, D. et al. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J. Am. Coll. Cardiol. 68, 2217–2228 (2016).
Oraii, A. et al. Atrial fibrillation ablation in heart failure with reduced vs preserved ejection fraction: a systematic review and meta-analysis. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2024.0675 (2024).
Chen, X., Zhang, X., Fang, X. & Feng, S. Efficacy and safety of catheter ablation for atrial fibrillation in patients with heart failure with preserved ejection fraction: a systematic review and meta-analysis. Front. Cardiovasc. Med. 11, 1423147. https://doi.org/10.3389/fcvm.2024.1423147 (2024).
Androulakis, E. et al. Catheter ablation for atrial fibrillation in patients with heart failure with preserved ejection fraction: a systematic review and meta-analysis. J. Clin. Med. 11 (2), 288. https://doi.org/10.3390/jcm11020288 (2022).
Mahalleh, M. et al. Heart failure with preserved ejection fraction and atrial fibrillation: catheter ablation vs. standard medical therapy a systematic review and meta-analysis. Heart Fail. Rev. 30 (1–15). https://doi.org/10.1007/s10741-024-10437-3 (2025).
Acknowledgements
This work was supported by the Key R&D Program of Xinjiang Uygur Autonomous Region (2022B03023).
Author information
Authors and Affiliations
Contributions
X.T.Z. and M.W. conceptualized the study. X.T.Z. and P.J.X. conducted the literature search and data extraction and management. X.T.Z. and M.W. performed data analysis. X.T.Z. and P.J.X assessed the study’s risk of bias and created the composite chart. X.T.Z. drafted the initial manuscript. X.T.Z. and B.P.T. revised and edited the manuscript. X.T.Z. and B.P.T. supervised the project. X.T.Z. handled project administration, and B.P.T. secured the funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, X., Wei, M., Xue, P. et al. Meta-analysis of comprehensive prognostic evaluation in patients with atrial fibrillation complicated by heart failure after catheter ablation. Sci Rep 15, 32785 (2025). https://doi.org/10.1038/s41598-025-16166-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16166-3