Abstract
a position paper by the Japanese Working Group for Respiratory Sarcopenia (JWGRS). However, the clinical values of probable RS remain unclear. Therefore, we conducted a longitudinal study to determine its impacts on short- and long-term all-cause mortality. Our data were extracted from the China Health and Retirement Longitudinal Study. A total of 5,006 participants were selected as a cohort in 2011, and followed up until 2020. According to the JWGRS, probable RS was defined as a coexistence of low respiratory muscle strength and low appendicular skeletal muscles (ASM) mass. Hazard ratios (HRs) for all-cause mortality and cause-specific mortality were calculated through Cox regression analyses. After an average of 9 years of follow up, 1,176 of the 5,006 participants (23.49%) died. Cox proportional hazard regression showed that low ASM mass (HR = 1.24, 95% CI = 1.09–1.41, P < 0.001), low respiratory muscle strength (HR = 1.31, 95% CI = 1.11–1.54, P = 0.001), and probable RS (HR = 1.31, 95% CI = 1.15–1.48, P < 0.001) were associated with increased all-cause mortality. However, when the follow-up period was shortened to 2 years, the association between probable RS and all-cause mortality became non-significant (P = 0.246), although it remained significant for respiratory-related mortality (HR = 3.16, 95% CI = 1.39–7.18, P = 0.006). Diagnosing probable RS and leading to intervention could significantly prevent and reduce the burden of long-term all-cause mortality.
Similar content being viewed by others
Introduction
Respiratory muscles, in common with many other appendicular skeletal muscles (ASM), are influenced by various factors such as aging, disease, inactivity, and malnutrition1. However, it is widely accepted that the loss of skeletal muscles does not always occur simultaneously or equally. For example, individuals with chronic lung diseases may experience the loss of both ASM and respiratory muscles, but the loss of respiratory muscles tends to be more significant2,3. As respiratory muscles are crucial for breathing, their loss can result in complications such as shortness of breath, exertional dyspnea, exercise intolerance, and decreased activities of daily living4. Additionally, a recent study by Morisawa et al.5. found that approximately 50% of community-dwelling older adults suffered from respiratory muscle weakness despite absence of systemic sarcopenia. Weakness in the respiratory muscles, diaphragm muscle in particular, can compromise airway clearance mechanisms (e.g., coughing and sneezing), putting people at a higher risk for developing pneumonia or other respiratory system infections6,7. Given that respiratory muscle wasting and weakness can lead to serious health complications, a deeper understanding of these conditions is crucial. In 2023, the Japanese Working Group for Respiratory Sarcopenia (JWGRS) introduced a reliable new definition of respiratory sarcopenia (RS) to facilitate systematic research8.
According to the JWGRS, RS was defined as a coexistence of low respiratory muscle strength and loss of respiratory muscle mass8. However, it should be noted that identifying low respiratory muscle mass remains difficult as there have not been sufficient data to quantitatively determine its cut-off point in clinical practice9. Considering this situation, the JWGRS added the concept of probable RS (defined as loss of ASM mass plus low respiratory muscle strength) if respiratory muscle mass was difficult to measure8. So far, the clinical values of probable RS remain unclear. Therefore, on the basis of a national study, we prospectively investigated the influences of probable RS on short- and long-term all-cause mortality in community-dwelling Chinese older adults aged ≥ 60. Additionally, as probable RS was a substitute for RS whose clinical values have been unfamiliar to us, our study also provides an new insight into the impact of RS on survival.
Methods
Study participants
The participants included in our study were abstracted from The China Health and Retirement Longitudinal Study (CHARLS) database. CHARLS is a prospective cohort initiated in 2011 recruiting socially and geographically representative Chinese adults aged ≥ 45 from 450 villages, 150 counties, and 28 provinces. A total of 17,705 adults were examined at baseline, and subsequently followed up every 2 or 3 years. The content of the examination involving demographic information, health status and functioning, health care and insurance, work retirement and pension, anthropometric measurements, and so on. Detailed information on the CHARLS has been reported in previous literature10. The protocol was approved by the Ethical Review Committee of Peking University (IRB00001052-11015). All participants provided written informed consent before the examination.
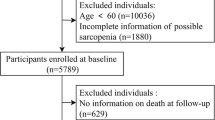
Up to now, a total of 5 waves of data have been released by the CHARLS project (wave 1, 2011y; wave 2, 2013y; wave 3, 2015y; wave 4, 2018y; wave 5, 2020y). Participants with the following criteria were excluded: (1) missing age or age < 60 in wave 1; (2) lack of key data such as gender, weight, height, peak expiratory flow rate (PEFR) or covariates in wave 1; (3) not participating in any follow-up from wave 2 to wave 5. Finally, a total of 5,006 community-dwelling Chinese adults aged ≥ 60 were chosen as a cohort for all-cause mortality and cause-specific mortality analyses. The detailed screening process is presented in Fig. 1.
Assessment of probable respiratory sarcopenia
According to the JWGRS, probable RS was defined as the presence of low respiratory muscle strength plus low ASM mass. In our study, PEFR was used to indicate low respiratory muscle strength, with a cut-off set at < 80% of the predicted value11. The predicted PEFR was calculated using the following formulas: PEFR (L/s) = 21.566 + 0.078 × H − 6.192 × ln(A) for men, and PEFR (L/s) = −22.741 + 6.901 × ln(H) − 0.090 × A for women, where A is age in years and H is height in centimeters12. These predictive formulas were derived from a study on elderly Chinese individuals aged 60–84 years in Jinan, a population that closely matches our study cohort, thereby minimizing bias due to population differences. During the measurement process, participants were asked to stand in an upright position, take as deep a breath as possible, and then blow as hard and as fast as they could into the peak flow meter (Everpure™ Peak Flow Meter, equipped with a disposable mouthpiece, manufactured by Shanghai Everpure Medical Plastic Co. LTD., Shanghai, China). PEFR was tested 3 times, and the best of the 3 records was selected as the PEFR value. For ASM, due to the fact that CHARLS did not measure it, we used the following formula for estimation, which has been validated by several studies showing strong agreement with values obtained from dual-energy X-ray absorptiometry (DXA)13,14,15:
where sex was set to 1 for men, 2 for women. According to the Asian Working Group for Sarcopenia (AWGS) 2019, low ASM mass for men and women were < 7.0 kg/m2 and < 5.4 kg/m216.
Outcome definition
In this study, the primary outcome was all-cause mortality, assessed during both the 2-year and 9-year follow-up periods. Cause-specific mortality, as a secondary outcome, was derived from the 2-year follow-up data and included deaths due to respiratory causes, as well as non-respiratory causes, which were classified into cancers, cardiovascular diseases (further categorized as coronary heart disease and stroke), and other medical causes.
Covariates
Variables that were mentioned in previous related studies16,17, or that were considered clinically relevant were selected as potential covariates, including sociodemographic characteristics, lifestyle factors, health status, and the components of sarcopenia. The sociodemographic variables included residential area (urban or rural), sex (men or women), age group (60–69 years, 70–79 years, or ≥ 80 years), marital status (married/married but separated, or unmarried/divorced/widowed), and education level (no formal education, primary school, middle school, and high school or above). Lifestyle factors included smoking status (no or yes), alcohol consumption (no or yes). The health status included the presence or absence of cardiovascular diseases, diabetes, cancer, chronic lung diseases, kidney diseases, liver diseases, other chronic conditions, and self-reported health (from “very good” to “very poor”). The components of sarcopenia included handgrip strength and physical performance. Low handgrip strength thresholds were defined as < 28 kg for men and < 18 kg for women18. Physical performance was assessed using Short Physical Performance Battery (SPPB), which included the walking test (assessing a combination of proximal leg strength, coordination and balance), the chair stand test (assessing proximal leg strength), and the tandem stand test (assessing balance)16. For the walking test and the chair stand test, scores ranged from 1 to 4 corresponding to the quartiles of time taken to complete the test in the total population (4 = best and 1 = worst performance); if the participant was not able to complete the test, the score was recorded as 0. For the tandem stand test, a score of 4 points was assigned if the participant was able to hold position for 10 s or longer; 2 points if able to hold on for 3–9 s. The score of 0 was assigned if participant held the position for less than 3 s or was not able to complete the test. The total score of the SPPB ranges from 0 to 12, with a score of 9 or lower being classified as low physical performance18.
Statistical analysis
The baseline characteristics of older adults are presented as numbers (percentages) according to the probable RS status. Statistical differences were compared by the chi-square test and Fisher exact test as appropriate. As the specific date and cause of death were only recorded in wave 2, the life table, stratified by ASM mass (low/normal to high), PEFR (low/normal to high), or probable RS status (yes/no) was used to characterize the change tendencies of all-cause mortality over 9 years of follow-up. The log-rank test was used to assess the equality of survival between the low ASM mass/low PEFR/probable RS and normal groups. Cox proportional hazards regression was performed to investigate the relationship between low ASM mass/low PEFR/probable RS and all-cause mortality after adjustment for potential confounding factors. The proportional hazards assumption was assessed using Schoenfeld residuals. Subgroup analyses were performed, and P-values for interaction were calculated to assess the consistency of associations across strata of key covariates, including chronic lung disease, sex, age group, and other confounders. Additionally, the exploratory analyses was conducted to investigate the influences of probable RS and its components on cause-specific mortality by using cox proportional hazards regression on the basis of cause of death database from wave 2. Data processing and analysis were performed using R version 4.3.0. All tests were 2-tailed, and P < 0.05 was considered statistically significant.
Results
In 2011, a total of 5,006 eligible older adults were included in our study. Among them, 1,450 (28.97%) were diagnosed with probable RS, 1,274 (25.45%) with sarcopenia, and 1,078 (21.53%) with both sarcopenia and probable RS. Table 1 shows the baseline characteristics according to probable RS status. Briefly, advanced age, women, being unmarried/divorced/widowed, lower education level, urban residence, chronic lung diseases, poor self-reported health, low handgrip strength, and poor physical performance were more prevalent in participants with probable RS (all P < 0.001). In contrast, the prevalence of cardiovascular diseases (P < 0.001) and diabetes (P < 0.001) was lower in those with probable RS. During an average of 9 years of follow-up, 1,176 participants (23.49%) died. The 9-year incidence of death was significantly higher in participants with probable RS compared to those without (32.69% vs. 19.74%, P < 0.001). Life table and log-rank analyses showed that participants with low ASM mass (HR = 1.66, 95% CI = 1.48–1.87, P < 0.001), low PEFR (HR = 1.66, 95% CI = 1.42–1.95, P < 0.001), and probable RS (HR = 1.75, 95% CI = 1.56–1.97, P < 0.001) had significantly shorter survival times than those without (Fig. 2).
Cox proportional hazards regression analyses further confirmed these findings (Table 2). In Model 1, after adjusting for PEFR, components of sarcopenia (ASM mass, handgrip strength, physical performance), chronic lung diseases, and other covariates, both low PEFR (HR = 1.31, 95% CI = 1.11–1.54, P = 0.001) and low ASM mass (HR = 1.24, 95% CI = 1.09–1.41, P < 0.001) remained significantly associated with increased 9-year all-cause mortality. There was no significant interaction between low ASM mass and low PEFR (P = 0.852; Table S1), suggesting that PEFR and ASM mass contribute additively, rather than synergistically, to mortality risk. In Model 2, which incorporated probable RS as an independent exposure, the adjusted hazard ratio was 1.31 (95% CI = 1.15–1.48, P < 0.001), indicating that probable RS independently predicted increased 9-year all-cause mortality.
Subgroup analyses revealed no significant interactions between probable RS and any of the included confounding variables (all P for interaction > 0.05; Table S4). Notably, when stratified by chronic lung disease status, probable RS remained significantly associated with increased 9-year all-cause mortality in both participants without (HR = 1.26, 95% CI = 1.09–1.45, P = 0.002) and with chronic lung disease (HR = 1.49, 95% CI = 1.15–1.94, P = 0.003), with no significant interaction observed (P for interaction = 0.161). These findings suggest that the association between probable RS and mortality is robust regardless of chronic lung disease status. Similarly, no significant interactions were detected between low ASM mass or low PEFR and any other potential confounders (Tables S2 and S3).
Due to the lack of cause-of-death information after wave 2, analyses of cause-specific mortality were restricted to the 2-year follow-up. During this period, 188 participants (3.76%) died (Table 3). Cox proportional hazard regression showed that, for all-cause mortality, neither probable RS nor its components were associated with an increased risk after 2 years. With regard to cause-specific mortality, only respiratory-related deaths were affected, with low PEFR (HR = 2.48, 95% CI = 1.10–5.64, P = 0.029) and probable RS (HR = 3.16, 95% CI = 1.39–7.18, P = 0.006) associated with an increased risk, while low ASM mass (HR = 1.34, 95% CI = 0.39–4.60, P = 0.642) showed no significant association.
Discussion
To our knowledge, this is the first longitudinal study to investigate the relationship between probable RS and all-cause mortality on the basis of a nationally representative sample. After a mean observation time of 9 years, multivariable analyses demonstrated that having probable RS or its components (low ASM mass and low PEFR) were associated with a 31%, 24% and 31% increased risk of all-cause mortality, respectively, in Chinese community-dwelling older adults. However, when the follow-up was reduced to 2 years, the association between probable RS and all-cause mortality was no longer significant, although it remained significant for respiratory-related mortality. These findings suggest that low ASM mass, low PEFR, and probable RS present a gradually accumulating health risk, which does not immediately affect all-cause mortality in the short term but contributes to an increase in long-term mortality.
Recently, a meta-analysis conducted by Zhou et al.19. has indicated that muscle wasting, defined as loss of muscle mass, was associated with 47% increased risk of all-cause mortality in participants aged ≥ 65 years, which is higher than the counterpart value in our study. Also, Zhou et al.19. showed that all-cause mortality risk was higher in participants with shorter follow-ups, which is completely different from our finding. Considering the generally cumulative nature of chronic diseases’ effects on mortality, their finding appears illogical, which may be resulted from the high proportion of participants aged ≥ 75 years in the low follow-up group. In line with our results, several studies found low PEFR a predictor of subsequent death in older adults during long-term follow-up20,21. However, Kera et al.22 reported that the relationship between low PEFR and all-cause mortality became insignificant after adjusting for covariates following a 5-year follow-up. The cut-off values for PEFR proposed by Kera et al.. were relatively low, which theoretically may have made it easier to observe a significant association between PEFR and all-cause mortality. One possible reason for this lack of significance could be the limited statistical power due to a small sample size—only 470 participants were included in their study, and 8 deaths occurred in the RS group over 5 years. Moreover, we explored whether the prognostic impact of probable RS simply reflected its overlap with conventional sarcopenia. Our results showed that both low PEFR and low ASM mass were independently associated with long-term mortality, and the absence of interaction between them (P = 0.852) suggests additive rather than synergistic effects. This indicates that probable RS captures the cumulative burden of respiratory and appendicular muscle decline, providing complementary prognostic value beyond conventional sarcopenia.
On the basis of cause-specific mortality analyses, we found that older adults with probable RS were confronted with a three-fold higher risk of respiratory mortality. The mechanisms for the association of probable RS with increased respiratory mortality may be attributed to airway clearance disturbance and decreased ventilatory reserve. Due to respiratory muscle weakness, older adults with probable RS were not only more likely to suffer from shortness of breath, but also unable to clear foreign pathogens, inflammation, secretions, or allergic factors effectively. Both dysfunctions may increase their susceptibility to various respiratory diseases. For example, Okazaki et al.6 reported that respiratory muscles weakness was associated with a high risk for pneumonia (OR:6.85, 95% CI:1.56–31.11, P = 0.011). Also, respiratory and peripheral muscle dysfunction are considered primary contributing factors for chronic obstructive pulmonary disease (COPD)23,24. It is estimated that in 2016 chronic respiratory diseases and respiratory infections were responsible for more than 61 million and 91 million years of life lost, respectively25. In turn, the onset or worsening of various respiratory diseases could exacerbate muscle wasting through multiple pathways: (1) respiratory diseases such as aspiration pneumonia could release lots of inflammatory cytokines (e.g., interlukine-6 and tumor necrosis factor-α), which is known as an important ground for catabolism in skeletal muscle26,27; (2) shortness of breath could lead to exercise intolerance, reduced physical activities, and decreased appetite and nutritional intake8,28,29; and (3) mechanically ventilated conditions were found to be associated with low muscle strength and muscle mass of the diaphragm30,31,32. Based on the vicious cycle, probable RS was also shown to be related to poor prognosis for respiratory diseases. Several studies of patients with COPD demonstrated that severity increased with the decreasing inspiratory muscle strength or diaphragm index33,34,35. Additionally, decreased paraspinal muscle area was found to be an independent risk factor to predict death in life-threatening pneumonia36. On the contrary, a considerable number of studies revealed that improving respiratory muscle mass and function by implementing all kinds of respiratory muscle training programs could have positive effects on the treatment of respiratory diseases37,38,39.
The present study has several strengths. First, our sample population was from a nationally representative longitudinal survey for the community-dwelling older adults, which made generalizability of our findings relatively high. Second, this is the first study to prospectively investigate the association of probable RS defined by JWGRS with all-cause mortality after adjustments for multiple confounding factors in Asia. Third, the present study provided new evidence for reducing long-term all-cause mortality through intervention of probable RS in the community-dwelling older adults.
The limitations of this study must be acknowledged. First, PEFR was used in our study to represent respiratory muscle strength due to its practicality in large-scale epidemiologic surveys. However, PEFR is influenced not only by respiratory muscle capacity but also by airway patency, underlying pulmonary conditions and participant effort, and may not fully reflect true inspiratory muscle strength (e.g., maximal inspiratory pressure). This limitation should be considered when interpreting our findings. Nevertheless, our stratified analysis showed that the association between probable RS and all-cause mortality remained consistent regardless of chronic lung disease status, suggesting that the predictive validity of PEFR in this context may be robust. Second, ASM mass was estimated using an anthropometric equation rather than directly measured by DXA. Although the predictive formula by Wen et al.40. demonstrates a strong correlation with DXA-derived values (adjusted R²=0.90, standard error = 1.63 kg) and has been validated in nearly 100 publications, it remains an indirect proxy and may introduce estimation errors at the individual level. This limitation is particularly important given that ASM is a core component in defining probable RS in our study. However, anthropometric equations offer a practical and low-cost alternative in large-scale epidemiological research, especially in resource-constrained settings like CHARLS. Moreover, previous studies using other anthropometric measures of muscle mass (e.g., mid-arm muscle circumference41 or calf circumference42 have reported HRs for mortality comparable to those derived from DXA, supporting the predictive validity of anthropometry-based approaches. Nevertheless, the potential bias introduced by estimation should be carefully considered when interpreting our findings, and future studies using DXA-based validation are warranted. Third, although residual confounding cannot be fully ruled out due to the absence of some important variables such as dietary intake and physical activity, we performed a sensitivity analysis adjusting for high-sensitivity C-reactive protein (hsCRP), a widely used marker of systemic inflammation, in a subset of 3,735 participants (Table S5). The results remained materially unchanged, suggesting that systemic inflammation alone is unlikely to account for the observed associations. Nevertheless, future studies incorporating more comprehensive Lifestyle and biological markers are needed to validate our findings. Fourth, because cause-of-death data were only available in wave 2, our analysis of cause-specific mortality was limited to the first two years of follow-up. Consequently, we were unable to evaluate long-term cause-specific mortality trends, particularly those related to respiratory causes. This limitation restricts the strength of causal inferences regarding specific causes of death. Future studies with extended follow-up are needed to validate these associations. Finally, since our study population consisted exclusively of community-dwelling older Chinese adults, the generalizability of our findings to institutionalized populations or individuals from other ethnic backgrounds remains uncertain. Future studies involving more diverse cohorts are needed to assess external validity and ensure broader applicability of the results.
Conclusions
In conclusion, our findings suggest that probable RS significantly increases the risk of long-term all-cause mortality, likely due to respiratory-related deaths, among community-dwelling older adults in China. These results highlight the need for diagnosing and intervening in probable RS.
Data availability
Details of how to access the CHARLS data and details of the data release schedule are available from https://charls.charlsdata.com/index/zh-cn.html.
References
Cruz-Jentoft, A. J. et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 48 (1), 16–31 (2019).
Mizusawa, H. et al. Evaluation of patients with chronic obstructive pulmonary disease by maximal inspiratory pressure and diaphragmatic excursion with ultrasound sonography. Respiratory Invest. 62 (2), 234–239 (2024).
Garcia, T. et al. Characteristics of skeletal muscle strength in subjects with interstitial lung disease. Arch. Phys. Med. Rehabil. (2024).
Nagano, A. et al. Respiratory sarcopenia and sarcopenic respiratory disability: concepts, diagnosis, and treatment. J. Nutr. Health Aging. 25 (4), 507–515 (2021).
Morisawa, T. et al. The relationship between sarcopenia and respiratory muscle weakness in Community-Dwelling older adults. Int. J. Environ. Res. Public. Health 18(24) (2021).
Okazaki, T. et al. Respiratory muscle weakness as a risk factor for pneumonia in older people. Gerontology 67 (5), 581–590 (2021).
Elliott, J. E., Greising, S. M., Mantilla, C. B. & Sieck, G. C. Functional impact of sarcopenia in respiratory muscles. Respir. Physiol. Neurobiol. 226, 137–146 (2016).
Sato, S. et al. Respiratory sarcopenia: A position paper by four professional organizations. Geriatr. Gerontol. Int. 23 (1), 5–15 (2023).
Miyazaki, S., Tamaki, A., Wakabayashi, H. & Arai, H. Definition, diagnosis, and treatment of respiratory sarcopenia. Curr. Opin. Clin. Nutr. Metabol. Care (2023).
Zhao, Y., Hu, Y., Smith, J. P., Strauss, J. & Yang, G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int. J. Epidemiol. 43 (1), 61–68 (2014).
Kera, T. et al. Validating respiratory sarcopenia diagnostic criteria by mortality based on a position paper by four professional organizations: Insights from the Otassha study. Geriatr. Gerontol. Int. (2024).
Tian, X. Y. et al. Spirometric reference equations for elderly Chinese in Jinan aged 60–84 years. Chin. Med. J. 131 (9), 1016–1022 (2018).
Dong, Y., Xi, Y., Wang, Y. & Chai, Z. Association between sarcopenia and frailty in middle-aged and elder population: findings from the China health and retirement longitudinal study. J. Global Health. 14, 04163 (2024).
Zhang, J., Jia, X., Li, Y., Li, H. & Yang, Q. The longitudinal bidirectional association between sarcopenia and cognitive function in community-dwelling older adults: findings from the China health and retirement longitudinal study. J. Global Health. 13, 04182 (2023).
Luo, Y. X. et al. Bidirectional transitions of sarcopenia States in older adults: the longitudinal evidence from CHARLS. J. Cachexia Sarcopenia Muscle. 15 (5), 1915–1929 (2024).
Eekhoff, E. M. W. et al. Relative importance of four functional measures as predictors of 15-year mortality in the older Dutch population. BMC Geriatr. 19 (1), 92 (2019).
Smith, M. et al. Peak flow as a predictor of cause-specific mortality in china: results from a 15-year prospective study of ~ 170,000 men. Int. J. Epidemiol. 42 (3), 803–815 (2013).
Chen, L. K. et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 21 (3), 300–7e2 (2020).
Zhou, H. H. et al. Association of muscle wasting with mortality risk among adults: A systematic review and meta-analysis of prospective studies. J. Cachexia Sarcopenia Muscle. 14 (4), 1596–1612 (2023).
Cook, N. R. et al. Peak expiratory flow rate and 5-year mortality in an elderly population. Am. J. Epidemiol. 133 (8), 784–794 (1991).
Fragoso, C. A., Gahbauer, E. A., Van Ness, P. H., Concato, J. & Gill, T. M. Peak expiratory flow as a predictor of subsequent disability and death in community-living older persons. J. Am. Geriatr. Soc. 56 (6), 1014–1020 (2008).
Kera, T. et al. Respiratory sarcopenia is a predictor of all-cause mortality in community-dwelling older adults-The Otassha study. J. Cachexia Sarcopenia Muscle. 14 (4), 1894–1899 (2023).
Henrot, P. et al. Main pathogenic mechanisms and recent advances in COPD peripheral skeletal muscle wasting. Int. J. Mol. Sci. 24(7) (2023).
Barreiro, E. & Gea, J. Molecular and biological pathways of skeletal muscle dysfunction in chronic obstructive pulmonary disease. Chronic Resp. Dis. 13 (3), 297–311 (2016).
Foreman, K. J. et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet (London England). 392 (10159), 2052–2090 (2018).
Bano, G. et al. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 96, 10–15 (2017).
Tu, H. & Li, Y. L. Inflammation balance in skeletal muscle damage and repair. Front. Immunol. 14, 1133355 (2023).
Baig, M. M. A., Hashmat, N., Adnan, M. & Rahat, T. The relationship of dyspnea and disease severity with anthropometric indicators of malnutrition among patients with chronic obstructive pulmonary disease. Pakistan J. Med. Sci. 34 (6), 1408–1411 (2018).
Komatsu, R. et al. Aspiration pneumonia induces muscle atrophy in the respiratory, skeletal, and swallowing systems. J. Cachexia Sarcopenia Muscle. 9 (4), 643–653 (2018).
Levine, S. et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N. Engl. J. Med. 358 (13), 1327–1335 (2008).
Hermans, G., Agten, A., Testelmans, D., Decramer, M. & Gayan-Ramirez, G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit. Care. (London, England). 14 (4), R127 (2010).
Grosu, H. B. et al. Diaphragm muscle thinning in subjects receiving mechanical ventilation and its effect on extubation. Respir. Care. 62 (7), 904–911 (2017).
Kim, N. S. et al. Respiratory muscle strength in patients with chronic obstructive pulmonary disease. Annals Rehabilitation Med. 41 (4), 659–666 (2017).
Singer, J. et al. Respiratory and skeletal muscle strength in chronic obstructive pulmonary disease: impact on exercise capacity and lower extremity function. J. Cardiopulm. Rehabil. Prev. 31 (2), 111–119 (2011).
Bakker, J. T. et al. Automated evaluation of diaphragm configuration based on chest CT in COPD patients. Eur. Radiol. Experimental. 8 (1), 87 (2024).
Guo, K. et al. Skeletal muscle depletion predicts death in severe community-acquired pneumonia patients entering ICU. Heart Lung: J. Crit. Care. 52, 71–75 (2022).
Li, Y., Ji, Z., Wang, Y., Li, X. & Xie, Y. Breathing exercises in the treatment of COPD: an overview of systematic reviews. Int. J. Chronic Obstr. Pulm. Dis. 17, 3075–3085 (2022).
Irina, B. P. et al. Respiratory muscle training program supplemented by a cell-phone application in COPD patients with severe airflow limitation. Respir. Med. 190, 106679 (2021).
Villelabeitia-Jaureguizar, K. et al. Low intensity respiratory muscle training in COVID-19 patients after invasive mechanical ventilation: A retrospective Case-Series study. Biomedicines 10(11) (2022).
Wen, X., Wang, M., Jiang, C. M. & Zhang, Y. M. Anthropometric equation for Estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pac. J. Clin. Nutr. 20 (4), 551–556 (2011).
Landi, F. et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from IlSIRENTE study. Age Ageing. 42 (2), 203–209 (2013).
Arango-Lopera, V. E., Arroyo, P., Gutiérrez-Robledo, L. M., Pérez-Zepeda, M. U. & Cesari, M. Mortality as an adverse outcome of sarcopenia. J. Nutr. Health Aging. 17 (3), 259–262 (2013).
Acknowledgements
We would like to acknowledge the China Health and Retirement Longitudinal Study (CHARLS) team for providing high quality, nationally representative data, which make it possible for our study.
Funding
The study has not been sponsored.
Author information
Authors and Affiliations
Contributions
Study design: CKK. Data collection: CQF and HMG. Data analysis: ZY. Result interpretation: ZY. Reporting & editing: ZY and CKK. Final approval of the version to be submitted: CKK, ZY, CQF, and HMG.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
CHARLS study is an open dataset. The CHARLS was approved by the Ethics Review Committee of Peking University (IRB 00001052–11015).
Human and animal rights statement
CHARLS study is an open dataset. All procedures performed in CHARLS study involving human participants were in accordance with the ethical standards of the Ethics Review Committee of Peking University (IRB 00001052–11015) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
All participants signed an informed consent.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, Y., Chen, Q., Huang, M. et al. Probable respiratory sarcopenia and 9-year mortality in community-dwelling older adults: the first longitudinal evidence from the CHARLS. Sci Rep 15, 33022 (2025). https://doi.org/10.1038/s41598-025-18511-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18511-y