Abstract
Thiophene derivatives are recognised for their notable biological and pharmacological properties. This study examines the electronic structure and antibacterial properties of 2-[(Trimethylsilyl)ethynyl]thiophene (2TSET), emphasising the effects of solvent environments. We utilised spectroscopic techniques such as Fourier transform infrared (FT-IR), ultraviolet-visible (UV-Vis), and Raman spectroscopy (FT-Raman) to verify the molecular geometry of 2TSET. The vibrational behaviour was analysed using generalised quantum mechanical force field methods. The electronic properties, including frontier molecular orbitals (HOMO-LUMO), were analysed in gas and different solvent phases to assess intramolecular charge transfer. The electronic structure was examined through projected density of states (PDOS) and crystal orbital overlap population (COOP) analyses to evaluate orbital contributions and bonding interactions. Molecular electrostatic potential (MEP), electron localisation function (ELF), and localised orbital locator (LOL) maps were employed to identify chemically reactive sites across various phase conditions. The compound’s drug-likeness was assessed through ADMET profiling and bioactivity scoring. Molecular docking studies demonstrated favourable binding interactions, with the highest binding energy. The tested compound (2TSET) exhibited the highest binding affinity toward 2J6M and 3ERT, with a docking score of -4.9 kcal/mol and − 4.6 kcal/mol, indicating strong potential for inhibitory activity.
Similar content being viewed by others
Introduction
Antibiotics, a typical drug, are employed to treat bacterial infections in human beings and other animals. Pathogenic bacteria effluents in water cause significant health issues to both humans and aquatic living systems. However, antibiotic-resistant bacteria have emerged due to the overconsumption of drugs and antibiotics1. Antibiotic-resistant bacteria have evolved defense mechanisms against several antibiotics, resulting in antibiotic-associated infections that are difficult to cure. To overcome this issue, various research efforts have been taken to develop a range of efficient antimicrobial drugs from herbal plant extracts. Numerous secondary metabolites, some of which have antibacterial efficiency, are produced by plants. The potential of these natural active ingredients has the potential to fight against drug-resistant bacteria and open up new therapeutic development opportunities2. The World Health Organization (WHO) reports that approximately 1.27 million fatalities occurred in 2022 worldwide caused by the microorganisms’ resistance to antibiotics. Therefore, developing non-toxic, environmentally less hazardous antibacterial drugs and water treatment agents is urgently needed.
Currently, a range of heterocyclic compounds with pharmacological activity are still regularly used in clinical settings3. Various organic compounds, including natural products, are majorly composed of heterocyclic ring-containing compounds. These heterocyclic rings could alter the molecular conformation, solubility, and physicochemical, pharmacological, and biological activities of the compounds. In biological and materials chemistry, heterocyclic molecules are important because of their electronegative character4. Among the heterocyclic molecules with intriguing medicinal chemistry potential, thiophene and its substituted derivatives are playing an essential role in using them in drug development. Because of its numerous therapeutic properties and uses in pharmaceutical chemistry and materials science, thiophene and its derivatives are receiving a lot of attention from the research and industrial sectors. Thiophene compounds are widely applied in effective therapy for various diseases5. Due to their biological and physiological characteristics, thiophene-related substances are considered an important derivative for designing different drug molecules. Thiophene cores are the primary active ingredients in a variety of medicinal drugs. Owing to the active functional characteristics, thiophene-containing compounds would effectively bind to the receptor and act as an efficient competitive inhibitor6.
Global Challenge: Conventional antibiotics are becoming less effective due to antibiotic resistance, which is a growing threat to global health. Novel Structures are needed: The urgent need to create new classes of antimicrobial agents with unique mechanisms of action is highlighted by this crisis. The Significance of Thiophene Derivatives The broad-spectrum biological activities of heterocyclic compounds, particularly thiophene derivatives, have garnered interest because of their antibacterial, antifungal, and anti-inflammatory properties. Their structural adaptability makes it simple to alter them to improve selectivity and activity. Study Focus: In order to address the problem of antibiotic resistance, our study investigates the synthesis and biological assessment of novel thiophene-based compounds.
These days, sophisticated computer programs are used to perform quantum chemical calculations on a wide variety of substances. DFT simulations offer crucial insights into molecule’s electronic structures7. The hybrid Becke3 (exchange) and Lee-Yang-Parr (correlation) functionals (B3LYP) were used in this work’s DFT calculations together with a modest 6-311 + G(d, p) and 6-311 + G(d,2p) basis set for 2-[(Trimethylsilyl)ethynyl]thiophene] (2TSET). The TD-DFT is to calculate theoretical electronic transitions (UV-Vis spectra) of the molecule both in the gas phase and in selected solvents8. The FT-IR vibrational frequency study was considered to be performed when no imaginary frequencies were obtained, representing that all of the geometry had been completely optimized without any geometric limitations9. The LOL, ELF, and NCI were carried out using Multiwfn 3.9 software. By analyzing the biological activities of the 2TSET, the pharmacological characteristics were ascertained by using a web-based program10. To improve the binding energies of ligands with proteins, molecular docking experiments were conducted utilizing the Auto dock tool, the Discovery Studio visualization, and PyMOL view software.
This study aims to use computational analyses based on DFT and experiments to examine the electronic and structural characteristics of the 2TSET molecule, to investigate how the solvent affects the stability and spectral behaviour of the molecule and to evaluate 2TSET’s molecular docking potential and its ability to interact with biological receptors, with an emphasis on its potential as a therapeutic candidate.
Materials and methods
Materials
The 2-[(Trimethylsilyl)ethynyl]thiophene molecule (2TSET) was procured from Sigma-Aldrich in India and used as received. Hi-Media supplied the bacterial culture media, which included Mueller Hinton Agar (MHA), Nutrient Broth (NB), Agar-Agar, and Nutrient Agar (NA). The antibacterial activity of the 2TSET was assessed against drug-resistant superbugs (DRSs) strains of Gram-positive (Bacillus filamentous) with NCBI number MN045186, and Gram-negative (Acinetobacter baumannii) with NCBI number MN045188 using the zone inhibition method.
Experimental methods
A Lamda 35 spectrophotometer was used to record the UV–Vis absorption spectra in the 200–800 nm wavelength range. To create a solution with a concentration of 1 × 10-5 mol·L-1, the compound was dissolved in spectroscopic-grade ethanol. To guarantee total dissolution, the solution was sonicated for five minutes. At room temperature, measurements were made in quartz cuvettes with a path length of 1 cm. The pure solvent was used for a baseline correction. A Perkin Elmer (Germany; Model: Spectrum II) FT-IR spectrometer was used to record Fourier-transform infrared (FT-IR) spectra at a resolution of 4 cm-1 and in the range of 4000–400 cm-1, with an average of 32 scans per sample. After being finely ground, the solid sample was combined with spectroscopic-grade KBr in a ratio of roughly 1:100 (sample: KBr). A hydraulic press was used to compress the mixture into a transparent pellet for five minutes at a pressure of ten tonnes. Transmission mode was used to record the spectra. To guarantee homogeneity for FT-Raman spectroscopic measurements, the solid sample was finely ground with an agate mortar and pestle. Without the use of a binder or matrix, 5–10 mg of the powdered compound was put into a sterile stainless-steel sample holder. In order to minimise scattering anomalies and ensure ideal laser focus, the surface was gently pressed to form a flat, uniform layer. To prevent interference from moisture or residues, no solvents or chemical treatments were used. A Bruker fitted with a Nd: YAG laser operating at 1064 nm and a laser power set to 150–250 mW at the sample was used to record the spectra at room temperature. To increase the signal-to-noise ratio, spectra were gathered between 100 and 4000 cm-1 with a spectral resolution of 4 cm-1. An average of 64 scans was taken. Every spectral measurement was carried out in a natural setting.
Antibacterial activity
The turbidity of DRS broth, following the Hi-Media guidelines, was measured using the 0.5 McFarland standards CFU/mL. The broth was then dispersed on the surface of the prepared MHA plates using a sterile cotton swab to ensure standardized growth11. Next, the plates were left in static condition for 15 min, after which wells with a diameter of 6 mm were formed in the plates. To evaluate the antimicrobial efficacy, the 2TSET was dissolved in DMSO and introduced into the wells at various concentrations, specifically 20, 40, 60, and 80 µg/mL12. For comparison, amoxicillin drug was used as the positive control. Subsequently, the plates containing the test solution and DRSs were placed in an incubator set at a temperature of 37 ± 2° for 24 h13. The extent of inhibition was then determined by employing the Hi-Antibiotic Scale-C to measure the zone of inhibition.
Computational study
Using DFT and the B3LYPand standard 6-311 + G(d, p) and 6-311 + G(d,2p) basis set of 2TSET, the quantum chemical calculation was driven from the Guassian09W package14. Gauss View 5.0 has been used to study the results of quantum chemical calculations. In our computational studies, solvation effects were treated using the Integral Equation Formalism Polarizable Continuum Model (IEFPCM). Specifically, we employed an implicit solvation model to simulate the solvent environment during the geometry optimization and energy calculations. The solvent used in the model was DMSO, ethanol, water and gas, and the calculations were performed using the DFT/B3LYP/6-311 + G(d,2p) level of theory in Gaussian 09 level of theory. The linear correlation approach was utilized to ascertain the scale factors by comparing the basic modes of theory and experiments. With the help of the multi-of program, the interatomic interactions in terms of an electron density ρ(r)’s topological characteristics can be determined. Using Multiwfn, the RDG map, LOL, and ELF were computed15. Using the Gaussian09W package at the B3LYP/6-311 + G(d,2p), NBO calculation was conducted to prove the intermolecular delocalization or hyperconjugation. The Gausssum2.2 software was used to display the DOS, PDOS, and COOP diagrams16. These diagrams are utilized to analyze the group contributions of molecular orbitals. The Auto-dock tool was employed to assess the molecular docking17.
Molecular docking procedure
The proteins 3ERT (Estrogen Receptor alpha) and 2J6M (Human Carbonic Anhydrase II) were selected based on their reported involvement in relevant biological pathways and previous literature suggesting their interaction with compounds structurally similar to our synthesized ligand. These targets are widely used in structure-based drug design studies due to their well-characterized binding sites and availability of high-resolution crystal structures. The docking grid was centered on the active site of each protein as identified from the co-crystallized ligand. For 3ERT and 2J6M, the grid box dimensions were set to 40 × 40 × 40 Å with a spacing of 0.375 Å. These dimensions were chosen to sufficiently cover the active site and surrounding residues. A total of 10 docking runs were performed for each ligand using the Lamarckian Genetic Algorithm (LGA), with 2.5 million energy evaluations per run to ensure thorough sampling of ligand conformations. The docking was performed using AutoDock 4.2, and the scoring function employed was the default AutoDock scoring function, which combines empirical free energy scoring with intramolecular torsional and desolvation penalties. This scoring function is well-validated and widely accepted in molecular docking studies.
Results and discussions
Molecular geometry optimization
One of the most important phases in quantum chemical computations is geometry optimization18. DFT at the B3LYP level uses the split valance basis set 6-311 + G(d,2p) to validate this. Figure 1 depicts the molecule formation and atom count of the 2TSET molecule19. Table 1 presents the optimized geometrical parameters. It is noted that the five-member ring’s C-C bond size in the 2TSET was determined from 1.368 Å to 1.4198 Å. The C-C bonds to the carbon next to the sulfur are typically 1.3784 Å and 1.368 Å, respectively. Similarly, the C-S bond length was determined to be about 1.7565 Å (S1-C2) and 1.7827 Å (S1-C5)20. However, as compared to thiophene, the 2TSET molecule showed a bond angle difference of about 2°21. The bond angles of the thiophene molecule are estimated to be approximately 93° at the sulfur, 109° at the C-C angle, and 114° at the bond angles of the other two carbons, respectively22. The predicted bond angle around the sulfur atom in the 2TSET is around 91.5°. Likewise, the computed C-C-S bond angles of around 110.3° (S1-C2-C3) and 120° (S1-C2-C9), respectively23. For the bond length, the RMSD values were computed and are shown in Table 1.
Vibrational analysis
The 2TSET molecule with 23 atoms can exhibit vibrations in 63 normal modes. Table 2 displays the theoretical and observed vibrational frequencies, respectively. Figures 2 and 3 illustrate the experimental and theoretical vibrational frequency of the 2TSET molecule24. A comprehensive elucidation of the typical conditions, as determined by the distribution of potential energy (PED), can be presented in the final column.
Determination of the CH3 vibration
The 2TSET contains a substituted side chain with methyl (− CH3) groups. The nine primary oscillations linked to each − CH3 group include symmetrical stretching, asymmetric stretching, in-plane stretching modes (specifically, the planar hydrogen stretching mode); symmetric (− CH3-symmetric deformation), and asymmetric (− CH3 asymmetric deformation) deformation states; in-plane (− CH3 ipr), from a plane rocking (− CH3 opr), and torsional (tCH3) states25. These oscillations are anticipated to govern the frequencies of each − CH3 group. A methyl group on an aromatic ring transforms an electron-donating substituent. The FT-IR and FT-Raman characterization results reveal the − CH3 asymmetric stretching mode 2TSET molecule at wavenumbers of 2959 and 2898 cm−1, and 2962 and 2852 cm−1, respectively. The scaled frequencies of B3LYP for 6-311 + G(d, p) are 3029, 2966, 2932, 2900, 2886, and 2856 cm−1. The theoretical PED of 97% was discovered at 3025, 2960, 2925, 2897, 2873, and 2854 cm-1 for 6-311 + G(d,2p). There is a significant correlation between the calculated theoretical values and the observed experimental values. Theoretical calculations identified symmetric stretching vibrations at 2835, 2812, and 2794 cm−1 using the 6-311 + G(d, p) method26. Experimental measurements using the FT-IR determined a vibration at 2789 cm−1, while FT-Raman identified vibrations at 2806 and 2791 cm−1. Additionally, PED 96% measurements revealed vibrations at 2830, 2810, and 2790 cm−1.
The corresponding theoretical values for 6-311 + G(d, p) are 1425, 1410, and 1380 cm−1, while for 6-311 + G(d,2p) are 1420, 1406, and 1370 cm−1, with a PED of 78%. The out-of-plane bending value for − CH3 was about 1419 cm−1 measured by using FT-IR. The in-plane bending of the methyl group was determined using FT-IR spectroscopy and the determined values are 1350 and 1299 cm−1. The estimated values for the 6-311 + G(d, p) basis set were 1354, 1330, and 1308 cm−1, while for the 6311 + G(d,2p) basis set, the estimated values were 1352, 1327, and 1300 cm−1. The percent error of deviation (PED) was found to be 76%. The out-of-plane rocking was determined experimentally using FT-IR and FT-Raman spectroscopy, respectively, and the values of 732 and 700 cm−1 for FT-IR, and 733 and 699 cm−1 for FT-Raman, respectively27. Theoretical calculations identified these modes at 6-311 + G(d, p) with 70% PED at frequencies of 738, 702, and 685 cm−1, and at 6-311 + G(d,2p) with 72% PED at frequencies of 735, 700, and 683 cm−1. An out-of-plane vibrations were observed by utilizing FT-IR at 414 cm−1 and FT-Raman at 416 cm−1, respectively (Figs. 2 and 3). Theoretical predictions indicate that for 6-311 + G(d, p) and 6-311 + G(d,2p), the expected modes are at 420 cm−1 and 339 cm−1, respectively. When considering 54% PED, the torsional vibration modes for 6-311 + G(d, p) and 6-311 + G(d,2p) were found to be 155, 145, and 138 cm−1, respectively.
Determination of the C-H vibration
Thiophene and its derivatives typically exhibit peaks in the region of 3120–3100 cm–1 due to − C-H stretching vibrations28. The − C-H stretching frequencies observed in this study are associated with the peaks detected at 3106 and 3078 cm–1 in the FT-IR spectra, and 3107 cm–1 in the FT-Raman spectra, respectively (Figs. 2 and 3). Theoretically, the scaled values with a PED contribution of 98% can be observed at 3112, 3091, and 3080 cm–1 for the B3LYP 6-311 + G(d, p) method, and 3008, 3087, and 3077 cm–1 for the 6-311 + G(d,2p) method.
Determination of the C-C vibration
The − C-C stretching of aromatic compounds is commonly associated with the range of 1650–1200 cm–1. The experimental FT-IR spectra showed multiple − C-C stretches at 1513 cm–1, while the FT-Raman spectra showed a − C-C stretch at 1514 cm–1. The theoretical values for the − C-C stretches were determined at 1520 cm–1 for 6-311 + G(d, p) and at 1515 cm–1 for 6-311 + G(d,2p), respectively. The spectral range of 1000–600 cm–1 exhibited the occurrence of the − C-C in-plane bending. The observed wavenumber at 643 cm–1 for 6-311 + G(d, p) and at 640 cm–1 for 6-311 + G(d,2p), respectively, corresponds to the measured − C-C in-plane bending modes at 641 cm–1 (FT-IR) and 640 cm–1 (FT-Raman) spectra, respectively. This signifies a 69% contribution from PED. The FT-IR and FT-Raman spectra indicated the presence of out-of-plane − C-C bending vibrations at 515 and 488 cm–1, respectively. Theoretically, the same modes for 6-311 + G(d, p) and 6-311 + G(d,2p) were estimated at frequencies of 521,496 cm–1 and, 512 and 485 cm–1, respectively (Figs. 2 and 3). The estimated wavenumbers and the experimental frequencies are in good agreement.
Determination of the Si-CH3 vibration
Typically, the − Si-CH3 bends symmetrically between 1240 and 1275 cm–1. For 6-311 + G(d, p) and 6-311 + G(d,2p), the computed peaks were determined at 1280, 1253, and 1238 cm–1 and 1276, 1250, and 1235 cm–1, respectively29. The experimental values are detected in FT-Raman at 1251 and 1236 cm–1 and FT-IR at 1250 cm–1, respectively (Figs. 2 and 3).
Determination of the C-S vibration
In the FT-IR spectrum, about 800–600 cm–1 range, the characteristic frequency of the − C-S stretching is seen as a faint band. In the FT-Raman spectrum, A very low intensity of the − C-S stretching band is observed in the present investigation is attributed to the symmetric stretching vibrations of the − C-S bond and is consistent with the value reported in the literature. It is known that the − C-S stretching vibrations mix with neighbouring modes, which are measured at 759 cm–1 in FT-IR and, 791 and 756 cm–1 in the FT-Raman spectra, respectively (Figs. 2 and 3). With PED 72%, these values were then computed at 798, 776 cm–1 for 6-311 + G(d, p) and 790, 760 cm–1 for 6-311 + G(d,2p).
HOMO and LUMO analysis
The characteristics of electron density contours, HOMO and LUMO provide a fair qualitative indicator of further excitation and the capacity to transport electrons and holes30. The neutral form of HOMO generally demonstrates characteristics that prevent subunits from joining together. In contrast, bonding characteristics exist between subunits in LUMO. Ionization potentials (I) and electron affinity (A) are directly correlated with the energy of the HOMO and LUMO, respectively31. The phrase “energy gap” pertains to the disparity in trajectory between the HOMO and the LUMO energies. This disparity is closely linked to the biological activity of a molecule. A high energy gap value denotes a tough molecule structure, whereas a low value suggests a delicate molecular structure. By determining the values of I and A, it can be determined to the global hardness (η) and electro-negativity (χ). The capacity of an atom or collection of atoms to take electrons is referred to as chemical softness. Table 3 lists these parameters for the solvents (ethanol, water, and DMSO) and gas phases’ FMO properties.
Figure 4 displays the forms and energies of the border molecular orbitals, HOMO and LUMO, which are estimated at the DFT/B3LYP/6-311 + G(d,2p) level. The molecular orbital diagram’s red and green colors correspond to the negative and positive phases, respectively32. The nature of HOMO is located on the whole molecule and partially located on C14, C13, and S11 and LUMO is ascribed around thiophene and partially around methyl of the compound. Consequently, for ethanol, gas, and water, the HOMO-LUMO energy gaps are 3.54 eV, and the DMSO energy gap is 3.55 eV, respectively. In this study, a huge energy gap suggests that the molecule’s order of stability is higher, and its reactivity is lower in the gas phase compared to the solvent phase (DMSO). As a result, the 2TSET molecule is more reactive in the solvent phase (ethanol and water); electro-negativity and hardness are crucial factors to consider when analyzing a compound’s chemical behavior33. The molecule’s hazardous character may be determined by its chemical softness.
Absorption properties
Due to the compound’s best solubility, stability, and reproducibility in DMSO, the experimental UV-vis spectrum was first recorded in this study. Our main goal at the time of the experimental measurements was to confirm the absorption properties in a stable, well-defined solvent environment. The differences in the theoretical UV-vis spectra between the various solvents were caused by the computational modelling step, in which the IEFPCM technique was used to simulate solvent effects and assess how polarity affected the electronic transitions. The purpose of these calculations was to predict the molecule’s photophysical behaviour in environments that might be pertinent for future applications, in addition to supplementing the experimental data. To analyze the electronic characteristics of the 2TSET molecule in its ground state, the B3LYP/6-311 + G(d,2p) method in ethanol was employed34. This method involves the TD-DFT calculations to determine the excitation energies and absorbance35. An experimental UV-vis spectrum was obtained at room temperature using ethanol solvent within the wavelength range of 200–800 nm. The experimental results demonstrated a significant interaction occurring at a wavelength of 281.70 nm. Figure 5 displays the UV-vis spectrum of the 2TSET molecule in the gas phase and different solvent phases, respectively. The absorption wavelength observed indicates that the electronic transition from the HOMO to the LUMO is primarily due to a (π - π*) transition, which accounts for 70.5% of the contribution36. The theoretical peak of this transition occurs at 284 nm, with the HOMO to LUMO transition contributing 79.1%. The experiment’s results perfectly validated the theoretically computed values. Figure 5 exhibits the UV-vis spectra of the 2TSET. About 70.5% of the observed absorption wavelength is attributed to the electronic transition occurring between the LUMO and the HOMO. This transition is attributed to the (π - π*) transition and reaches its theoretical peak at 284 nm, where HOMO to LUMO contributes 79.1%. The computed theoretical values agreed well with the experiment values.
Molecular electrostatic potential map
The three-dimensional charge distribution of molecules is depicted by molecular electrostatic potential (MEP) maps, sometimes referred to as electrostatic potential energy maps (ESP). Finding the molecule’s reactive location is the goal of ESP research37. This mapping study allows to see areas of a molecule that are charged differently. It is feasible to ascertain how molecules interact with one another by sensing the charge distribution. Molecular structure and its physicochemical properties can be observed with great benefit through the application of molecular electrostatic potential (MEP) mapping. Figure 6 displays the MEP surfaces that were mapped for the 2TSET investigation using the B3LYP/6-311 + G(d, p) level in gas and various solvent phases, respectively38. The MEP surface displays regions with varying electron density, with somewhat electron-rich regions represented by the red color, electron-poor regions represented by the blue color, and partially positive charge regions represented by the yellow color. The different electrostatic potential values at surfaces are indicated by different colors; surfaces with a negative MEP are depicted as red due to a high electron density, while surfaces with a positive MEP are depicted as blue due to a low electron density. The electrostatic potential’s red, orange, yellow, and blue colors indicate electron positivity and negativity, respectively. Consequently, the location of the structure’s interactions with nearby molecules in the crystal structure is anticipated using MEP data39. The MEP map of the 2TSET molecule illustrates that the range of values for red to blue are − 7.533 to 7.533, −7.535 to 7.535, −7.575 to 7.575, and − 7.532 to 7.532 for DMSO, ethanol, gas, and water respectively. When it comes to the 2TSET, the light blue color highlights the hydrogen while the red color is mostly focused on carbon. In the MEP plot, a higher electrostatic potential energy means there is a higher positive charge, which means there are fewer electrons than there should be. On the other hand, a lower electrostatic potential means there are more electrons than there should be.
NBO analysis
The NBO method is one of the approaches for examining charge transfer inside molecules. As the interaction between the electron donor and acceptor develops, the stabilization of energy becomes more and more prominent40. Interactions between the contributor and receiver bonds were investigated using a second-order Fock matrix41. maybe in this approach, the amount of energy consumed in the molecule, and the donor-acceptor interaction can be computed using the following formula. The equation can be used to find a greater E(2) value.
where the orbital occupancy is indicated by qi. The diagonal elements are Ei and Ej, while the off-diagonal NBO Fock matrix element is F(i, j). σ, π, and lone pair interactions are three possible forms of interactions in a molecule42. Therefore, the σ intermolecular conjugations are as follows, as indicated in Table 4. All three rings have three − C-C transitions with a σ→σ∗ transition with stabilization energy ranges of 4.76 to 6.86 Kcal/mol. The shift in π is: There are nine π → π* in each of the three rings, and their stabilization energies range from 8.31 to 39.38 Kcal/mol. Because lone pair LP (1) C2 and anti-bonding acceptor BD*(2)S1-C5 have intramolecular interactions, a very significant value of 93.57 Kcal/mol was achieved.
Dual descriptor analysis
The mechanism of electrons travels within the molecule and how donor and acceptor pairs are created is explained by the distribution of charges among the atoms. Mulliken charges alter polarizability, dipole moment, electronic properties, and other attributes43. Table 5 shows the Mulliken atomic charges. The values of the hydrogen atoms are almost equal and positive. With the highest negative charge (−0.826918) and the greatest ability to draw electrons, atom C13 is the most attractive44. Similarly, atom C5 has the lowest positive charge (0.088264) while silicon has the largest positive charge (1.235486) shown in Fig. 7.
DOS, and PDOS study
The distribution and contribution of atomic orbitals close to the Fermi level are revealed by the PDOS analysis. For instance, strong metal-ligand orbital interactions, which are suggestive of good complex stability, are suggested by the substantial contribution of metal d-orbitals and ligand p-orbitals close to the HOMO-LUMO gap. The bonding nature between the metal centre and donor atoms (such as N, O, and S) is further supported by the COOP analysis. In the HOMO–LUMO region, bonding interactions are indicated by positive COOP values, while weak or antibonding interactions are suggested by small or negative values. Stable coordination complex formation is facilitated by these bonding characteristics. From a chemical reactivity perspective, the overlap of donor (ligand) and acceptor (metal) orbitals enhances charge transfer, which may facilitate redox activity or catalytic behavior. From the perspective of biological activity, stronger orbital interactions could increase the compound’s bioavailability or binding affinity for biological targets (like proteins or DNA), especially through improved π-backbonding or electron delocalisation that can stabilise it in a biological setting.
The DOS, PDOS, OPDOS, and COOP of the 2TSET are represented graphically in Fig. 8. These visualizations illustrate the molecular orbitals and their influence on chemical bonding. DOS diagrams are primarily used to depict MO compositions and their involvement in chemical bonding45. This is achieved by utilizing OPDOS curves, also known as COOP diagrams in the scientific literature. A molecule’s orbital levels are referred to as its density of states (DOS) at a certain energy level. This describes the molecular orbital system as a whole and provides details on population analysis for each orbital. The HOMO is shown by the green line, while the LUMO is shown by the red line46.
The OPDOS or COOP diagrams explain the bonding, anti-bonding, or nonbonding characteristics of the interaction between two orbitals, atoms, or groups. The OPDOS number reflects the type of interaction (bonding, anti-bonding, or nonbonding) based on whether there is a positive or negative overlap population. The height of the OPDOS indicates the level of advantage that the molecular orbital has in forming the chemical bond47. The bonding interaction over the overlap of Si with trimethyl (-CH3) is represented by a positive (red line) in the OPDOS diagram, while the anti-bonding interaction is represented by a negative (blue line) towards the overlap of Si with -CH3. Certain orbitals of interaction between particular groups expose these interactions. At the boundary, the surrounding orbital might display quasi-degenerate energy levels.
Non-covalent interaction
It is common practice to evaluate non-covalent, intramolecular, and intermolecular interactions48. In the region furthest from the molecule, where the electron density is decreasing exponentially to zero, the reduced density gradient (RDG) shows substantial positive values. Incomparable systems, regions with a large concentration of electrons will exhibit RDG values that are close to zero, indicating the presence of both covalent bonding and non-covalent interactions49. This is utilized to differentiate between various forms of engagement by resolving the following issues. To comprehend the interactions, one must analyze the sum of three eigenvalues derived from the electron density Hessian matrix along the three principal axes with the least amount of variation, comparable to the equation where (r)< (r)< (r).
In Fig. 9, the graph indicates that the following three interactions are explained by (r). Sign(λ2)ρ > 0 denotes non-bonding, which is strong repulsive interactions denoting steric effect in rings (represented by red color), sign(λ2)ρ ≈ 0 corresponds to weakly interactive regions, namely van der Waals forces, and such region is shown in green. Bonding is indicated by strong attractive interactions, which are represented by a blue color50. Weak, decreased gradient, and low-density zones are used to classify non-covalent interactions. In the current investigation, destabilizing repulsive interactions that exhibit a substantial steric effect are indicated by the red color found in the carbon ring and the center of the thiophene. The strong, attractive hydrogen bond interaction that surrounds silicon molecules is indicated by the blue color. Figure 10 depicts the steric effect and hydrogen interactions, whereas Fig. 9 shows the 2D graph.
ELF and LOL analysis
The ELF is a useful tool for determining molecular aromaticity, understanding chemical bonding in transition metallic complexes, and directly visualizing the repulsion effect caused by the Pauli exclusion principle51. This can be achieved by measuring the surplus of nearby kinetic energy. The color markers in Fig. 11 range from blue to red, representing the ELF scale values from 0.0 to 1.0. The color gradients of the ELF maps were employed to confirm the existence of both bonding and nonbonding electrons. The presence of concentrated bonding and nonbonding electrons between the C2-C9, C9-C20, C2-C3, and C4-C5 atoms creates a distinct red zone, which contributes to the confinement of the higher ELF region52. The electron depletion zone, which exhibits low ELF, is indicated by the narrow blue circle encircling the C2, C9, and C10 atoms. The 2TSET molecule’s strong repulsion exists between the atoms C4 and C5.
Localized molecular orbitals are those that are concentrated in a small area of space. A two-dimensional depiction of a localized orbital locator (color shading and contour maps) with a scale range of 0.0 to 0.8 is shown in Fig. 12. The spatially bounded molecular orbitals represented by B3LYP/6-311 + G(d,2p) serve as the basis for the distribution of localized orbital locators53. The red color indicates the point at which the gradient of localized orbitals reaches its maximum because of overlapping. A red area with high LOL values represents the region surrounding the hydrogen atom. Conversely, a blue circle surrounding the carbon atoms indicates the presence of an electron depletion area between the valence and inner shells. LOL presents a clearer and more pronounced image than ELF.
ADME and antibacterial studies
The computational models are widely used in drug discovery research. One molecule may satisfy all the requirements to be licensed as a medicine among many thousands to millions of others54. More than half of these molecules fail the physicochemical properties tests; hence, studies of ADME qualities considerably assist the drug development procedures. The new Swiss ADME web tool offers rapid, reliable predictive models for physicochemical attributes, pharmacokinetics, drug-likeness, and medicinal chemistry friendliness. Figure 13 shows that the pink part of the hexagon on the left is the physicochemical space optimum for oral bioavailability, the white area is unsuitable, and the red lines show where the specific drug falls55. A hexagon can be found utilizing the domain borders of XLOGP, molecular weight, topological polar surface area (TPSA), Log S, a fraction of Csp3, and several rotating bonds. This hexagon matches lipophilicity (LIPO), size (SIZE), polarity (POLAR), solubility (INSOLU), unsaturation (INSATU), and flexibility (FLEX), suggesting the 2TSET molecule has settled in an oral bioavailability zone. TableS1 lists drugs satisfying the Lipinski rule: oral medications must have ≤ 5 hydrogen bond donors; ≤ 10 hydrogen bond acceptors; Log P of ≤ 5; and a molecular weight of ≤ 500. Moreover, medication bioavailability is thought to be mostly dependent on topological polar surface area (TPSA) evaluated as well56. Calculation findings (Fig. 14) reveal that 2TSET molecule can block the following top enzyme families and receptors: Primary active transport, nucleus receptor, membrane receptor, protease, kinase, phosphodiesterase, family AG protein-coupled receptor, oxidoreductase, voltage-gated ion channel, and cytochrome P450. It is important to carefully consider 2TSET’s limitations, including its moderate solubility, potential metabolic interactions, and limited brain penetration, even though computational models show promising drug-likeness and ADMET profiles. These characteristics make it a good candidate for the development of oral non-CNS drugs; however, additional in vitro and in vivo pharmacokinetic testing is necessary. Its potential is supported by a comparison with drugs that are structurally and pharmacokinetically similar, but it also emphasises the necessity of meticulous lead optimisation. The B. filamentosus caused 18 mm, while A. baumannii showed 12 mm as the highest zone of inhibition.
Boiled EGG
Many instances of unsuccessful drug development can be related to inadequate pharmacokinetics and bioavailability, as well as issues with toxicity and effectiveness. Two important pharmacokinetic activities that are crucial for evaluating different stages of the drug development process are gastrointestinal absorption and brain permeability57. The Brain or Intestinal Estimate D permeation system (Boiled Egg) is a suggested precise predictive model that calculates the lipophilicity and polarity of tiny compounds. Due to the model’s fast speed, accurate precision, and consistent graphical performance, predictions for both brain and intestinal permeation may be derived from the same two physicochemical descriptors58. These descriptors are then directly converted through molecular architecture. Boiled eggs have multiple applications, such as assessing the potential of drug candidates for development and screening chemical libraries in the first phases of drug discovery. Based on Fig. 15, the white zone represents the area where molecules have the greatest degree of absorption in the gastrointestinal tract, while the yellow region (yolk) indicates the area with the highest likelihood of entering the brain. The presence of red dots signifies chemicals that are predicted by the P-glycoprotein to be non-excreted from the brain.
Molecular docking
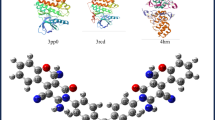
The 3ERT and 2J6M receptor macromolecules were converted to pdb format after being downloaded from the Protein Data Bank at http://www.rcsb.org/pdb. To locate the residues in the receptor, the cavity must be identified. The offline Autodock program was downloaded from https://www.cgl.ucsf.edu/chimera/ and used to determine the cavity59. It was possible to isolate receptor macromolecules from ligands, solvents, and nonstandard residues. The Discovery Studio 4.0 program was used to separate macromolecules from unneeded molecules60. The Bioavailability Radar enables a first glance at the drug-likeness of a molecule is presented in Table S1. The separation’s output was saved in PDB format. The PubChem website (http://pubchem.ncbi.nlm.nih.gov) provides a download for the ligand structure of the generated molecule61. Using Autodock Tools, this was optimized by adjusting the number of action torsion and changing the format to mol file. The grid box was set up to know the location of the binding site when the receptor preparation was being done by adding hydrogen polar, and the format was changed to pdbqt form. This file was saved on the computer’s C: disc in a single folder62. Utilizing Autodock Vina, the molecular docking process was carried out. Autodock Vina was run using the command prompt program, and the ligands and receptors that were already on drive C: copied and converted in the form of notepad were stored with the name conf.txt. The free energy value of the binding docking data was extracted from the log.txt output, which allowed for an examination of the study’s conclusions. Figure 16 depicts the docking structure of the 2TSET molecule63. A hydrogen bond forming between the target protein and the 2TSET molecule is indicated by the dashed lines in the image. Protein 3ERT has three hydrogen bonds with ALA743, LEU844, and MET 793 with bond distances of 4.81, Å 5.60 Å, and 4.13 Å. However, 2J6M protein has two hydrogens with LEU391 and PHE404 with bond distances of 5.65 Å and 4.25 Å. Docking studies of human estrogen receptor alpha in the 2TSET molecule are shown in Table 6.
Our synthesised compound showed binding affinities of −4.9 kcal/mol with the 2j6m protein and − 4.6 kcal/mol with the 3ert protein, according to the docking analysis. As natural or optimised inhibitors for these targets, the native ligands demonstrated noticeably higher binding affinities of − 7.78 kcal/mol (2j6m) and − 10.20 kcal/mol (3ert). Even though the synthesised compound’s binding energies are low, they nevertheless show a positive interaction inside the binding pocket. The ligand interacts with important residues through hydrophobic and aromatic stacking interactions, forming, and may help modulate the target. Compounds with docking scores between − 4 and − 6 kcal/mol have been shown to still demonstrate detectable in vitro and in vivo activity, especially when their pharmacokinetic characteristics, solubility, and bioavailability are favourable. As a result, even though our compound binds less strongly than the native ligands, its interaction profile indicates that it might be a good candidate for additional optimisation.
Conclusion
The aromatic heterocyclic thiophene derivative 2TSET has unique structural, electronic, and pharmacokinetic properties that support its potential as a bioactive compound, according to the combined experimental and theoretical studies. Electronic structure studies showed favourable charge distribution, chemical stability, and electron delocalisation, while spectroscopic and computational analyses verified its stability across gas and solvent phases. While ADME profiling showed good gastrointestinal absorption and blood–brain barrier permeability without P-glycoprotein efflux liability, molecular electrostatic potential mapping revealed active electrophilic and nucleophilic sites. The energy flow from the HOMO to LUMO has been shown by the PDOS and OPDOS diagrams, respectively. The HOMO-LUMO gap is determined for the 2TSET molecule which indicates the chemical stability in both gas and solvent phases. The localization and delocalization of electrons are identified by topological analyses like ELE and LOL, respectively. The compound’s capacity to bind to the 2J6M target protein with a significant binding affinity was further validated by molecular docking. All of these findings point to 2TSET as a promising scaffold for additional development and optimisation in the direction of targeted treatments.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Change history
16 October 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-24146-w
References
Krishnan, M. et al. Synthesis of Bimetallic BiPO(4)/ZnO Nanocomposite: Enhanced Photocatalytic Dye Degradation and Antibacterial Applications. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24031947 (2023).
Subramanian, H., Krishnan, M. & Mahalingam, A. Photocatalytic dye degradation and photoexcited anti-microbial activities of green zinc oxide nanoparticles synthesized via sargassum muticum extracts. RSC Adv. 12, 985–997. https://doi.org/10.1039/D1RA08196A (2022).
Feng, X. et al. Enhancement of Mass Transfer Process for Photocatalytic Reduction in Cr(VI) by Electric Field Assistance. Int. J. Mol. Sci. https://doi.org/10.3390/ijms25052832 (2024).
Maulani, B. I. G., Rasmi, D. A. C. & Zulkifli, L. Isolation And characterization of endophytic bacteria from Mangrove rhizophora mucronata lam. And antibacterial activity test against some pathogenic bacteria. J. Phys. Conf. Ser. 1402, 33038. https://doi.org/10.1088/1742-6596/1402/3/033038 (2019).
Sharma, V., Chitranshi, N. & Agarwal, A. K. Significance and biological importance of pyrimidine in the microbial world. Int. J. Med. Chem. 2014, 202784. https://doi.org/10.1155/2014/202784 (2014).
Samuel, Y., Garg, A., Mulugeta, E. & Synthesis, D. F. T. Analysis, and evaluation of antibacterial and antioxidant activities of sulfathiazole derivatives combined with in Silico molecular Docking and ADMET predictions. Biochem. Res. Int. 2021, 7534561. https://doi.org/10.1155/2021/7534561 (2021).
Malmström, J. et al. The antioxidant profile of 2,3-Dihydrobenzo[b]furan-5-ol and its 1-Thio, 1-Seleno, and 1-Telluro analogues. J. Am. Chem. Soc. 123, 3434–3440. https://doi.org/10.1021/ja0035811 (2001).
Parakkal, S. C., Datta, R., Muthu, S., Alharbi, N. S. & Abbas, G. Solvent-solute interaction, thermodynamic behaviour, structural, chemical and anti-cancer biological properties of 3(2H)-furanone derivatives. J. Mol. Liq. 390, 123185. https://doi.org/10.1016/j.molliq.2023.123185 (2023).
Shah, R. & Verma, P. K. Therapeutic importance of synthetic thiophene. Chem. Cent. J. 12, 137. https://doi.org/10.1186/s13065-018-0511-5 (2018).
Ibrahim, S. M., Saeed, A. M., Elmoneam, W. R. A. & Mostafa, M. A. Synthesis and characterization of new schiff base bearing bis(pyrano[3,2-c]quinolinone): efficient cationic dye adsorption from aqueous solution. J. Mol. Struct. 1284, 135364. https://doi.org/10.1016/j.molstruc.2023.135364 (2023).
Aydinli, S. G. et al. Synthesis, characterization and investigation of antibacterial and antifungal activities of novel 1,3-butadiene compounds. Synth. Commun. 50, 3234–3244. https://doi.org/10.1080/00397911.2020.1799010 (2020).
Abdou, A., Synthesis, S., Docking, M. & Vibrational Spectroscopy, D. F. T. Exploration, antibacterial and antifungal activity of new Fe(III), Co(II) and Ni(II) hetero-ligand complexes. J. Mol. Struct. 1262, 132911. https://doi.org/10.1016/j.molstruc.2022.132911 (2022).
Rajkumar, G. & Sundar, R. Biogenic one-step synthesis of silver nanoparticles (AgNPs) using an aqueous extract of persea Americana seed: characterization, phytochemical screening, antibacterial, antifungal and antioxidant activities. Inorg. Chem. Commun. 143, 109817. https://doi.org/10.1016/j.inoche.2022.109817 (2022).
Frisch, M. J. et al. (2009). Gaussian 09, Revision B.01, Gaussian 09, Revis. B.01, Gaussian, Inc., Wallingford CT 1–20. citeulike-article-id:9096580.
Lu, T. & Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592. https://doi.org/10.1002/jcc.22885 (2012).
Fazilath Basha, A., Liakath Ali Khan, F., Muthu, S. & Raja, M. Computational evaluation on molecular structure (Monomer, Dimer), RDG, ELF, electronic (HOMO-LUMO, MEP) properties, and spectroscopic profiling of 8-Quinolinesulfonamide with molecular docking studies. Comput. Theor. Chem. 1198, 113169. https://doi.org/10.1016/j.comptc.2021.113169 (2021).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: automated Docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791. https://doi.org/10.1002/jcc.21256 (2009).
Bakheit, A. H., Abuelizz, H. A. & Al-Salahi, R. Crystallographic analysis, Hirshfeld surface investigation, and DFT calculations of 2-phenoxy-triazoloquinazoline molecule: implications for drug design. J. Mol. Struct. 1319, 139436. https://doi.org/10.1016/j.molstruc.2024.139436 (2025).
Gobinath, P. et al. Design, synthesis of new 4,5-dibenzylidene-9,10-diphenyl-1,2,7,8,9,10 hexahydroacridine-3,6-dione derivatives using extract of vitexnegundo: cytotoxic activity & molecular Docking study. Chem. Phys. Impact. 8, 100483. https://doi.org/10.1016/j.chphi.2024.100483 (2024).
Rajimon, K. J., Elangovan, N., Amir, A. & Thomas, R. Schiff bases from Chlorine substituted anilines and salicylaldehyde: synthesis, characterization, fluorescence, thermal features, biological studies and electronic structure investigations. J. Mol. Liq. 370, 121055. https://doi.org/10.1016/j.molliq.2022.121055 (2023).
Bakheit, A. H. et al. Crystallographic Analysis and Theoretical Investigation of Intermolecular Interactions and Antimicrobial Activity of Synthesized 1-(3-Chloropropyl)Piperidin-1-Ium Tetraphenylborate. ChemistrySelect 10, e202404452. https://doi.org/10.1002/slct.202404452 (2025).
Priya, C. G. et al. Fluorescence property and solvent effect on m -bromosalicylaldehyde derivative; insights from synthesis, characterization, antimicrobial activity and computational studies. Chem. Phys. Impact. 7, 100323. https://doi.org/10.1016/j.chphi.2023.100323 (2023).
Bakheit, A. H., Abuelizz, H. A. & Al-Salahi, R. Hirshfeld Surface Analysis and Density Functional Theory Calculations of 2-Benzyloxy-1,2,4-triazolo[1,5-a] quinazolin-5(4H)-one: A Comprehensive Study on Crystal Structure, Intermolecular Interactions, and Electronic Properties. Crystals https://doi.org/10.3390/cryst13101410 (2023).
Elangovan, N. et al. Electronic structure and biological activity screening of the schiff base 4-((4-Hydroxy-3-Methoxy-5-Nitrobenzylidene)Amino)-N-(Pyrimidin-2-yl)Benzene sulfonamide from 5-Nitrovaniline and sulphadiazene, polycycl. Aromat. Compd. 0, 1–18. https://doi.org/10.1080/10406638.2021.1991392 (2021).
Elangovan, N., Sowrirajan, S., Alzahrani, A. Y. A., Rajendran Nair, D. S. & Thomas, R. Fluorescent azomethine by the condensation of sulfadiazine and 4-Chlorobenzaldehyde in solution: synthesis, characterization, solvent interactions, electronic structure, and biological activity prediction. Polycycl. Aromat. Compd. 0, 1–22. https://doi.org/10.1080/10406638.2023.2216833 (2023).
Elangovan, N. et al. Structural, electronic features, photoluminescence property, antimicrobial activity and spectral properties of 5-bromosalicylaldehyde derivative. J. Mol. Struct. 1294, 136355. https://doi.org/10.1016/j.molstruc.2023.136355 (2023).
Muthukumar, R., Karnan, M., Elangovan, N., Karunanidhi, M. & Sankarapandian, V. Synthesis, experimental antimicrobial activity, theoretical vibrational analysis, quantum chemical modeling and molecular Docking studies of (E) -4- (b enzylideneamino) b enzenesulfonamide. J. Mol. Struct. 1263, 133187. https://doi.org/10.1016/j.molstruc.2022.133187 (2022).
Elangovan, N., Yousef, S., Sowrirajan, S., Rajeswari, B. & Nawaz, A. Photoluminescence property and solvation studies on sulfonamide; synthesis, structural, topological analysis, antimicrobial activity and molecular Docking studies. Inorg. Chem. Commun. 155, 111019. https://doi.org/10.1016/j.inoche.2023.111019 (2023).
Gobi, T., Elangovan, N., Sowrirajan, S., Islam, M. S. & Sirajunnisa, A. FT-Raman), topology and molecular Docking studies of. J. Mol. Struct. 1296, 136805. https://doi.org/10.1016/j.molstruc.2023.136805 (2024).
Elangovan, N. et al. Synthesis, solvent role, absorption and emission studies of cytosine derivative. Heliyon 10, e28623. https://doi.org/10.1016/j.heliyon.2024.e28623 (2024).
Jayachitra, R. et al. Synthesis, computational, electronic spectra, and molecular Docking studies of 4-((diphenylmethylene)amino)-N-(pyrimidin-2-yl)benzenesulfonamide. J. Indian Chem. Soc. 100, 100836. https://doi.org/10.1016/j.jics.2022.100836 (2023).
Ahalya, S., Santhi, R., Elangovan, N., Arumugam, N. & Almansour, A. I. Synthesis, antimicrobial activity and computational studies on (E) -N- (2 ((4-chlorobenzylidine) amino) ethyl) napthalen-1-amine. Next. Res. https://doi.org/10.1016/j.nexres.2024.100023 (2024).
Ganesan, T. S. et al. Spectroscopic, Computational(DFT), quantum mechanical studies and protein-ligand interaction of schiff base 6,6-((1,2-phenylenebis(azaneylylidene))bis(methaneylylidene))bis(2-methoxyphenol) from o-phenylenediamine and 3- methoxysalicylaldehyde. J. Indian Chem. Soc. 99, 100713. https://doi.org/10.1016/j.jics.2022.100713 (2022).
Elangovan, N. & Arumugam, N. Structural, spectral, pharmacokinetics analysis (in-Silico), Drug-Likeness, NCI analysis (ELF, LOL, IRI & DORI) & molecular Docking computations of 2-Hydroxy 2-Phenyl acetophenone a DFT approaches, polycycl. Aromat. Compd. 0, 1–33. https://doi.org/10.1080/10406638.2024.2406931 (2024).
Elangovan, N. et al. Theoretical investigation on solvents effect in molecular structure (TD-DFT, MEP, HOMO-LUMO), topological analysis and molecular Docking studies of N-(5-((4-Ethylpiperazin-1-yl)Methyl)Pyridin-2-yl)-5-Fluoro-4-(4-Fluoro-1-Isopropyl-2-Methyl-1H-Benzo[d] imi, polycycl. Aromat. Compd. 0, 1–24. https://doi.org/10.1080/10406638.2023.2254896 (2023).
Kanchana, S. et al. Computational investigation of solvent interaction (TD-DFT, MEP, HOMO-LUMO), wavefunction studies and molecular Docking studies of. Chem. Phys. Impact. 7, 100263. https://doi.org/10.1016/j.chphi.2023.100263 (2023).
Elangovan, N. & Sowrirajan, S. Heliyon synthesis, single crystal (XRD), Hirshfeld surface analysis, computational study (DFT) and molecular Docking studies of (E) -4- ((2-hydroxy-3, 5-diio. Heliyon 7, e07724. https://doi.org/10.1016/j.heliyon.2021.e07724 (2021).
Manivel, S. et al. Schiff base (Z)-4-((furan-2-ylmethylene)amino) benzenesulfonamide: synthesis, solvent interactions through hydrogen bond, structural and spectral properties, quantum chemical modeling and biological studies. J. Mol. Liq. 350, 118531. https://doi.org/10.1016/j.molliq.2022.118531 (2022).
Elangovan, N. & Arumugam, N. Solvent role in molecular structure level, structural and topology investigations, molecular Docking and antimicrobial activity studies on sulpha drug derivative. J. Mol. Struct. 1303, 137570. https://doi.org/10.1016/j.molstruc.2024.137570 (2024).
Geethapriya, J. et al. Synthesis, characterization, computational, excited state properties, wave function and molecular Docking studies of (E)-1-(perfluorophenyl)-N-(p-tolyl) methanimine. J. Indian Chem. Soc. 99, 100785. https://doi.org/10.1016/j.jics.2022.100785 (2022).
Kanagavalli, A. et al. Synthesis, characterization, computational, excited state properties, wave function, and molecular Docking studies of (E)-4-((2-hydroxybenzylidene)amino)N-(thiazol-2-yl) benzenesulfonamide. J. Indian Chem. Soc. 100, 100885. https://doi.org/10.1016/j.jics.2023.100885 (2023).
Elangovan, N., Alzahrani, A. Y., Ajithkumar, G., J, R. K. & Thomas, R. Investigation of molecular structure, topological and molecular Docking studies of a novel anticancer drug pacritinib, polycycl. Aromat. Compd. 0, 1–28. https://doi.org/10.1080/10406638.2023.2249575 (2023).
Ajayi, T. J. & Shapi, M. Solvent-free mechanochemical synthesis, Hirshfeld surface analysis, crystal structure, spectroscopic characterization and NBO analysis of Bis(ammonium) Bis((4-methoxyphenyl) phosphonodithioato)-nickel (II) dihydrate with DFT studies. J. Mol. Struct. 1202, 127254. https://doi.org/10.1016/j.molstruc.2019.127254 (2020).
Hamidinasab, M., Bodaghifard, M. A. & Mobinikhaledi, A. Synthesis of new, vital and Pharmacologically important Bis phthalazine-triones using an efficient magnetic nanocatalyst and their HF and NBO investigation. J. Mol. Struct. 1200, 127091. https://doi.org/10.1016/j.molstruc.2019.127091 (2020).
Janeoo, S., Reenu, A., Saroa, R., Kumar, H. & Kaur Computational investigation of bioactive 2,3-diaryl Quinolines using DFT method: FT- IR, NMR spectra, NBO, NLO, HOMO-LUMO transitions, and quantum-chemical properties. J. Mol. Struct. 1253, 132285. https://doi.org/10.1016/j.molstruc.2021.132285 (2022).
Kateris, N., Jayaraman, A. S. & Wang, H. HOMO-LUMO gaps of large polycyclic aromatic hydrocarbons and their implication on the quantum confinement behavior of flame-formed carbon nanoparticles. Proc. Combust. Inst. https://doi.org/10.1016/j.proci.2022.07.168 (2022).
Karpagakalyaani, G., Magdaline, J. D. & Chithambarathanu, T. Comparative spectral (FT-IR, FT-Raman, UV) investigations, HOMO–LUMO, NBO and in-silico Docking analysis of nikethamide, Niazid and 2-Mercaptonicotinic acid. J. Mol. Struct. 1252, 132032. https://doi.org/10.1016/j.molstruc.2021.132032 (2022).
Shobana, D., Sudha, S., Ramarajan, D. & Dimić, D. Synthesis, crystal structure, spectral characterization and Hirshfeld surface analysis of (E)-N′-(3-ethoxy-4-hydroxybenzylidene)-4-fluorobenzohydrazide single-crystal – a novel NLO active material. J. Mol. Struct. 1250, 131856. https://doi.org/10.1016/j.molstruc.2021.131856 (2022).
Dhanalakshmi, M., Balakrishnan, C., Vijayasri, M. & Parthiban, S. Synthesis, structural characterization, and Hirshfeld surface analysis of novel NLO active 1-methyl-1,3,5,7-tetraazaadamantan-1-ium 3-carboxy-5-nitrobenzoate hydrate. J. Mol. Struct. 1269, 133740. https://doi.org/10.1016/j.molstruc.2022.133740 (2022).
Bensafi, T. et al. Synthesis, characterization and DFT calculations of linear and NLO properties of novel (Z)-5-benzylidene-3-N(4-methylphenyl)-2-thioxothiazolidin-4-one. J. Sulfur Chem. 42, 645–663. https://doi.org/10.1080/17415993.2021.1951729 (2021).
SangeethaMargreat, S. et al. Synthesis, spectroscopic, quantum computation, electronic, AIM, wavefunction (ELF, LOL) and molecular Docking investigation on (E)-1-(2,5-dichlorothiophen-3-yl)-3-(thiophen-2-yl)-2-propen-1-one. Chem. Data Collect. 33, 100701. https://doi.org/10.1016/j.cdc.2021.100701 (2021).
M.J. Pramila, D.A. Dhas, I.H. Joe, S. Balachandran, G. Vinitha, Structural insights,spectral, flourescence, Z-scan, C-H… O/N-H… O hydrogen bonding and AIM, RDG, ELF,LOL, FUKUI analysis, NLO activity of N-2(Methoxy phenyl) acetamide, J. Mol. Struct.1272 (2023) 134140. https://doi.org/https://doi.org/10.1016/j.molstruc.2022.134140.
Sukanya, R., Aruldhas, D., Hubert Joe, I. & Balachandran, S. Spectroscopic and quantum chemical computation on molecular structure, AIM, ELF, RDG, NCI, and NLO activity of 4-VINYL benzoic acid: A DFT approach. J. Mol. Struct. 1253, 132273. https://doi.org/10.1016/j.molstruc.2021.132273 (2022).
Izuchukwu, U. D. et al. Synthesis, vibrational analysis, molecular property investigation, and molecular Docking of new benzenesulphonamide-based Carboxamide derivatives against plasmodium falciparum. J. Mol. Struct. 1269, 133796. https://doi.org/10.1016/j.molstruc.2022.133796 (2022).
Kumar, H., Dhameja, M., Kurella, S., Uma, A. & Gupta, P. Synthesis, in-vitro α-glucosidase Inhibition and molecular Docking studies of 1,3,4-thiadiazole-5,6-diphenyl-1,2,4-triazine hybrids: potential leads in the search of new antidiabetic drugs. J. Mol. Struct. 1273, 134339. https://doi.org/10.1016/j.molstruc.2022.134339 (2023).
kerkour, R., Chafai, N., Moumeni, O. & Chafaa, S. Novel α-aminophosphonate derivates synthesis, theoretical calculation, molecular docking, and in Silico prediction of potential Inhibition of SARS-CoV-2. J. Mol. Struct. 1272, 134196. https://doi.org/10.1016/j.molstruc.2022.134196 (2023).
Semenets, A. P. et al. Synthesis, docking, and biological evaluation of novel 1-benzyl-4-(4®5-sulfonylidene-4,5-dihydro-1H-1,2,4-triazol-3-yl)pyrrolidin-2-ones as potential nootropic agents. Eur. J. Med. Chem. 244, 114823. https://doi.org/10.1016/j.ejmech.2022.114823 (2022).
Amalraj, S. D., Palapetta, S. C. & Harichandran, G. A facile one-pot synthesis, computational and molecular Docking studies of benzimidazole and benzothiazole compounds using amberlite IRA 400-Cl resin as green/reusable catalyst. J. Mol. Struct. 1268, 133704. https://doi.org/10.1016/j.molstruc.2022.133704 (2022).
Rajaraman, D., Anthony, L. A., Nethaji, P. & Vallangi, R. One-pot synthesis, NMR, quantum chemical approach, molecular Docking studies, drug-likeness and in-silico ADMET prediction of novel 1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(furan-2-yl)-4,5-diphenyl-1H-imidazole derivatives. J. Mol. Struct. 1273, 134314. https://doi.org/10.1016/j.molstruc.2022.134314 (2023).
Sowrirajan, G. A. S., Elangovan, N. & Manoj, K. P. E)-4-((4-Bromobenzylidene) Amino)-N-(Pyrimidin-2-yl) benzenesulfonamide from 4-Bromobenzaldehyde and sulfadiazine, synthesis, spectral (FTIR, UV–Vis), computational (DFT, HOMO–LUMO, MEP, NBO, NPA, ELF, LOL, RDG) and molecular Docking studies. Polycycl. Aromat. Compd. 42, 7616–7631. https://doi.org/10.1080/10406638.2021.2006245 (2022).
Hamed, M. M. et al. Synthesis, biological evaluation, and molecular Docking studies of novel diclofenac derivatives as antibacterial agents. J. Mol. Struct. 1273, 134371. https://doi.org/10.1016/j.molstruc.2022.134371 (2023).
Lalpara, J. N. et al. Water promoted one pot synthesis of Sesamol derivatives as potent antioxidants: DFT, molecular docking, SAR and single crystal studies. Polycycl. Aromat. Compd. https://doi.org/10.1080/10406638.2022.2083194 (2022).
Geesi, M. H. et al. Synthesis, antimicrobial evaluation, crystal structure, Hirschfeld surface analysis and Docking studies of 4-[2-(1-methyl-1H-imidazol-2-ylsulfanyl)-acetylamino]-benzenesulfonic acid. J. Mol. Struct. 1265, 133425. https://doi.org/10.1016/j.molstruc.2022.133425 (2022).
Acknowledgements
The Authors extend their appreciation to Taif University٫ Saudi Arabia, for supporting this work through project number (TU-DSPP-2025-16).
Funding
This research was funded by Taif University, Saudi Arabia, Project No. (TU-DSPP-2025-16).
Author information
Authors and Affiliations
Contributions
Vaithilingam Sasikala: Formal analysis, Validation.Saminathan Kayarohanam; Data curation, Formal analysis.V. Balachandran: Software, Validation.Merfat S. Al-Sharif: Funding acquisition, Project administration, Resources, Software, Supervision.Samy F. Mahmoud: Data curation, Formal analysis, Validation.Natarajan Elangovan: Data curation, Formal analysis, Investigation, Supervision, writing – original draft, Writing – review & editing.Ling Shing Wong; Software, Validation.Sinouvassane Djearamane; Investigation, Software, Validation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors in the Abstract, Materials and methods, and Conclusion sections. Full information regarding the corrections made can be found in the correction for this Article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sasikala, V., Kayarohanam, S., Balachandran, V. et al. Solvent-driven spectroscopic and quantum chemical evaluation of 2-[(trimethylsilyl) ethynyl]thiophene with molecular docking insights. Sci Rep 15, 31851 (2025). https://doi.org/10.1038/s41598-025-16607-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16607-z