Abstract
Considering neo-angiogenesis as one of the most important hallmarks of solid tumors and a key promoter of recurrence, perfusion studies represent a modality to investigate tumor’s vascularization and to identify its influence on chemotherapy efficacy. To analyze neoplastic tissue adjacent to radiofrequency thermal-ablation (RFA) areas to demonstrate early perfusion anomalies and to suggest the synergic role of ablative techniques with chemotherapy treatments. In this prospective study, a total of 10 mice were subjected to a colon-carcinoma cell implantation and after the tumor colonization the RFA procedure was performed. All mice underwent a 7T MRI including 2D single-echo single-shot Dynamic-Contrast-Enhanced (DCE) sequences with intravenous injection of Gadolinium-based contrast agent (CA), before and after 72 h from RFA treatment. Data-driven segmentation of the tumor mass enabled the identification of three ROIs with peculiar pharmacokinetic profiles of CA uptake: VTA, viable tumor area; PNA, partial necrotic area; CNA, complete necrotic area. Within these ROIs, permeability (K-trans and Extravascular-extracellular volume, Ve) and perfusion (Time to Peak, TTP) indices were evaluated with non-parametric paired samples Wilcoxon tests. Robust increase of Ve (PNA and VTA, p = 0.014, p < 0.001, respectively) and TTP (VTA, p < 0.01) is consistent with severe cellular and vascular damage induced by RFA. Perfusion MRI enables robust detection of significant modulation of the microvasculature within 72 h from radiofrequency induced ablation in a murine model of colon-carcinoma. This opens a potentially new time window for the evaluation of therapeutic effects of RFA-enhanced chemotherapy.
Similar content being viewed by others
Introduction
Radiofrequency ablation (RFA) has emerged as a minimally invasive and effective treatment option for various solid tumors, particularly in hepatic primary malignancies and liver metastases. It is routinely employed in clinical settings to achieve local tumor control in patients who are not candidates for surgical resection or systemic therapy1. Moreover, RFA plays an alternative or complementary role in hepatic tumor treatments2,3; in particular, combined treatments such as neoadjuvant chemotherapy followed by liver resection have been widely applied strategy to resectable colorectal cancers; however, few studies investigated a possible synergic role of chemotherapy regimens followed by RFA4. RFA is based on the direct application of energy-based treatment to promote destruction of focal malignant lesions and is commonly used in a wide range of malignancies1. Thermal-ablation procedure is known to determine tissue composition changes and vascularization anomalies around and inside the ablation zone characterized by coagulative necrosis. Inadequately treated tumors remain with high risk of recurrence and the knowledge of changes in the periphery of the tumor and in the twilight zone around thermal ablation is low. Strategies to improve the tumor destruction volume should be promoted and the understanding of perfusional and histological changes background is essential5.
Although histopathology remains the gold standard for assessing tissue composition following RFA, several limitations have been reported. In particular, the post-ablation tissue environment is characterized by heterogeneous zones of necrosis, edema, and viable tumor, which can be challenging to distinguish with conventional staining alone6. Moreover, histological sampling is inherently spatially limited and may not capture the full extent or distribution of residual viable tumor7,8. These limitations can reduce the accuracy of therapeutic efficacy assessment, especially during the early phase following RFA, when tissue remodeling is dynamic and still evolving.
Incomplete RFA is of particular clinical relevance, as residual tumor tissue at the ablation margins can lead to local recurrence and reduced treatment efficacy. Understanding the biological and vascular changes occurring after incomplete ablation through advanced imaging is therefore essential to improving therapeutic outcomes9. Conventional imaging modalities, such as ultrasound, CT, and standard MRI sequences, often fail to provide a precise assessment of ablation completeness in the early post-procedural phase. To address these issues, dynamic contrast-enhanced MRI (DCE-MRI) and relaxometry have emerged as valuable tools for assessing parameters of tissue perfusion and permeability changes10. These parameters are the volume transfer constant of contrast agent (K-trans) which quantifies the permeability-surface area from the plasma to the extravascular-extracellular space, the extravascular-extracellular volume fraction (Ve) and the time-to-peak (TTP) which reflects the time at contrast-enhanced concentration reaches its maximum11,12. They offer quantitative, spatially-resolved biomarkers that reflect microvascular integrity, extracellular matrix remodeling, and interstitial fluid dynamics. These imaging-derived metrics, differing from conventional MRI, provide functional information that complements histological findings, improving the characterization of post-ablation tissue and potentially identifying viable tumor areas that might benefit from additional therapy. In addition, T1 and T2 relaxometry provide quantitative, non-contrast-based measurements of tissue microstructure8, sensitive to changes in water content, necrosis, and extracellular matrix alterations9. Their integration into post-ablation imaging may enhance the interpretation of treatment effects and support non-invasive monitoring strategies. We hypothesize that RFA-induced alterations in tumor vascular permeability could enhance the delivery and efficacy of chemotherapeutic agents, providing a rationale for combined modality treatment.
Aim of the study was to evaluate early microenvironment changes caused by hyperthermia after RFA based on a murine model of colorectal cancer with DCE-MRI and to analyze and confirm histological abnormalities in the tissue composition.
Materials and methods
All experimental procedures of the study involving animals were performed according with experimental guidelines committee on animal research and by the Italian Ministry of Health (n.378/2020-PR). We confirm that all experimental protocols were approved by University of Verona, in compliance with relevant ethical regulations. All methods are reported in accordance with the ARRIVE guidelines to ensure transparency and reproducibility.
Population study
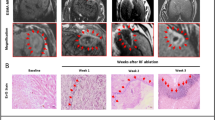
A total of 16 homozygous male mice (Harlan Laboratories Udine, Italy) were maintained under standard environmental conditions (temperature, humidity, hygiene, 12/12 hours light/dark cycle, with water and food ad libitum) and veterinary control, according to the italian law (DLgs. 26, 4TH March 2014) and the European legislation (2010/63/EU). Animals were housed in IVC cages (Isolated Ventilated Cages, Tecniplast, Italy) enriched with nesting houses and materials for nest building. All mice received a 1–1,5% Isoflurane anesthesia and 2*106 HT-29 colorectal human cells were subcutaneously inoculated. Human colorectal adenocarcinoma HT-29 cells (Cat. No. HTB-38) were purchased from ATCC (Manassas, VA, USA). HT-29 cell line was cultured with fetal bovine serum, 1% penicillin/streptomycin 1:1 and 1% of L-Glutamine 200mM (GIBCO, Thermo Fisher Scientific, MA, USA) at 37 °C humidified air with 5% CO2. As the mass reached 1 cm of diameter, the RFA procedure was performed. An additional monitoring control group of 4 mice did not receive RFA and was allowed to grow the tumor spontaneously until day 29 (d29) after the inoculation. All in vivo experiments were performed using experimental procedures designed to minimize pain in animals, and imaging and thermal ablation procedures were carried out under gaseous anesthesia through inhalation of isoflurane. This was an exploratory study without prospective power analysis. Sample size was based on previous similar studies, ethical feasibility, and the available budget under a Joint Project Grant. A flowchart of the study design, animal allocation, timepoints, and exclusions is shown in Fig. 1.
CONSORT-style flowchart summarizing the experimental design. Timepoints were defined as follows: t0 = day 21 post-cell injection (baseline MRI for both groups); t2 = 72 h post-RFA (day 24) for the RFA group, and day 29 for the control group. Both groups underwent MRI at t0 and t2, followed by histological analysis at sacrifice.
Radiofrequency ablation technique
The equipment for the ablation procedure was kindly provided by RF Medical VER SAN & DAFNE company (Verona, Italy). The RF ablation procedure was performed under ultrasound guide. A 17 Gauge electrode with 1 mm uninsulated tip was used and reached an intra-tumoral ablative temperature of 90 °C, maintained with a 0–10-Watt power amplifier. Ablation parameters were controlled by the system based on real-time impedance monitoring, ensuring consistent necrosis while limiting collateral thermal damage.
MR imaging protocol
All mice underwent MRI examination using a 7T MRI Bruker system (Pharmascan 70/16) (Bruker, Karlsruhe, Germany) before (t0) and after 72 h (t72) from RFA treatment. The MRI system was equipped with a 16 cm bore horizontal magnet, a 3.5 cm i.d. bird-cage volumetric coil, gas anesthesia, heated animal bed and vital parameters measure systems. MRI protocol is detailed in Table 1. Perfusion study was performed with intravenous injection of contrast enhancement medium (0.5 mmol/ml, Magnevist, Bayer Schering Pharma AG, Berlin, Germany) at 100µM/kg dose. Tumor growth was monitored with a T2w TSE 3D SE SS acquired at different time points from HT-29 cells injection: day 1 (d1), day 7 (d7), day 14 (d14), day 21 (d21), day 29 (d29), day 35 (d35), day 43 (d43) and day 50 (d50). Animals were considered for RFA treatment when the tumor volume reached the threshold value of 100ul.
Data analysis
Raw Bruker images were converted into NIfTI format using Bru2Nii13,14, enabling compatibility with widely used open-source tools for medical image analysis and visualization. Subsequent image analysis was performed using tools from the FMRIB Software Library (FSL v6.0.5), including fslstats15, which was used to extract quantitative parameters from the registered volumes. All images were manually reviewed and segmented using ITK-SNAP v4.2.016, an open-source software for image visualization and segmentation. The NIfTI format was adopted throughout the analysis for compatibility with these tools and standardized image orientation. In particular, three dimensional binary masks to include the whole tumor mass before and after RFA were manually drawn on 3D T2w RARE by a trained operator with ITK-SNAP17, as showed in Fig. 1. All subsequent analysis (morphometry, relaxometry, perfusion and permeability) were performed at subject level within the subject specific tumor mask.
For reproducibility and integration with future workflows, we refer to Quantiphyse, an open-source tool for quantitative image analysis18.
Statistical analysis of DCE-MRI parameters (K-trans, Ve, and TTP) between RFA and Monitoring groups was performed using a paired Wilcoxon test. Tumor growth rates were calculated as the slope of a linear fit between two timepoints (t0 and t2), corresponding to pre- and post-treatment in the RFA group, and matched timepoints in the monitoring group.
Morphometry
To assess the macroscopic effect of RFA, gross volume of tumors was evaluated before and after the treatment. Tumor volumes, expressed in µl, were obtained from the tumor masks with fslstats19. Changes between tumor size before and after treatment and between tumoral volumes of control- and RFA- groups were calculated. Volume measurements were used to quantify the macroscopic tumor response to RFA and to serve as a reference point for interpreting the imaging and histological changes observed.
Relaxometry
Voxel-wise fitting of T1 and T2 relaxation curves was performed using a custom MATLAB function (R2020a, MathWorks, Natick, MA) of variable-TR, multi-echo images at time t0 and t72. Quantitative T1 and T2 values were averaged over the whole tumor mask and compared with a paired sample t-test.
Dynamic contrast enhanced MRI
Quantitative maps of perfusion and permeability were obtained using the Quantiphyse platform Extended Tofts Model12, which implements the extended Tofts model for pharmacokinetic modeling of contrast enhancement. Voxel-wise time–signal intensity curves were fitted to extract the volume K-trans and the Ve. The arterial input function (AIF) was estimated using the Parker model, based on semi-automatic identification of a vascular input region. The TTP was calculated as the time point corresponding to the maximum value in the contrast enhancement curve for each voxel. FSL MELODIC was used to apply an Independent Component Analysis (ICA) to cluster regions with maximally independent temporal modulation of signal intensity20. The deterministic clustering algorithm was constrained to 3 independent components and labeled as follows: Viable Tumor Area (VTA), a highly vascularized region with fast wash-in; Partial Necrotic Area (PNA), a edematous region with medium wash-in; Complete Necrotic Area (CNA), a necrotic region with slow perfusion21. Independent components were used to extract average values of perfusion and permeability parameters and then were tested with a paired sample Wilcoxon test.
Tissue sampling and peroxidase-immunohistochemistry analysis
After completion of the experimental procedures, animals were sacrificed by inhalation of Isofluorane overdose. Isofluorane was purchased from MERIAL Italia spa, Milano, Italy. After excision, tumors were fixed in 4% formaldehyde and processed by embedding in paraffin. Sections were cut to 7 μm thickness, mounted on polylysine-coated microscope slides, and processed for histology. To evaluate tissue damage and morphology, sections were stained with hematoxylin and eosin.
For the peroxidase-immunohistochemistry analysis, sections were deparaffinized and endogenous peroxidase activity was blocked by incubating the slides in 3% H2O2 in methanol for 30 min. Subsequently, the sections were incubated with CD31 primary antibodies (BD PharMingen, CA, USA) diluted 1:400, and the immunoreaction was detected using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA). Sections processed as above described but without the primary antibodies were used as negative controls.
Results
The study finally included 10 mice out of 16. These animals had successfully completed the experimental protocol with two MR sessions before and after RFA. Other animals were sacrificed due to excessive tumor growth or did not survive the short time window between imaging sessions.
Morphometry
Pre- and post-ablation mean volumetric data showed a tumor growth within 72 h hours after RFA. Before RFA the mean volume was 299 ± 178 µl; after RFA the mean volume was 319 ± 214 µl. Volumetric data of each mouse are reported in Supplementary Data. Compared to the control group, the RFA group showed a decrease of the growth rate of about 30%.
Relaxometry
No significant differences were found in T1 and T2 relaxometry data over the whole tumor mass (p = 0.387 and p = 0.086, respectively. Results are described in Supplementary Material.
Dynamic contrast enhanced MRI
A representative data driven segmentation and corresponding temporal profiles of signal enhancement are reported in Fig. 2. Average K-trans showed a global increase in the whole tumor compartments after RFA, with the most relevant increase in the VTA, as illustrated in Fig. 3, panel (a) CNA and PNA also showed a three- to four-fold increase at t72 wrt t0. However, due to elevated inhomogeneity and between subjects’ variability, the observed trend remained non statistically significant. When considering Ve, a strong positive effect was reported on PNA and VTA, with a marked increase after RFA (p = 0.014 and p = 0.002, respectively), as showed in Fig. 3, panel (b) A similar condition was observed in the evaluation of the TTP, with a significant increase in VTA (p = 0.002), a substantial, although non-significant increase in PNA and negligible variation in CNA as illustrated in Fig. 3, panel (c) Perfusion and permeability parameters were not predictive of tumor growth, as the correlation between K-trans, Ve, TTP and tumor volume before and after RFA was not significant.
Representative components for the three compartments superimposed to the post-contrast images are shown in Panel a (CNA: Complete Necrotic Area, top row; PNA: Partial Necrotic Area, middle row; VTA: Viable Tumor Area, bottom row). Panel b shows the averaged temporal profile of signal enhancement compared to baseline signal (t=-1). The time point of contrast-agent injection is marked by a dashed vertical line at t = 0; the shaded area is obtained with the stretched-exponential model (SEM). Data are expressed as mean ± standard deviation.
Histological
The outer portion of the treated tumor has a relevant presence of tumor cells and contains bundles of connective tissue with large sizes. Extensive areas of necrotic-edematous (NE) tissue alternating with partially necrotic (PN) areas are detectable in the inner portion of the tumor.
The outer portion of the control tumors has a significant number of uniformly distributed tumor cells, and bundles of connective tissue with smaller sizes than those observed in the treated tumors. Also in the control tumor, the inner portion is characterized by the presence of necrotic-edematous (NE) areas surrounded by a reduced presence of cells. Figures 4, panel A, shows a histological slice of representative treated tumor excised 72 h after the RFA treatment and stained with Hematoxylin and Eosin. Figure 4, panel B, is relative to a size matched control.
RFA-induced alteration of permeability and perfusion parameters within the tumor mass for each of the three compartments considered. From left to right: k-trans; extracellular volume fraction (Ve); time to peak (TTP). CNA: Complete Necrotic Area; PNA: Partial Necrotic Area; VTA: Viable Tumor Area. Data are shown as mean ± standard deviation. Statistical significance was assessed using paired Wilcoxon tests. Levels of significance: *(p < 0.05); ***(p < 0.01).
In treated tumors, the analysis of immunohistochemistry revealed the presence of a rim that is rich in CD31+ blood vessels. The black arrows in Figs. 5, panel A, panel B and panel E, indicate this rim. Nevertheless, a limited presence of blood vessels in the internal area of the tumor can be seen in Figs. 5, panel C and panel D. Qualitatively, this rim overlaps with the highly vascularized viable tumor area (VTA) observed in MRI. Conversely, in the control tumor, blood vessels are more homogeneously distributed in both the internal and external areas, as clearly demonstrated in Figs. 6, Figs 7.
Representative histological slices of treated (panel A) and control (panel B) tumors, stained with Hematoxylin and Eosin. (*) in Panel A mark large bundles of connective tissue within the tumor tissue. (+) in Panel B mark smaller bundles of connective tissue within the tumor tissue. NE = necrotic-edematous areas. PN = partially necrotic areas.
Representative histological slices of a tumor obtained 72 h after RFA treatment, stained with anti-CD31 antibody. CD31 + blood vessels appear in brown. Panel A and Panel B show entire tumor sections including both peripheral and central regions. The peripheral rim displays a dense presence of CD31 + vessels (black arrows), which corresponds to the Viable Tumor Area (VTA) observed in DCE-MRI, while the central area shows reduced vascularity. Panel C and Panel D focus on the central portion of the tumor, characterized by a limited vascularization. These deeper regions show areas of partial necrosis (PNA) and complete necrosis (CNA). Panel E provides a magnified view of the VTA, emphasizing the presence of numerous CD31 + vessels (black arrows).
Representative histological slice of a control tumor stained with anti-CD31 antibody. CD31 + blood vessels (brown) are homogeneously distributed throughout both central and peripheral regions, without the formation of a vascularized rim as seen in treated mice: Panel A and Panel B show panoramic views of the tumor, encompassing both central and peripheral regions, where CD31 + vessels are uniformly distributed. Panel C and Panel D illustrate higher-magnification views of the central tumor area, confirming consistent vascular density across the tissue. Panel E presents a detailed close-up from the peripheral zone, further demonstrating the absence of any localized vascular rim.
Discussion
Percutaneous treatments are increasingly used to manage different malignancies, with the purpose of being potentially curative. RFA procedures cause coagulative necrosis, but few studies investigated the histological correlation with imaging perfusion characteristics due to architectural tissue changes and vascularization anomalies around the ablation zone.
Despite heterogeneous gross tumor volumes before and after thermal ablation, morphometric analysis revealed an overall tumor growth within 72 h of RFA treatment. However, the RFA group exhibited a significantly reduced tumor growth rate compared to the control group. This finding is consistent with the biological effects of partial necrosis and vascular alterations observed post-RFA, although no direct causal relationship can be inferred from this study. The volumetric assessment provided macroscopic evidence of treatment response and supported the interpretation of microstructural and perfusion changes seen in MRI and histology.
The overall tumor dimension on histological analysis in RFA-group is mainly related to widespread areas of coagulative necrotic-edematous tissue in the inner portion and relevant number of cancer cells with large bundles of connective tissue in its outer portion. On the other hand, the whole tumor volume in the control group is either composed of necrotic-edematous areas in the inner portion (caused by rapidly aggressive growing of cancer foci) and tumor cells with smaller bundles of connective tissue in the outer portion. Relative increasing volume of cancer after RFA could be both related to diffuse coagulative and edematous necrotic heat-derived changes subsequently and gradually replaced from connective tissue in the ablated area.
Relaxometry was included in the imaging protocol to evaluate possible changes in tumor microstructure following RFA. T1 and T2 values are sensitive to the presence of free water, protein content and cellularity, and have been shown to vary in response to necrosis, hemorrhage, and inflammation9. Although the observed differences did not reach statistical significance in our small cohort, these parameters offer valuable, non-invasive biomarkers for characterizing early post-RFA tissue modifications, and may be useful in combination with DCE-MRI for future studies.
This study was specifically designed to investigate the effects of incomplete RFA, which is a clinically relevant scenario and it mimics residual tumor viability, allowing for the characterization of perfusion changes at the tumor periphery.
Concerning perfusion and permeability tumor state in CNA, PNA and VTA, no significant correlation between tumor size and Ktrans was achieved. Nevertheless, a considerable Ktrans increase was found in each region, mostly in VTA after RFA which includes viable neoplastic tissue. Also, Ve, which represents the extracellular space, increases in both VTA and PNA (p = 0.002, p = 0.014). Also, TTP demonstrates a significant rise in VTA and PNA (p = 0.002) in the RFA-group. Perfusion and permeability parameters modification reflects the vessel heat-damage of RFA; in particular, increasing of Ktrans, mostly in VTA and PNA, could be ascribed to a faster transition of contrast agent from the intravascular to the extravascular space. Also, the increasing of Ve and TTP in VTA and PNA represent the widening of the interstitial space and the vascular damage after RFA. Indeed, MRI perfusion findings are explained by histological and immunohistochemistry analysis, which confirm a different vascular and stromal architecture. Histology supports the MRI findings. In the periphery of the ablation zone, the immunohistochemistry analysis reveals a CD31+ blood vessel rim which spatially corresponds to the highly VTA observed at MRI; however, a more homogeneous distribution of CD31+ blood vessel is found in the control-group. Moreover, increasing values of Ve and TTP in the RFA-group could be explained with the widening of the interstitial space and the presence of large size bundles of connective tissue that cannot be found in the control-group, where bundles of connective tissue are smaller in size. Thus, quantitative perfusion parameters reflect the architectural tissue changes of ablated areas within 72 h from RFA.
Our DCE-MRI findings indicate a significant increase in K-trans and Ve values in the VTA and PNA regions post-RFA, suggesting enhanced vascular permeability and extracellular space. Considering a possible human application, assessment of perfusion changes in the ablated-zone and their quantification with DCE-MRI could help to identify a viable cell area with improved permeability and a potential target for further therapeutic approaches after thermal-ablation. Few studies investigated possible synergic effects of RFA on chemotherapy regimens4. In this regard, Yang W. et al. found that RFA with heat target delivery chemotherapy with lyso-thermosensitive liposomal doxorubicin facilitated the tumor coagulation necrosis without additional toxicity22. Chen Y. et al., found significant advantages in controlling disease progression with combined RFA and chemotherapy treatment, with a quick recovery after the procedure and fewer serious complications compared to hepatectomy23. In clinical settings, Wang et al.24 demonstrated that the combination of RFA with lenvatinib and sintilimab in patients with unresectable hepatocellular carcinoma significantly improved progression-free survival and objective response rates compared to systemic therapy alone. These outcomes are partly attributed to the increased tumor vascular permeability following RFA, which may facilitate greater intratumoral accumulation of therapeutic agents. Indeed, RFA induces coagulative necrosis, releasing tumor antigens and promoting local inflammatory infiltration, which may enhance antitumor immune responses. Additionally, sublethal thermal injury at the periphery of the ablation zone can increase vascular permeability and stimulate the expression of angiogenic and hypoxia-related markers such as VEGF and HIF-1α, potentially improving the delivery and efficacy of chemotherapeutic agents. Such microenvironmental changes may potentiate the efficacy of chemotherapy when administered in conjunction with RFA, supporting the hypothesis of a synergistic mechanism between these treatments. Our findings reinforce this hypothesis. The observed vascular enrichment in the peripheral tumor regions post-RFA suggest a more permissive microenvironment for drug delivery, representing an optimal context for the administration of cytotoxic or immune-modulating agents. Effectiveness of combined therapies depends on different factors, such as neoplastic mass size and patient’s comorbidities, but this tissue modification could affect the choice of targeted drugs needed to reach the response rate and the adoption of alternative chemotherapy regimens for patients eligible to RFA treatment.
Although this study prioritized DCE-MRI to assess early vascular and permeability changes following RFA, diffusion-weighted imaging (DWI) could provide additional information on tumor cellularity and residual viability. The exclusion of DWI was motivated by technical limitations in scan time and the need to preserve animal stability under prolonged anesthesia. Nevertheless, ADC values have been shown to correlate with treatment response, and guidelines25 recommend its inclusion in post-ablation follow-up. Future studies will consider integrating DWI into multiparametric MRI protocols.
Our findings suggest that post-RFA functional imaging may play a pivotal role in identifying residual viable tissue and altered tumor microenvironments and this could support decision-making for timing and personalization of adjuvant therapies in clinical scenario. Integrating imaging biomarkers such as K-trans or T1 relaxation times into clinical follow-up protocols may help optimize therapeutic strategies in patients undergoing RFA for solid tumors.
This study had several limitations. Further examinations could be done considering a larger subject’s sample monitored for a longer time, to evaluate the biological process evolution due to the RFA treatment in a wider time scale. Another limitation was the absence of a formal power analysis. Moreover, CD31 staining visualizes overall microvascular patterns, but does not allow for the discrimination between newly formed angiogenic vessels and structurally persistent vasculature. The observed enrichment of CD31 + vessels at the tumor periphery may result from a combination of angiogenesis, inflammatory neovascular remodeling, and residual viable tumor vasculature. Future studies will include co-staining for α-SMA to identify pericyte-covered mature vessels, as well as immunohistochemical and molecular profiling for VEGF and HIF-1α to more precisely characterize the angiogenic and hypoxic responses to sublethal ablation.
Conclusion
Remarkable modification of perfusion and permeability state of tumor’s growth were found after RFA, according to the neoplasia’s inner features. The observed increases in vascular permeability and extracellular space, especially in the viable tumor rim post-RFA, suggest the existence of a time-sensitive therapeutic window for adjunctive treatments. These findings support the use of functional imaging biomarkers to guide post-ablation therapy planning and to optimize the timing of systemic treatments. Future clinical studies should explore whether integrating DCE-MRI into clinical workflows could enhance the efficacy of combination therapies and improve patient stratification in colorectal liver metastases and other solid tumors treated with RFA.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- Ve:
-
extravascular-extracellular-volume
- PNA:
-
partial necrotic area
- VTA:
-
viable tumor area
- RFA:
-
radiofrequency thermal ablation
- TTP:
-
time-to-peak
- DCE:
-
dynamic-contrast-enhanced
- CA:
-
contrast agent
- NE:
-
necrotic edematous
- AIF:
-
arterial-input-function
- ICA:
-
independent component analysis
References
Ahmed, M. Image-guided tumor ablation: standardization of terminology and reporting Criteria-A 10-year update, Radiology. Oct 273 (1), 241–260. https://doi.org/10.1148/radiol.14132958 (2014).
Yang, G. et al. The prognosis of radiofrequency ablation versus hepatic resection for patients with colorectal liver metastases: A systematic review and meta-analysis based on 22 studies. Int. J. Surg. 87, 105896 https://doi.org/10.1016/j.ijsu.2021.105896 (2021).
Wang, L. J. et al. Radiofrequency ablation versus resection for technically resectable colorectal liver metastasis: A propensity score analysis. World J. Surg. Oncol. 16 (1). https://doi.org/10.1186/s12957-018-1494-3 (Oct. 2018).
Wang, Z. M., Chen, Y. Y., Chen, F. F., Wang, S. Y. & Xiong, B. Peri-operative chemotherapy for patients with resectable colorectal hepatic metastasis: A meta-analysis. Eur. J. Surg. Oncol. 41 (9), 1197–1203 https://doi.org/10.1016/j.ejso.2015.05.020 (2015).
Goldberg, S. N. et al. Percutaneous radiofrequency tissue ablation: Does perfusion-mediated tissue cooling limit coagulation necrosis? J. Vasc. Interv. Radiol. 9 (1 I), 101–111 https://doi.org/10.1016/S1051-0443(98)70491-9 (1998).
Lu, D. S. K. et al. Radiofrequency ablation of hepatocellular carcinoma: Treatment success as defined by histologic examination of the explanted liver, Radiology 234 (3), 954–960 https://doi.org/10.1148/radiol.2343040153 (2005).
Shady, W. et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes-a 10-year experience at a single center. Radiology 278 (2), 601–611. https://doi.org/10.1148/radiol.2015142489 (Feb. 2016).
Vogl, T. J. et al. Thermal ablation of liver metastases from colorectal cancer: radiofrequency, microwave and laser ablation therapies. Radiologia Med. 119 (7), 451–461. https://doi.org/10.1007/s11547-014-0415-y (2014).
Olthof, S. C. et al. Single-centre survival analysis over 10 years after MR-guided radiofrequency ablation of liver metastases from different tumour entities. Insights Imaging. 13 (1). https://doi.org/10.1186/s13244-022-01178-8 (Dec. 2022).
Lubner, M. G., Brace, C. L., Hinshaw, J. L. & Lee, F. T. Microwave tumor ablation: mechanism of action, clinical results, and devices. Aug https://doi.org/10.1016/j.jvir.2010.04.007 (2010).
Cuenod, C. A. & Balvay, D. Perfusion and vascular permeability: basic concepts and measurement in DCE-CT and DCE-MRI. Elsevier Masson SAS. https://doi.org/10.1016/j.diii.2013.10.010 (2013).
Tofts, P. S. et al. Estimating Kinetic Parameters from Dynamic Contrast-Enhanced T 1-Weighted MRI of a Diffusable Tracer (Standardized Quantities and Symbols, 1999).
GitHub - neurolabusc/Bru2Nii. Bruker ParaVision to NIfTI conversion [Online]. Available: https://github.com/neurolabusc/Bru2Nii Accessed: May 25, 2025.
Ioanas, H. I. et al. An Automated Open-Source Workflow for Standards-Compliant Integration of Small Animal Magnetic Resonance Imaging Data, Front Neuroinform 14, https://doi.org/10.3389/fninf.2020.00005 (2020).
FSL utilities. [Online]. Available: https://fsl.fmrib.ox.ac.uk/fsl/docs/#/utilities/fslutils Accessed May 25, 2025.
ITK-SNAP Home. [Online]. Available: https://www.itksnap.org/pmwiki/pmwiki.php Accessed May 25, 2025.
Yushkevich, P. A., Gao, Y. & Gerig, G. ITK-SNAP: An interactive tool for semi-automatic segmentation of multi-modality biomedical images, in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Institute of Electrical and Electronics Engineers Inc., Oct. pp. 3342–3345. https://doi.org/10.1109/EMBC.2016.7591443 (2016).
Quantiphyse — Quantiphyse documentation. [Online]. Available: https://quantiphyse.readthedocs.io/en/latest/index.html Accessed May 22, 2025.
Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. FSL, Neuroimage 62 (2), 782–790 https://doi.org/10.1016/j.neuroimage.2011.09.015 (2012).
MELODIC. [Online]. Available: https://fsl.fmrib.ox.ac.uk/fsl/docs/#/resting_state/melodic Accessed May 22, 2025
Moon, J. et al. Correlation of quantitative dynamic contrast-enhanced MRI with microvascular density in necrotic, partial necrotic, and viable liver tumors in a rabbit model. J. Appl. Clin. Med. Phys. 17 (5), 418–427. https://doi.org/10.1120/jacmp.v17i5.6314 (2016).
Yang, W. et al. Thermosensitive liposomal doxorubicin plus radiofrequency ablation increased tumor destruction and improved survival in patients with medium and large hepatocellular carcinoma: A randomized, double-blinded, dummy-controlled clinical trial in a single center, J. Cancer Res. Ther. 15 (4), 773–783 https://doi.org/10.4103/jcrt.JCRT_801_18 (2019).
Chen, Y. et al. Neoadjuvant Chemotherapy Followed by Radiofrequency Ablation May Be a New Treatment Modality for Colorectal Liver Metastasis: A Propensity Score Matching Comparative Study, Cancers (Basel) 14 (21), https://doi.org/10.3390/cancers14215320 (2022).
Wang, X. et al. The efficacy and safety of radiofrequency ablation combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: a real-world study. BMC Cancer. 24 (1). https://doi.org/10.1186/s12885-024-12779-5 (Dec. 2024).
Maas, M. et al. Follow-up after radiological intervention in oncology: ECIO-ESOI evidence and consensus-based recommendations for clinical practice. Insights Imaging. 11 (1). https://doi.org/10.1186/s13244-020-00884-5 (Dec. 2020).
Funding
This project was co-funded by the Joint Project 2018 (JPVR18MXR8) of the University of Verona, of which the Department of Radiology was one of the recipients of the funds allocated. Further details about the Joint Projects initiative can be found at https://www.univr.it/it/joint-projects.All authors confirm that this funding does not influence the scientific integrity or the findings of this study, and there are no additional financial or non-financial interests directly related to the subject matter discussed in this manuscript.
Author information
Authors and Affiliations
Contributions
M.D., S.T., P.M. N.C and C.B. designed the research. D.C., I.M., M.G. contributed to data acquisition and image processing. N.C. and R.D.R contributed to the development of imaging protocols and technical optimization. M.G., D.C, S.T. and A.N. provided statistical analysis and methodological insights. F.V., F.M. and P.M. contributed to the anatomical and histological interpretation of imaging data. F.V., A.N. e P.M. contributed to the in vivo animal experimentation on the animal modelsM.D., S.P. and C.B. provided clinical expertise and selection criteria. M.G., D.C, M.T., L.T and S.T analyzed the data. S.T. and P.T. developed computational tools for data processing. M.D., D.C., L.T. M.T., S.T., F.M. and B.M. wrote the manuscript. B.M., A.N., S.T., S.P., R.D.R and P.M. critically reviewed and revised the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
D’Onofrio, M., Tomaiuolo, L., Tambalo, S. et al. 7T MRI and histology reveal early tissue and perfusion changes after radiofrequency ablation in a murine colorectal cancer model. Sci Rep 15, 32993 (2025). https://doi.org/10.1038/s41598-025-18068-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-18068-w