Abstract
In this study, graphene oxide (GO) was synthesized via a modified low-temperature Hummer’s method and incorporated into a poly (ethylene oxide) (PEO) matrix to fabricate PEO/GO nanocomposite thin films using a casting technique. This work explores how GO incorporation affects the structural and optical properties of PEO, with emphasis on its suitability for optical switching and other optoelectronic applications. The films were prepared with varying GO concentrations and characterized using XRD, UV–Vis spectroscopy, and other standard techniques. XRD confirmed successful GO incorporation by revealing intercalation-induced structural changes. Optical transmittance dropped from 88% (pure PEO) to 18% (7 wt% GO), demonstrating strong UV-blocking ability. UV–Vis analysis showed a red-shift in the absorption edge with increasing GO content, and Tauc’s method indicated a reduction in the optical bandgap from 5.82 eV to 2.12 eV. Key optical constants improved significantly: the refractive index rose from 1.72 to 2.21, the absorption edge decreased from 5.63 to 2.27 eV, and the extinction coefficient increased with GO loading. Wemple–DiDomenico analysis revealed a decrease in dispersion energy (from 13 to 12.7 eV) and oscillator energy (from 6.52 to 3.29 eV). Nonlinear optical properties also improved, with third-order susceptibility increasing from 0.57 × 10⁻⁵ to 2.98 × 10⁻⁵ esu and nonlinear refractive index from 4.4 × 10⁻⁵ to 5.2 × 10⁻⁵ at 7 wt% GO. Drude model analysis showed a substantial rise in the carrier concentration-to-effective mass ratio from 1.50 × 10⁵⁴ to 11.58 × 10⁵⁴ m⁻³·kg⁻¹. These enhancements demonstrate that GO-doped PEO films are promising candidates for next-generation optical switching and optoelectronic devices.
Similar content being viewed by others
Introduction
In recent years, graphene and its composites have attracted substantial research interest owing to their remarkable physical and chemical properties, so these materials are being explored across a wide range of applications, including batteries, supercapacitors, catalysis, sensors, and electronic devices1. Among these, graphene has shown particular promise as an electrode material in supercapacitors due to its high surface area and excellent conductivity. Graphene oxide (GO), a chemically modified derivative of graphene, has become especially noteworthy, so structurally, GO retains the hexagonal carbon network characteristic of graphene but differs due to the presence of various oxygen-containing functional groups such as hydroxyl (-OH), alkoxy (C–O–C), carbonyl (C = O), and carboxyl (-COOH) groups2 so these oxygen-based groups impart GO with unique physicochemical properties. For instance, the hydrophilic nature of these functional groups enhances GO’s solubility in a range of organic solvents3. Additionally, the weaker van der Waals forces between GO sheets compared to pristine graphene enhance its compatibility with polymer matrices, also the GO’s hydrophilicity, primarily resulting from carbonyl and carboxyl functionalities, also facilitates its dispersion in aqueous media4 so this combination of dispersibility and reactivity makes GO an adaptable material that can be readily functionalized. A particularly significant feature of GO is its ability to form covalent bonds with other substances, especially polymers, through the reactive oxygen functionalities on its surface, so this property enables the synthesis of GO-based composites, where GO interacts chemically with polymer matrices, resulting in robust interfacial bonding5. Polymers, another class of widely utilized materials, are typically characterized by high dielectric constants but low electrical conductivity in their pure form, however, their properties can be tailored through the incorporation of suitable fillers into the polymer matrix6 also the extent and nature of these modifications are influenced by several variables, including the type of polymer-filler system used, the filler concentration, and external stimuli such as electric fields7. A wide range of polymer/GO nanocomposites has been investigated, employing various polymeric matrices such as polyurethane8 PMMA-PVA9 PVA10 Methylcellulose11 Chitosan12 PEG13 polystyrene14 PVC15 PEO/PVP16, and polypyrrole17. Among these, (PEO), a semicrystalline polymer, has gained particular attention for its potential in bio-electrochemical applications18 however, a notable limitation of PEO in such applications lies in its reduced optical and electrical performance above its melting temperature. To address this drawback, researchers have explored the formation of PEO-based nanocomposites by incorporating various nanofillers, so these include Al2O319, TiO220, nanoceramic Li4Ti5O1221, and AgNO3 NPs22 so the inclusion of these nanostructures enhances interactions with the polymer’s surface functional groups, which in turn improves thermal stability above the polymer’s melting point23,24 consequently, the integration of different nanofillers into the PEO matrix has proven to be an effective strategy for enhancing its physicochemical properties and broadening its potential for use in diverse technological applications25,26.
What distinguishes the present study is the strategic incorporation of graphene oxide (GO) into poly(ethylene oxide) (PEO) via a straightforward, scalable casting technique, yielding flexible PEO/GO nanocomposite films with tunable optical and electronic functionalities, also the nanocomposites were synthesized by dispersing GO in a mixed solvent system of acetonitrile and distilled water (5:1) at 80–90 °C, followed by uniform blending with PEO at varying GO concentrations (1–7 wt%), so this simple yet effective approach ensured excellent GO dispersion, minimized aggregation, and produced films with enhanced interfacial interactions. The uniqueness of this work lies in its comprehensive assessment of how GO incorporation systematically modulates the optical, electronic, and nonlinear optical properties of PEO, also UV-Vis spectroscopic analysis revealed significant enhancement in light absorption and a remarkable red-shift in the absorption edge with increasing GO content, leading to a reduction in both direct and indirect band gaps—down to 2.12 eV at 7 wt% GO, so this optical tunability is critical for designing materials suitable for UV shielding, photodetectors, and energy harvesting devices, furthermore, a pronounced decline in transmittance (from 88 to 18%) with increasing GO loading highlights the nanocomposite’s potential as a UV-blocking layer in optoelectronic and photovoltaic systems, also the corresponding increase in the refractive index and extinction coefficient, attributed to GO’s high polarizability and localized π-electron interactions, reinforces the applicability of these films in photonic devices, optical modulators, and waveguides. A deeper exploration of dispersion behavior using Drude theory enabled us to extract crucial parameters such as charge carrier concentration-to-effective mass ratio (N/m*), plasma frequency (ωp), relaxation time (τ), optical mobility (µopt), and optical conductivity (ρopt). Notably, the N/m* value surged from 1.50 × 10⁵⁴ in pristine PEO to 11.578 × 10⁵⁴ at 7 wt% GO, confirming increased free carrier density and enhanced charge transport, simultaneously, the significant drop in ρopt indicates improved conductivity-making these films promising candidates for advanced optoelectronic circuitry and energy storage layers.
Importantly, the study also sheds light on the nonlinear optical (NLO) behavior of these nanocomposites, also the third-order susceptibility (χ⁽³⁾) and nonlinear refractive index (n₂) values increased significantly with GO content, with χ⁽³⁾ reaching 2.979 × 10⁻⁵ at 7 wt% GO-nearly a fivefold improvement over pure PEO, so these enhancements, driven by GO’s delocalized π-electron systems and their strong interaction with the polymeric host, offer exciting potential for optical switching, signal modulation, and photonic computing platforms, finally this study delivers a significant scientific contribution by demonstrating how a low-cost, scalable fabrication method can be used to create multifunctional PEO/GO nanocomposite films with customizable optical, electrical, and nonlinear responses, also the findings pave the way for their application in next-generation electronics, flexible optical devices, and high-performance nonlinear optical components.
Polyethylene oxide (PEO), while valued for its film-forming ability and flexibility, faces several limitations that restrict its use in optoelectronic applications. It exhibits low optical absorption and a wide bandgap, making it inefficient for light-harvesting devices27. Additionally, its poor charge transport, low dielectric constant, and lack of inherent nonlinear optical properties limit its functionality in advanced photonic systems. PEO also suffers from high crystallinity, which affects uniformity and ionic conductivity, along with weak thermal and mechanical stability27. Moreover, its sensitivity to environmental conditions, such as moisture absorption, can degrade device performance and reliability. These limitations necessitate material modifications, such as doping with functional nanomaterials like graphene oxide, to enhance its optoelectronic properties. Recent efforts have focused on enhancing these properties through the incorporation of nanomaterials such as GO, which possesses excellent electronic and optical characteristics. While previous studies have reported on the general behavior of GO or PEO-based composites, comprehensive investigations into the optical and nonlinear optical performance of GO-doped PEO films remain limited. Despite these efforts, most previous studies have focused only on a few optical or electrical parameters such as absorption and bandgap energy28. The aim of the present work is to systematically study the effect of GO incorporation on the structural and optical properties of PEO, including parameters such as absorption, transmittance, reflectivity, optical bandgap, and nonlinear optical coefficients (χ¹, χ³, and n2). By doing so, this study provides new insights into the design of GO–PEO composite materials with tailored optical responses, offering potential for use in optoelectronic devices, optical switching systems, and photonic technologies. The results of the current study establish that GO insertion into PEO is crucial to improve both linear and nonlinear optical properties of PEO composite. Clear D and G bands in Raman spectra and obvious shifting in UV-vis absorption is an evidence for the modification of optical properties of PEO upon addition of GO material.
Experimental techniques
Synthesis of graphene oxide (GO)
A mixture solution of sulfuric acid (H2SO4) and phosphoric acid (H3PO4) at a volume ratio of 9:1 (30:3.3 ml) was prepared. The mixture was stirred at room temperature for 15 min and then 1.0 g of graphite powder together with 6.0 g of potassium permanganate (KMnO4) were added to the solution slowly with continuous stirring at 3.0 °C for an additional time of 30 min. Subsequently the mixture was allowed to stir continuously at 38–40 °C for a further 30 min. Next 100 ml of deionized water was added peroxide and 10 ml of 30% hydrogen peroxide (H2O2) was added at ambient temperature for 20 min to terminate the reaction. The obtained mixture was divided into 8 separate 80 ml centrifuge tubes for cleaning. For each washing step, the cleaning was carried out using deionized (DI) water alternately with centrifugation at 3000 rpm for 30 min. These processes were reiterated twice. Finally, the completed product was dried for 24 h at 80 °C in an oven. Scheme 1 illustrates the GO manufacturing process.
Preparation of PEO/GO nanocomposite films
PEO/GO nanocomposite films were prepared by casting technique. 4.0 g of PEO powder was dissolved in acetonitrile and distilled water at a volume ratio of 5:1 (125:25 ml) was prepared at 80–90 ℃ under stirring to obtain a homogenous viscous solution. A homogenous PEO/GO aqueous suspension was achieved by gradually adding the GO aqueous suspension to the PEO solution under vigorous stirring. The GO content was adjusted to 1, 3, 5, and 7 wt% against the quantity of PEO powder. The aqueous PEO/GO suspensions were poured into glass Petri dishes and allowed to air dry at room temperature. Scheme 2 shows a schematic of the PEO/GO nanocomposite film preparation process using the casting technique.
Characterization techniques
We have used several different techniques to investigate the samples such as X-Ray Diffraction (XRD), Ultraviolet–Visible (UV-VIS), Attenuated Total Reflectance - Fourier Transform Infrared (ATR-FTIR) and Raman Spectroscopy. The ultraviolet-visible (UV-Vis) absorption spectra of the prepared films were recorded on a UV-vis spectrometer (V-570, Jasco, Japan) with the scanning range from 180 to 1000 nm. XRD patterns were recorded using Empyrean X-ray diffractometer, (PANalytical, Netherland) with operating current and voltage of 40 mA and 40 kV, respectively. The samples were scanned with a beam of monochromatic CuKα X-radiation of wavelength (λ = 1.5406 Ǻ), and the glancing angles X-ray diffraction was in the range of 5° ⩽ 2θ ⩽ 80° with a step size of 0.1°.
Results and discussion
FTIR spectrum of PEO/GO nanocomposites
Fourier Transform Infrared (FTIR) spectroscopy was employed to identify the major functional groups and to investigate potential molecular interactions between PEO and GO in the prepared nanocomposite films. The FTIR spectra ranging from 3000 cm−1 to 400 cm⁻¹ were recorded and presented in Fig. 3. For pristine PEO, two prominent absorption bands were observed at 1107.4 cm⁻¹ and 2870.32 cm⁻¹. The band at 1107.4 cm⁻¹ corresponded to the C–O–C stretching vibration, characteristic of the ether linkages in the semi-crystalline PEO backbone29,30. The absorption peak at 2870.32 cm⁻¹ is probably due to the asymmetric stretching vibration of CH₂ groups, typically associated with the aliphatic segments of PEO chains29. The FTIR spectrum of synthesized GO displays a strong absorption band at 1725.9 cm⁻¹, corresponding to the stretching vibration of carbonyl (C = O) groups, and another distinctive band at 1051.8 cm⁻¹, assigned to the stretching of C–O bonds, likely from epoxy or alkoxy functionalities. These bands confirmed the presence of oxygen-containing groups introduced during the oxidation of graphite, as expected from the Hummers’ method31,32. The FTIR spectra of the PEO/GO nanocomposite films (HPEOGO1, HPEOGO3, HPEOGO5, and HPEOGO7) exhibited all the main bands associated with pristine PEO, indicating that the primary chemical structure of the polymer is preserved in the presence of GO (Fig. 1). However, two notable spectral changes were observed. First, the intensity of the C–H asymmetric stretching band at 2870.32 cm⁻¹ showed a gradual decrease with increasing GO content. This attenuation may suggest a partial restriction in the vibrational freedom of CH₂ groups due to interactions between the GO nanosheets and the PEO matrix33,34. Second, the intensity of the C–O–C stretching band at 1107.4 cm⁻¹ increased upon GO incorporation (Fig. 2). This enhancement can be interpreted as evidence of intermolecular interactions and a potential increase in local ordering or alignment of PEO chains in the presence of GO, which may also be associated with modifications in the polymer’s crystallinity32. No new bands were introduced in the FTIR spectra of the composite films, suggesting that the interaction between GO and PEO is primarily physical rather than chemical. The observed spectral changes corroborate the successful formation of the nanocomposite structure through non-covalent interactions, such as Van der Waals forces or possible hydrogen bonding, without altering the fundamental chemical architecture of the polymer matrix34. Finally, the FTIR analysis confirmed the incorporation of GO into the PEO matrix and revealed subtle yet significant molecular interactions that influence the polymer’s vibrational characteristics and structural organization.
XRD analysis
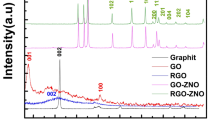
The XRD patterns of the prepared PEO/GO nanocomposite films with varying GO content (1, 3, 5, and 7 wt%) are shown in Fig. 3. In addition, pure PEO polymer film exhibits distinct diffraction peaks at 2θ = 19.374° and 23.704°, which correspond to the (120) and (112) crystallographic planes, respectively, confirming the semi-crystalline nature of the polymer matrix. Consequently, these diffraction features are consistent with previously reported data for PEO-based systems35,36. Incorporation of GO into the PEO matrix leads to noticeable changes in the diffraction pattern. Notably, a new diffraction peak emerges in the nanocomposite films with the addition of GO, so this peak, which appears at approximately 2θ = 7.024° in HPEOGO1, becomes progressively more intense in HPEOGO3, HPEOGO5. It can be observed that the most prominent peak in HPEOGO7 at 2θ = 7.194° is attributed to the (001) crystallographic plane of GO. Furthermore, the presence of this peak confirms the successful integration of GO into the PEO matrix and the preservation of its layered structure during the composite formation. In the case of HPEOGO1, the GO-related peak exhibits very low intensity, suggesting a minimal degree of GO restacking or poor crystallinity at lower concentrations. As the GO content increases, the intensity of the (001) peak becomes more pronounced, particularly in HPEOGO3 and HPEOGO7, indicating a higher content and better structural ordering of GO within the polymer matrix. Therefore, this observation suggests that GO is well incorporated and partially retains its layered structure within the PEO phase. Furthermore, the diffraction peaks corresponding to PEO (120) and (112) planes remain visible across all compositions, although a slight reduction in their intensity is observed with increasing GO content, thus the relative decrease in peak intensity may be attributed to the disruption of PEO’s crystalline domains due to the presence of GO nanosheets, which interact with PEO chains via hydrogen bonding and restrict their regular packing. The overall trend implies a partial reduction in the crystallinity of the PEO matrix upon the incorporation of GO, consistent with similar polymer/GO systems reported in literature34.
Raman spectral analysis
Raman spectroscopy was employed to investigate the structural integrity and molecular interactions within the PEO/GO nanocomposite films, so the Fig. 4 presents the Raman spectra of pristine PEO, synthesized GO, and nanocomposites with varying GO loadings (HPEOGO1, HPEOGO3, HPEOGO5, and HPEOGO7). The Raman spectrum of pristine PEO displays a notable band at 2802.03 cm⁻¹, which is attributed to the C–H stretching vibration within the PEO backbone, so this band reflects the characteristic vibrational mode of the PEO chains and is commonly associated with the semi-crystalline structure of the polymer, in contrast, the Raman spectrum of synthesized GO shows two prominent bands: the D band at 1285.44 cm⁻¹ and the G band at 1527.91 cm⁻¹37. The D band arises from the breathing modes of sp² carbon atoms in disordered rings, indicative of defects and functional groups introduced during the oxidation of graphite38. The G band corresponds to the in-plane stretching vibration of sp²-hybridized carbon atoms (E2g phonon mode) and is characteristic of graphitic structures39,40. Based on the Raman spectrum, the intensity ratio of the D to G band (ID/IG) was calculated and found to be approximately 0.84, so this value is in good agreement with those reported in the literature (R1), confirming a moderate level of structural disorder and the successful oxidation of graphite to GO. Upon incorporation of GO into the PEO matrix, both D and G bands are retained in the Raman spectra of the nanocomposite films. Their positions remain unchanged, and their intensity increases with higher GO content, indicating the preservation of GO’s structural framework within the polymer matrix and successful formation of the composite39a noticeable evolution is observed in the PEO-related Raman band as GO content increases. In the HPEOGO1 film, the C–H stretching band shifts to a higher wavenumber at 2919.85 cm⁻¹, however, its intensity decreases progressively with increasing GO content and nearly disappears in the HPEOGO7 film. This reduction in intensity may be attributed to strong interfacial interactions, such as hydrogen bonding, between PEO chains and GO nanosheets, which restrict the molecular motion of PEO and suppress its Raman-active modes41. The persistent D and G bands, along with the diminishing PEO band, suggest physical incorporation of GO within the polymer matrix without covalent functionalization, so these observations are in agreement with earlier studies reporting that the presence of carbon-based nanofillers influences the local environment of polymer chains and modifies their vibrational behavior through non-covalent interactions38,41.
UV-Visible spectroscopy
Absorbance spectra of PEO and doped GO
One of the most important techniques for interpreting and analyzing optical properties is the use of UV absorption. For this purpose, we used UV light in the range of 200 to 1100 nm. Initially, the PEO polymer showed a small abroad peak at 252 nm due to its very low absorption capacity. Based on molecular orbital theory, PEO might undergo electronic transition from unsaturated bonds and at 4.92 eV attributed to \(\:n\:\to\:\:{\pi\:}^{*}\) from carbonyl C–O bonds42. Initially, when the GO doping was 1%, we observed a slight change in absorption, indicating the presence of GO in the PEO polymer matrix, which consists of several oxygen-active sites such as epoxy, hydroxyl, and carboxyl groups increased the absorption capacity43. This is evidence that new energy levels have been created as a result of this interaction. The lack of motion of the highest wavelength indicates an interaction between the polymer and GO. This transition is attributed to \(\:n\:\to\:\:{\pi\:}^{*}\) in the oxygenated functional groups in GO. However, when the doping increased to 3%, the interaction between GO and PEO increased, further shifting the absorption peak. These electron transitions are caused by \(\:\pi\:\:\to\:\:{\pi\:}^{*}\) in the range of 264 nm, especially in conjugated aromatic systems44. More doping means more interactions. When the doping reaches 5%, it is more red-shifted. This may be due to localized electronic transitions45. At 443 nm and UV visible, a complete sharp band is formed when the doping is maximum 7%, all variations from UV-absorption can be seen in Fig. 5a.
This change in the structure of PEO makes its optical properties suitable for applications such as optical devices, UV sensors, and flexible electronics. This is because the transmittance is reduced. It makes the material highly resistant to UV light, through absorption and enhanced reflectivity. Their benefits include extending the life of devices with this material in their components and improving the durability of devices, and preventing the deterioration of electronic components. However, if it does not have a high responsiveness to UV light, it will have a high transmission capacity, causing degradation of the polymer and reducing its durability. As shown in Fig. 5b and c, the transmittance decreased, when the GO doping was the highest, the minimum transmittance at the wavelength of 452 nm was 18%, while for pure PEO the transmittance in this range was 88%. Through the application of this relationships, we calculated the reflectivity (R = 1-T-A), where T and A represents the transmittance and absorbance. Increased reflectivity makes the materials suitable for applications, for example by reducing the absorption of undesirable light, greater reflectivity may improve energy production in applications photodetectors, Light Emitting Diodes (LEDs), and solar panels46,47. It is also a great aid to thermal management due to thermal radiation, especially in coating applications48. PEO/CaTiO3 nanocomposite films exhibit significantly lower absorption and reflectivity compared to the results obtained in the present study42.
Absorption coefficients
Both crystalline and non-crystalline materials’ band gap energies and structures may be deduced from their optical absorption spectra. Absorption coefficient is one of the helpful optical characteristics. It may be thought of as measuring how much light a material can absorb. The incoming radiation fraction absorbed per thickness of absorbent was used to derive this49. Figure 6 shows the absorbance coefficient of many PEO films and PEO films doped with different amounts of GO. Applying the subsequent the formula, we performed the simple calculation of the absorption coefficient (α)50.
Here A is the absorbance and t are the sample thickness. The change in molecular configuration might be associated with the reason of an increase in (α) when the GO concentration increases. Put simply, it facilitated the transfer of charges inside complex systems involving metal ions, which includes the host polymer51. Table 1 shows absorption-edge values extracted by linear extrapolation to zero absorption. Increasing GO concentration from 1 to 7 wt% lowers the absorption edge from 5.63 to 2.27 eV. When the amount of dopant is increased, the absorption edge is lowered, which may be understood as better interchain interaction among the chains of the polymer composite. Conjugation stacking became denser as a result. In simpler terms, shifting the absorption edge has the potential to create novel effective trap levels inside the optical band gap. As a result, in these altered configurations, electrons slid from the highest valence band to the lowest conduction band52. The optical measurements obtained for PEO by Kumar et al.53 were comparable to the ones used in the present study.
Bandgap energy calculations
The bandgap, which defines the energy barrier between the conduction and valence bands, is one of the most basic features that determine the electrical and optical behavior of solids50. The bandgap size is the determining factor in how much energy a solid can take in light efficiently and is used to classify solids as insulators and semiconductors54. Optoelectronic components and photocatalytic processes are two examples of areas where materials’ light interactions are crucial. UV-vis measurements, together with the use of the so-called Tauc plot, have become a well-established and perhaps the most popular technique for calculating bandgap values55. In this case, the direct or indirect bandgap values are calculated by fitting the observed absorbance using simple power-law equations. The third Since UV-vis spectroscopy is both quick and easy to use, it offers immediate access to the bandgap of solid materials and allows for rapid examination of the material’s absorption behavior, in comparison to other analytical methods56.
B is a constant, h is Planck’s constant, and m is power factor. Based on the value of m, we can decide whether the electron transition is a direct 2 or an indirect 1/2 band gap, as shown in Fig. 7a and b. Indirectness is more likely to occur because in this case both the hole momentum and the electron change as the electron transitions from the valence band to the conduction band. This occurs because the symmetry required for direct electronic transitions is reduced in the absence of an adequately defined crystal structure. Having hybrid sp3 domains, GO can produce localized energy states within the band structure of the polymer matrix. These mid-gap states, which act as centers or recombination traps, are responsible for narrowing the band gap. There is a correlation between increasing GO content and an increasing number of structural disorders and defect states. As shown in the XRD section, the crystallinity decreased with increasing GO NPs. By allowing transitions with lower energy, these additional electronic levels gradually reduce the width of the band gap over the spectrum. Pure PEO exhibits a band gap energy of 5.82 eV at direct transmission and 5.56 eV at indirect transmission. However, at 7% doping, the band gap is 2.51 and 2.12 eV for direct and indirect, respectively, as shown in Table 1. This result agrees well with those which was found in previous studies, in which the lowest band gap energies were 3.8 and 2.2 eV for direct and indirect when Ag NPs were added to PEO57.
Refractive index characterization
The extinction coefficient (\(\:k)\) quantifies the percentage of light that is absorbed or scattered for every unit of distance along the penetration medium. We can use the relation to estimate it from the values of α and λ58.
The samples that were studied have their extinction coefficients shown against their energy levels in Fig. 8a. It shows that as the energy increases, so does the extinction coefficient. The fact that the extinction coefficient grows as the energy level rises indicates that the amount of light that is absorbed as well. As energy increases above 4 eV, the extinction coefficient drops, indicating a reduction in the percentage of light lost to absorption and scattering. One of the most important parameters of optical properties is the refractive index. Because it depends on other important parameters, such as the dielectric constant, density of the material, phase, and group velocity. For this reason, we calculated the refractive index (n) using the following law59.
As shown in Fig. 8b, GO can completely control the refractive index of PEO polymer through different concentrations. Raising the refractive index can increase the ability of the material to capture light. It can be used in applications such as solar cells and solar panels because it increases light absorption and improves efficiency by converting more light into electrical energy60. Most of the light is absorbed rather than transmitted or reflected, which gives better results. A high refractive index can improve the variability in sensor signals61. This improves responsiveness, making it suitable for optical communications. In most photovoltaics and sensors films are used, adjusting the optical pathways and interference patterns in stacks of thin films relies significantly on the refractive index62. The reactive index of the PEO and doped GO NPs, which is excessively doped, is higher than that of PEO polymer, as shown in Fig. 3b. It consists of many functional groups such as epoxy, hydroxyl, and carboxylic, which are polymerizable, and have \(\:\pi\:-\)electrons in the sp2 hybridization of carbon structures. When an external electric field is applied to these functional groups, they increase polarization. When the GO NPs is the highest, the refractive index is the highest. Because the density of the composite is higher, it interacts more with light. Therefore, when the amount of GO NPs is low or 1%, the polarization capacity is low. The main reasons for these changes can be attributed to several factors, including increased UV-Vis absorption, denser structure, decreased bandgap, hydrogen bonding, and interactions as shown in FTIR.
Dielectric properties
The optical characteristics and the electrical band structure are highly correlated63. A distinguishing feature of the optical characteristics of absorbing isotropic materials is a complicated dielectric function that depends on the wavelength (λ)64. Figuring out the real and imaginary parts of the dielectric function allows one to find important optical functions65. To be more precise, the electronic polarizability and electronic absorption of the material are linked to the real component \(\:{\epsilon\:}_{r}\left(w\right)\) and the imaginary component \(\:{\epsilon\:}_{i}\left(w\right)\) correspondingly66. In most cases, a thorough description of the optical characteristics may be achieved with knowledge of the complicated dielectric function:
How to store electrical energy when an external electric field is applied to the material and the polarizability, capacitance, and ability of the material to interact with light depends on the real part. When the amount of GO in the polymer is high, it increases the refractive index, density, and polarization of the material, making it suitable for applications such as filters, waveguides, and optical modulators. In applications such as solar cells and photovoltaic devices, it can improve the charge distance between electrons and holes. This is due to reduced recombination and conversion of more light into energy, as can be seen in Fig. 4a. Strong density of polarizable functional groups (such as –OH, –COOH, and epoxy) of GO will improve dipole polarization in the composite. More localized electronic states are brought in when GO concentration rises, hence improving electronic polarizability. These effects increase the dielectric constant’s imaginary and real parts related to energy loss or absorption and energy storage respectively. Pure PEO has a semi-crystalline structure; yet, the ordered arrangement of PEO chains is disrupted by GO nanosheets. By means of hydrogen bonding and van der Waals forces, GO interacts with the PEO matrix to disturb polymer chain alignment and crystalline domain growth. As the GO content rises, the polymer therefore achieves a more amorphous structure and reduces general crystallinity. Greater reorientation of dipoles in response to an external electric field is made possible in amorphous areas by the disordered and more flexible chains. This improved polarization raises the \(\:{\epsilon\:}_{r}\), therefore reflecting the capacity of the material to store electric energy. Particularly in cases of GO presence, amorphous areas bring disorder sites and localized electronic states. These imperfections enhance light absorption (more electronic transitions), hence increasing the \(\:{\epsilon\:}_{i}\), which is connected to energy loss. If we compare this result with the results of the effect of V2O5 on the PEO matrix, the highest real dielectric constant value has reached 7, which corresponds to this result. However, the \(\:{\epsilon\:}_{i}\) is higher because it reached \(\:10\times\:{10}^{-4}\) at a concentration of wt% V2O5, but in this study, the highest level is \(\:16\times\:{10}^{-4}\)18.
Direct band gap transitions and indirect band gap transitions both influence \(\:\epsilon\:(\omega\:\)), however the latter category is less significant than the former due to the mediation of phonons65. Previous studies showed that the optical band gap could not be reliably calculated using the KT and Taucs equations due to the large number of formulaic variables67. Modern methods for investigating optical band gaps, on the other hand, are more precise and easier to analyses data with, according to the available research67,68. We postulate that optical dielectric loss is an essential variable for precise optical band gap estimation. Recent developments in quantum physics and various models constructed for band gap research provide the basis of our hypothesis. From a quantum mechanical point of view, the photon-electron interaction within the system may be described by looking at dependent upon time perturbations of the ground electronic state.
The change from an occupied to an unoccupied state occurs as a result of photon absorption or emission. The form that follows may be used to represent the imaginary component \(\:{\epsilon\:}_{i}\left(\omega\:\right)\) of the dielectric function69:
The incident photon frequency is denoted as \(\:\left(\omega\:\right)\)69 the conduction band as C and the valence band as V, and the vectors u and k, which represent the incident electric field polarization and the reciprocal lattice vector, respectively, in the previous section. The spectrum linked to the \(\:{\varepsilon\:}_{i}\left(\omega\:\right)\:({\varepsilon\:}_{i}=2nk)\) is shown in Fig. 9b. Optical band gaps were determined by finding the points where the x-axis of Fig. 9’s linear components intersected. Bandgap energy findings value in this system are very close to bandgap energy in indirect bandgap energy. On this basis, we can confirm that the transition type is indirect. However, for pure PEO, because of the high crystallinity, the bandgap energy is roughly equivalent to the bandgap energy of the direct transition. So, this confirms that the system went to amorphous due to GO doping. When there is a higher concentration of GO, there is an increase in the number of structural disorder and defect states, as shown in XRD section. These newly discovered electronic levels make it possible for transitions to occur at lower energies, which further narrows the bandgap, increase polarization, and UV-vis absorption. PEO doped with tin titanate (SnTiO3) exhibits a much lower optical dielectric constant due to reduced absorption and a shift of the absorption spectrum toward the visible range, especially when compared with our results70.
Wemple DiDomenico model
To interpret and analyze the optical dispersion properties of the material, the Wemple DiDomenico (W-D) method is the main method. This model is based on the fact that the optical properties of materials can be represented by a single oscillator model, through both dispersion energy (\(\:{E}_{d}\)) and oscillator energy (\(\:{E}_{o}\)) parameters71. where the energy of the oscillator is related to the bandgap energy or the average energy required for electron transport. However, the scattered energy is related to the ability of the material to change the refractive index when exposed to a beam, or refers to the retention of optical transitions between bands. The correlation between n and the oscillator strength under the band gap in the low absorption region is articulated by the single oscillator model introduced by DiDomenico and Wemple72.
By recording the dependence of the refractive index-related variable \(\:{\left({n}^{2}-1\right)}^{-1}\) and the \(\:{\left(hv\right)}^{2}\), the parameters of the oscillator may be determined, as shown by Eq. (10). The value of (\(\:{{E}_{o}/E}_{d}\)) is shown by the Y-intercept of this straight-line Fig. 10a. As shown in Table 2, the value of the single oscillator energy decreased, which is due to the decrease in bandgap energy caused by the interaction between GO and PEO, due to the properly localized state and changes in the structure of the polymer. However, the dispersion energy increased with increasing GO doping, which is due to the increased polarization ability, as mentioned in the absorption section, that GO has a number of functional groups with high interaction potential73.
Based on the two equations we calculated the first and third optical moment (M−1 and M−3), through the use of the respective values of Ed and Eo. Due to the further increase in the price of Ed, the values of M−1 and M−3 have increased. The increase in the value of Ed in Eo and its further conversion is the main reason for the increase in M−1 and M−3 with increasing GO doping. This is due to the increased polarization capacity and changes compared to the bandgap energy. These results are entirely consistent with the results when V2O5 was added to the PEO matrix in different proportions18. In addition, the static refractive index \(\:n\left(0\right)\) and single oscillator energy (\(\:f)\) may be calculated using the equations that follows concerning the WDD model and given the values of the dispersion energies, \(\:{E}_{d}\) and \(\:{E}_{o}\)74.
Λ is the wavelength of the incident light. The (1/\(\:{S}_{o}\)) slope and \(\:(1/{S}_{o}{\lambda\:}_{o})\) intercept define the inverse linear connection between \(\:{\left({n}^{2}-1\right)}^{-1}\) and \(\:{\lambda\:}^{-2}\). Figure 10b shows how average single oscillator wavelength \(\:{\lambda\:}_{o}\), and average single oscillator strength \(\:{S}_{o}\) are related75.
Furthermore, by rewriting Eq. (15) in the manner that follows, the relevance of the previous components may have been inferred from a visual representation76.
\(\:{\lambda\:}_{o}\) had the lowest value when the GO doping reached the highest value of 7 W%, which may be due to several reasons including the occurrence of more electron delocalization in the SP2 of GO region. That is, electron transitions occurred at high energies, and there was a blueshift in the optical response of the material. The increase in \(\:{S}_{o}\) value is due to the increased level of interaction with light by the increase of GO in the PEO polymer. The enhanced S0 indicates increased polarization, electron delocalization and active dipole in conjugate carbon domains and functional groups GO.
Dispersion characterization
In order to further investigate the optical properties, we can determine some other important parameters by knowing the charge carrier concentration. For this purpose, we can find \(\:N/{m}^{*}\) by using the following Eq. 77.
Equation (23) is the relationship between the dielectric constant and the refractive index with the concentration of the substance. c is the speed of light and \(\:{\epsilon\:}_{0}\) is the absolute permittivity in vacuum. \(\:{\epsilon\:}_{\infty\:}\) is the intersection of the straight line with the Y-axis. Figure 11a represent the relationship between \(\:{\epsilon\:}_{r}\) and \(\:{\lambda\:}^{2}\).
We can write Eq. (23) as follows, and make connections with the plasma frequency \(\:{w}_{p}\)75.
Through calculating the \(\:{\epsilon\:}_{i}\) component with values of N/m*, the Drude free electron theory may readily estimate several more critical features, including plasma frequency (\(\:{w}_{p}\)), relaxation time (\(\:\tau\:\)), and optical resistivity (\(\:\rho\:\))77:
Based on the results in Fig. 11b between \(\:{\epsilon\:}_{i}\) and \(\:{\lambda\:}^{3}\), we can find the relaxation time. Then we can do optical mobility (\(\:{\mu\:}_{opt})\:\)and optical resistivity (\(\:{\rho\:}_{\text{o}\text{p}\text{t}})\:\)calculations through the following Eq78..
As shown in Table 3, the relaxation time increased because the GO consisted of a number of functional groups such as trap sites for the charge carriers. It makes recombination between charge carriers less likely. It increases the time spent in the excited state, increasing these functional groups increases the trap site and increases the relaxation time. Another point is the increased disorder in the composite’s structure. It reduces the order of the charge carriers. Increased polarizability enables the material to store more energy and interact more effectively with light. This makes the relaxation time and the return of the material to the equilibrium point take longer. When the density of the material increases due to GO doping, the number of charges increases, which makes them easier to transport, leading to increased delocalization due to the conjugated π-electron system. This is a major factor in the increase in optical mobility.
Non-linear optical characterization
Equation (32) is used to calculate the \(\:{\chi\:}^{\left(1\right)}\) and \(\:{\chi\:}^{\left(3\right)}\) values of the PEO and GO films that are described in Table 279.
Interactions between the dopants and the polymer may improve the local electric field, generate new energy levels, and raise \(\:{\chi\:}^{\left(3\right)}\)80. The nonlinear refractive index (\(\:{n}_{2}\)) is affected by the polarizability of the molecules, which is in turn affected by the chemical structure of the polymer molecules81. Equation (33) may be used to compute the \(\:{n}_{2}\), a critical parameter for determining the appropriate applications for the produced polymer films82.
Table 4 shows that the nonlinear refractive index (\(\:{n}_{2}\)) rose along with the polarizability of the polymer molecules due to the increasing concentration of GO. Adding GO to PEO matrix improves the materials’ linear and nonlinear refractive indices. Consequently, optoelectronic applications might greatly benefit from the produced films. Table 4 reports the usage of certain optical properties (\(\:{E}_{d}\), \(\:{E}_{i}\), \(\:{n}_{o}\), \(\:{\chi\:}^{\left(3\right)}\), and \(\:{n}_{2}\)) of PEO doped GO composite polymer films for comparison with prior research. Compared to earlier research, the value of \(\:{E}_{d}\) in this one is lower. The preference for PEO doped GO films for optoelectronic applications is shown by the fact that the values of \(\:{n}_{o}\), \(\:{\chi\:}^{\left(3\right)}\), and \(\:{n}_{2}\) are larger than those reported in prior research. For example, pristine and irradiated PEO/NiO nanostructured films \(\:{\chi\:}^{\left(3\right)}\) were in the range of 10–19 56. r-GO/120 mW, the value of \(\:{\chi\:}^{\left(3\right)}\) is \(\:-4.332\times\:\:{10}^{-9}\) and the value of n2 is \(\:1.12\:\times\:\:{10}^{-13}\)83. A promising and quickly developing area is the enhancement of polymer films’ nonlinear refractive index. This region is very promising for advancements in photonics, communication, and other areas because to the unusual mix of material features and different applications. It is an intriguing place to keep an eye on. Polymer films with enhanced optical characteristics for various uses may be created by enhancing the nonlinear refractive index. By directing more light into the solar cell’s active region, the high nonlinear refractive index could increase the cell’s efficiency. Nonlinear optical phenomena, like as second harmonic generation (SHG), become more pronounced as the nonlinear refractive index increases. Using this, one may be able to control the amount and angle of light entering the solar cell, which might increase the efficiency of light collecting and conversion. In addition, the carbon nanotubes (CNTs) included in the nanofillers have a light-scattering effect, which means that more light will reach the solar cell’s active layer and more photons will be absorbed. This has the potential to improve light harvesting as well. Metal complexing is one of many ways to improve the nonlinear optical properties of polymers, but the results are minimal. For example, Pb-MC in chitosan, \(\:{\chi\:}^{\left(3\right)}\) has been reduced to \(\:{10}^{-14}\)84. But in this paper, it is \(\:{10}^{-6}\). This proves the importance of this approach.
We reviewed several previous studies on PEO doped with composites and found that most reported bandgap energies remain relatively higher than the values obtained in our study. For instance, in prior literature, the bandgap energy of PEO-composites typically ranges from 3.2 to 4.73 eV depending on the concentration and dispersion of doping concentration, as shown in Table 5. However, in our study, the bandgap energy was significantly reduced, which indicates a stronger electronic interaction between the PEO matrix and the GO nanosheets. This reduction in bandgap is critical, as it facilitates enhanced charge transfer and optical absorption in the visible region, making the material more suitable for optoelectronic applications. Furthermore, our investigation revealed distinct nonlinear optical (NLO) behaviors in the PEO-GO composites, such as a higher nonlinear refractive index and third-order susceptibility (\(\:{\chi\:}^{\left(3\right)}\)) values. These properties were not prominently observed or reported in earlier studies. The presence of GO enhances local field effects and induces a more pronounced polarization response under high-intensity light, contributing to improve nonlinear optical characteristics. Therefore, the combination of reduced \(\:{E}_{g},\:{E}_{edge}\) and enhanced nonlinear optical properties in our samples suggests superior performance and applicability in optoelectronic devices, such as optical limiters, modulators, and photonic switches. These findings not only confirm the effectiveness of our material preparation approach but also demonstrate the novelty and potential of our PEO-GO composites compared to previous research.
Conclusion
In conclusions GO was successfully inserted into PEO matrix using a modified low-temperature Hummer’s method. The FTIR analysis further confirmed molecular interactions between PEO and GO, as evident from slight peak shifts and intensity changes in characteristic vibrational bands. No new peaks appeared, indicating no chemical bonding between GO and PEO. The interaction is mainly physical, involving hydrogen bonding and van der Waals forces. The structural analysis via XRD confirmed the presence of the characteristic (001) GO diffraction peak, indicating successful intercalation and uniform dispersion within the polymer matrix. Increased GO content led to reduced peak intensity, confirming decreased crystallinity. This is due to GO disrupting the regular packing of PEO chains. In the Raman spectroscopy, the D and G bands of GO remained, while PEO bands diminished, confirming physical incorporation of GO without covalent functionalization. Transmittance decreased notably with higher GO content, indicating strong UV-blocking capability. The optical bandgap narrowed from 5.82 eV (pure PEO) to 2.12 eV (7 wt% GO), confirming enhanced light absorption. The increase in the extinction coefficient and refractive index, and significant enhancement in third-order susceptibility (χ³) and nonlinear refractive index (n₂) was observed reaching 30 × 10⁻⁶ and 5.2 × 10⁻⁵, respectively, at 7 wt% GO. These improvements confirm the suitability of GO–PEO composites for use in optoelectronic and nonlinear optical devices, indicates that the incorporation of GO substantially improved the optical density and polarizability of the nanocomposites. The Drude model analysis revealed that carrier concentration, optical conductivity, and plasma frequency increased with GO content, indicating enhanced charge transport and segmental motion. These tunable structural, optical, and electronic properties make PEO/GO nanocomposites excellent candidates for optoelectronic applications such as flexible photonic devices, UV filters, and transparent conductive films. The ability to tailor these properties via GO concentration offers a scalable and cost-effective route for advanced multifunctional materials.
Data availability
Data will be available from the corresponding author upon request.
References
Ghosh, T. N., Pradhan, S. S., Sarkar, S. K. & Bhunia, A. K. On the incorporation of the various reduced graphene oxide into Poly (vinyl alcohol) nano-compositions: comparative study of the optical, structural properties and magnetodielectric effect. J. Mater. Sci.: Mater. Electron. 32, 19157–19178 (2021).
Pendolino, F. & Armata, N. Graphene oxide in environmental remediation process. (2017).
Dimiev, A., Kosynkin, D. V., Alemany, L. B., Chaguine, P. & Tour, J. M. Pristine graphite oxide. J. Am. Chem. Soc. 134, 2815–2822 (2012).
Ahmad, R. T. M., Hong, S. H., Shen, T. Z. & Song, J. K. Water-assisted stable dispersal of graphene oxide in non-dispersible solvents and skin formation on the GO dispersion. Carbon 98, 188–194 (2016).
Wadhwa, H., Kandhol, G., Deshpande, U. P., Mahendia, S. & Kumar, S. Thermal stability and dielectric relaxation behavior of in situ prepared Poly (vinyl alcohol)(PVA)-reduced graphene oxide (RGO) composites. Colloid Polym. Sci. 298, 1319–1333 (2020).
Akbaş, A. M., Tataroğlu, A., Altındal, Ş. & Azizian-Kalandaragh, Y. Frequency dependence of the dielectric properties of Au/(NG: PVP)/n-Si structures. J. Mater. Sci.: Mater. Electron. 32, 7657–7670 (2021).
Al-Muntaser, A., Alzahrani, E., Asnag, G. & Yassin, A. Tailoring structural, optical, and dielectric properties of pvc/pmma/ps/zno nanocomposites for capacitive energy storage applications. ECS J. Solid State Sci. Technol. 14, 033001 (2025).
Liang, J. et al. Infrared-triggered actuators from graphene-based nanocomposites. J. Phys. Chem. C. 113, 9921–9927 (2009).
Kadhim, M. A. & Al-Bermany, E. New fabricated PMMA-PVA/graphene oxide nanocomposites: structure, optical properties and application. J. Compos. Mater. 55, 2793–2806 (2021).
Al-Muntaser, A. et al. Investigation of structural and optical characteristics of pva/crystal Violet dye composites for flexible smart optoelectronic applications. J. Polym. Res. 31, 311 (2024).
Yassin, A., Abdelrazek, E., Abdelghany, A. & Abdallah, E. Incorporated au/se nanoparticles into HPMC/CMC blend for enhancing structural, optical and morphological properties: hybrid nanocomposites for optoelectronic applications. Opt. Mater. 154, 115721 (2024).
Dhayal, V. et al. Optical and electrical properties of biocompatible and novel (CS–GO) polymer nanocomposites. Opt. Quant. Electron. 53, 1–13 (2021).
Abdelamir, A. I. & Al-Bermany, E. & Sh Hashim, F. in Journal of Physics: Conference Series. 022029 (IOP Publishing).
Suresh, I. Synthesis, characterization and optical properties of graphene oxide–polystyrene nanocomposites. Polym. Adv. Technol. 26, 214–222 (2015).
Taha, T. & Saleh, A. Dynamic mechanical and optical characterization of pvc/fgo polymer nanocomposites. Appl. Phys. A. 124, 600 (2018).
Koduru, H. et al. Characterization of PEO/PVP/GO nanocomposite solid polymer electrolyte membranes: microstructural, thermo-mechanical, and conductivity properties. Ionics 24, 3459–3473 (2018).
Bora, C. & Dolui, S. Fabrication of polypyrrole/graphene oxide nanocomposites by liquid/liquid interfacial polymerization and evaluation of their optical, electrical and electrochemical properties. Polymer 53, 923–932 (2012).
Khasraw SS, Mamand DM, Saeed SR, Hama PO, Hassanzadeh A, Aziz DM, Mohammed PA, Abdulwahid RT, Aziz SB. PEO based nanocomposite with improved structural and optical properties. Journal of Inorganic and Organometallic Polymers and Materials., 1–27 (2025).
Dhatarwal, P. & Sengwa, R. Nanofiller controllable optical parameters and improved thermal properties of (PVP/PEO)/Al2O3 and (PVP/PEO)/SiO2 nanocomposites. Optik 233, 166594 (2021).
Saeed, A. et al. Enhanced optical and electrical properties of PEO/PMMA/TiO2 nanocomposites for optoelectronic applications. Opt. Mater. 157, 116402 (2024).
Al-Muntaser, A. et al. Structural, morphological, optical, electrical and dielectric features based on nanoceramic Li4Ti5O12 filler reinforced PEO/PVP blend for optoelectronic and energy storage devices. Ceram. Int. 49, 18322–18333 (2023).
AlSulami, F. M. H. et al. Structural, optical, dielectric and electrical properties of flexible nanocomposite based on naalg/peo composite and AgNO3 NPs for energy storage applications and optoelectronic devices. Opt. Mater. 152, 115515 (2024).
Samir, M. A. S. A., Alloin, F., Sanchez, J. Y. & Dufresne, A. Cellulose nanocrystals reinforced Poly (oxyethylene). Polymer 45, 4149–4157 (2004).
Samir, M. A. et al. POE-based nanocomposite polymer electrolytes reinforced with cellulose whiskers. Electrochim. Acta. 50, 3897–3903 (2005).
Zhang, H., Xia, H. & Zhao, Y. Optically triggered and spatially controllable shape-memory polymer–gold nanoparticle composite materials. J. Mater. Chem. 22, 845–849 (2012).
Kim, J., Kim, B., Kim, E., Park, H. & Jeong, H. Synthesis and shape memory performance of polyurethane/graphene nanocomposites. Reactive Funct. Polym. 74, 16–21 (2014).
Hanash, F. et al. Study of optical, thermal, electrical, and impedance properties of Li4Ti5O12-Based PEO/SA biopolymer blend electrolytes for Lithium-Ion batteries. ECS J. Solid State Sci. Technol. 14, 023011 (2025).
Saber, S., El-Sayed, S. & El Sayed, A. M. Influence of Eu3 + on the structural, optical and electrical properties of PEO–PVA: dual bandgap materials for optoelectronic applications. J. Mater. Sci.: Mater. Electron. 34, 406 (2023).
Al-Shamari, A., Abdelghany, A., Alnattar, H. & Oraby, A. Structural and optical properties of PEO/CMC polymer blend modified with gold nanoparticles synthesized by laser ablation in water. J. Mater. Res. Technol. 12, 1597–1605 (2021).
Noor, S., Ahmad, A., Talib, I. & Rahman, M. Y. Morphology, chemical interaction, and conductivity of a PEO-ENR50 based on solid polymer electrolyte. Ionics 16, 161–170 (2010).
Kumar, N. A., Gambarelli, S., Duclairoir, F., Bidan, G. & Dubois, L. Synthesis of high quality reduced graphene oxide nanosheets free of paramagnetic metallic impurities. J. Mater. Chem. A. 1, 2789–2794 (2013).
Huang, Y. et al. Preparation and swelling properties of graphene oxide/poly (acrylic acid-co-acrylamide) super-absorbent hydrogel nanocomposites. Colloids Surf., A. 401, 97–106 (2012).
Ali, H. E. A novel optical limiter and UV–Visible filters made of Poly (vinyl alcohol)/KMnO4 polymeric films on glass-based substrate. J. Mater. Sci.: Mater. Electron. 30, 7043–7053 (2019).
Abdullah, O. G., Aziz, S. B. & Rasheed, M. A. Structural and optical characterization of PVA: KMnO4 based solid polymer electrolyte. Results Phys. 6, 1103–1108 (2016).
Polu, A. R. & Rhee, H. W. The effects of LiTDI salt and POSS-PEG (n = 4) hybrid nanoparticles on crystallinity and ionic conductivity of PEO based solid polymer electrolytes. Sci. Adv. Mater. 8, 931–940 (2016).
Azli, A., Manan, N. & Kadir, M. Conductivity and Dielectric Studies of Lithium Trifluoromethanesulfonate Doped Polyethylene Oxide-Graphene Oxide Blend Based Electrolytes. Advances in Materials Science and Engineering 145735 (2015). (2015).
Yassin, A., Mohamed, A. R., Abdelghany, A. & Abdelrazek, E. Enhancement of dielectric properties and AC electrical conductivity of nanocomposite using Poly (vinyl chloride-co-vinyl acetate-co-2-hydroxypropyl acrylate) filled with graphene oxide. J. Mater. Sci.: Mater. Electron. 29, 15931–15945 (2018).
Guo, P., Song, H. & Chen, X. Electrochemical performance of graphene nanosheets as anode material for lithium-ion batteries. Electrochem. Commun. 11, 1320–1324 (2009).
Dresselhaus, M., Dresselhaus, G., Jorio, A., Souza Filho, A. & Saito, R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 40, 2043–2061 (2002).
Dresselhaus, M. & Eklund, P. Phonons in carbon nanotubes. Adv. Phys. 49, 705–814 (2000).
Chen, S. et al. Interface manipulation of composite solid polymer electrolyte with polydopamine to construct durable and fast Li + conduction pathways. ACS Appl. Energy Mater. 6, 1989–2000 (2023).
Aziz, S. B. et al. Characteristics of PEO incorporated with CaTiO3 nanoparticles: structural and optical properties. Polymers 13, 3484 (2021).
Alexa, A., Rahnenführer, J. & Lengauer, T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22, 1600–1607 (2006).
Rajoba, S. J., Sartale, S. D. & Jadhav, L. D. Investigating functional groups in GO and r-GO through spectroscopic tools and effect on optical properties. Optik 175, 312–318 (2018).
Koduru, H. K. et al. in Journal of Physics: Conference Series. 012006 (IOP Publishing).
Dai, B. et al. Piezo-phototronic effect on photocatalysis, solar cells, photodetectors and light-emitting diodes. Chem. Soc. Rev. 50, 13646–13691 (2021).
Wang, W. & Qi, L. Light management with patterned micro-and nanostructure arrays for photocatalysis, photovoltaics, and optoelectronic and optical devices. Adv. Funct. Mater. 29, 1807275 (2019).
Wang, X. et al. Thermal control property and corrosion resistance of PEO coatings on AZ91 Mg alloy. Surf. Coat. Technol. 393, 125709 (2020).
Fox, M. Optical Properties of SolidsVol. 3 (Oxford University Press, 2010).
Klein, J. et al. Limitations of the Tauc plot method. Adv. Funct. Mater. 33, 2304523 (2023).
Muhammad, F. F., Aziz, S. B. & Hussein, S. A. Effect of the Dopant salt on the optical parameters of PVA: nano 3 solid polymer electrolyte. J. Mater. Sci.: Mater. Electron. 26, 521–529 (2015).
Aziz, S. B., Rasheed, M. A. & Abidin, Z. H. Optical and electrical characteristics of silver ion conducting nanocomposite solid polymer electrolytes based on Chitosan. J. Electron. Mater. 46, 6119–6130 (2017).
Kumar, K. K. et al. Investigations on the effect of complexation of NaF salt with polymer blend (PEO/PVP) electrolytes on ionic conductivity and optical energy band gaps. Phys. B: Condens. Matter. 406, 1706–1712 (2011).
Grundmann, M. Physics of SemiconductorsVol. 11 (Springer, 2010).
Scholz, F. Compound Semiconductors: Physics, Technology, and Device Concepts (Jenny Stanford Publishing, 2017).
Althubiti, N., Atta, A., Al-Harbi, N., Sendi, R. K. & Abdelhamied, M. Structural, characterization and linear/nonlinear optical properties of oxygen beam irradiated peo/nio composite films. Opt. Quant. Electron. 55, 348 (2023).
Aziz, S. B. et al. Structural, thermal, morphological and optical properties of PEO filled with biosynthesized ag nanoparticles: new insights to band gap study. Results Phys. 13, 102220 (2019).
Naccarato, F. et al. Searching for materials with high refractive index and wide band gap: A first-principles high-throughput study. Phys. Rev. Mater. 3, 044602 (2019).
Aziz, S. B. Modifying Poly (vinyl alcohol)(PVA) from insulator to small-bandgap Polymer: A novel approach for organic solar cells and optoelectronic devices. J. Electron. Mater. 45, 736–745 (2016).
Carron, R. et al. Refractive indices of layers and optical simulations of Cu (In, Ga) Se2 solar cells. Sci. Technol. Adv. Mater. 19, 396–410 (2018).
El Amrani, A. et al. Determination of the suitable refractive index of solar cells silicon nitride. Superlattices Microstruct. 73, 224–231 (2014).
Al-Ghamdi, A. et al. Effects of laser irradiation on optical properties of amorphous and annealed Ga15Se81In4 and Ga15Se79In6 chalcogenide thin films. J. Alloys Compd. 505, 229–234 (2010).
Taib, M., Yaakob, M., Hassan, O. & Yahya, M. in IEEE Symposium on Business, Engineering and Industrial Applications. 13–17 (IEEE). 13–17 (IEEE). (2012).
Kymakis, E. & Amaratunga, G. A. Optical properties of polymer-nanotube composites. Synth. Met. 142, 161–167 (2004).
Cui-E, H., Zhao-Yi, Z., Yan, C., Xiang-Rong, C. & Ling-Cang, C. First-principles calculations for electronic, optical and thermodynamic properties of ZnS. Chin. Phys. B. 17, 3867 (2008).
Nasr, T. B., Maghraoui-Meherzi, H., Abdallah, H. B. & Bennaceur, R. First principles calculations of electronic and optical properties of Ag2S. Solid State Sci. 26, 65–71 (2013).
Thutupalli, G. & Tomlin, S. The optical properties of thin films of cadmium and zinc Selenides and tellurides. J. Phys. D. 9, 1639 (1976).
Srivastava, N. & Tiwari, T. New trends in polymer electrolytes: a review. e-Polymers 9, 146 (2009).
Li, L. et al. First principles calculations of electronic band structure and optical properties of Cr-doped ZnO. J. Phys. Chem. C. 113, 8460–8464 (2009).
Muhammed, D. S. et al. Optical dielectric loss as a novel approach to specify the types of electron transition: XRD and UV-vis as a non-destructive techniques for structural and optical characterization of PEO based nanocomposites. Materials 13, 2979 (2020).
Hassanien, A. S. & Akl, A. A. Influence of composition on optical and dispersion parameters of thermally evaporated non-crystalline Cd50S50 – xSex thin films. J. Alloys Compd. 648, 280–290 (2015).
Aly, K. A. On the study of the optical constants for different compositions of Sn x (GeSe) 100 – x thin films in terms of the electronic polarizability, electronegativity and bulk modulus. Appl. Phys. A. 120, 293–299 (2015).
Mamand, D. M., Hussen, S. A. & Aziz, S. B. Green approach to synthesis polymer composites based on Chitosan with desired linear and non-linear optical characteristics. Sci. Rep. 15, 3130 (2025).
Khan, T. M. & BiBi, T. Compatibility and optoelectronic of ZnSe nano crystalline thin film. Chin. Phys. B. 21, 097303 (2012).
Alzaid, M., Qasem, A., Shaaban, E. & Hadia, N. Extraction of thickness, linear and nonlinear optical parameters of Ge20 + xSe80-x thin films at normal and slightly inclined light for optoelectronic devices. Opt. Mater. 110, 110539 (2020).
Shehap AM, Akil DS. Structural and optical properties of TiO 2 nanoparticles/PVA for different composites thin films. International Journal of Nanoelectronics & Materials. 9 (1), (2016).
Abdel-Baki, M., Wahab, F. A. A. & El-Diasty, F. Optical characterization of xTiO2–(60 – x) SiO2–40Na2O glasses: I. Linear and nonlinear dispersion properties. Mater. Chem. Phys. 96, 201–210 (2006).
Hassanien, A. S., Sharma, I. & Sharma, P. Optical and dispersion studies of thin S35-xGe15SnxTe50 films: assessment of some physical parameters of samples. Phys. Scr. 98, 045911 (2023).
Keru, G., Ndungu, P. G. & Nyamori, V. O. A review on carbon nanotube/polymer composites for organic solar cells. Int. J. Energy Res. 38, 1635–1653 (2014).
Divya, R., Manikandan, N., Girisun, T. S. & Vinitha, G. Investigations on the structural, morphological, linear and third order nonlinear optical properties of manganese doped zinc Selenide nanoparticles for optical limiting application. Opt. Mater. 100, 109641 (2020).
Innocenzi, P. & Lebeau, B. Organic–inorganic hybrid materials for non-linear optics. J. Mater. Chem. 15, 3821–3831 (2005).
Bredas, J. L., Adant, C., Tackx, P., Persoons, A. & Pierce, B. Third-order nonlinear optical response in organic materials: theoretical and experimental aspects. Chem. Rev. 94, 243–278 (1994).
Tao, Y., Zhang, X., Wang, H., Nie, Z. & Pan, D. Tuning electrical conductivity and ultrafast optical nonlinearity of Reduced-GO films ablated by femtosecond laser direct writing. Molecules 30, 348 (2025).
Mamand DM, Hussen SA, Aziz SB. Drude-Lorentz classical oscillator model and Tauc’s approach to study the localized density of states and energy band gap of polymer composites based on chitosan integrated with green synthesized Pb-metal complexes: Improvement of linear and non-linear optical parameters. International Journal of Biological Macromolecules. 143978 (2025).
Hameed, T. A., Mohamed, F., Abdelghany, A. & Turky, G. Influence of SiO2 nanoparticles on morphology, optical, and conductivity properties of Poly (ethylene oxide). J. Mater. Sci.: Mater. Electron. 31, 10422–10436 (2020).
Alzahrani, H. S., Al-Sulami, A. I., Alsulami, Q. A. & Rajeh, A. A systematic study of structural, conductivity, linear, and nonlinear optical properties of PEO/PVA-MWCNTs/ZnO nanocomposites films for optoelectronic applications. Opt. Mater. 133, 112900 (2022).
Aziz, S. B. et al. Optical properties of pure and doped PVA: PEO based solid polymer blend electrolytes: two methods for band gap study. J. Mater. Sci.: Mater. Electron. 28, 7473–7479 (2017).
Sengwa, R. J., Choudhary, S. & Dhatarwal, P. Nonlinear optical and dielectric properties of TiO2 nanoparticles incorporated PEO/PVP blend matrix based multifunctional polymer nanocomposites. J. Mater. Sci.: Mater. Electron. 30, 12275–12294 (2019).
Menazea, A. One-Pot pulsed laser ablation route assisted copper oxide nanoparticles doped in PEO/PVP blend for the electrical conductivity enhancement. J. Mater. Res. Technol. 9, 2412–2422 (2020).
Acknowledgements
The authors gratefully acknowledge financial support for this study from University of Sulaimani, University of Charmo, and the University of Raparin. The authors also express their gratitude to the support of Princess Nourah bint Abdulrahman University (Saudi Arabia) through the University Researchers Supporting Project number PNURSP2025R58.
Author information
Authors and Affiliations
Contributions
“D. M. Mamand, D. M. Aziz, S.S. Khasraw, A.G.S. AlAzzawi, and S. I. AlSaeedi, conducted the experiments, performed data analysis, and contributed to the initial draft of the manuscript. S. B. Aziz and J. Hassan were responsible for administration, supervision, and improvement of the manuscript. All authors reviewed the manuscript.”
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mamand, D.M., Aziz, D.M., Khasraw, S.S. et al. Improved optical characteristics of PEO polymer integrated with graphene oxide. Sci Rep 15, 32225 (2025). https://doi.org/10.1038/s41598-025-16778-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16778-9
Keywords
This article is cited by
-
Comprehensive enhancement of PVC nanocomposites through Al2O3 for advanced optoelectronics
Scientific Reports (2026)
-
Interconnected and Layered Morphological Structure of Polycarbazole-Vanadium Pentoxide Nanocomposites for the Application of OLED as ETL Material
Journal of Inorganic and Organometallic Polymers and Materials (2025)