Abstract
Determine whether APOE gene polymorphism is associated with hypoperfusion intensity ratio (HIR) in acute ischemic stroke (AIS) patients with large vessel occlusion (LVO). Continuously reviewed hospitalized LVO-AIS patients. According to whether the patients carried APOE allele ε 4, they were divided into 2 groups: ε4 carriers and non-ε4 carriers. CTP assessed HIR and infarct core (IC) volume. Good collaterals were defined as HIR < 0.4 and poor collaterals were defined as HIR ≥ 0.4. The patients were divided into two groups based on their HIR value: the HIR < 0.4 group and the HIR ≥ 0.4 group. IC volume was a rCBF < 40% volume. NIHSS at admission assessed stroke severity. A total of 101 patients with LVO-AIS were enrolled, including 82 patients with HIR < 0.4 and 19 patients with HIR ≥ 0.4. Among the patients with HIR < 0.4, 10 were ε4 carriers (12.20%), while among those with HIR ≥ 0.4, 8 were ε4 carriers (42.11%). The proportion of ε4 carriers was significantly higher in the HIR ≥ 0.4 patients group (P = 0.006). In all enrolled patients, there were 83 non-ε4 carriers and 18 ε4 carriers. The IC volume in ε4 carriers was significantly higher than that in non-ε4 carriers (P = 0.003). The NIHSS score in ε4 carriers was significantly higher than that in non-ε4 carriers (P = 0.004). Binary logistic regression showed that APOE ε4 was an independent risk factor for poor collaterals (OR = 6.00, 95%CI: 1.80, 20.02, P = 0.004). Multiple linear regression showed HIR had a significant positive effect on IC volume (B = 167.70, P < 0.001) and NIHSS score (B = 8.53, P = 0.014). APOE ε4 is an independent risk factor for poor collaterals.
Similar content being viewed by others
Introduction
Hypoperfusion intensity ratio (HIR) is automatically calculated from perfusion imaging which offers an estimation of cerebral blood perfusion over time and defined as the ratio between volumes with time-to-maximum of the tissue residue function (Tmax) > 10 s and > 6s1. HIR has been suggested to be a measure of microvascular tissue perfusion and tissue collateral status2. Apolipoprotein E (APOE) is a polymorphic glycoprotein encoded by APOE gene that is involved in the growth, maintenance, and regeneration of the peripheral and central nervous system during development and after injury3. Animal studies have demonstrated that APOE gene polymorphism can affect the formation of collateral circulation during embryogenesis and the growth of collaterals post-disease through vascular endothelial growth factor (VEGF)4,5,6,7,8. Currently, there is no study on the impact of APOE gene polymorphism on HIR in acute ischemic stroke (AIS) patients with large vessel occlusion (LVO). Previous studies have shown that poor collaterals in LVO-AIS patients were associated with larger infarct volume, higher risk of death, and poor functional prognosis9,10, but these studies did not use HIR for quantitative assessment of collateral status. To sum up, this study intends to use HIR to evaluate the collateral status of brain tissue, clarify the effect of APOE gene polymorphism on HIR, and investigate the effects of HIR on the infarct core (IC) volume and stroke severity.
Methods
This study was a retrospective case-control study, and the study protocol was approved by Medical Ethics Committee of Shaanxi Provincial People’s Hospital (Fund number: SPPH-LLBG-17-3.2). All patients or their legally authorized representatives signed the informed consent. This study performed in accordance with the Declaration of Helsinki.
Patients
Continuously reviewed hospitalized patients in the Department of Neurology of Shaanxi Provincial People’s Hospital from September 2022 to August 2024. Inclusion criteria: (1) Age between 18 and 80 years; (2) The time from the onset of the disease to hospital admission was within 24 h; (3) CTA showed internal carotid artery (ICA) occlusion or the middle cerebral artery (MCA) M1 or M2 segment occlusion; (4) completed Computed tomography (CT) perfusion imaging (CTP) and Computed tomography angiography (CTA) examination; (5) completed APOE gene examination. Exclusion criteria: (1) Have contraindications to iodinated contrast agents; (2) poor quality of CT images to assess HIR and vessels occlusion; (3) patients with hemorrhagic transformation after infarction.
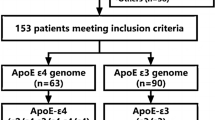
According to whether the patients carried APOE allele ε4, they were divided into 2 groups: ε4 carriers (ε3/ε4 and ε4/ε4) and non-ε4 carriers (ε2/ε3 and ε3/ε3). The patients were also divided into two groups based on their HIR value: the HIR < 0.4 group and the HIR ≥ 0.4 group.
Clinical assessment
Demographic information included age, gender, height, weight, diabetes, hypertension, hyperlipidemia, atrial fibrillation (AF), coronary heart disease, stroke history, transient ischemic attack (TIA) history, smoking and drinking history were collected. Data of homocysteine (HCY), glycated hemoglobin (HBALC), triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), hemoglobin (HGB) and Uric acid (UA) were also collected. The data of National Institutes of Health Stroke Scale (NIHSS) at admission was collected as a quantifiable scale to assess stroke severity and neurological status at admission11. Trained nursing professionals measured height and weight, allowing for the computation of BMI as the weight in kilograms divided by the square of the height in meters.
Imaging acquisition
CTP was performed using a 320-slice spiral CT scanner (TOSHlBA, Aquilion One, Japan). The patient was supine during the examination. CT scan was performed first, parameters were set as follows: tube current 150 mA, tube voltage 120 kV, speed 0.75s/turn, spacing 9 mm, layer thickness 9 mm. Then, the whole brain CTP examination was performed. The patient was injected with ioprosamide injection (Bayer Pharmaceutical, National Drug Approval number H10970417, specification: 100mL: 37 g)50rnL through the elbow vein at a speed of 5mL/S and a delay of 5s for perfusion scan, parameters were set as follows: tube current 125 mA, tube voltage 80 kV, speed 0.4 S/ turn, interval time 5s, layer thickness 5 mm, interval distance 5 mm, cycle scan 15 times. The relevant data were recorded and imported into the workstation, and the images were analyzed by the bundled software to measure cerebral blood flow (CBF) and the volume of brain tissue with a Tmax delay in the infarcted hemispheres.
CTA was performed using Siemens SOMATOM Definition -Flash dual source CT. Patient was scanned from the basis cranii to the calvarium, including the entire brain structure. Parameters were set as follows: tube voltage 200Kev, layer thickness 0.5 mm, interval distance 0.5 mm. The enhancement was performed with threshold triggered scan, ROI was placed in the ascending aorta, and the CT threshold was set to 100HU. The enhancement was performed with threshold triggered scan, ROI was placed in the ascending aorta, and the CT threshold was set to 100HU. An automatic high-pressure syringe was used to infuse iodohexyl alcohol into the elbow vein at an injection rate of 4.0mL/s and a dosage of 1.5mL/kg, followed by the infusion of 50mL saline at the same injection rate. The original image data was transmitted to the post-processing workstation, and the CTA map is obtained by 3D imaging.
Imaging processing and analysis
Infarct core (IC) volume, low perfusion volume and ischemic penumbra volume (mismatch volume) were obtained by CTPDoc (Shukun Technology Co., Ltd., China, version 1.0) software2. IC volume was a rCBF < 40% volume1,12. The total hypoperfused tissue volume is the volume of brain tissue with a Tmax delay of > 6s. The ischemic penumbra volume is a mismatched volume of the low perfusion volume minus the IC volume. HIR was defined as the volume of ischemic brain tissue with a Tmax delay of > 10 s divided by the volume of brain tissue with a Tmax delay of > 6 s. Favorable (good) collaterals were defined as HIR < 0.4 and poor collaterals were defined as HIR ≥ 0.413.The patients were divided into two groups based on their HIR value: the HIR < 0.4 group and the HIR ≥ 0.4 group.
The CTA was used to evaluate unilateral cerebral artery occlusion, which was defined as a 100% cross-sectional truncation of the vascular lumen14.
Statistical analysis
Statistical analysis was performed using SPSS 24.0, and P < 0.05 was considered statistically significant. Shapiro-Wilk test was used to test the normality of continuous variables. Normal distribution data were represented by mean ± standard deviation (SD), and independent sample t test was used for comparison between groups. Non-normal distribution data were represented by median (interquartile range (IQR)), and Mann-Whitney U test was used for comparison between groups. Categorical variables were represented by proportion (%), Pearson χ2 test and Fisher exact probability method were used for comparison between groups. Binary logistic regression was used to test risk factors for poor collaterals. Multiple linear regression was used to test the risk factors for HIR, IC volume and NIHSS at admission.
Results
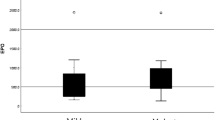
A total of 101 patients with LVO-AIS were enrolled in our study, including 83(82.18%) non-ε4 carriersand 18(17.82%) ε4 carriers. There were 51(61.45%) males in non-ε4 carriers and 16(88.89%) males in ε4 carriers, the proportion of males in ε4 carriers was significantly higher than that in non-ε4 carriers (P = 0.026). The LDL-C in non-ε4 carriers (2.31 ± 0.71) was significantly higher than that in ε4 carriers(3.05 ± 0.68)(P<0.001). The median (IQR) NIHSS score was 5(3,9) in non-ε4 carriers and 10.5(6.75,16.75) in ε4 carriers, the NIHSS score in ε4 carriers was significantly higher than that in non-ε4 carriers (P = 0.004). There were 11(13.25%) patients with poor collaterals in non-ε4 carriers and 8(44.44%) patients in ε4 carriers (Fig. 1), the proportion of poor collaterals in ε4 carriers was significantly higher than that in non-ε4 carriers (P = 0.006). The median (IQR) HIR in non-ε4 carriers was 0.15 (0.04, 0.33) and in ε4 carriers was 0.35(0.17, 0.56), and the HIR in ε4 carriers was significantly higher than that in non-ε4 carriers (P = 0.012). The median (IQR) IC volume was 38 (20.3, 70.9) ml in non-ε4 carriers and 87.85 (50.38, 114.35) ml in ε4 carriers, the IC volume in ε4 carriers was significantly higher than that in non-ε4 carriers (P = 0.003). Details were shown in Table 1.
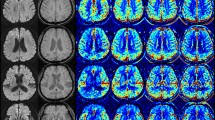
The red area is Tmax > 10s area, and the green area is Tmax > 6s area. (A) depicts a patient with a right internal carotid artery occlusion (ICAO) who is a carrier of the ε4 allele, exhibiting poor collateral circulation with a HIR of 0.44. (B) shows a patient with a left ICAO and a carrier of the ε3 allele, possessing favorable collateral circulation and an HIR of 0.02.
There were 82 patients with HIR < 0.4 and 19 patients with HIR ≥ 0.4, as shown in Table 2. Among the patients with HIR < 0.4, 10 were ε4 carriers (12.20%), while among those with HIR ≥ 0.4, 8 were ε4 carriers (42.11%). The proportion of ε4 carriers was significantly higher in the HIR ≥ 0.4 patients group (P = 0.006), as detailed in Table 2. In the HIR < 0.4 group, the IC volume was 40.00 (19.93, 69.47), whereas in the HIR ≥ 0.4 group, it was 90.50 (27.00, 176.00). The IC volume was significantly larger in the HIR ≥ 0.4 group (P = 0.004), as shown in Table 2.
After adjusting for gender and age, binary logistic regression showed that APOE ε4 was an independent risk factor for poor collaterals (HIR ≥ 0.4) (OR = 6.00, 95%CI: 1.80, 20.02, P = 0.004),as shown in Table 3. This means that compared with ε3 carriers, ε4 carriers have a risk of poor collaterals (HIR ≥ 0.4) that is 6 times higher.
After adjusting for gender, age and IC volume, binary logistic regression showed that APOE ε4 was an independent risk factor for poor collaterals (HIR ≥ 0.4) (OR = 4.65, 95%CI: 1.20, 18.04, P = 0.026), as shown in Table 3. This means that compared with ε3 carriers, ε4 carriers have a risk of poor collaterals (HIR ≥ 0.4) that is 4.65 times higher.
Multiple linear regression tested the effects of age, gender and APOE ε4 on HIR. The final model was statistically significant (F = 3.64, P = 0.015, corrected R2 = 0.07), and HIR had a significant positive effect on IC volume (B = 0.16, P = 0.002), as shown in Table 3. This means that after controlling for age and gender, when the independent variable APOE genotype changed from ε3 carriers to ε4 carriers, the average value of the dependent variable HIR increased by 0.16. This indicates that the HIR value of ε4 carriers is significantly higher than that of ε3 carriers.
Multiple linear regression tested the effects of age, gender, HIR and APOE ε4 on IC volume. The final model was statistically significant (F = 11.28, P < 0.001, corrected R2 = 0.29), and HIR had a significant positive effect on IC volume (B = 167.70, P < 0.001), as shown in Table 3.This means that after controlling for age, gender and APOE genotype, for every 0.1 unit increase in HIR, the IC volume would increase by 16.77 ml. For every 1 unit increase in HIR, the IC volume would increase by 167.70 ml. This indicates that the change in HIR has a significant impact on the IC volume.
Multiple linear regression tested the effects of age, gender, HIR, IC volume, ischemic penumbra volume, the side of artery occlusion and APOE ε4 on NIHSS score. The final model was statistically significant (F = 3.19, P = 0.005, corrected R2 = 0.13), and HIR had a significant positive effect on NIHSS score (B = 8.53, P = 0.014), as shown in Table 3. This means that after controlling for age, gender, APOE genotype and other independent variables, for every 0.1 unit increase in HIR, the NIHSS score would increase by 0.853 points. For every 1 unit increase in HIR, the NIHSS score would increase by 8.53 points. This indicates that the change in HIR has a significant impact on NIHSS.
Discussion
The proportion of APOE ε4 carriers in the global world is 23.9% and in normal cognition people in coastal areas of China is 19.54%15,16. In our study, there were 83 (82.18%) non-ε4 carriers and 18 (17.82%) ε4 carriers, indicating that the ε3 allele was still the most common APOE allele, and the proportion of APOE ε4 allele was slightly lower than that in the normal cognition population. In our study, ε4 carriers were more likely to have poor collaterals, and the probability of poor collaterals was 6 times higher in ε4 carriers than in non-ε4 carriers (OR = 6.00, 95%CI: 1.80, 20.02, P = 0.004). APOE ε4 can significantly change the signal pathways related to cholesterol homeostasis and transport17, thereby affecting collateral circulation18. In our study, the LDL-C in ε4 carriers was significantly higher than that in non-ε4 carriers, thereby poor collaterals of ε4 carriers may affected by high LDL-C. In addition, APOE ε4 may affect collateral blood perfusion through other factors such as changes in cholinergic pathway. Cerebrovascular reactivity is regulated by parasympathetic and sympathetic nerve activities through the central cholinergic system, and APOE ε4 may affect cerebrovascular reactivity by changing the cholinergic pathway, further affect cerebral blood flow and cerebral perfusion in patients19,20. Cardiogenic embolism, atherosclerotic plaque rupture with in situ thrombosis and artery-to-artery embolization were the pathogenesis of our enrolled patients with cerebral artery occlusion. APOE can affect both the formation of collateral circulation in the embryo and the growth of collateral circulation after the disease onset4,5,6,7,8, in our study, where large artery atherosclerosis was the predominant etiology of cerebral vessel occlusion, the APOE ε4 allele potentially impaired compensatory collateral formation during the progression of atherosclerotic stenosis, thereby contributing to poorer collateral circulation (HIR ≥ 0.4) in ε4 carriers. The collateral circulation of ε4 carriers should theoretically be worse, and our study also verified this theory.
When the cerebral artery is occluded, poor collaterals can lead to reduced collateral blood flow, which further leads to aggravation of ischemia and increase of infarct volume in the territory supplied by occluded vessel. When the collaterals is good, the collateral blood flow can save ischemic penumbra tissue and avoid increasing the infarct volume in the territory supplied by occluded vessel21. The results of multiple linear regression in our study showed that HIR significantly positively affected the IC volume (B = 167.70, P < 0.001), indicating that the IC volume increased with the HIR increased. HIR represents collateral circulation and microperfusion of brain tissue, so the results of this study proved that the IC volume is larger when the collateral circulation is poor.
Patients with poor collaterals have more severe stroke22,23, which may be related to infarct volume, brain edema, intracranial pressure and reperfusion24,25,26. Higher NIHSS scores are associated with more severe stroke11.The results of multiple linear regression in our study showed that HIR significantly positively affected NIHSS (B = 8.53, P = 0.014), indicating that the higher the HIR, the higher the NIHSS score, the more severe stroke, but the multiple linear regression also showed that the IC volume did not affect the NIHSS score (P = 0.397). Therefore, stroke severity in our study may be related to other factors such as brain edema caused by poor collaterals.
This is a retrospective study, there were relatively fewer patients with poor collaterals in our study, the results of our study need to be verified in a larger cohort. Our study could not clarify the etiological mechanism of poor collaterals in ε4 carriers, and animal experiments should continue to clarify the influence of APOE gene on collateral circulation in future.
Conclusion
APOE ε4 is an independent risk factor for poor collaterals.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Busto, G. et al. Hypoperfusion intensity ratio correlates with collaterals and predicts outcome and infarct volume in acute ischemic stroke patients. Eur. J. Clin. Invest. e14264 (2024).
Winkelmeier, L. et al. Hypoperfusion intensity ratio is correlated with the risk of parenchymal hematoma after endovascular stroke treatment. Stroke 54, 135–143 (2023).
Paternoster, L., Martínez González NAn, Lewis, S. & Sudlow, C. Association between Apolipoprotein E genotype and carotid Intima-Media thickness May suggest a specific effect on large artery atherothrombotic stroke. Stroke 39, 48–54 (2008).
Chilian, W. M. & Pung, Y. F. Vascular endothelial growth factor and the collateral circulation. Circul. Res. 103, 905–906 (2008).
Clayton, J. A., Chalothorn, D. & Faber, J. E. Vascular endothelial growth Factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circul. Res. 103, 1027–1036 (2008).
Couffinhal, T. et al. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE-/- mice. Circulation 99, 3188–3198 (1999).
Troidl, K. et al. (2020)The lipopeptide MALP-2 promotes collateral growth. Cells 9(4):997 .
van Royen, N. et al. Local monocyte chemoattractant protein-1 therapy increases collateral artery formation in Apolipoprotein E-deficient mice but induces systemic monocytic CD11b expression, neointimal formation, and plaque progression. Circul. Res. 92, 218–225 (2003).
Patel, S. D. & Liebeskind, D. Collaterals and elusive ischemic penumbra. Translational Stroke Res. 14, 3–12 (2022).
Gersing, A. S. et al. Clinical outcome predicted by collaterals depends on technical success of mechanical thrombectomy in middle cerebral artery occlusion. J. Stroke Cerebrovasc. Dis. 26, 801–808 (2017).
Lyden, P. Using the National institutes of health stroke scale. Stroke 48, 513–519 (2017).
Bivard, A., Spratt, N., Levi, C. & Parsons, M. Perfusion computer tomography: imaging and clinical validation in acute ischaemic stroke. Brain: J. Neurol. 134, 3408–3416 (2011).
Guenego, A. et al. Hypoperfusion intensity ratio is correlated with patient eligibility for thrombectomy. Stroke 50, 917–922 (2019).
Malhotra, K., Goyal, N. & Tsivgoulis, G. Internal carotid artery occlusion: pathophysiology, diagnosis, and management. Curr. Atheroscler. Rep. 19 (10), 41 (2017).
Wang, Y-Y. et al. The proportion of APOE4 carriers among Non-Demented individuals: A pooled analysis of 389,000 Community-Dwellers. J. Alzheimers Dis. 81, 1331–1339 (2021).
Jia, L. et al. The APOE ε4 exerts differential effects on Familial and other subtypes of alzheimer’s disease. Alzheimer’s Dement. 16, 1613–1623 (2020).
Blanchard, J. W. et al. (2022)APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 611:769–779 .
Ma, S. et al. (2022)Combination of High-Density lipoprotein cholesterol and lipoprotein(a) as a predictor of collateral circulation in patients with severe unilateral internal carotid artery stenosis or occlusion. J. Clin. Neurol. 18(1):14–23 .
Mahley, R. W. & Rall, S. C. Jr(2000)Apolipoprotein E: Far more than a lipid transport protein. Annu. Rev. Genom. Hum. Genet. 1:507–537 .
Piccarducci, R. et al. (2023)Apolipoprotein E ε4 triggers neurotoxicity via cholesterol accumulation, acetylcholine dyshomeostasis, and PKCε mislocalization in cholinergic neuronal cells. Biochimica et biophysica acta (BBA) -. Mol. Basis Disease 1869(7):166793 .
Sallustio, F. et al. CT angiography-based collateral flow and time to reperfusion are strong predictors of outcome in endovascular treatment of patients with stroke. J. Neurointerventional Surg. 9, 940–943 (2017).
Liu, R., Gao, M. & Zhao, X. Evaluation of collateral circulation in patients with internal carotid artery occlusion: A clinical and ultrasonographic multicenter study. Vascular Med. 29, 707–715 (2024).
Chen, L. et al. Importance of computed tomography perfusion on assessing collateral circulation and prognosis of patients with acute anterior circulation large vessel occlusion after endovascular therapy. SLAS Technol. 29 (4), 100139 (2024).
Faizy, T. D. et al. et al(2021)Perfusion imaging-based tissue-level collaterals predict ischemic lesion net water uptake in patients with acute ischemic stroke and large vessel occlusion. J. Cereb. Blood Flow. Metabolism 41:2067–2075 .
Berthezène, Y. et al. Collateral circulation assessment within the 4.5 h time window in patients with and without DWI/FLAIR MRI mismatch. J. Neurol. Sci. 394, 94–98 (2018).
Villringer, K. et al. The association between recanalization, collateral flow, and reperfusion in acute stroke patients: A dynamic susceptibility contrast MRI study. Front. Neurol. 10, 1147 (2019).
Funding
This study was supported by General Project of Key R&D Program in Shaanxi Province - Social Development Field (2022SF-032), Shaanxi Traditional Chinese Medicine Technology Project (SZY-KJCYC-2023-032), Xi’an Science and Technology Plan Project (24YXYJ0153), Shaanxi Provincial Natural Science Foundation (2022JM-533, 2022JM-483), and Scientific and technological personnel support plan funded project in Shaanxi Provincial People’s Hospital (2021BJ-25, 2021YJY-26).
Author information
Authors and Affiliations
Contributions
NaLiang wrote the main manuscript text and Feng Jiang prepared figure 1 . All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
This study was a retrospective case-control study, and the study protocol was approved by Medical Ethics Committee of Shaanxi Provincial People’s Hospital (Fund number: SPPH-LLBG-17-3.2).
Informed consent
All patients or their legally authorized representatives signed the informed consent. This study performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liang, N., Jiang, F., Wang, L. et al. The hypoperfusion intensity ratio associates with APOE gene polymorphism in acute ischemic stroke patients with large vessel occlusion. Sci Rep 15, 32288 (2025). https://doi.org/10.1038/s41598-025-16237-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16237-5