Abstract
Salinity is a significant challenge for grapevines growing in arid and semiarid regions. In this study, ‘Castellana Negra’ plants were subjected to NaCl stress (90 mM) for 75 days and treated with silicon (Si) and proline to evaluate their potential in mitigating the effects of salinity. Si was administered through irrigation, while proline was applied as a foliar spray. Analysis of the results (P < 0.05) indicated that salt stress reduced leaf fresh weight (75%), but Si and proline limited this reduction (57% and 54%, respectively, compared to control). Also, Si improved total chlorophyll (chl t, 46%) and carotenoids (car, 49%), while proline increased chl t (72%) and car (52%), compared to their levels in only NaCl-stressed plants. Additionally, salt stress increased abscisic acid (ABA) in 39%, but Si maintained its levels, and proline reduced it (42%) in comparison to control. Similar trends were observed for salicylic acid and jasmonic acid, where Si and proline helped normalize their levels. Moreover, salt exposure raised Na+ concentrations; however, Si and proline reduced this accumulation. Overall, data show that Si and proline can regulate hormonal, nutritional and physiological processes, and may be considered promising tools for enhancing grapevine resilience to salinity.
Similar content being viewed by others
Introduction
Salinity is one of the most significant environmental threats for agricultural productivity worldwide, particularly in arid and semi-arid regions. The higher salt ion concentrations disrupt plant growth and development, leading to a decline in both crop yield and quality1. Thereby, the excess of salt in the soil solution alters the osmotic balance between plant roots and the soil, creating an osmotic stress like drought and an ionic toxicity stress2. In this context, grapevine plants subjected to salt stress have been reported to prove leaf necrosis, reduced photosynthesis and chlorophyll fluorescence, lower chlorophyll content and Rubisco activity, as well as disturbed Na+ and K+ balance3.
To cope with salinity impact, plants activate salt stress signaling pathways to mitigate the harmful effects of excess Na+ and other ions. In this sense, the presence of Ca2+, phytohormones, such as abscisic acid (ABA), ethylene, and salicylic acid (SA), triggers a cascade of signal transduction reactions, helping the plant adaptation to saline conditions4. Thus, grapevine genotypes that exhibit greater tolerance to salt stress have been shown to accumulate higher levels of ABA under such conditions5. Additionally, grapevines that accumulate higher amounts of proline and soluble sugars are reported to exhibit reduced growth inhibition and increased foliar concentrations of chlorophyll and carotenoids6.
ABA has been involved in the regulation of numerous physiological processes, including seed maturation, bud dormancy, growth, leaf senescence and subsequent abscission, net photosynthetic rate, gene expression, and triggering adaptive responses against salinity, drought, and temperature stresses7,8. In grapevines, root ABA synthesis in response to water stress and its transport through the xylem to the leaves, or stress-induced ABA mobilization within the leaf, play a key role in regulating the stomatal response9. In the same way, the exogenous application of ABA enhances plant growth in grapevines, promoting the increase in leaf area, leaf number and shoot biomass. As well, this treatment increases the levels of proline, soluble sugars, and the antioxidant activity10.
On the other hand, SA acts as a signaling molecule in plants, affecting responses to salinity by regulating growth, photosynthesis, and ion uptake11,12. Particularly, in grapevines, SA can help counteract the adverse effects of salinity by reducing the accumulation of toxic ions and enhancing the uptake of essential nutrients13. Moreover, it has been shown that the application of SA mitigates the adverse impacts of salt stress on photosynthesis and growth. This improvement is induced by enhanced accumulation of glycine betaine and methionine, reduced ethylene production, and a strengthened antioxidant defense system14. Furthermore, exogenous SA increases plant tolerance by enhancing photosynthesis, proline, soluble sugars, antioxidant system, and gene transcription15.
In the same way, jasmonic acid (JA) has been involved in plant responses to salinity16,17. Additionally, exogenous JA application mitigates the salt injuries in seedling development and photosynthetic activity18, and significantly decreases Na+ content19.
Although the roles of ABA, SA, and JA in plant responses to salinity are well-documented, the involvement of indole-3-acetic acid (IAA) remains less understood, with limited studies addressing this aspect. Research has shown that exogenous IAA application increases sugar content in grape berries20. Exogenous IAA mitigated the harmful effects of salinity on strawberry seedlings by enhancing Na+ flux, reducing Na+ accumulation to maintain ion balance, protecting root growth, and improving nutrient uptake, ultimately improving photosynthetic efficiency21. Foliar application of IAA overcomes the decline in growth rate and yield caused by salinity, regulated foliar proline and glycine betaine levels, and enhanced antioxidant enzyme activities under salt stress22.
To alleviate the effects of salinity on crop development and production, the role of Si in enhancing plant responses to salt stress has been widely studied in various species, including grapevines23. For instance, the application of Si through foliar spray decreases Na+ absorption and content in shoots in shoots of tomato plants against salt stress24. In this context, the role of Si in mitigating salt stress involves several mechanisms. Likewise, as shown by Zuccarini25, Si reduces the apoplastic movement of Na+ from roots to shoots, thereby promoting a higher K+/Na+ ratio, which is beneficial for plant health under salt stress conditions. Furthermore, the decline of Na+ absorption and the preservation of optimum K+/Na+ ratio induced by Si plays an important role in plant salt tolerance26.
Regarding the involvement of proline in plant responses to abiotic stress, it is well-established that plants accumulate proline as a compatible osmolyte in the cytoplasm to maintain pH, membrane integrity, protein structure, antioxidant enzyme activity, and water status. Proline also regulates cellular redox balance and osmotic pressure in response to salt and other abiotic stresses27,28,29. In grapevines, the application of proline enhances its endogenous concentration and the superoxide dismutase (SOD) activity in response to salt stress30. Si application has also been shown to decline proline content in grapevine rootstocks31. In addition, Gou et al.32 show that integrative application of silicon and/or proline markedly improves the growth and yield of Sweet corn under salinity, along with increases in photosynthetic pigments, free proline, K+/Na+ ratio, and the antioxidant activities among others, thereby suggesting their potential role in increasing the acclimation of this species to saline soil conditions.
It is currently evident that the impact of salinity on plants is primarily due to an excess of salt ions in the soil solution, which disturbs the balance of essential mineral elements needed for optimal plant metabolism. In this context, K+ is the key inorganic solute that regulates guard cell turgor and stomatal opening. Thus, adequate K+ levels are crucial for its quick movement between the apoplast, cytosol, and vacuoles, enabling effective stomatal control33. Salinity also decreases K+ and Ca2+ uptake due to increased Na+ levels34,35, but Si can counteract this effect by enhancing K+ and Ca2+36. In grapevine ‘Cabernet Sauvignon’ young plants, salt stress raises leaf Na+, however, the addition of Si decreases its level23. In the same way, Si induces the sequestration of Na+ in the vacuoles, regulating its accumulation in the chloroplasts37. Furthermore, the supplementation of Si enhances K+/Na+ ratio, ionic concentration, and nutrient balance38. Regarding the effect of Si on mineral nutrient accumulation under salinity conditions, numerous experiments reported an increase of K+, Ca2+, Mg2+, Fe2+, Mn2+, Zn2+, Cu2+, and B induced by Si in plants exposed to salinity stress39,40,41,42.
Taken together, grapevines are frequently exposed to environmental stresses that hinder their growth and development. Moreover, osmotic stress, high salinity, and nutrient imbalances can lead to increased production of ROS, which accelerate plant senescence and reduce both photosynthesis and growth43. In addition, salinity disrupts several physiological processes, including phytohormone biosynthesis, accumulation of compatible solutes, and production of photosynthetic pigments, among others. In this sense, the response of grapevines to salt stress can vary depending on the genotype, as well as the intensity and duration of exposure. Likewise, to improve the tolerance of grapevine to salinity, exogenous chemical treatments are assessed and satisfactory results obtained.
Therefore, the objective of this study was to assess the effects of salt stress (90 mM NaCl) applied over a 75-day period on young ‘Castellana Negra’ grapevine plants, focusing on morphological, physiological, nutritional, and hormonal responses. Additionally, this research aimed to elucidate the effectiveness of exogenous Si and proline in mitigating salt stress in this genotype, with an especial emphasis on phytohormones, mineral nutrient dynamics, and key physiological attributes related to stress resilience.
Materials and methods
Plant material and growth conditions
Grapevine ‘Castellana Negra’ (‘CasNeg’) plants were reproduced from cuttings collected from pruned shoots in field-grown vineyards located in the University of La Laguna at an altitude of 578.26 m above sea level, with coordinates 28º 28’ 39.02’’ N latitude and 16º 19’ 19’’ W longitude. Initially, these cuttings were put in 1 L plastic bags to stimulate rooting and sprouting, then transferred to 5 L plastic pots filled with a substrate (Profi-Substrat, Gramoflor, Valencia, Spain,) in a greenhouse setting. To enrich the soil, each pot received 60 g of granular fertilizer (ProTurf®, N, P, K fertilizer with Ca and Mg: 12-5-20 + 2 CaO + MgO) 15 days before the experiment began. When the plants reached one-year-old, they were pruned to retain 2–3 buds on each of the two shoots per plant. The experiment ran for 75 days, with environmental conditions monitored throughout: temperatures averaged between 15 and 32 °C, relative humidity ranged from 55–85%, and the maximum photosynthetically active radiation (PAR) reached approximately 1200 µmol m⁻² s⁻¹.
Salinity stress and chemical applications
In the experimental system, plants were randomly distributed into four blocks, each comprising all four treatments, resulting in 16 plants per treatment and four biological replicates per treatment group. The treatments were applied as follows: (a) control, plants watered with fresh water; (b) NaCl stress, plants irrigated with a water solution containing 90 mM NaCl; (c) NaCl stress + silicon (Si, applied as calcium silicate via irrigation), plants irrigated with a water solution containing 90 mM NaCl plus Si (10 mM) for four weeks (one treatment of Si a week, 1 L per plant) from the start of the experiment; and (d) NaCl stress + proline (10 mM), plants irrigated with a water solution containing 90 mM NaCl plus proline (applied as a foliar spray) for four weeks (one treatment of proline a week) from the beginning of the experiment. Proline was dissolved in a 5% (v/v) water solution, and to improve thorough wetting of the leaves and achieve optimal foliar coverage, few drops of 0.05% Tween-20 was added to each mixture. Additionally, control and NaCl-stressed plants (treatments b and c) were sprayed in parallel with distilled water containing 0.05% Tween-20 when proline was applied (treatment d). Irrigation of all the plants with saline and non-saline water was carried out by providing each plant with 1 L of water, three times weekly, over a period of 75 days. Si (calcium silicate, 12–22% CaO and ≥ 87% SiO2 basis) and proline were purchased from Sigma-Aldrich, Madrid, Spain.
Growth and sample collection
To evaluate plant growth, the fresh weight of all functional leaves per plant was measured at the conclusion of the trial, 75 days after the onset of treatments (DAT). Following this, leaf samples from at least four plants per treatment were frozen in liquid nitrogen, freeze-dried, or stored at −80 °C, for further analysis.
Analyses of photosynthetic pigments
To 0.05 g (m0) of powdered frozen leaf tissue, 5 mL (V0) of an 8:2 acetone/water solution was added. This mixture was then homogenized using a T 25 digital Ultra-Turrax (IKA-Werke, Staufen, Germany) homogenizer set at 10,000 rpm for 1 mn. Subsequently, the homogenate was centrifuged at 4500 rpm and 4 °C for 30 mn to separate phases. The resulting supernatants were carefully transferred into spectrophotometer cuvettes, and absorbance measurements were recorded at wavelengths of 663, 647, and 470 nm. Using adapted equations according to Lichtenthaler and Buschmann44, concentrations of chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophylls (Chl t), and carotenoids (Car) were calculated:
Chl a (mg·g−1 DW) = (12.25·Aλ = 663 nm – 2.79·Aλ = 647 nm)·V0/(m0·1000).
Chl b (mg·g−1 DW) = (21.50·Aλ = 647 nm – 5.10·Aλ = 663 nm)·V0/(m0·1000).
Chl t (mg·g−1 DW) = Chl a + Chl b.
Car (mg·g−1 DW) = (5.05·Aλ = 470 nm + 2.08·Aλ = 663 nm – 9.21·Aλ = 647 nm)·V0/(m0·1000).
Proline determination
A 0.05 g sample of dry, ground leaf tissue (DW) was extracted with 5 mL of 3% w/v sulfosalicylic acid (Sigma-Aldrich, Madrid, Spain) and homogenized using a T 25 digital Ultra-Turrax (IKA-Werke, Staufen, Germany). The homogenized mixture was then centrifuged at 4,500 rpm and 4 °C for 45 mn to separate the supernatant, from which proline content was analyzed according to Bates et al.45. For this, 1 mL of the supernatant was mixed with 2 mL of glacial acetic acid and ninhydrin reagent (Sigma-Aldrich, Madrid, Spain) in a 1:1 (v/v) ratio. The reaction was conducted in a 100 °C water bath for 1 h and subsequently cooled on ice for 15 mn. Absorbance was measured in the organic phase at 520 nm using a Genova Plus Spectrophotometer (Jenway, Bibby Scientific, Chelmsford, UK). A standard curve, prepared using commercial proline (Sigma-Aldrich, Madrid, Spain), was used to determine the final proline concentration in each sample.
Phytohormone determinations
The foliar concentrations of ABA, JA, SA, and IAA were measured following a previously established protocol46. Briefly, 50 mg of dry, powdered leaf material was homogenized in 2 mL of ultrapure water using a mill ball grinder (MillMix 20, Domel Železniki, Slovenia). Internal standards were added to the mixture, including 25 ng of [2H6]-ABA, [13C6]-SA, dehydrojasmonic acid (DHJA), and 2.5 ng of [2H5]-IAA. After homogenization, samples were centrifuged at 4000 × g for 10 mn at 4 °C. The resulting supernatants were collected, and the pH was adjusted to between 2.8 and 3.2 using 80% acetic acid. The extract was then partitioned twice with 2 mL of diethyl ether (Fisher Scientific, Hampton, NH, USA), and the organic layer was separated and evaporated to dryness under vacuum in a centrifuge concentrator (Speed Vac, Jouan, Saint Herblain Cedex, France). The dried residue was reconstituted in 0.5 mL of 10:90 methanol/H2O by sonication. This solution was then filtered through 0.22 μm polytetrafluoroethylene membrane syringe filters (Albert S.A., Barcelona, Spain) and analyzed using an ultraperformance liquid chromatography system (Acquity SDS; Waters Corp., Milford, MA, USA). Chromatographic separation was performed on a reversed-phase C18 column (Gravity, 50 × 2.1 mm, 1.8-µm particle size; Macherey–Nagel GmbH, Düren, Germany) with a methanol gradient (both containing 0.1% acetic acid) at a flow rate of 300 µL min − 1. Phytohormone levels were quantified with a TQS triple quadrupole mass spectrometer (Xevo TQ-S, Waters Corp., Milford, MA, USA) using an orthogonal Z-spray electrospray ion source. Calibration curves from commercial standards injected into the UPLC-MS system enabled quantification of the hormones in the samples. Data were analyzed using MassLynx v4.1 software, and results represent the mean of three biological replicates per experimental condition.
Analyses of mineral elements
Ground leaf tissues (0.2 g DW) collected 75 days after treatment (DAT) were analyzed for ion content. The leaf powder was incinerated in a muffle oven at 450 °C, and the resulting ashes were dissolved in an aqueous HCl solution for mineral element analysis. Cations, including K+, Ca2+, Mg2+, Na+, Fe2+, Mn2+, Cu2+, and Zn2+, were quantified according to Perkin-Elmer47, using inductively coupled plasma atomic emission spectroscopy (ICP-AES). Additionally, boron content was assessed using the azomethine H colorimetric method48.
Statistical analysis
Statistical analysis was conducted using IBM® SPSS® Statistics software, version 29.0.1.0 for Windows (IBM Corporation, Armonk, NY, USA). All the variables studied here met the assumptions of normality (as determined by the Shapiro-Wilk test) and homogeneity of variance (as indicated by Levene’s test) were analyzed using one-way ANOVA, followed by Duncan’s post hoc test for mean separation. Statistical significance was set at p < 0.05.
Results and discussion
Leaf growth
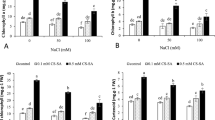
Leaf growth was assessed by means of the fresh weight obtained from the total of functional leaves per plant collected at the end of the experimental period in ‘CasNeg’ (Fig. 1). The average leaf FW was 76.24 g in the control plants, 18.95 g in NaCl-stressed plants, 32.44 g in plants treated with NaCl plus Si, and 35.32 g in those treated with NaCl plus proline. Thus, the applied salt stress significantly decreased leaf biomass by about 75% compared to control. However, the supply of Si and proline induced a lower reduction of leaf weight (around 57% and 54%, respectively) with respect to control, indicating that the percentage of FW decrease induced by NaCl in plants treated with proline and Si is lower than such decrease observed in plants only subjected to NaCl (75%). Additionally, this growth parameter was 42% and 46% higher in plants subjected to NaCl-stressed plants treated with Si and those treated with proline, respectively compared to plants only exposed to NaCl. Therefore, Si and proline appear to alleviate the negative effect of salt stress on leaf biomass and the effects of these treatments were statistically similar. In this context, it has been previously shown that the treatments of potted plants of ‘Cabernet Sauvignon’ with 100 mM NaCl considerably decrease the plant height, leaf-area expansion rates and dry weights; however, exogenous Si improves these growth parameters23. In addition, Si alleviates the negative effects of salt stress on shoot growth by reducing Na+ concentration in 1103P (V. Berlandieri x V. Rupestris), and B in 41B (V. Vinifera x V. Berlandieri) and 1103P grapevine rootstocks31. In the same way, proline application (100 mg L−1) to grapevine ‘Rasha’ cuttings exposed to salt stress increases leaf fresh and dry weight30. In other species, the Si supply against salinity stress also enhances gas exchange parameters39.
Leaf fresh weight (FW) per plant in grapevine ‘Castellana Negra’ measured at the end of the trial (75 days after treatment, DAT). Values are presented as means ± standard errors, calculated from all leaves collected per plant (n = 16 plants per treatment). The treatments are designated as control (CT), NaCl (90 mM), NaCl (90 mM) + Si (10 mM), and NaCl (90 mM) + proline (10 mM). Different letters denote significant differences between treatments (p < 0.05) as determined by ANOVA and Duncan’s test.
Photosynthetic pigment variations
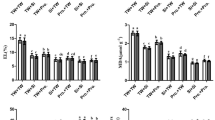
The photosynthetic pigments were evaluated through chlorophyll a (chl a) (Fig. 2A), chlorophyll b (chl b) (Fig. 2B), total chlorophyll (chl t) (Fig. 2C) and carotenoids (car) (Fig. 2D) in the sampled leaves at the end of the trial. NaCl-stress significantly reduced chl a (66%), chl b (68%), chl t (67%) and car (63%) contents compared to control. The addition of Si to irrigation water enriched with NaCl improved the levels of chl b (54%), chl t (46%) and car (49%) but did not significantly alter chl a content compared to plants watered with NaCl alone. In turn, the foliar application of proline to salt-stressed plants significantly increased the concentration of chl a compared to control (38%), NaCl (79%), NaCl + Si (66%) treatments. Additionally, exogenous proline increased the contents of chl b and chl t in NaCl-stressed plants to control level and improved the car concentration around 52% compared to its level in only salt-stressed plants, but in this case the treatment of NaCl + proline maintained lower content of car (22%, compared to control). To sum up, exogenous application of proline and Si significantly improved chlorophyll and carotenoid concentrations compared to plants subjected to NaCl stress alone. Proline had a notably greater effect than Si on chl a and chl t accumulation, while both compounds showed similar effects on chl b and car levels. Furthermore, the enhancement of these photosynthetic pigments may contribute to improved photosynthetic activity and biomass accumulation, as indicated by the smaller reduction in FW (Fig. 1) observed in treated plants compared to those exposed solely to salt stress. In this context, Gohari et al.30 reported that proline treatment increases foliar chl a and b contents in grapevine ‘Rasha’ cuttings against salinity stress. Under short-term NaCl-stress (10 days), Chl a and Chl t contents decrease in leaves of ‘Cabernet Sauvignon’ compared to control; however, exogenous Si relieved such decline23. In addition, Liang et al.49 report that silicon might protect the photosynthetic apparatus leading to the rise of salt tolerance.
Concentrations of Chl a (A), Chl b (B), Chl t (C) and Car (D) measured in leaves harvested at 75 DAT, with data expressed as means ± standard errors (n = 4 plants per treatment). The treatments were designated as control (CT), NaCl (90 mM), NaCl (90 mM) + Si (10 mM), and NaCl (90 mM) + proline (10 mM). Within each variable, different letters denote significant differences (p < 0.05) as determined by ANOVA and Duncan’s test.
Proline production
Seventy-five days after treatment initiations, irrigation with saline water increased proline content around 20% compared to the control (Fig. 3). The application of Si did not alter proline levels compared to NaCl alone; however, stressed plants treated with proline showed a lower concentration of this endogenous metabolite, with a decrease of 10% compared to NaCl and 11% compared to NaCl + Si. Additionally, the exogenous application of proline increased its endogenous content by about 11% compared to the control. These findings are consistent with other experimental systems, showing increased proline levels in response to salt stress; however, Si application has been shown to decline proline content in grapevine rootstocks31. In grapevine ‘Rasha’ cuttings, exogenous proline (100 mg L⁻¹) enhances proline production and SOD activity under excessive salinity30. In contrast, Si (Na2SiO3) supplementation increases proline and glycine betaine accumulation under salt and drought stress in mung bean36. This suggests that the chemical composition or formulation of the applied silicon may influence its effectiveness in modulating plant physiological and biochemical responses to salt stress.
Concentrations of proline measured in leaves harvested at 75 DAT, with data expressed as means ± standard errors (n = 4 plants per treatment). The treatments were designated as control (CT), NaCl (90 mM), NaCl (90 mM) + Si (10 mM), and NaCl (90 mM) + proline (10 mM). Different letters denote significant differences between treatments (p < 0.05) as determined by ANOVA and Duncan’s test.
Phytohormones changes
Salt stress increased ABA accumulation in ‘CasNeg’ leaves by approximately 39% compared to the control at 75 DAT (Fig. 4A). The addition of Si to water irrigation maintained ABA levels similar to the control. However, foliar proline treatment reduced ABA concentration by about 57% in plants subjected to NaCl stress alone and by 27% in plants irrigated with NaCl + Si. Moreover, exogenous proline even lowered ABA levels by around 29% compared to the control. The mediator role and the increase of ABA biosynthesis against salinity and other abiotic stress conditions have been already reported in several plant species7,8. The data suggests that applying Si and proline effectively helps maintain or reduce ABA levels, respectively, thereby enhancing plant responses to salt stress. In contrast, JA exhibited an opposite response to ABA under salt stress (Fig. 4B). Data showed that the highest concentration of JA (10 ng g⁻¹ DW) was found in the leaves of control plants, while NaCl-stressed plants showed a significantly lower concentration, with a 92% reduction compared to non-stressed plants. In this context, it has been reported that JA levels increase briefly within a few hours of stress before returning to baseline by 30 h, while ABA shows a more gradual increase, beginning around the same time or shortly after the JA peak50. Thus, since the low JA concentration in our experiment corresponds to 75 days after the onset of salt stress, the data may suggest that an early increase in JA levels likely occurred at the beginning of the stress. To determine the precise pattern of JA changes under salinity in grapevine, it would be valuable to analyze samples at 24-hour intervals in future experiments to capture the initial peak of this hormone. Additionally, JA levels were higher in plants treated with NaCl + Si (69%) and NaCl + proline (51%) compared to NaCl alone. Further research19 found that JA concentrations were consistently lower in salt-sensitive cultivars compared to their salt-tolerant counterparts in rice. Exogenous JA application improved salt tolerance in wheat seedlings by reducing membrane lipid damage, enhancing root structure, increasing ABA, JA, and SA levels. Additionally, antioxidant systems, hormone regulation, and transcriptional factors contributed to this JA-mediated tolerance51. On the other hand, SA followed a similar trend as ABA, with a significant accumulation (32%) in the leaves of plants irrigated with NaCl solution compared to the control (Fig. 4C). The accumulation of SA induced by salt stress seems to be positive for ‘CasNeg’ tolerance to salinity. In this sense, exogenous SA has been reported to enhance glycine betaine accumulation, strengthen the antioxidant system, and reduce ethylene synthesis, ultimately improving growth and photosynthesis in mung bean under salt stress14. In addition, exogenous SA improves plant tolerance by enhancing photosynthesis and antioxidant systems, increasing proline and soluble sugar levels, and promoting gene transcription15. The supply of silicon to NaCl-enriched water did not alter SA concentration relative to the control. However, exogenous proline combined with NaCl significantly reduced SA levels by approximately 51% compared to NaCl alone, and by 29% compared to the control. This result might suggest that proline reinforces plants’ ability to withstand salt stress and subsequently contributes to mediating SA biosynthesis. Finally, the foliar IAA levels, regardless of the treatments, ranged from 2.4 to 3.5 ng g⁻¹ DW (Fig. 4D). Thus, neither salt stress nor the application of Si and proline altered the accumulation of this hormone, suggesting that the applied salinity did not cause severe damage, and that active tissue growth was maintained throughout the experimental period. These findings align with those of Cackett et al.52, who reported that IAA levels increase in response to salt stress due to enhanced biosynthesis, further supporting the role of IAA in promoting growth under ionic stress in Arabidopsis. Nevertheless, Lang et al.53 stated that NaCl stress (100 mM) decreases IAA concentration, while the application of Si recovered such content in Glycyrrhiza uralensis. It has also been reported that the application of IAA alleviates the negative impacts of salinity by increasing Na+ flux, which limited Na+ buildup to sustain ion balance. This treatment protects root development, enhances nutrient absorption, and leads to increased photosynthetic efficiency21. Regarding the hormonal changes, it is important to highlight that data presented here revealed a parallel increase in ABA (Fig. 4A) and SA (Fig. 4C) levels in plants treated solely with NaCl compared to the control. Also, exogenous Si did not significantly affect the accumulation of either phytohormone. However, the application of proline significantly reduced the concentrations of both ABA and SA. These results support the hypothesis that proline plays a palliative role in mitigating salt stress, potentially through the modulation of ABA and SA accumulation under stress conditions.
Foliar concentration of ABA (A), JA (B), SA (C) and IAA (D) in grapevine ‘Castellana Negra’ plants determined at the end of the trial (75 days after treatment, DAT). Values are presented as means ± standard errors, determined from leaves collected per plant (n = 4 plants per treatment). The treatments were designated as control (CT), NaCl (90 mM), NaCl (90 mM) + Si (10 mM), and NaCl (90 mM) + proline (10 mM). Within each variable, different letters denote significant differences (p < 0.05) as determined by ANOVA and Duncan’s test.
Foliar mineral elements
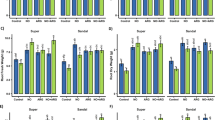
Salt stress, applied alone or in combination with Si or proline, increased foliar K+ content by ca. 55%, 58%, and 60%, respectively, compared to the control (Fig. 5A). Therefore, the addition of Si and proline did not significantly affect the accumulation of this macronutrient compared to NaCl stress alone. In contrast, salinized water reduced Ca2+ concentration by 24% compared to the control (Fig. 5B), and the exogenous supply of Si or proline produced a similar effect to salt stress alone. Additionally, neither salinity nor the treatments with Si and proline affected foliar Mg2+ levels, which remained close to the control (Fig. 5C). In other studies, salinity has been shown to reduce K+ and Ca2+ uptake due to increased Na+ accumulation34,35; however, Si has been found to enhance K+ and Ca2+ levels under these conditions36. K+ has been identified as the primary inorganic solute responsible for changes in guard cell turgor, which in turn controls stomatal opening. Therefore, maintaining an adequate K+ concentration is essential for its rapid exchange between the apoplast, cytosol, and vacuoles to regulate stomatal aperture effectively33. In our study, salt stress whether applied alone or with Si and proline resulted in a significant increase in the K+/Ca2+ and K+/Mg2+ ratios compared to control (Table 1). These findings may suggest a preferential uptake of K+ in this cultivar under salinity conditions.
Foliar concentration of K+ (A), Ca2+ (B), Mg2+ (C) and Na+ (D) in grapevine ‘Castellana Negra’ plants determined at the end of the trial (75 days after treatment, DAT). Values are presented as means ± standard errors, determined from leaves collected per plant (n = 4 plants per treatment). The treatments were designated as control (CT), NaCl (90 mM), NaCl (90 mM) + Si (10 mM), and NaCl (90 mM) + proline (10 mM). Within each variable, different letters denote significant differences (p < 0.05) as determined by ANOVA and Duncan’s test.
On the other hand, it is interesting to remark that salt stress markedly increased the accumulation of Na+ in 93% respect to control (Fig. 5D). However, the applied Si and proline significantly reduced Na+ concentration by around 22% and 39%, respectively compared to NaCl stress alone. In this case, proline seems to be slightly more effective in reducing Na+ accumulation compared to Si. Thus, plants treated with proline accumulated 23% lower Na+ content than those treated with Si. In accordance with our findings, Qin et al.23 reported a significant increase of foliar Na+ in young ‘Cabernet Sauvignon’ plants exposed to salt stress, and the supply of Si decreases such accumulation. In this sense, Si application enhances the sequestration of Na+ within the vacuoles, leading to the regulation of its excessive increase in the chloroplasts37. Also, Si supply induces an optimum K+/Na+ ratio, ionic concentration, and nutrient balance38. In the same way, Hurtado et al.41 indicate that Si improves the buildup of K+ and Ca2+ compared to Na+, leading to the enhancement of growth and yield in sorghum and sunflower. Our findings are consistent with the increase of K+ but not with Ca2+ in the grapevine ‘CasNeg’. Furthermore, it has been shown that an excess of Na+ elevates the Na+/K+ ratio, disrupting ionic and nutritional homeostasis34. In our experimental system, salinity significantly decreased the K+/Na+ ratio in plants exposed to NaCl. However, the application of Si and proline tended to slightly increase this ratio compared to salt stress alone (Table 1). Although the differences among all salinity treatments were not statistically significant (p < 0.05), exogenous proline resulted in a higher K+/Na+ ratio, suggesting a potentially greater capacity to mitigate the ratio reduction caused by NaCl. Similarly, proline supply also showed a tendency to increase the Ca2+/Na+ ratio relative to NaCl treatment alone, although this effect was not statistically significant.
Regarding leaf micronutrients (Table 2), salt stress increased Fe2+ concentration by 29% relative to the control. Nevertheless, Si addition significantly decreased the buildup of this element by about 54% and 36% with respect to NaCl stress and control, respectively. Similarly, exogenous proline largely decreased its content around 65% and 51% in salt-stressed and non-stressed plants. In this line, the effects of Si and proline on reducing foliar Fe2+ accumulation followed a similar trend, although the percentage of reduction differed. This variation is expected, as the two compounds function through distinct mechanisms. For instance, Si can limit Fe2+ uptake by restricting apoplastic flow as reported in rice roots54. In addition, under Fe2+ deficiency, Si initially enhances root Fe2+ acquisition by upregulating related genes, but over time, this response diminishes as Si improves the plant’s overall Fe2+ status55. In contrast, proline is known to support Fe2+ homeostasis under stress conditions. However, in our experimental system, salt-stressed plants already exhibited elevated Fe2+ concentrations, indicating that additional Fe2+ was not required. In this context, proline may help regulate and prevent excessive Fe2+ accumulation. Likewise, salinity increased Mn2+ accumulation by 31% compared to the control; however, the application of Si maintained the content of this element as in non-salinized plants. In this sense, the plants treated with proline statistically accumulated a concentration of Mn2+ similar to the salt-stressed ones. Cu2+ and Zn2+ showed comparable contents and trend between control, NaCl-stressed, and NaCl + Si treated plants; however, proline addition increased their concentrations by about 74% and 33%, respectively, compared to control. Finally, salt-stressed plants showed a similar boron content to non-stressed plants, although plants treated with Si and proline accumulated approximately 40% and 34% more boron than control plants. Therefore, salt stress did not decrease micronutrient concentrations; on the contrary, it either increased or maintained them at levels comparable to the control. This suggests that this cultivar may tolerate these conditions without experiencing nutrient deficiencies. These results coincide at least partially with previous studies reporting an increase of K+, Ca2+, Mg2+, Fe2+, Mn2+, Zn2+, Cu2+, and B induced by Si in plants exposed to salinity stress42,43,44,45.
In conclusion, the data highlight the involvement of several key factors, such as phytohormones, photosynthetic pigments, proline, and essential mineral nutrients in improving osmotic adjustment, which helps modulate the response of ‘CasNeg’ grapevine to salt stress. The findings further suggest that Si and proline play a significant role in regulating grapevine responses, and that their application may enhance the salinity tolerance of sensitive genotypes, serving as practical tools for managing grapevine in saline soils.
Data availability
The data from this study cannot be shared publicly due to privacy considerations. However, all experimental results analyzed in this study are available upon request at jmahou@ull.edu.es.
References
Han, Y. & Li, X. Current progress in research focused on salt tolerance in vitis vinifera L. Front. Plant. Sci. 15, 1353436. https://doi.org/10.3389/fpls.2024.1353436 (2024).
Munns, R. & Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant. Biol. 59, 651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911 (2008).
Lu, X. et al. Grapevine (Vitis vinifera) responses to salt stress and alkali stress: transcriptional and metabolic profiling. BMC Plant. Biol. 22, 528. https://doi.org/10.1186/s12870-022-03907-z (2022).
Zhao, S. et al. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 22, 4609. https://doi.org/10.3390/ijms22094609 (2021).
Upreti, K. & Murti, G. Response of grape rootstocks to salinity: changes in root growth, polyamines and abscisic acid. Biol. Plant. 54, 730–734. https://doi.org/10.1007/s10535-010-0130-z (2010).
Fozouni, M., Abbaspour, N. & Baneh, H. D. Leaf water potential, photosynthetic pigments and compatible solutes alterations in four grape cultivars under salinity. Vitis 51, 147–152. https://doi.org/10.5073/vitis.2012.51.147-152 (2012).
Cutler, S. R., Rodriguez, P. L., Finkelstein, R. R. & Abrams, S. R. Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant. Biol. 61, 651–679. https://doi.org/10.1146/annurev-arplant-042809-112122 (2010).
Sah, S. K., Reddym, K. R. & Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant. Sci. 7, 571. https://doi.org/10.3389/fpls.2016.00571 (2016).
Pantin, F., Simonneau, T. & Muller, B. Coming of leaf age: control of growth by hydraulics and metabolics during leaf ontogeny. New. Phytol. 196, 349–366. https://doi.org/10.1111/j.1469-8137.2012.04273.x (2012).
Stevens, R. M., Harvey, G., Norton, S. & Frahn, W. Over-canopy saline sprinkler irrigation of grapevines during different growth stages. Agric. Water Manag. 101, 62–70. https://doi.org/10.1016/j.agwat.2011.09.003 (2011).
Khodary, S. E. A. Effect of Salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt-stressed maize plants. Inter J. Agric. Biol. 6, 5–8 (2004).
Gunes, A. et al. Effects of exogenously applied Salicylic acid on the induction of multiple stress tolerance and mineral nutrition in maize (Zea Mays L). Arch. Agron. Soil. Sci. 51, 687–695. https://doi.org/10.1080/03650340500336075 (2005).
Amiri, J., Eshghi, S., Tafazoli, E., Kholdebarin, B. & Abbaspour, N. Ameliorative effects of Salicylic acid on mineral concentrations in roots and leaves of two grapevine (Vitis vinifera L.) cultivars under salt stress. Vitis 53, 181–188. https://doi.org/10.5073/vitis.2014.53.181-188 (2014).
Khan, M., Asgher, M. & Khan, N. A. Alleviation of salt-induced photosynthesis and growth Inhibition by Salicylic acid involves Glycine betaine and ethylene in mung bean (Vigna radiata L). Plant. Physiol. Bioch. 80, 67–74. https://doi.org/10.1016/j.plaphy.2014.03.026 (2014).
Li, Q. et al. Salicylic acid alleviates Zn-induced Inhibition of growth via enhancing antioxidant system and glutathione metabolism in alfalfa. Ecotoxicol. Environ. Saf. 265, 115500. https://doi.org/10.1016/j.ecoenv.2023.115500 (2023).
Abouelsaad, I. & Renault, S. Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. J. Plant. Physiol. 226, 136–144. https://doi.org/10.1016/j.jplph.2018.04.009 (2018).
Ahmad, B., Raina, A., Naikoo, M. I. & Khan, S. Role of methyl jasmonates in salt stress tolerance in crop plants. In: Plant Signaling Molecules (ed. Elsevier) 371–384The Netherlands, (2019).
Javid, M. G., Sorooshzadeh, A., Moradi, F., Sanavy, M., Allahdadi, I. & S. A. M. & The role of phytohormones in alleviating salt stress in crop plants. Aust J. Crop Sci. 5, 726 (2011).
Kang, D. J. et al. Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J. Agron. Crop Sci. 191, 273–282. https://doi.org/10.1111/j.1439-037X.2005.00153.x (2005).
Deytieux-Belleau, C., Gagne, S., L’Hyvernay, A., Donèche, B. & Geny, L. Possible roles of both absicisic acid and indol-acetic acid in controlling grape berry ripening process. J. Int. Sci. Vigne Vin. 41, 141–148. https://doi.org/10.20870/oeno-one.2007.41.3.844 (2007).
Zhang, R. et al. Auxin alters sodium ion accumulation and nutrient accumulation by playing protective role in salinity challenged strawberry. Plant. Physiol. Biochem. 164, 1–9. https://doi.org/10.1016/j.plaphy.2021.04.008 (2021).
Shahzad, K. et al. Exogenous application of indole-3-acetic acid to ameliorate salt induced harmful effects on four eggplants (Solanum melongena L.) varieties. Sci. Hortic. 292, 110662. https://doi.org/10.1016/j.scienta.2021.110662 (2022).
Qin, L., Kang, W., Qi, Y., Zhang, Z. & Wang, N. The influence of silicon application on growth and photosynthesis response of salt stressed grapevines (Vitis vinifera L). Acta Physiol. Plant. 38, 68. https://doi.org/10.1007/s11738-016-2087-9 (2016).
Hernández-Salinas, M. et al. Silicon enhances the tolerance to moderate NaCl-salinity in tomato grown in a hydroponic recirculating system. J. Plant. Nutr. 45, 413–425. https://doi.org/10.1080/01904167.2021.1963772 (2021).
Zuccarini, P. Effects of silicon on photosynthesis, water relations and nutrient uptake of Phaseolus vulgaris under NaCl stress. Biol. Plant. 52, 157–160. https://doi.org/10.1007/s10535-008-0034-3 (2008).
Yin, L. et al. Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant. Cell. Environ. 39, 245–258. https://doi.org/10.1111/pce.12521 (2016).
Ozden, M., Demirel, U. & Kahraman, A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 119, 163–168. https://doi.org/10.1016/j.scienta.2008.07.031 (2009).
Hayat, S. et al. Role of proline under changing environments: a review. Plant. Signal. Behav. 7, 1456–1466. https://doi.org/10.4161/psb.21949 (2012).
Yan, K. et al. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol. Plant. 35, 2867–2878. https://doi.org/10.1007/s11738-013-1325-7 (2013).
Gohari, G. et al. Enhanced tolerance to salinity stress in grapevine plants through application of carbon quantum Dots functionalized by proline. Environ. Sci. Pollut Res. Int. 28, 42877–42890. https://doi.org/10.1007/s11356-021-13794-w (2021).
Soylemezoglu, G., Demir, K., Inal, A. & Gunes, A. Effect of silicon on antioxidant and stomatal response of two grapevine (Vitis vinifera L.) rootstocks grown in Boron toxic, saline and Boron toxic-saline soil. Sci. Hortic. 123, 240–246. https://doi.org/10.1016/j.scienta.2009.09.0053 (2009).
Gou, C. et al. Integrative application of silicon and/or proline improves sweet corn (Zea Mays L. saccharata) production and antioxidant defense system under salt stress condition. Sci. Rep. 13, 18315. https://doi.org/10.1038/s41598-023-45003-8 (2023).
Andrés, Z. et al. Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc. Natl. Acad. Sci. 111, E1806–E1814 (2014). https://doi.org/10.1073/pnas.1320421111
Assaha, D. V. M., Ueda, A., Saneoka, H., Al-Yahyai, R. & Yaish, M. W. The role of na++ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 8, 509. https://doi.org/10.3389/fphys.2017.00509 (2017).
Wang, X. S. & Han, J. G. Effects of NaCl and silicon on ion distribution in the roots, shoots and leaves of two alfalfa cultivars with different salt tolerance. Soil. Sci. Plant. Nutr. 53, 278–285. https://doi.org/10.1111/j.1747-0765.2007.00135.x (2007).
Ahmad, P. et al. Silicon (Si) supplementation alleviates NaCl toxicity in mung bean [Vigna radiata (L.) Wilczek] through the modifications of physiobiochemical attributes and key antioxidant enzymes. J. Plant. Grow. Regul. 38, 70–82. https://doi.org/10.1007/s00344-018-9810-2 (2019b).
Bosnic, P., Bosnic, D., Jasnic, J. & Nikolic, M. Silicon mediates sodium transport and partitioning in maize under moderate salt stress. Environ. Exp. Bot. 155, 681–687. https://doi.org/10.1016/j.envexpbot.2018.08.018 (2018).
Almeida, D. M., Oliveira, M. M. & Saibo, N. J. Regulation of na++ and K+ homeostasis in plants: towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 40, 326–345. https://doi.org/10.1590/1678-4685-gmb-2016-0106 (2017).
Etesami, H. & Jeong, B. R. Silicon (Si): review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 147, 881–896. https://doi.org/10.1016/j.ecoenv.2017.09.063 (2018).
Alzahrani, Y., Kuşvuran, A., Alharby, H. F., Kuşvuran, S. & Rady, M. M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 154, 187–196. https://doi.org/10.1016/j.ecoenv.2018.02.057 (2018).
Hurtado, A. C. et al. Silicon alleviates sodium toxicity in sorghum and sunflower plants by enhancing ionic homeostasis in roots and shoots and increasing dry matter accumulation. Silicon 13, 475–486. https://doi.org/10.1007/s12633-020-00449-7 (2021).
Liu, B., Soundararajan, P. & Manivannan, A. Mechanisms of silicon-mediated amelioration of salt stress in plants. Plants 8, 307. https://doi.org/10.3390/plants8090307 (2019).
Keller, M. E. Constraints and Stress Physiology (ed. Keller, M). The Science of Grapevines 267–341(Academic Press, (2015).
Lichtenthaler, H. M. (ed Buschmann, C.) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr. Protocols Food Anal. Chem. 1 F431–F438 https://doi.org/10.1002/0471142913.faf0403s01 (2001).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant. Soil. 39, 205–207. https://doi.org/10.1007/BF00018060 (1973).
Durgbanshi, A. et al. Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography-electrospray tandem mass spectrometry. J. Agri Food Chem. 53, 8437–8442. https://doi.org/10.1021/jf050884b (2005).
Perkin-Elmer. Analytical Methods for Atomic Absorption Spectrometry (Perkin-Elmer Corporation (USA, 1996).
Gaines, T. P. & Mitchell, A. Boron determination in plant tissue by the azomethine H method. Commun. Soil. Sci. Plant. Anal. 10, 1099–1108. https://doi.org/10.1080/00103627909366965 (1979).
Liang, Y., Nikolic, M., Bélanger, R., Gong, H. & Song, A. Silicon in Agriculture: From Theory to Practice (ed. Springer) ISBN 978-94-017-9978-2 (Netherlands, 2015).
De Ollas, C., Hernando, B., Arbona, V. & Gómez-Cadenas, A. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol. Plant. 147, 296–306. https://doi.org/10.1111/j.1399-3054.2012.01659.x (2013).
Zhu, M. et al. Jasmonic acid pretreatment improves salt tolerance of wheat by regulating hormones biosynthesis and antioxidant capacity. Front. Plant. Sci. 13, 968477. https://doi.org/10.3389/fpls.2022.968477 (2022).
Cackett, L. et al. Salt-Specific gene expression reveals elevated auxin levels in Arabidopsis Thaliana plants grown under saline conditions. Front. Plant. Sci. 13, 804716. https://doi.org/10.3389/fpls.2022.804716 (2022).
Lang, D. et al. Silicon promotes seedling growth and alters endogenous IAA, GA3 and ABA concentrations in Glycyrrhiza uralensis under 100 mM NaCl stress. J. Hortic. Sci. Biotechnol. 94, 87–93. https://doi.org/10.1080/14620316.2018.1450097 (2018).
Becker, M., Ngo, N. S. & Schenk, M. K. A. Silicon reduces the iron uptake in rice and induces iron homeostasis related genes. Sci. Rep. 10, 5079. https://doi.org/10.1038/s41598-020-61718-4 (2020).
Pavlovic, J. et al. Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root Apoplast. New. Phytol. 198, 1096–1107. https://doi.org/10.1111/nph.12213 (2013).
Acknowledgements
This research was supported by funding of the Departamento de Ingeniería Agraria y del Medio Natural and the Vicerrectorado de Investigación y Transferencia, Universidad de La Laguna. UPLC-MS equipment used for hormonal analyses was provided by the SCIC of the Universidad Jaume I.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Permissions and licenses
The grapevine collection of ‘Castellana Negra’ belongs to the University of La Laguna, Spain, and no special permissions or licenses are required to collect this material.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Urbano-Gálvez, A., López-Climent, M.F., Gómez-Cadenas, A. et al. Effectiveness of exogenous silicon and proline on mediating grapevine ‘castellana negra’ responses to salt stress. Sci Rep 15, 32751 (2025). https://doi.org/10.1038/s41598-025-17312-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17312-7