Abstract
The role of systemic inflammation in the pathogenesis of respiratory diseases is increasingly recognized, but the relationship between individual inflammatory markers, lung function and respiratory symptoms is not well established. We studied 1,238 adults participating in the first follow-up of the Burden of Obstructive Lung Disease (BOLD) cohort study in Sweden, Norway, Iceland, and Estonia in 2019–2021. Systemic inflammation was assessed using total white blood cell (WBC) count and WBC sub-populations (neutrophils, lymphocytes, monocytes, eosinophils, basophils and neutrophil-to-lymphocyte ratio (NLR)). In regression models adjusted for gender, age, smoking status, body mass index and study site, all WBC sub-populations, except for lymphocytes, were associated with chronic airflow obstruction (CAO) and wheeze. In never smokers, increased neutrophils were associated with reduced lung volumes (FEV1 β coef. with 95%CI -1.95 (-3.33, -0.57) p = 0.006; FVC − 1.94 (-3.18, -0.69) p = 0.002), but not CAO. Only increased basophils were associated with CAO in never smokers (OR with 95%CI 2.70 (1.28, 5.72) p = 0.009). In former smokers, increased neutrophils, monocytes and eosinophils were significantly associated with reduced FEV1, reduced FVC, CAO and wheeze. In current smokers, inflammatory markers were associated with cough (neutrophils OR with 95%CI 1.49 (1.10, 2.01) p = 0.010; monocytes 1.24 (0.99, 1.55) p = 0.058; basophils 2.56 (1.12, 5.86) p = 0.026). Systemic inflammation may be related to both obstructive and restrictive respiratory impairment in the general population, with associations that vary by smoking status.
Similar content being viewed by others
Introduction
Systemic inflammation is involved in the pathogenesis of several respiratory diseases1,2,3,4. White blood cell (WBC) total count and sub-populations are easily obtainable biomarkers that can reflect systemic inflammation.
In cross-sectional and longitudinal studies, blood eosinophils have been associated with reduced lung function5,6,7. They are considered to have a pathogenetic role in type 2-high asthma and, to a lesser extent, chronic obstructive pulmonary disease (COPD)8. Blood neutrophils have been associated with lower forced expiratory volume in the 1 st second (FEV1), forced vital capacity (FVC), and FEV1/FVC in the general population5, and severity and phenotype of asthma and COPD in clinical studies9,10.
Few studies have explored the relationship between other WBC sub-populations and lung function in general population samples, and the findings were not conclusive11,12,13. Two studies reported inverse associations of blood monocytes and basophils with FEV111,13, while another study found an association with monocytes but not basophils12. Some have also reported a relationship between blood lymphocytes and reduced FEV112,13, but not others11. Limited information is also available regarding the association between systemic inflammation and respiratory symptoms11,14,15.
Smoking status is an important factor to consider in these associations. Higher blood counts of eosinophils, neutrophils and monocytes have been described in current smokers16,17,18 and cigarette smoking is the major determinant of COPD and related symptoms such as cough, phlegm and dyspnoea. Increased WBCs, however, can also be found in asthma and it is increasingly recognized that COPD and reduced lung volumes can be present in never smokers19,20.
The main aim of this study was to investigate the association of circulating WBCs with lung function and respiratory symptoms in adults from four Northern European countries participating in the first follow-up of the Burden of Obstructive Lung Disease (BOLD) cohort study. We hypothesized the presence of distinct profiles of associations between inflammatory markers, lung function indices (FEV1, FVC, FEV1/FVC, chronic airflow obstruction) and respiratory symptoms (cough, phlegm, wheezing, dyspnoea), and that these associations vary by smoking status (current, former, never smokers).
Methods
Study population
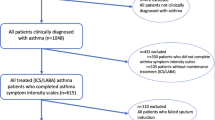
The BOLD cohort study was designed to estimate the prevalence of chronic obstructive lung disease and identify its risk factors across several world regions21,22. At baseline, representative samples of non-institutionalized adults aged 40 years or older were identified from the general population around sites with at least 150,000 inhabitants. Participants were selected from a random sample of the population according to a predefined site-specific sampling strategy. The current analysis is based on data collected in four North European sites [Uppsala (Sweden), Bergen (Norway), Reykjavik (Iceland), Tartu (Estonia)] between 2019 and 2021 during the first follow-up of the participants seen at baseline. Participants provided information on demographics, smoking habits and respiratory symptoms through a standardized questionnaire and performed spirometry to test their lung function. In the four North European sites, participants also provided blood samples. Participants were included in this study if they had completed the core study questionnaire and had availability of the blood sample. Participants were excluded if they had died, emigrated, were institutionalized, could not be contacted, actively refused to participate or had contraindications for lung function testing. The number of participants at the baseline and follow-up stages is reported in Table S1. All sites received approval from their local ethics committee and all participants provided informed consent. The follow-up study was also approved by Imperial College London Research Ethics Committee (ref. 17IC4272). The research was conducted in accordance with the Declaration of Helsinki.
Respiratory symptoms
Respiratory symptoms consisted of cough and phlegm in the absence of cold (defined as affirmative answer to the questions “Do you usually cough when you don’t have a cold?” and “Do you usually bring up phlegm from your chest, or do you usually have phlegm in your chest that is difficult to bring up when you don’t have a cold?”, chronic cough and chronic phlegm (“Do you cough on most days for as much as three months each year?” and “Do you bring up this phlegm on most days for as much as three months each year?”), wheeze (“Have you had wheezing or whistling in your chest at any time in the last 12 months?”), and dyspnoea (defined as ≥ 2 on the modified Medical Research Council (mMRC) dyspnoea scale). These definitions were based on the standardized BOLD questionnaire used in previous research21,23.
Lung function
Spirometry was performed with a ndd EasyOne spirometer (ndd Medizintechnik, Zurich, Switzerland) before and after bronchodilation with 200 micrograms of salbutamol through a spacer. All spirometry curves were checked centrally at the BOLD Operations Centre, and to be considered useable, tests had to include at least three acceptable curves (no hesitation, complete blow, no artefact affecting lung function readings), with the two best blows being within 200mL of each other. Predicted values of FEV1, FVC, and FEV1/FVC were calculated using NHANES reference equations24. Airflow obstruction was defined as FEV1/FVC < LLN (lower limit of normal, defined as 5th percentile) according to ERS/ATS 2005 guidelines25. We used post-bronchodilatory values to define chronic airflow obstruction (CAO) in order to exclude subjects with reversible airflow obstruction.
White blood cell (WBC) counts
Blood samples were collected to measure inflammatory markers. Total WBC and WBC sub-populations (neutrophils, lymphocytes, monocytes, eosinophils, and basophils) were measured with automated hematological analyzers. The neutrophil-to-lymphocyte ratio (NLR) was the ratio between neutrophils and lymphocytes26.
Statistical analysis
Data in the descriptive analyses was presented as mean ± SD, median (IQR) and %, as appropriate. We compared variables across study sites with ANOVA for continuous normally distributed variables, Kruskal-Wallis test for continuous non-normally distributed variables and chi-square test for categorical variables. Multivariable linear regression was used to analyze the association between inflammatory markers and post-bronchodilatory FEV1, FVC and FEV1/FVC % of predicted as continuous variables. Beta coefficients with 95% confidence intervals (CI) indicate the average change in lung function per one-unit increase of inflammatory markers. Multivariable logistic regression was used to analyze the association of inflammatory markers with CAO and respiratory symptoms (categorical variables). Odds ratios (ORs) with 95% CI indicate the odds of the outcome per one-unit increase of inflammatory markers.
All analyses were adjusted for gender, age (continuous), smoking status (categorized as never, former, current), body mass index (BMI, categorized as < 18.5, 18.5–24.9, 25.0–29.9, ≥ 30.0 kg/m2) and study site. We further investigated the role of smoking status in the association between inflammatory markers and outcomes using stratified analyses and interaction tests. In supplementary material, we reported the results of the unadjusted analyses. In sensitivity analyses, we tested if the associations between inflammatory markers, lung function and respiratory symptoms were comparable across study sites by performing random-effects meta-analysis. Missing data on covariates were minimal (smoking status n = 9, BMI n = 4) and they were not imputed. Analyses were conducted on complete data. Stata version 18 (StataCorp LLC) was used to analyze the data.
Results
Characteristics of the study population
We obtained data from 1,238 participants. The mean age was 68 years and there were slightly more men (53%) than women. Of the participants, n = 533 (43%) were never smokers, n = 548 (45%) were former smokers and n = 148 (12%) were current smokers (Table 1). About 10% had CAO, one in five reported wheezing within the last 12 months, and chronic cough was reported by one in ten. Mean total WBC were 6.1±2.1 × 109/L and median blood eosinophils were 0.18 (0.13) x 109/L.
Characteristics stratified by smoking status are reported in Tables S2–S4. The total WBC, neutrophils, lymphocytes, monocytes, eosinophils and basophils counts were higher in current smokers, followed by former smokers and never smokers. Current smokers had lower FEV1, FVC and FEV1/FVC than former and never smokers and a higher prevalence of respiratory symptoms and CAO (25.8% vs. 10.3% vs. 5.8%).
Inflammatory markers and lung function
In the total population (Fig. 1), increased neutrophils (OR with 95%CI 1.26 (1.07, 1.49) p = 0.005), monocytes (1.25 (1.10, 1.42) p = 0.001) and eosinophils (1.16 (1.02, 1.32) p = 0.022) were positively associated with CAO and negatively associated with FEV1 and FVC. Increased basophils were positively associated with CAO (OR with 95%CI 2.10 (1.36, 3.24) p = 0.001) and negatively associated with FEV1 (β coef. with 95%CI −1.45 (−3.66, 0.75) p = 0.196), but not with FVC (0.30 (−1.59, 2.19) p = 0.756).
Associations between inflammatory markers and post-bronchodilatory lung function in the total population (β coefficients and odds ratios (OR) per unit with 95% confidence intervals (CI) adjusted for gender, age, body mass index, smoking status and study site). Abbreviations: FEV1 = forced expiratory volume in the 1 st second, FVC = forced vital capacity, CAO = chronic airflow obstruction (defined as post-bronchodilatory FEV1/FVC values below 5th percentile), WBC = white blood cells, NLR = neutrophil-lymphocyte ratio. Units of measurement: total WBC, neutrophils, lymphocytes 109/L; monocytes, eosinophils, basophils 1010/L.
When stratified by smoking status (Table 2), in never-smokers, increased neutrophils and the NLR were associated with reduced FEV1 (β coef. with 95%CI −1.95 (−3.33, −0.57) p = 0.006, and − 2.14 (−3.79, −0.50) p = 0.011, respectively) and reduced FVC (β coef. with 95%CI −1.94 (−3.18, −0.69) p = 0.002, and − 1.62 (−3.10, −0.13) p = 0.033, respectively), but not CAO. Only increased basophils were associated with CAO (OR with 95%CI 2.70 (1.28, 5.72) p = 0.009) in never-smokers. In former smokers, multiple inflammatory markers (total WBC, neutrophils, monocytes, eosinophils) were significantly associated with reduced FEV1, reduced FVC, and CAO. A similar tendency was observed in current smokers, although these associations did not reach statistical significance likely due to the small size of the subgroup.
Inflammatory markers and respiratory symptoms
In the total population (Fig. 2), increased neutrophils were associated with presence of all respiratory symptoms (cough and phlegm in the absence of a cold, chronic cough, chronic phlegm, wheeze and dyspnoea). Increased eosinophils were associated with cough in the absence of a cold (OR with 95%CI 1.14 (1.04, 1.24) p = 0.005), phlegm in the absence of a cold (1.17 (1.06, 1.29) p = 0.002), and wheeze (1.26 (1.14, 1.39) p < 0.001). Increased basophils were associated with cough in the absence of a cold (1.29 (0.96, 1.74) p = 0.097), wheeze (1.69 (1.22, 2.35) p = 0.002), and dyspnoea (1.74 (0.95, 3.19) p = 0.073).
Associations between inflammatory markers and respiratory symptoms in the total population (odds ratios (OR) per unit with 95% confidence intervals (CI) adjusted for gender, age, body mass index, smoking status and study site). Abbreviations: mMRC = modified Medical Research Council dyspnoea scale, WBC = white blood cells, NLR = neutrophil-lymphocyte ratio. Units of measurement: total WBC, neutrophils, lymphocytes 109/L; monocytes, eosinophils, basophils 1010/L.
When stratified by smoking status (Table 3), associations of inflammatory markers with respiratory symptoms were more numerous in current and former smokers compared to never smokers. In never smokers, increased neutrophils and the NLR were associated with cough in the absence of a cold and dyspnoea, while increased eosinophils were associated with cough and phlegm in the absence of a cold. In former smokers, all inflammatory markers, except for lymphocytes, were associated with wheeze, and neutrophils were associated with all respiratory symptoms. In current smokers, inflammatory markers were predominantly associated with cough and phlegm, especially basophils (OR with 95%CI: chronic cough 3.98 (0.89, 17.86) p = 0.071, chronic phlegm 6.12 (1.36, 27.56) p = 0.018).
Interactions
We examined the interaction of smoking status in the association between inflammatory markers and outcomes in Tables S5 and S6. A significant interaction was identified in the association between total WBC and all lung function indices, with former smokers having larger negative associations of total WBC with FEV1 (p < 0.001) and FVC (p = 0.012), and larger positive associations with CAO (p = 0.030) (Table S5). A similar interaction was also found in the association of lymphocytes (p = 0.044) and eosinophils (p = 0.038) with FEV1.
With regard to respiratory symptoms (Table S6), we found a significant interaction of smoking status in the association between total WBC and cough and phlegm without a cold (larger positive effect in current smokers, p = 0.005 and p = 0.046, respectively). Another significant interaction was found in the association of total WBC (p = 0.007), neutrophils (p = 0.007) and the NLR (p = 0.025) with wheeze, with larger positive effects in former smokers. Finally, there were significant interactions in the association between basophils and chronic cough and chronic phlegm (larger positive effect in current smokers, p = 0.043 and p = 0.002, respectively).
Unadjusted analyses
The main analyses were adjusted for gender, age, body mass index and study site. Unadjusted analyses of the association between inflammatory markers, lung function and respiratory symptoms are reported in Table S7 and S8. The results were overall consistent with the adjusted analyses.
Variation across study sites
The associations of inflammatory markers with lung function (Figures S1–S8) and respiratory symptoms (not shown) calculated with random-effect meta-analysis demonstrated non-significant low to moderate heterogeneity across study sites.
Discussion
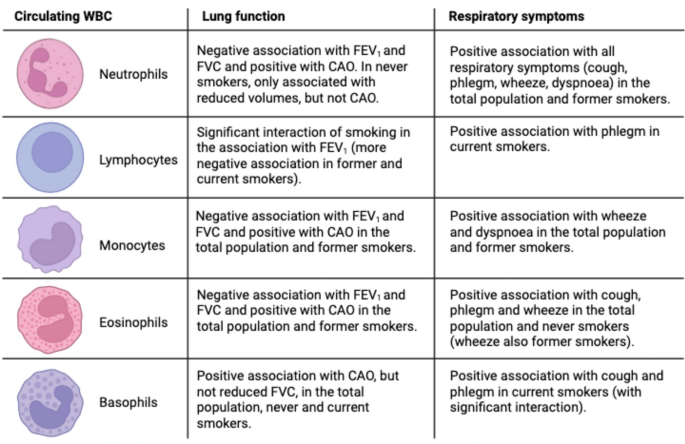
In this study, we found significant associations between circulating markers of systemic inflammation, lung function and respiratory symptoms in a general population cohort from Northern Europe, with different patterns according to smoking status. The main findings are summarized in Fig. 3.
Summary of the findings of the study: associations between inflammatory markers and outcomes (lung function, respiratory symptoms), stratified by smoking status. Image created with BioRender.com. Abbreviations: WBC = white blood cells, FEV1 = forced expiratory volume in the 1 st second, FVC = forced vital capacity, CAO = chronic airflow obstruction, defined as post-bronchodilatory FEV1/FVC values below 5th percentile.
Elevated blood neutrophils were associated with all respiratory symptoms (cough and phlegm – both without a cold and chronic –, wheeze, and dyspnoea), reduction of all lung function indices (FEV1, FVC, FEV1/FVC) and presence of CAO. This is in keeping with the established role of neutrophilic inflammation in the pathogenesis of obstructive lung disease, primarily COPD27, but also subtypes of asthma9. Lewis et al.11 also found an association between blood neutrophil levels and persistent cough, phlegm, dyspnoea, and reduced FEV1, but not wheeze, in a British general population sample. Nerpin et al.5 found an inverse relationship between blood neutrophils and pre-bronchodilatory FEV1, FVC, FEV1/FVC in the NHANES 2007–08 and 2009–10 surveys. When stratified by smoking status, the associations of neutrophils with reduced FEV1 and FVC – but not airflow obstruction – persisted in never-smokers. This suggests that neutrophilic inflammation can be related to lung restriction independently of cigarette smoking. Restrictive spirometric patterns are known to be related to metabolic syndrome, diabetes and obesity, with evidence that the reduction in lung function may precede the other manifestations28. The pathogenetic link is thought to be systemic inflammation, although the exact mechanisms are unknown28. In metabolic diseases, neutrophils release enzymes like myeloperoxidase and neutrophil elastase which promote insulin resistance and inflammation29. It is possible that these mechanisms are also implicated in the pathogenesis of low lung function.
Elevated blood eosinophils were associated with cough and phlegm in the absence of a cold, wheeze, reduced FEV1, reduced FVC, and presence of CAO. These findings also align with Lewis et al.11, although their study also evidenced an association with persistent cough. The association between eosinophils and wheeze in our study was present both in never and former smokers, while the association with reduced lung function was found only in former smokers, and the one with cough and phlegm only in never smokers. This might reflect the multiple aetiologies behind the findings, such as asthma, COPD, their overlap, infections, and potentially other conditions. It is well established that eosinophilia is related to type-2 inflammation, with eosinophils produced in response to IL-5 and further regulated in response to IL-4 and IL-1330. Type-2 inflammation is a feature of asthma and of a subset of subjects with COPD, although this role is more controversial31. There can be multiple differences in the nature of type-2 inflammation between asthma and COPD, such as the type of inflammatory mediators, the role of IgE in mast cell activation and the pathophysiology of mucus hypersecretion32. In our study, it is possible that the association between eosinophils and reduced lung function in former smokers might reflect type-2 inflammation in COPD, while the association with cough and phlegm in never smokers might reflect type-2 inflammation in asthma.
We also evidenced the association of circulating basophils with wheeze, dyspnoea, CAO and reduced FEV1, but not FVC. This might suggest that basophils are involved in airflow obstruction, but not restriction. The association with CAO was present both in never and current smokers, although the significance of the latter was affected by the small sample size. In current smokers, basophils were also related to cough and phlegm. Lewis et al.11 also evidenced significant associations between circulating basophils and wheeze, dyspnoea, and reduced FEV1, but not cough and phlegm, although their study did not stratify for smoking status. Only a few studies have explored the role of basophils in COPD and asthma. Winter et al. found that a mast cell/basophil gene signature measured in sputum was associated with eosinophilic inflammation and lower lung function in severe asthma33 and COPD34. Jogdand et al.35 described both eosinophils and basophils in histological lung COPD samples, with tissue density that increased with disease severity. Mast cells and basophils originate from common precursors. Recent studies hypothesized that mast cells could be a link between COPD and asthma36,37. It is possible that basophils may be related to airflow obstruction in never smokers in our study with IgE-dependent mechanisms typical of asthma32, while they may be related to airflow obstruction and mucus hypersecretion in current smokers with IgE-independent mechanisms typical of COPD38.
We found associations of circulating monocytes with wheeze, dyspnoea, reduced FEV1, reduced FVC and CAO. In stratified analyses, the association with reduced lung function was more evident in former smokers. Similarly, Halper-Stromberg et al.39 reported cross-sectional and longitudinal associations of monocytes with reduced FEV1 in the COPDGene and ECLIPSE cohorts, which consist of former and current smokers. Other studies confirmed associations of increased monocytes with reduced lung function in population-based samples11,12 and also reported associations with persistent cough and phlegm11. Blood monocytes can be recruited into the lung and differentiate into alveolar macrophages, key cells in COPD pathogenesis, which are further activated by cigarette smoking amplifying the inflammatory process40.
We did not find significant associations between blood lymphocyte counts and lung function in the total population, which is in line with the study of Lewis et al.11. However, we found a significant association between increased lymphocytes and phlegm in current smokers, confirmed by a significant interaction. Smoking status also modified the association between lymphocytes and FEV1, with more severe lung function impairment in former and current smokers. This might support a role for increased blood lymphocytes in COPD pathogenesis, although it has also been reported lower cross-sectional FEV1 and greater FEV1 decline in current and former smokers with baseline lymphopenia39. One potential explanation for this difference could be the severity of the disease, as the mean FEV1 in the COPDGene and ECLIPSE cohorts39 was much lower than in our study. Our study also examined the NLR, an index that can reflect inflammation associated with neutrophilia and impaired immune response associated with reduced lymphocytes10,26,41. The NLR has been proposed as a marker of activity and severity of COPD and a predictor of COPD exacerbations. In our study, the NLR showed a profile of association with lung function and respiratory symptoms substantially in line with that of neutrophils.
At present, only blood eosinophils are used as a biomarker of respiratory disease in clinical practice, specifically in the diagnosis and management of COPD42 and asthma43. Our study confirmed their relationship with reduced lung function and presence of respiratory symptoms (cough, phlegm, wheeze). In addition, our study showed a significant association between circulating basophils, lung function and respiratory symptoms, which to our knowledge has been described before only in a British population cohort11. In particular, they were related to airflow obstruction in the whole population, and to cough and phlegm in current smokers. We hypothesize that they might be helpful in differentiating, together with eosinophils, type-2 inflammation in asthma and COPD based on IgE-dependent and Ig-E-independent mechanisms, respectively. This possibility should be confirmed in future studies with other demographics and geographical settings, as well as mechanistic studies.
Our study also suggested that different markers of systemic inflammation may be related to different types of lung function impairment. We observed that circulating neutrophils were related to both airflow obstruction and reduced lung volumes (FEV1 and FVC) in the total population and in former smokers. In never-smokers, they were related to reduced FEV1 and FVC, but not CAO. Accordingly, they could reflect both obstructive and restrictive respiratory disease. The hypothesis of neutrophilic systemic inflammation linked to lung restriction would need to be confirmed in future studies using static lung volumes to assess restrictive impairment. Blood eosinophils and basophils, on the other hand, appeared to be related to CAO and reduced FEV1 to a larger extent than FVC, supporting a predominant role in airflow obstruction rather than restriction.
The strengths of our study include the general population sample, the use of standardized protocol and questionnaires across the study sites and the conduction of spirometry by trained and certified technicians. In addition, all spirometry curves were visually inspected centrally, and only those that passed quality control were used in this study. Compared to previous studies, our study investigated a larger number of outcomes (multiple lung function variables and respiratory symptoms) and it was multi-center, which increases generalizability. Our study also has limitations, including self-reporting respiratory symptoms, which may be affected by recall bias, and the fact that the mean age of our population was 68 years, which impedes the extrapolation of our findings to younger age groups. However, the demographics of the population may provide unique insights on the relationship between systemic inflammation and respiratory health in older adults on whom there is little data. The cross-sectional design does not allow us to infer causality; longitudinal studies are needed to establish temporal relationships between the variables involved. We did not have allergic sensitisation data on the participants, which would have been useful to consider in the current study. The proportion of current smokers in this population was low and this might have affected the significancy of the results. We focused on certain markers of systemic inflammation because we were interested in the individual contribution of WBC sub-populations, but other markers such as the systemic immune inflammation index (SII), the systemic inflammation response index (SIRI), or the aggregate index of systemic inflammation (AISI) may be worth exploring in future studies44.
In conclusion, larger counts of different WBC sub-populations are associated with different lung function indices and respiratory symptoms in the general population. Novel findings of our study include an association of blood basophils with CAO in the general population and with cough and phlegm in current smokers. Together with eosinophils, they might be useful in differentiating the type of inflammation in asthma and COPD. Blood neutrophils were related to reduced FEV1 and reduced FVC – but not CAO – in never smokers, suggesting a potential pathogenetic role in restrictive lung disease. These findings warrant further investigation in future studies.
Data availability
Data can be available on a collaborative basis upon reasonable request. Data requests can be directed to Dr. Christer Janson (christer.janson@medsci.uu.se).
References
McKarns, S. C. et al. Correlation of hematologic markers of inflammation and lung function: a comparison of asymptomatic smokers and nonsmokers. Hum. Exp. Toxicol. 15, 523–532 (1996).
Gan, W. Q., Man, S. F., Senthilselvan, A. & Sin, D. D. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 59, 574–580 (2004).
Wouters, E. F., Reynaert, N. L., Dentener, M. A. & Vernooy, J. H. Systemic and local inflammation in asthma and chronic obstructive pulmonary disease: is there a connection? Proc. Am. Thorac. Soc. 6, 638–647 (2009).
Sanchez-Azofra, A. et al. Inflammation biomarkers in OSA, COPD and COPD/OSA overlap syndrome. J Clin. Sleep. Med. 19, 1447–1456 (2023).
Nerpin, E. et al. Systemic inflammatory markers in relation to lung function in NHANES. 2007–2010. Respir. Med. 142, 94–100 (2018).
Mogensen, I. et al. Blood eosinophil level and lung function trajectories: cross-sectional and longitudinal studies in European cohorts. ERJ Open. Res. 6, 00320–2020. (2020).
Lee, S. H., Ahn, K. M., Lee, S. Y., Kim, S. S. & Park, H. W. Blood Eosinophil Count as a Predictor of Lung Function Decline in Healthy Individuals. J Allergy Clin Immunol Pract ; 9: 394–399 e391. (2021).
Singh, D. et al. Blood eosinophils and chronic obstructive pulmonary disease: A global initiative for chronic obstructive lung disease science committee 2022 review. Am. J. Respir Crit. Care Med. 206, 17–24 (2022).
Flinkman, E. et al. Association between blood eosinophils and neutrophils with clinical features in Adult-Onset asthma. J. Allergy Clin. Immunol. Pract. 11, 811–821e815 (2023).
Lee, H. et al. Association of the Neutrophil-to-Lymphocyte ratio with lung function and exacerbations in patients with chronic obstructive pulmonary disease. PLoS One. 11, e0156511 (2016).
Lewis, S. A. et al. The relation between peripheral blood leukocyte counts and respiratory symptoms, atopy, lung function, and airway responsiveness in adults. Chest 119, 105–114 (2001).
McKeever, T., Saha, S. & Fogarty, A. W. The association between systemic inflammatory cellular levels and lung function: a population-based study. PLoS One. 6, e21593 (2011).
Wu, X. et al. Circulating white blood cells and lung function impairment: the observational studies and Mendelian randomization analysis. Ann. Med. 53, 1118–1128 (2021).
Schwartz, J. & Weiss, S. T. Peripheral blood leukocyte count and respiratory symptoms. Ann. Epidemiol. 3, 57–63 (1993).
Schwartz, J. & Weiss, S. T. Prediction of respiratory symptoms by peripheral blood neutrophils and eosinophils in the first National nutrition examination survey (NHANES I). Chest 104, 1210–1215 (1993).
Hartl, S. et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur Respir J. 55, 1901874 (2020).
Amaral, R. et al. The influence of individual characteristics and non-respiratory diseases on blood eosinophil count. Clin. Transl Allergy. 11, e12036 (2021).
Smith, M. R. et al. Smoking status and differential white cell count in men and women in the EPIC-Norfolk population. Atherosclerosis 169, 331–337 (2003).
Yang, I. A., Jenkins, C. R. & Salvi, S. S. Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir Med. 10, 497–511 (2022).
Burney, P., Knox-Brown, B. & Amaral, A. F. S. Small lung syndrome: the need to reclassify chronic lung disease. Lancet Respir Med. 11, 405–406 (2023).
Buist, A. S. et al. The burden of obstructive lung disease initiative (BOLD): rationale and design. COPD: J. Chronic Obstr. Pulmonary Disease. 2, 277–283 (2009).
Amaral, A. F. S. et al. Cohort profile: burden of obstructive lung disease (BOLD) study. Int. J. Epidemiol. 52, e364–e373 (2023).
Janson, C. et al. The impact of COPD on health status: findings from the BOLD study. Eur. Respir J. 42, 1472–1483 (2013).
Hankinson, J. L., Odencrantz, J. R. & Fedan, K. B. Spirometric reference values from a sample of the general U.S. Population. Am. J. Respir Crit. Care Med. 159, 179–187 (1999).
Pellegrino, R. et al. Interpretative strategies for lung function tests. Eur Respir J. 26, 948–968 (2005).
Ellingsen, J. et al. Neutrophil-to-lymphocyte ratio, blood eosinophils and COPD exacerbations: a cohort study. ERJ Open. Res. 7, 00471–2021 (2021).
Barnes, P. J. Inflammatory endotypes in COPD. Allergy 74, 1249–1256 (2019).
Godfrey, M. S. & Jankowich, M. D. The vital capacity is vital: epidemiology and clinical significance of the restrictive spirometry pattern. Chest 149, 238–251 (2016).
Herrero-Cervera, A., Soehnlein, O. & Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 19, 177–191 (2022).
Wechsler, M. E. et al. Eosinophils in health and disease: A State-of-the-Art review. Mayo Clin. Proc. 96, 2694–2707 (2021).
Polverino, F. & Sin, D. D. Type 2 airway inflammation in COPD. Eur Respir J. 63, 2400150 (2024).
Beech, A. et al. Type 2 inflammation in COPD: is it just asthma? Breathe (Sheff). 20, 230229 (2024).
Winter, N. A. et al. Sputum mast cell/basophil gene expression relates to inflammatory and clinical features of severe asthma. J. Allergy Clin. Immunol. 148, 428–438 (2021).
Winter, N. A., Gibson, P. G., McDonald, V. M. & Fricker, M. Sputum gene expression reveals dysregulation of mast cells and basophils in eosinophilic COPD. Int. J. Chron. Obstruct Pulmon Dis. 16, 2165–2179 (2021).
Jogdand, P. et al. Eosinophils, basophils and type 2 immune microenvironments in COPD-affected lung tissue. Eur Respir J. 55, 1900110 (2020).
Tesfaigzi, Y. et al. Does chronic obstructive pulmonary disease originate from different cell types?? Am. J. Respir Cell. Mol. Biol. 69, 500–507 (2023).
Higham, A., Dungwa, J., Pham, T. H., McCrae, C. & Singh, D. Increased mast cell activation in eosinophilic chronic obstructive pulmonary disease. Clin. Transl Immunol. 11, e1417 (2022).
Walter, S. & Walter, A. Basophil degranulation induced by cigarette smoking in man. Thorax 37, 756–759 (1982).
Halper-Stromberg, E. et al. Systemic markers of adaptive and innate immunity are associated with chronic obstructive pulmonary disease severity and spirometric disease progression. Am. J. Respir Cell. Mol. Biol. 58, 500–509 (2018).
Barnes, P. J. Alveolar macrophages as orchestrators of COPD. COPD 1, 59–70 (2004).
Gao, X. et al. Association of neutrophil to lymphocyte ratio with pulmonary function in a 30-Year longitudinal study of US veterans. JAMA Netw. Open. 3, e2010350 (2020).
Global Strategy for the Diagnosis. Management, and Prevention of Chronic Obstructive Pulmonary Disease 2025. [cited 2025 Jul 28]. Available from: https://goldcopd.org/
Global Strategy for Asthma Management and Prevention. [cited 2025 Jul 28]. (2025). Available from: https://ginasthma.org/
Wang, C., Liao, X., Chen, J., Lan, Y. & Wen, J. Systemic inflammation partially mediates the association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and chronic cough. Lipids Health Dis. 24, 85 (2025).
Funding
Open access funding provided by University of Bergen. The BOLD baseline study was supported by grants to the coordinating centres in London (UK) and Portland (USA) by the Wellcome Trust (085790/Z/08/Z). Local support was provided by: the Swedish Heart and Lung Foundation, the Swedish Association against Heart and Lung Diseases, GlaxoSmithKline Sweden (Uppsala, Sweden); the Norwegian Ministry of Health’s Foundation for Clinical Research, Haukeland University Hospital’s Medical Research Foundation for Thoracic Medicine (Bergen, Norway); Landspítali-University Hospital-Scientific Fund, GlaxoSmithKline Iceland, AstraZeneca Iceland (Reykjavik, Iceland); GlaxoSmithKline, AstraZeneca, Estonian Science Foundation ETF6889 (Tartu, Estonia). The BOLD follow-up study was supported by grants to the coordinating centre in London (UK) by the UK Medical Research Council (MR/R011192/1) and to the local sites in European countries by AstraZeneca AB (ESR-17-13417). The Icelandic BOLD follow-up study was supported by Rannís: The Icelandic Centre for Research (Grant number 185348-053). L.C. acknowledges support from: Den norske legeforening (Trelasthandler A. Delphin og hustrus legat), Norsk forening for lungemedisin (grant sponsored by AstraZeneca), and University of Bergen (mobility grant).
Author information
Authors and Affiliations
Contributions
L.C.: Conceptualization, Formal analysis, Writing - Original Draft, Writing - Review & Editing, Visualization. A.A.: Data curation, Methodology, Writing - Review & Editing, Project administration. B.B.: Resources, Methodology, Writing - Review & Editing. T.G.: Resources, Methodology, Writing - Review & Editing. R.J.: Resources, Methodology, Writing - Review & Editing. A.M.: Resources, Methodology, Writing - Review & Editing. R.N.: Resources, Methodology, Writing - Review & Editing. C.J.: Conceptualization, Data curation, Methodology, Formal analysis, Writing - Review & Editing, Supervision, Project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cestelli, L., Amaral, A.F.S., Benediktsdottir, B. et al. The association between systemic inflammation, lung function and respiratory symptoms in the BOLD study in Northern Europe. Sci Rep 15, 30929 (2025). https://doi.org/10.1038/s41598-025-16776-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16776-x