Abstract
Breast milk serves as a critical source of nutrition for infants and significantly impacts their developmental trajectories and health outcomes throughout their lives. This study investigates the role of breast milk-derived stem cells (BMSCs) and their potential involvement in recovery following brain ischemia. Prior research has demonstrated that BMSCs can migrate into the digestive tract of nursing offspring and subsequently differentiate into various cell types, including neurons and astrocytes within the brain. Our study aims to elucidate whether these differentiated BMSCs contribute to cellular recovery in response to ischemic events in a mouse model. Utilizing a cerebral ischemia/reperfusion (I/R) model, we found that maternal transfer of BMSCs through breastfeeding allows these cells to reach the central nervous system (CNS) of offspring and persist into adulthood. Notably, following ischemic injury, we observed enhanced recruitment and differentiation of these BMSCs into neuronal and glial lineages specifically in the peri-infarct region. This study represents the first evidence of a natural stem cell-mediated repair mechanism within the injured CNS facilitated by maternal breastfeeding, revealing significant implications for therapeutic strategies targeting maternal-neonatal interactions. Our findings suggest that BMSCs may play a protective role in the aftermath of cerebral ischemic injury. These findings reveal a natural, maternally derived stem cell-mediated repair mechanism in the injured CNS, highlighting BMSCs’ dual role in development and pathology in adulthood.
Similar content being viewed by others
Introduction
Breast milk is an essential source of nutrition playing a role in development and future health of infants later in life1. Postnatal breastfeeding has been shown to reduce the prevalence of many diseases, especially infectious diseases, in infants2. The nutritional composition of breast milk includes macro and micronutrients like protein, fat, vitamins, minerals, bioactive molecules, and various types of cells3,4. Ductal, alveolar, and epithelial cells from mammary gland have also been observed in breast milk as well as stem cell populations5,6,7. Several studies also demonstrated that these breast milk stem cells (BMSCs) can transfer into breast milk via cell migration or mechanical effects of breastfeeding7,8,9,10,11. These cells can differentiate into various functional cells such as myoepithelial cells, hepatocytes, osteoblasts, and neuron-like cells7,12. There has also been a plethora of studies showing that these cells pass through the digestive system of the offspring and can migrate to different parts and organs of offspring and differentiate into functional cells8,13,14,15,16.
In our previous study, we showed that breast milk cells can transfer and differentiate into neurons and astrocytes in various brain regions of breastfed pups even when they reached adulthood8. However, it remains unclear whether differentiated BMSCs participate in responses to pathological conditions. Since they can differentiate into the central nervous system (CNS) cells, it is important to understand their role in CNS pathologies, such as brain ischemia. Brain ischemia, the occlusion or rupture of the vessels supplying the brain, is one of the leading causes of disability and death worldwide, and long-term care of patients is usually required due to brain injury related motor and psychological debilitations17,18,19. Understanding acute and long term structural pathophysiological processes and recovery after injury is necessary for finding novel targets for treatment. Neurogenic activity increases after cerebral ischemia, leading progenitors originating from the subventricular zone to redirect toward damaged brain tissue, which contributes to glial scar formation19,20,21,22.
In this context, we aimed to observe whether natural transfer of BMSCs through breastfeeding in infancy participate in the recovery process after cerebral ischemia in mice. Here we show that BMSCs not only reach the offspring’s CNS under physiological conditions, but also survive into adulthood and differentiate into neurons, astrocytes, and oligodendrocytes. Remarkably, following ischemic injury, we observed a significantly increased recruitment and neural differentiation of these maternally transferred BMSCs in the peri-infarct region. This is the first study to unveil a previously unrecognized, naturally occurring stem cell-mediated repair mechanism in the injured CNS, opening new avenues for maternal-neonatal therapeutic strategies. However, the mechanisms of recruitment and differentiation of these cells to the injured site remains to be elucidated.
Materials and methods
Animals and experimental groups
All experiments were conducted in conformity with institutional guidelines with European Economic Community Council Directive 86/609, reported in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guideline, and approved by the Animal Research Ethics Committee of İstanbul Medipol University. FVB-Tg(Prism)1989Htz/J (#018068) (Tg-Prism) and FVB/NJ (#001800) (WT) mice were obtained from Jackson Laboratory (ME, USA). Both Tg-Prism and WT mice aged 8–16 weeks weighed between 25 and 30 g. Tg-Prism mice express three distinct fluorophores in specific subsets of cells in the brain: a cerulean fluorescent protein (CFP) under the control of the Mobp promoter in oligodendrocytes, DsRedMax under the control of the Aldh1l1 promoter in astrocytes, and a YFP-tagged ribosomal protein L10A (Rpl10a) under the Snap25 promoter in neurons23. Animals were kept on a 12-hour light/dark cycle with ad libitum access to food and water in temperature and humidity-controlled cages.
Experimental groups were designed as follows; (i) Wild-type Breastfeeding Group (WT-BF), newborn WT pups were breastfed by WT mothers (n = 10), (ii) Prism Breastfeeding Group (Prism-BF), newborn WT pups were breastfed by Tg-Prism mothers (n = 10), (iii) Prism Breastfeeding Ischemia Group (Prism-BF I/R) newborn WT pups were breastfed by Tg-Prism mothers and undergone cerebral ischemia/reperfusion operation at P60 (n = 10). After the one-month nursing period, foster mothers and pups were separated in all groups. At P112, mice that were used for clearing and imaging were anesthetized with an intraperitoneal injection of ketamine (Pfizer, 100 mg/kg) and xylazine (10 mg/kg) to prepare for trans-cardiac perfusion. Mice, that were used in flow cytometry experiments, were euthanized using carbon dioxide (CO₂) delivered via a gradually rising concentration method. Specifically, 100% CO₂ was introduced into the euthanasia chamber at a fill rate of approximately 20–30% of the chamber volume per minute. The gas was administered via the top of the chamber, ensuring uniform exposure. Mice were monitored continuously and confirmed dead by cessation of respiration and lack of reflexes before being removed. Five brain tissues from each group were used for microscopy, while five were used for flow cytometry.
Middle cerebral artery occlusion/reperfusion (MCAO/R)
The MCAO/R method was adapted from previously published work24,25,26. 2-month old mice of Prism-BF/IR group were kept under gas anaesthesia (1% isoflurane, 30% O₂, remainder N₂O) delivered via a nose cone using an animal anaesthesia system (E-Z Systems) while their body temperature was maintained at 36.5–37 °C using a feedback-controlled heating system during the MCAO/R period. Laser Doppler flowmetry (Perimed, Sweden) with a 0.5 mm fibre optic probe was used to monitor changes in cerebral blood flow instantaneously during occlusion and reperfusion. The fibre optic probe was attached with dental adhesive directly on intact skull of the injured hemisphere (2 mm posterior and 6 mm lateral from Bregma). Monofilament technique was used to implement a MCAO model. After incision in the neck region, carotid arteries were isolated and ligated with 6 − 0 silk thread (Doğsan, Turkey). A microvascular clip (FE691, Aesculap, USA) was briefly placed on internal carotid, and common carotid artery was cut with vascular scissors, and then 7 − 0 thick monofilament (701934PK5Re, Doccol, USA) was inserted approximately 9 mm into internal carotid artery through incision. After 30 min, monofilament was removed to initiate reperfusion, carotid arteries were permanently ligated to prevent postoperative arterial bleeding and ensure hemostasis. Anaesthesia was discontinued and animals were placed back into their cages to recover.
Functional recovery tests
Functional neurological recovery was evaluated with behavioural tests performed on the 3rd, 14th, 28th and 42nd days after MCAO/R injury. The first behavioural tests were performed 1 day before the operation to establish a baseline as described previously27. For the Hand Pull/Grip Strength test, a spring-loaded Newton-meter with a triangular steel wire attached was used. The left paws were fixed with medical tape, and the grip strength of the right front paw was measured. Motor Coordination was evaluated with rotarod test. Mice were placed on a rotating cylinder that gradually accelerated from 4 to 40 rpm over 245 s, and the duration of retention on the cylinder was recorded. Results were compared across groups and time points.
Flow cytometry
Sample preparations were carried out as described previously8. Briefly, mice were sacrificed with CO2 followed by swift decapitation. The ischemic (ipsilateral) and contralesional (contralateral) hemispheres of brain samples were separately cut into 1 mm sections with a razor blade on brain matrix (WPI, USA) and incubated in Hibernate-A (Gibco, US) medium with Papain (4 mg/ml) (P4762, Sigma, US) for one hour at 30 °C, and then triturated vigorously. Samples were centrifuged for 5 min at 500 g, supernatant was discarded, and the pellet was resuspended in 1X PBS. To detect oligodendrocytes (CFP), neurons (YFP) and astrocytes (DsRed), 405 nm, 488 nm and 561 nm excitation lasers and emission filter sets with compensation setup were used accordingly. As for gating strategy, forward scatter (FSC) and side-scatter (SSC) plots were used to eliminate cell debris and doublets. Then, nuclear stain (DRAQ-5) was used to select cells with nuclei. After that CFP, DsRed and YFP expressing cells were identified. Brains from WT and Tg-Prism mice were used as negative and positive controls respectively, to determine gating parameters and thresholds for all cell populations. Percentage of cells with each fluorescent signal in I/R groups were analysed across ipsilateral and contralateral hemispheres of each mouse. To validate cell types and respective fluorescent signals, cells were sorted and collected in separate tubes. Sorted cells were examined under laser scanning confocal microscope (LSM 780, Carl Zeiss, Germany) for further confirmation.
Tissue clearing and immunohistochemistry (IHC)
The mice were anesthetized with an intraperitoneal injection of ketamine (Pfizer, 100 mg/kg) and xylazine (10 mg/kg). Animals were perfused transcardially with 0.1 M PBS with heparin (10 U/ml of heparin, Nevparin, Mustafa Nevzat) following with 4% paraformaldehyde (PFA, pH 7.4) in PBS. Brain tissues were collected and postfixed in 4% PFA for 3 days at 4 °C. Tissues were washed with PBS for 1 h before sectioning coronally to 1 mm slices using a vibratome (Leica, Germany) and stored in PBS until staining. For IHC staining, the samples were incubated in blocking solution (0.1 M PBS with 1% goat serum, 3% bovine serum albumin, 0.3% sodium azide, 0.2% Triton X-100) overnight at 37 °C and washed with PBS for 1 h. Then, the samples were incubated with conjugated primary antibodies (1:500 anti-GFAP-Alexa488 for astrocytes (53-9892-82, ThermoFisher Scientific), 1:100 anti-MBP-Alexa488 for oligodendrocytes (836505, Biolegend), 1:200 anti-GFP-Alexa594 for neurons (A-21312, ThermoFisher Scientific) for 3 days at 37 °C. After washing with PBS for 1 day, sections were incubated with TO-PRO™-3 (T3605, ThermoFisher Scientific) at 1:1000 concentration in PBS for 5 h at room temperature and washed again with PBS. After IHC staining, uDISCO tissue clearing method described by Pan et al.28 was used on coronal brain sections. Firstly, tissues were dehydrated in tert-butanol (360538, Sigma-Aldrich)/distilled water solution series for 1 h in each following concentration (v/v); 30%, 50%, 70%, 80%, 90%, 96% and pure (%100) tert-butanol at 37 °C. Then, pure dichloromethane (270997, Sigma-Aldrich) was used as delipidation agent for 15 min at room temperature. Finally, a mixture of benzyl alcohol (24122, Sigma-Aldrich), benzyl benzoate (W213802, Sigma-Aldrich) and diphenyl ether (A15791, Alpha Aesar) (BABB-D10, at a ratio of 3:6:1, respectively) adding 0.4% DL-alpha-tocopherol (A17039, Alpha Aesar) was used as a refractive index (RI:1.561) matching solution. Tissues were incubated in BABB-D10 at room temperature until samples became optically transparent.
Imaging
All cleared brain samples and sorted cells from flow cytometry were imaged using laser scanning confocal microscope (LSM 780, Carl Zeiss, Germany) in a glass bottomed chamber. CFP, DsRed, and YFP signals were imaged using excitation lasers at 405 nm, 561 nm, and 514 nm, respectively. Cell subtypes were validated as oligodendrocytes, astrocytes, and neurons by immunolabeling with anti-MBP, anti-GFAP, and anti-GFP antibodies, and detected using 488 nm, 488 nm, and 594 nm excitation lasers, respectively. Imaging was performed on brain sections using 10x/0.4NA, 20x/0.8NA, or 40x/1.4NA Plan-Apochromat objectives. Z-stack and x, y tile images were taken from striatum for analysis. The number of each cell type were counted and compared for ipsilateral and contralateral striatum in each group.
Statistical analysis
Statistical analyses of flow cytometry and microscopy data were performed using one-way ANOVA in GraphPad Prism version 10.1.0 for Windows (GraphPad Software, San Diego, CA, USA) to compare ipsilateral and contralateral brain regions across all groups. Data are presented as mean ± S.E.M values, p values < 0.05 were considered significant unless stated otherwise.
Results
Tg-Prism mice can be used for identifying different cell types in brain
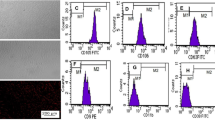
We investigated differentiation of BMSCs in healthy and injured nervous tissue via 1-month of breastfeeding of Tg-Prism mice mothers to wild-type pups. Two-month-old breastfed mice were subjected to ischemia by MCAO/R at 2 months of age (Fig. 1a). Brain tissues were collected 52 days after I/R. To confirm that the fluorescent proteins expressed in distinct cell types of Tg-Prism mice can be visualized following tissue clearing, coronal brain sections from adult Tg-Prism mice were imaged using confocal microscopy. The expression of CFP, YFP, and DsRed was validated in oligodendrocytes, neurons, and astrocytes, respectively (Fig. 1). Gating strategy for the flow cytometry analysis was determined using resuspended brain tissues from wild-type and Tg-Prism mice. Single cells were identified using FSC/SSC analysis to avoid debris in tissue suspensions in addition to nuclear staining (DRAQ5) and singlets were considered for further analysis. To optimize flow cytometry parameters for Tg-Prism mice, a gating strategy was established using dissociated brain tissues from both wild-type and Tg-Prism mice (Supplementary Fig. 1). Single cells were identified based on forward and side scatter (FSC/SSC) to exclude debris, complemented by nuclear staining with DRAQ5, and singlets were selected for downstream analysis (Supplementary Fig. 2, Fig. 1c).
The distribution ratio of each distinct cell type was calculated according to the same gating strategy among all the samples. The percentages of each cell type from brain samples of Tg-Prism mice were determined as 6.98%±0.45, 9.11%±2.02 and 33.07%±6.52 for oligodendrocytes, neurons, and astrocytes respectively (Fig. 1c).
Experimental setup and characterization of Tg-Prism mice. (a) Left and middle panels summarize the breeding and breastfeeding strategy. WT pups are breastfed by Tg-Prism mothers for 1 month and subjected to MCAO/R at 2 months of age. (b) Representative confocal microscopy image of a brain slice from adult Tg-Prism mice expressing fluorescent proteins in three different cell types: oligodendrocyte (CFP), neuron (YFP) and astrocyte (DsRed). (c) Flow cytometry analysis of Tg-Prism brain tissues. Top panel shows the YFP + cells (neurons) in Tg-Prism brain compared to WT brain samples. Bottom panel shows the CFP+ (oligodendrocytes) and DsRed+ (astrocytes) cells in Tg-Prism brain compared to WT brain samples. The graph illustrates the distribution percentages of different cell types analysed by flow cytometry in brain tissues in Tg-Prism mice and WT controls (n = 3).
BMSCs can migrate into brain and differentiate into oligodendrocytes, neurons, and astrocytes
WT pups breastfed by Tg-Prism mothers were examined to detect if BMSCs can migrate to the brain tissues under normal conditions. Whole brains were collected for both flow cytometry and microscopy analysis at P122. Microscopy revealed BMSC-derived fluorescent signals in various brain regions, which were confirmed by antibody staining (Fig. 2a). Fluorescent protein-expressing cells were detected in Prism-BF mice but not in WT-BF controls, including YFP + neurons (1.46 ± 0.42), CFP + oligodendrocytes (1.73 ± 0.39), and DsRed + astrocytes (3.80 ± 0.67) (Fig. 2d. Flow cytometry analysis confirmed the presence of these BMSC-derived cells in adult Prism-BF mice, with cell percentages of YFP + neurons (0.016 ± 0.003), CFP + oligodendrocytes (0.035 ± 0.005), and DsRed + astrocytes (0.052 ± 0.01) (Fig. 2b, c). BF group consistently showed a fluorescence expressing cells, whereas all values in the WT group were zero across all biological replicates.
Differentiation of BMSCs into CNS cells in breastfed wild-type mice. (a) Immunofluorescence images showing BMSC origin YFP+, CFP + and DsRed + cells in cleared brain sections that were stained against anti-GFP, anti-MBP and anti-GFAP for neurons, oligodendrocytes, and astrocytes respectively. (b) Flow cytometry analysis of brain tissues. Left panel shows the YFP + cells (neurons) and the right panel shows the CFP+ (oligodendrocytes) and DsRed+ (astrocytes) cells. (c) Quantitative analysis of flow cytometry of 2-months old Prism-BF compared to WT-BF (n = 5). (d) Quantitative analysis of confocal microscopy images of oligodendrocytes, neurons, and astrocytes obtained from three randomized ROIs (each 8 × 106µm2) per animal in the striatum of 2-months old Prism-BF compared to WT-BF (n = 5). The mean value of the three ROIs was calculated for each animal and used as a single biological replicate in the analysis, error bars represents standard deviation.
MCAO/R-Induced brain injury enhances migration and differentiation of BMSCs in CNS
We induced MCAO/R injury in 2-month old Prism-BF I/R group to detect if any BMSCs migrate to the injury site and differentiate into CNS cells (Supplementary Fig. 3). Fifty-two days after ischemia, the infarct zone was clearly distinguishable as autofluorescence signal in cleared brain sections of the ipsilateral hemisphere (Fig. 3a). Immunohistochemistry and confocal analysis revealed the presence of BMSC-derived neurons, oligodendrocytes, and astrocytes in both the ipsilateral and contralateral hemispheres (Fig. 3b, c). While no significant difference was observed in the number of neurons (ipsi: 0.93 ± 0.15; contra: 0.40 ± 0.16) and oligodendrocytes (ipsi: 1.46 ± 0.35; contra: 0.53 ± 0.16) between hemispheres, the number of astrocytes (ipsi: 14.60 ± 1.62; contra: 1.86 ± 0.50) was significantly higher in the ipsilateral hemisphere (Fig. 3f). Flow cytometry analysis confirmed the presence of BMSC-derived neurons, oligodendrocytes, and astrocytes in both the ipsilateral and contralateral hemispheres (Fig. 3d). A statistically significant increase was detected in the percentages of neurons (ipsi: 0.11 ± 0.01; contra: 0.03 ± 0.008) and astrocytes (ipsi: 0.49 ± 0.05; contra: 0.14 ± 0.03) in the ipsilateral hemisphere compared to the contralateral side, while no significant difference was observed in oligodendrocyte (ipsi: 0.14 ± 0.03; contra: 0.13 ± 0.02) percentages (Fig. 3e).
Differentiation of BMSC into CNS cells in MCAO induced breastfed wild-type mice. (a) Representative image showing TO-PRO3 staining for cleared brain section and autofluorescence indicating infarct zone after MCAO/R. (b,c) Immunofluorescence images show YFP+, CFP+, and DsRed + cells in cleared brain sections after MCAO/R in both the ipsilateral and contralateral hemispheres, stained using anti-GFP, anti-MBP and anti-GFAP antibodies to identify neurons, oligodendrocytes, and astrocytes respectively. (d) Flow cytometry analysis of ipsilateral and contralateral hemispheres. Top panels show the YFP + cells (neurons) and the bottom panels show the CFP+ (oligodendrocytes) and DsRed+ (astrocytes) cells. (e) Quantitative analysis of flow cytometry of contralateral and ipsilateral hemispheres in Prism-BF I/R group (n = 5). (f) Quantitative analysis was performed on confocal microscopy images of oligodendrocytes, neurons, and astrocytes obtained from three randomized ROIs (each 8 × 10⁶ µm³) per animal in the striatum of both contralateral and ipsilateral hemispheres in the Prism-BF I/R group (n = 5). The mean value of the three ROIs was calculated for each animal and used as a single biological replicate in the analysis, error bars represent standard deviation.
Discussion
Breast milk has long been recognized for its nutritional and immunological benefits in early development; however, its potential as a source of therapeutically active stem cells has only recently begun to attract scientific attention. In this study, we aimed to explore the capacity of BMSCs to differentiate into various CNS cell types in breastfed offspring, and their behaviour and distribution following ischemic brain injury.
Stroke, being the one of leading causes of death according to World Health Organisation reports, leads to functional disruptions in motor and psychological abilities and necessitates long term care for patients17. As brain ischemic injury, the whole pathophysiological picture during brain stroke29does not yet have a final cure, only tissue plasminogen activator (tPA) thrombolysis and adjuvant treatments with limited time window. It is known that the integrity of the blood-brain barrier is impaired, and its permeability increases after stroke, and neurogenesis is triggered by endogenic factors30. There is important evidence that neural stem and progenitor cells trigger proliferation after cerebral ischemia31. BMSCs are highlighted as a valuable cell source due to their stem cell potential and ease of accessibility. Besides its nutritional content, breast milk also contains various cell types including immune cells like leukocytes32epithelial cells from mammary glands, milk producing lactocytes9and progenitor cells expressing neuroectodermal stem cell markers like Nestin and Cytokeratin 5 33. Previous studies have shown that these cells are transferred to neonates along with other essential nutrients and have the potential to integrate into a variety of tissues. The transfer of lymphocytes and antigens from mother to offspring has been reported13,34. However, the most distinct finding was a previous study in which we demonstrated BMSCs pass through the brain tissue of offspring and differentiate into neurons and astrocytes8. In this study, we show that BMSCs can differentiate into oligodendrocytes as well as neurons and astrocytes in the brain of the breastfed offspring. To better observe post-ischemic structural changes in cell populations, we performed a brain ischemia-reperfusion injury model in mice and investigated the fate and presence of cells in CNS that were transferred through breastfeeding. We showed that these cells can accumulate at the injury site and take part in the cellular process after ischemic brain injury, especially around the pericontusional area.
The increase in neural cell numbers, especially astrocytes, in the injury site indicates BMSCs’ contribution to the glial cell response and cellular reorganization of brain tissue following injury. This finding is of great importance in terms of the relationship of cells passing from mother’s milk to the suckling pups in post-injury processes in the brain. This observation reveals that breast milk cells do not only have a role in normal physiological functioning during development but also play a part in pathological processes in adulthood, which highlights its importance for further investigation. However, even though more oligodendrocytes were detected in microscopic analysis in ipsilateral hemisphere in comparison to the contralateral in Prism-BF I/R group, oligodendrocytes did not show a statistically significant increase as astrocytes in terms of glial response to the injury. Remyelination after ischemic injury is crucial for recovery. Studies showed that although oligodendrocyte precursor cells (OPCs) were known to be recruited into ischemic striatum after injury, only limited number of OPCs can differentiate into mature oligodendrocytes35,36,37. The lack of detectable oligodendrocyte changes in our study may reflect temporal or methodological constraints. As Tg-Prism mice express CFP under the control of mOBP promoter in oligodendrocytes, which identifies mature oligodendrocytes, may not capture transient or early-stage oligodendrocyte precursor cell (OPC) activity. Future studies extending the observation period or incorporating OPC-specific markers (e.g., NG2, PDGFRα) could clarify this dynamic.
An alternative approach to our study design could have involved the inclusion of a non-breastfed pup group, potentially allowing for a more comprehensive assessment of the role of BMSCs in modulating cellular or pathophysiological processes. However, establishing such a group presents significant challenges, as breast milk contains not only cells but also a multitude of bioactive molecules essential for development. The exclusion of breastfeeding could lead to nutritional deficiencies, potentially confounding the results by introducing adverse developmental effects. Consequently, the creation of a formula-fed control group devoid of breastfeeding would not accurately reflect physiological or developmental norms. As a result, our analysis is restricted to monitoring processes specifically associated with milk-derived cells, precluding the ability to investigate the developmental outcomes in the absence of breastfeeding.
To sum up our findings, we have shown that BMSCs are involved not only in normal physiological processes but also in pathological conditions within the CNS. For the first time, it has been demonstrated that these cells participate in cellular responses following brain injury. Our results provide evidence that BMSCs can differentiate into various functional cell types in both healthy and injured adult brain tissue, highlighting their potential as therapeutic agents. Single-cell transcriptomic profiling of BMSCs at different post-injury time points could help reveal their dynamic cellular states and functional trajectories. These mechanistic insights will not only deepen our understanding of maternal cell-derived repair processes but also help refine the therapeutic potential of BMSCs in regenerative medicine.
Data availability
All relevant data and resources can be found within the article and its supplementary information.
References
Camacho-Morales, A. et al. Breastfeeding contributes to physiological immune programming in the newborn. Front. Pediatr. 9, 744104. https://doi.org/10.3389/fped.2021.744104 (2021).
Gartner, L. M. et al. Breastfeeding and the use of human milk. Pediatrics 115, 496–506. https://doi.org/10.1542/peds.2004-2491 (2005).
Ballard, O. & Morrow, A. L. Human milk composition: nutrients and bioactive factors. Pediatr. Clin. North. Am. 60, 49–74. https://doi.org/10.1016/j.pcl.2012.10.002 (2013).
Walker, A. Breast milk as the gold standard for protective nutrients. J. Pediatr. 156, 3–7. https://doi.org/10.1016/j.jpeds.2009.11.021 (2010).
Doerfler, R. et al. Characterization and comparison of human and mouse milk cells. Plos One. 19, e0297821. https://doi.org/10.1371/journal.pone.0297821 (2024).
Gleeson, J. P. et al. Profiling of mature-stage human breast milk cells identifies six unique lactocyte subpopulations. Sci. Adv. 8, eabm6865. https://doi.org/10.1126/sciadv.abm6865 (2022).
Hassiotou, F. et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells. 30, 2164–2174. https://doi.org/10.1002/stem.1188 (2012).
Aydın, M., Yiğit, E. N., Vatandaşlar, E., Erdoğan, E. & Öztürk, G. Transfer and integration of breast milk stem cells to the brain of suckling pups. Sci. Rep. 8, 14289. https://doi.org/10.1038/s41598-018-32715-5 (2018).
Hassiotou, F. & Geddes, D. Anatomy of the human mammary gland: current status of knowledge. Clin. Anat. 26, 29–48. https://doi.org/10.1002/ca.22165 (2013).
Thomas, E. et al. Receptor activator of NF-κB ligand promotes proliferation of a putative mammary stem cell unique to the lactating epithelium. Stem Cells. 30, 1255–1264. https://doi.org/10.1002/stem.1092 (2012).
Thomas, E., Zeps, N., Rigby, P. & Hartmann, P. Reactive oxygen species initiate luminal but not basal cell death in cultured human mammary alveolar structures: a potential regulator of Involution. Cell. Death Dis. 2, e189. https://doi.org/10.1038/cddis.2011.69 (2011).
Shackleton, M. et al. Generation of a functional mammary gland from a single stem cell. Nature 439, 84–88. https://doi.org/10.1038/nature04372 (2006).
Cabinian, A. et al. Transfer of maternal immune cells by breastfeeding: maternal cytotoxic T lymphocytes present in breast milk localize in the peyer’s patches of the nursed infant. PLoS One. 11, e0156762. https://doi.org/10.1371/journal.pone.0156762 (2016).
Hassiotou, F. & Hartmann, P. E. At the dawn of a new discovery: the potential of breast milk stem cells. Adv. Nutr. 5, 770–778. https://doi.org/10.3945/an.114.006924 (2014).
Ninkina, N. et al. Stem cells in human breast milk. Hum. Cell. 32, 223–230. https://doi.org/10.1007/s13577-019-00251-7 (2019).
Zhou, L. et al. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunology 101, 570–580. https://doi.org/10.1046/j.1365-2567.2000.00144.x (2000).
Donnan, G. A., Fisher, M., Macleod, M. & Davis, S. M. Stroke Lancet 371, 1612–1623, https://doi.org/10.1016/s0140-6736(08)60694-7 (2008).
Feigin, V. L. et al. Global, regional, and National burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 20, 795–820. https://doi.org/10.1016/S1474-4422(21)00252-0 (2021).
Moskowitz, M. A., Lo, E. H. & Iadecola, C. The science of stroke: mechanisms in search of treatments. Neuron 67, 181–198. https://doi.org/10.1016/j.neuron.2010.07.002 (2010).
Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z. & Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8, 963–970. https://doi.org/10.1038/nm747 (2002).
David-Bercholz, J., Kuo, C. T. & Deneen, B. Astrocyte and oligodendrocyte responses from the subventricular zone after injury. Front. Cell. Neurosci. 15, 797553. https://doi.org/10.3389/fncel.2021.797553 (2021).
Parent, J. M., Vexler, Z. S., Gong, C., Derugin, N. & Ferriero, D. M. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol. 52, 802–813. https://doi.org/10.1002/ana.10393 (2002).
Dougherty, J. D., Zhang, J., Feng, H., Gong, S. & Heintz, N. Mouse transgenesis in a single locus with independent regulation for multiple fluorophores. PLoS One. 7, e40511. https://doi.org/10.1371/journal.pone.0040511 (2012).
Beker, M. C. et al. Age-Associated resilience against ischemic injury in mice exposed to transient middle cerebral artery occlusion. Mol. Neurobiol. 60, 4359–4372. https://doi.org/10.1007/s12035-023-03353-4 (2023).
Caglayan, B. et al. Evidence that activation of P2X7R does not exacerbate neuronal death after optic nerve transection and focal cerebral ischemia in mice. Exp. Neurol. 296, 23–31. https://doi.org/10.1016/j.expneurol.2017.06.024 (2017).
Kilic, U. et al. Particular phosphorylation of PI3K/Akt on Thr308 via PDK-1 and PTEN mediates melatonin’s neuroprotective activity after focal cerebral ischemia in mice. Redox Biol. 12, 657–665. https://doi.org/10.1016/j.redox.2017.04.006 (2017).
Kilic, E. et al. HMG-CoA reductase Inhibition promotes neurological recovery, Peri-Lesional tissue remodeling, and contralesional pyramidal tract plasticity after focal cerebral ischemia. Front. Cell. Neurosci. 8, 422. https://doi.org/10.3389/fncel.2014.00422 (2014).
Pan, C. et al. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat. Methods. 13, 859–867. https://doi.org/10.1038/nmeth.3964 (2016).
Dirnagl, U., Iadecola, C. & Moskowitz, M. A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 22, 391–397. https://doi.org/10.1016/s0166-2236(99)01401-0 (1999).
Lin, R. et al. Stepwise impairment of neural stem cell proliferation and neurogenesis concomitant with disruption of blood-brain barrier in recurrent ischemic stroke. Neurobiol. Dis. 115, 49–58. https://doi.org/10.1016/j.nbd.2018.03.013 (2018).
Knotek, T., Janeckova, L., Kriska, J., Korinek, V. & Anderova, M. Glia and neural stem and progenitor cells of the healthy and ischemic brain: the workplace for the Wnt signaling pathway. Genes (Basel). 11. https://doi.org/10.3390/genes11070804 (2020).
Hassiotou, F. et al. Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clin. Transl Immunol. 2, e3. https://doi.org/10.1038/cti.2013.1 (2013).
Fan, Y., Chong, Y. S., Choolani, M. A., Cregan, M. D. & Chan, J. K. Unravelling the mystery of stem/progenitor cells in human breast milk. PLoS One. 5, e14421. https://doi.org/10.1371/journal.pone.0014421 (2010).
Dutta, P. et al. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood 114, 3578–3587. https://doi.org/10.1182/blood-2009-03-213561 (2009).
Sozmen, E. G. et al. White matter stroke induces a unique Oligo-Astrocyte niche that inhibits recovery. J. Neurosci. 39, 9343–9359. https://doi.org/10.1523/jneurosci.0103-19.2019 (2019).
Zhao, H. et al. Effects of the transcription factor Olig1 on the differentiation and remyelination of oligodendrocyte precursor cells after focal cerebral ischemia in rats. Mol. Med. Rep. 20, 4603–4611. https://doi.org/10.3892/mmr.2019.10713 (2019).
Han, B. et al. Integrating Spatial and single-cell transcriptomics to characterize the molecular and cellular architecture of the ischemic mouse brain. Sci. Transl Med. 16, eadg1323. https://doi.org/10.1126/scitranslmed.adg1323 (2024).
Acknowledgements
We sincerely thank Research Institute for Health Sciences and Technologies (SABITA) and Experimental Animal Centre of Istanbul Medipol University (MEDITAM) for providing resources to carry out this study.
Funding
This research was funded by the Scientific and Technological Research Council of Turkey (TÜBİTAK), grant number 118S506.
Author information
Authors and Affiliations
Contributions
E.N.Y., S.S, A.S.S and M.S.A wrote the main manuscript text, E.N.Y and M.S.A prepared figures, E.N.Y., S.S, A.S.S, E. V, A.B.C, T.K. and M.S.A contributed experiments, G.O and M.S.A conceptualized the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yİğİt, E.N., Serdengeçtİ, S., Sezer, A.S. et al. Breast milk stem cells integrate into cellular remodeling and functional differentiation in the ischemic brain. Sci Rep 15, 35422 (2025). https://doi.org/10.1038/s41598-025-18917-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18917-8