Abstract
The primary objective of the present study was to explore the effect of ultrasound on the encapsulation potential of a ternary blend of wall materials (Whey Protein Concentrates: Maltodextrin: Gum Arabic; WPC: MD: GA) for adequate protection of flaxseed oil. Combined effects of sonication treatment time and variable composition of wall materials on the emulsion properties (droplet size, Polydispersity Index and Zeta Potential and viscosity) and subsequent spray-dried microcapsules of flaxseed oil were investigated. The best-formulated emulsion, with average droplet sizes of 406.28 nm, exhibited pseudoplastic behaviour. Microcapsules were obtained with the highest encapsulation rates of 90.27% with 4 min of sonication treatment with WPC: MD: GA present in ratio of 0.20:0.50:1.5. The microcapsules’ flow properties were also found to be optimum. Morphological assessment of microcapsules indicated uniform spherical and continuous wall formation. The microcapsules’ oxidative stability was significantly higher even after 56 days of storage, and accelerated oxidative stability rates also increased manifolds after encapsulation. In vitro gastrointestinal digestion results revealed that designed conditions retarded release under gastric conditions, with less than 10% release of oil. However, higher (56% release) bioaccessibility rates were favoured after sequential exposure to intestinal conditions. Therefore, the study outcomes demonstrated the critical implications of designing encapsulation systems for lipid-soluble bioactive for their practical and targeted, controlled delivery in various food products.
Similar content being viewed by others
Introduction
Omega-3 fatty acids (α-linolenic acid [ALA]) are essential fatty acids and are majorly restricted to seafood, except for a few vegetable oils (flaxseed and canola). They confer beneficial health effects on some cancers, coronary heart disease, and hormonal and neurological disorders1. However, despite their essential role in human health and development, their deficiency is quite common in vegetarians and non-seafood eaters. On the contrary, there has been a rapid rise in ω−6 fatty acid-rich oils (sunflower, soybean, and groundnut) consumption, which has been proven to affect the metabolic homeostasis of ɷ−6:ɷ−3 fatty acids, resulting in the shift from 1:1 to 20:12. This fatty acid composition alteration corresponds to a significant rise in obesity and overweight. Moreover, the eicosanoid metabolic products from arachidonic acid are formed in higher amounts than those originating from ɷ−3 fatty acids, which leads to developing atheromas, thrombus, and other allergic and inflammatory disorders, resulting in cell proliferation. As a result of the increased blood viscosity, vasoconstriction, vasospasm, and cell proliferation, a diet high in omega-6 fatty acids transforms the physiological state into prothrombotic, proinflammatory, and pro-aggregatory3.
Flaxseed oil is obtained from the plant of flax (Linum usitatissimim) and contains about 57% ALA of its total fatty acid composition. Its consumption has been proven to have a protective effect against type 2 diabetes (T2D), as in a study, the T2D risk was lowered by 21%4. In addition, a cohort study by Orchard et al.5 observed a 54% lower risk of hip bone fractures with omega-3 fatty acids consumption (1.39 g/day), depicting their protective role against bone fracturing. Despite this, its amalgamation into commercial food products is still challenging due to low oxidative stability, higher rancidity rate, off-flavour, poor storage stability, and toxic peroxides. Thus, a feasible means of shield must be explored for its commercial application. Simultaneously, introducing a protecting layer surrounding the lipid droplets, encapsulation, has been proven to reduce oils’ sensitivity to oxygen. Encapsulation is the process of enclosing, packing, and sealing a solid, liquid, or gaseous (core) item within a secondary, more stable material for protection and controlled release6. Spray drying is the most prevalent process for encapsulating oils in food. The composition and nature of the wall materials affect/control the encapsulated material’s properties because of their interfacial functionality6. Numerous biopolymers have been utilized as wall material in spray drying, especially hydrocolloids such as gum arabic (GA), exhibiting low viscosity, high solubility, and good emulsification. However, because of supply fluctuations and rising pricing, the researchers are looking for alternate wall materials that may replace them or be used in conjunction with other polymers. Maltodextrin (MD), a hydrolyzed starch, has several advantages, such as a cheap rate, neutral odour and flavour, low viscosity at high solids, and superior oxidation resistance, but it also has a limited emulsifying ability. As a result, MDshould be used in conjunction with other surface-active biopolymers such as GA, modified starches, etc7. Proteins for encapsulation offer an additional advantage as they possess a high emulsification capacity. In addition, proteins are excellent for oxidation protection due to lower oxygen permeability8. Thus, proteins, carbohydrates, and gum blends tend to form more stable and efficient emulsions and, therefore, are explored widely. As for reservatrol, the whey protein concentrates (WPC) and GA blend improved emulsion stability9. Recent developments in microencapsulation have featured the effectiveness of multi-component wall materials consisting of a protein, polysaccharide and gums for enhancing the stability of oil in water emulsions and the oxidative stability of encapsulated lipids10. In a recent study by Sukumar et al.11, they demonstrated that a multilayered emulsion system, employing WPC, GA and Xanthan Gum (XG) for the encapsulation of chia seed oil yielded high encapsulation efficiency (93%) and superior oxidative stability in spray dried microcapsules obtained. Likewise, earlier also Carneiro et al.12 revealed that ternary wall combination matrices containing WPC, MD and GA significantly reduced particle size, with improved powder flowability and enhanced lipid protection when carried encapsulation of fish oil and grape seed oil. Various studies have explored similar combination of biopolymer combinations using ultrasonically assisted emulsification for the production of nano and microencapsulated powders with improved shelf life and functional delivery properties (Sharma et al.6; Chen et al.13, Khatkar et al.14, Mehta et al.15). Despite these advancements, flaxseed oil encapsulation using ternary wall components still remained unexplored relatively, even though its high alpha linolenic acid content exhibits higher susceptibility to oxidative degradation. Therefore, this study’s objective is to address this gap by encapsulating flaxseed oil using ternary wall system of WPC, MD and GA with ultrasonic assisted emulsification employed to enhance droplet dispersion, promote better interfacial adsorption and emulsions stability prior to spray drying. Further this research work also explores the synergistic effects of different combination of ternary wall material on enhancing the potential bioavailability of the encapsulated flaxseed oil and ultimate aim of optimizing the most effective carrier system for omega 3 fatty acid delivery in functional food applications.
Methods
Flaxseed oil® (cold-pressed) was purchased from Sattvic Foods (Sattvic et al., India) and was kept at 4 °C in an amber-coloured bottle. The WPC was procured from Fonterra (New Zealand); Maltodextrin (DE < 20) and gum Arabic were purchased from Chemical Drug House (Delhi). Chemicals, reagents, and enzymes like pepsin (1:10000 per min, 10000 NF U/mg) and pancreatin (4 NF/USP, porcine pancreas) were procured from Hi-Media Laboratories (Mumbai, Maharashtra).
Emulsion Preparation
Flaxseed oil emulsions (containing 6% oil) were stabilized with different proportions of wall materials (WPC, MD, and GA), as presented in Table S1 and the composition is designated as C-1, C-2 and C-3. The oil-to-wall material ratio was kept constant (1:4) with the final total solids (30% w/w) to provide the industrial feasibility. WPC, MD, and GA solutions were prepared separately by allowing their dispersion in distilled water and dissolved by continuous stirring at a magnetic stirrer (600 rpm) overnight. Emulsions were created by mixing different wall material solutions, followed by the subsequent drop-wise addition of oil. Emulsions prepared were then subjected to continuous mixing for 1 h at 1025 rpm. Ultrasonic treatment using probe sonicator (Cole Parmer, Model CV X750, probe diameter 13 mm) operating at power of 1200 W with varying times (0, 2, 4, and 6 min)was given to emulsions at an amplitude 50%, frequency 20 kHz, pulsation regime 5 sON and 2 s OFF, and at a temperature 25 °C. Before spray drying, the emulsions were immediately stored in a refrigerator (7–8 °C).
Spray drying
The spray drier (Model-SPD-P-111, Technosearch Instruments, Mumbai, India) was utilized for drying. A peristaltic pump was used to feed (4.0 mL/min) emulsion to the dryer. The compressed drying air flow rate was kept constant at 1.17 kg/cm2, and the pressure was set at 0.008 MPa. The temperature of the entrance air was 180 °C, while the output air temperature was 75–80 °C. The oil microcapsules (loaded with ɷ−3 fatty acid)were transferred to amber-coloured bottles and kept under refrigeration.
Nanoemulsions characterization (Average droplet size, polydispersity index (PDI), and zeta potential)
A Nano Series 6.3—Zetasizer (Malvern Instruments Ltd., UK) was used to determine the oil droplet size and zeta potential in all emulsions on the subsequent day of preparation. The emulsion (1 mL) was diluted 200 times with distilled water, mixed thoroughly, and transferred into plastic cuvettes and capillary cells to measure size and zeta potential.
Viscosity
The viscosity (in triplicate) was determined using a rheometer (MCR 72, Anton Paar, Germany) with parallel plate geometry (50 mm diameter) having a Peltier system-controlled temperature of 25 °C and a gap of 0.2 mm at a 0.1–100 s −1 forced shear rate. 1 mL sample was injected into the emulsion rheometer, and the emulsions’ consistency (K, Pa.sn) and flow behaviour indices (η) were determined using a power-law model. The power-law model parameters were used to fit the obtained data using the following formula: σ = Kγn, where σ is the shear stress (Pa), K is the consistency coefficient (Pa.s), γ is the shear rate (s − 1), and n is the power-law exponent.
Microcapsules characterization (Moisture content, water activity and physical characteristics)
For moisture content, 2.0 g encapsulated powder was dried in the oven at 105 ± 2 °C until it reached a consistent weight16. The Aqualab Pawkit water activity meter (METER, USA) was used to measure water activity.
Physical characteristics
The bulk density (ρB) and tapped density (ρT) of oil microcapsules (3 gm) were estimated as per Quispe-Conori et al.16. The flowing characteristics of microcapsules were evaluated with’s index (compressibility), Compressibility Index(free-flowing properties), and the Hausner ratio (HR), which indicates cohesiveness using the method of Turchiuli et al.17. The dispersibility (%) and wettability (min) of microcapsules were determined using the methods of Thiengnoi et al.18 and Teo et al.19, respectively. A Color Reader CR-10 (Konica Minolta Sensing Inc.) was used to measure the coordinates of the colour (L*, a* and b* values).
Encapsulation efficiency (EE)
The method described by Alcantra et al.20 was followed with slight modifications. This is based on separating oil present on the matrix surface and determining the encapsulated oil present within the core. 2 g of powder was weighed in falcon tube, followed by addition of 10 mL of hexane, homogenized in a vortex stirrer successively centrifuged at 5000 rpm for 15 min. The collected supernatant was discarded and 5 mL of d.w. is added to the pallet obtained, vortexed for 1 min. 25 mL of a mixture of hexane/isopropanol solution (3:1 v/v), vortexed for 3 min and centrifuged at 5000 rpm for 20 min again. The supernatant containing organic phase was pipetted using Pasteur pippete and transferred to a pre weighed Erlenmeyer flask. The extraction process of organic phase was repeated thrice with hexane/isopropanol solution and supernatants were collected in the same flask, followed by their evaporation at 60 C in a circulating air oven and oil contents were determined gravimetrically according to the following equation
Morphology
The powder morphology was examined by a scanning electron microscope (SEM) (JEOL, Model JSM 6100) operating at an accelerating voltage of 10.0 kV. The powders were placed on aluminium stubs using double-sided tape and were then observed for image acquisition.
In-vitro release kinetics
Simulated digestive fluid including gastric (SGF) and intestinal (SIF) were prepared according to international consensus report (Minekus et al.21 with their detailed composition in Table S2 attached in supplementary file. Firstly, 3 g of micro encapsulated flaxseed oil capsule were dispersed in 10mL of ddw. In the same dispersion, 7.5 mL SGF was added followed by addition of porcine pepsin (25,000 U/mL) and 5 µL 0.3 M CaCl2 solution and 0.695 mL Milli-Q water were added to the mixture, pH adjustment were carried to maintain pH of 2.0 by using 1 M HCL. The mixture was incubated for 2.0 h at 37 C with continuous shaking at 300 rpm.
For simulated intestinal digestion, 10 mL of gastric digesta was mixed with 10 mL SIF solution, followed by addition of 5 mL SIF containing pancreatin (800 U/mL). To this dispersion, 2.5 mL 160 mM fresh bile,0.04 mL of 0.3 M CaCl2 solution and 1.31 mL Milli-Q water was added. pH adjustments to pH 6.8 of the above mixture were made using 1 M NaOH. It was agitated at 100 rpm for 2.5 h.
The amount of released oil was measured at the end of each digestion stage (Errate et al.22 with minor modifications. Each digested sample was mixed with 75 mL hexane and the mixture was vortexed for 1 min. Then, the hexane containing oil was filtered into a pre-weighted flask through a filter paper (Whatman No. 4). The filtrate was evaporated using a rotary evaporator to remove hexane and recover the oil. The recovered oil was heated at 105 °C for 30 min to ensure complete removal of residual solvent and the oil mass was measured. The amount of oil released from the digested microcapsule was expressed as percentage of the total oil of the intact microcapsule.
Oxidative stability (Peroxide value, rancimat test: accelerated oxidative stability)
Flaxseed microcapsules’ oxidative stability was determined weekly during storage (45 °C) for 56 days. The standard method was adopted for measuring the peroxide value (PV)23. 1 g of powdered sample was weighed into a test tube and dispersed in 5 mL of water and vortex for 15 min till complete dissolution. 400 µL portion was extracted and stirred with 1.5 mL of a isooctane/isopropanol (2:1) mixture to extract the oil. The phases were separated by centrifugation (3500 rpm for 5 min) and the upper phase was collected for analysis. 300 microL aliquot of extraction medium was taken and 9.6 mL of chloroform/methanol (7:3) mixture was added. For the development, 50 µL of a solution of FeCl2.4H2O, 40 mg dissolved in 10 mL 3.7% HCl) and 50 µL of 30% ammonium thiocyanate (7.5 g dissolved in 25 mL distilled water) were added. Samples were shaken and incubated in dark for 5 min and then the absorbance was taken at 500 nm. Standard curve of Fe3+ (1–25 µg) was used to determine PV.
Rancimat accelerated test was used to estimate the oxidative stability of flaxseed oil before and after microencapsulation using Rancimat 873 (Metrohm, Herisau, Switzerland) at 110 °C and 20 mL air/h. The induction period (IP), defined as the time interval corresponding to the inflexion point of the conductivity versus the time curve, was examined. In each reaction tube, 2 g of powder sample (microencapsulated oil) and a pure flex seed oil sample (control) were weighed. The analyses were carried out in triplicate.
Statistical analysis
All experiments were repeated using newly produced materials three times, and results are shown as averages and standard deviations (SD). The statistical analyses were carried out using one-way analysis of variance (ANOVA) in SPSS v.20.0 software (SPSS, USA), and mean values of distinct replies were analyzed using Tukey’s b comparison test, with p values < 0.05 regarded as substantially different.
Results and discussion
Nanoemulsions characterization (droplet size, zeta potential, PDI, and viscosity)
The emulsion droplet average means diameter, zeta potential, and PDI are presented in Table 1. The emulsion’s average droplet size was found to vary significantly (p < 0.05) and ranged between 406 and 1958 nm. The wall materials’ composition and sonication duration affected the sizes significantly (p < 0.05). Maximum droplet sizes were found in emulsions formed with E2 containing WPC: MD: GA in the ratio of 1.5:0.5:0.1 (C-2), followed by emulsions formulated with C-1 and C-3 with corresponding droplet sizes of 1958 nm, 1768 nm, and 1702 nm, respectively, that was due to no ultrasonication. Larger droplet sizes in C-2 could have resulted from WPC’s higher proportion in wall composition makeup, which indirectly indicates the protein aggregates’ adsorption or proteins’ multilayers on the oil-water interface that could have led to increased droplet sizes24. Another explanation is that proteins are attracted to the hydrophobic groups on the wall material solids, causing them to clump together, such as the presence of whey proteins’ -SH groups, which could have promoted interaction between adjacent particles by disulfide bridge formation. Similar observations were reported by Premi and Sharma25 that the increasing proportion of WPC in WPC: MD containing emulsion from 25:75 to 75:25 significantly raised the emulsion’ droplet size from 3.58 to 4.92 μm. Increased sonication time had a significant (p < 0.05) but inverse effect on the nanoemulsions’ droplet size. At C-1, on increasing the exposure time to 2 min, the droplet sizes were reduced from 1768.08 nm to 652.38 nm, corresponding to a decrease of 63.11%.
Further increases in sonication time to 4 min and a parallel decline in the emulsions’ droplet sizes were also observed. A significant reduction in droplet sizes was observed for all three compositions, up to 4 min. Beyond that, no comparable reductions were noted, indicating the optimum time of 4 min for decreasing the droplet sizes to form emulsions. The percentage decrease in sizes with increased treatment duration was comparable, representing a total reduction of 74.66%, 76.14%, and 73.13% for C-1, C-2, and C-3, respectively. The high energy given to the emulsion with high shock waves encourages increased shear of the dispersed droplets, thereby lowering their size. Similarly, Alcantara et al.20 reported a similar decrease in chia oil nanoemulsion droplet size from 1.34 μm to 0.48 μm on increasing the sonication time from 1 min to 3 min. Emulsions varied from 0.491 to 1.03 (Table 1) and showed excellent droplet variability among emulsions. The effect of wall material composition on PDI was insignificant except for C-3, where slightly lower (0.850) values were observed for the E3 sample (no ultrasonication). However, all the samples recorded a significant decline in the PDI with increased sonication time. The decreased PDI can be illustrated by the increased energy density transferred to the emulsion systems, which could have broken down the droplets’ size into a homogenous distribution.
Figure 1 depicts the particle size distribution of the microcapsules of the best emulsions from each composition. A uni-modal distribution was observed in all three emulsions, representing a narrower size distribution of particles and excellent homogeneity in sizes. The particle size was found to be varied between 250 and 510 nm, 400–900 nm and 300–520 nm for E7, E11, and E9, respectively, with the predominant peak observed to be approximately 460 nm, 600 nm, and 400 nm, respectively, confirming their particle sizes obtained with DLS. The emulsions formulated with different ternary systems as encapsulating materials showed negative zeta potential and were found to vary between − 32.45 and − 15.45 mV. High negative zeta potential values were imparted by emulsions containing C-1 (1:1:0.15) with values ranging from − 32.45 mV to −29.44 mV, exhibiting higher stability than emulsions with C-2 (−23.92 to −28.13 mV). In contrast, emulsions formulated with C-3 had less harmful zeta potential (−15.45 mV to −18.79 mV). Such a difference can be corroborated by the higher proportion of GA (1:1:0.15) in C-1, owing to its better emulsifying properties, stabilizing nature, and the presence of hostile carboxylic groups in the carboxylated form that resulted in stable emulsions and led to maximum encapsulation. In their study, Premi and Sharma25 also observed such results as increased gum arabic concentration in different binary systems containing MD/GA and WPC/GA resulted in more negative zeta potential values (−32 to −41 mV) with less observed aggregation. An increased duration of sonication treatment from 0 to 6 min had no significant effect on the zeta potential, and very few variations were seen.
Figure 2 shows the flow curves for all emulsions generated by apparent viscosity vis-à-vis shear rate (1–100 s–1). All emulsions were observed to exhibit non-Newtonian, more precisely shear-thinning pseudoplastic behaviour, as the viscosity of emulsions decreased with increased shear rates, typical rheology of nanoemulsions. All emulsions exhibited higher viscosities at lower shear rates (10–20 s–1) irrespective of their composition. This behaviour was further confirmed as their flow behaviour index (n) values were found to vary from 0.255 to 0.745, demonstrating their shear-thinning behaviour. All emulsions’ consistency index (k) values ranged from 3.270 to 11.40 Pa.s (Table 1). The total solids content of all the emulsions was similar. However, significant differences in viscosities were observed, and the highest was exhibited with C-2 (24–26 mPa.s), followed by C-3 (20–24 mPa.s) and the least were observed for C-1 (17–18.2 mPa.s). Higher viscosities of C-2 could be ascertained by the higher proportion of WPC in the wall materials, as proteins are well known for their water-binding and retention abilities, which might have partly contributed to the increased viscosity. The viscosity of emulsion formulated with C-3 was comparable, as a higher proportion of GA(thickener) was present, increasing viscosity. However, the presence of MD at a higher level could have diluted the thickening effect of GA. Several reasons ascertain the shear-thinning of food emulsions: the alterations in the particles’ spatial distribution by the shear field and the deformation and disruption of flocs.
Moreover, Özbek and Ergönü26 also observed similar pseudoplastic rheological behaviour. Furthermore, they also confirmed the WPC-GA complex’s synergistic effect in increasing the emulsion’s viscosity. Hence, the present results agree with their observation whereby they formulated cold-pressed pumpkin seed oil emulsions with variable WPC/MD/GA levels. Increased exposure of emulsions to ultrasonic treatment affected the viscosity of emulsions significantly (p < 0.05) as it reduced viscosity for all three emulsions. At C-1, the consistency coefficient (k) decreased from 4.38 to 3.69 Pa.sn after 2 min exposure. However, increasing exposure time to 4 and 6 min was found to have no significant effect (p > 0.05) on the k values of emulsions. Similarly, at C-2, a similar trend in the k values was observed. Emulsions formulated with C-3 were significantly affected with increased exposure time to 2, 4, and 6 min, decreasing the k value from 11.4 to 7.25 Pa.sn. Such behaviour can be explained by the decreased droplet sizes of the emulsions on increased exposure to sonication treatment. This can be explicated by the Taylor equation, which signifies an inverse relationship between the radius of emulsion droplet size and their apparent viscosity. Similar observations were reported by Qayum et al.27), who also observed a significant decrease in k value from 0.0164 Pa.sn to 0.011 Pa.sn on ultrasonic emulsification of α-lactalbumin and soybean oil emulsion.
Microcapsule characterization (moisture and water activity, physical properties)
The microcapsules’ moisture content was found to vary between 2.19 and 3.60% (Table 1). The wall material’s compositional makeup and the sonication durations significantly affected the moisture content (p < 0.05). Comparatively higher moisture contents (3.02–3.60%) were observed for microcapsules containing higher proportions of WPC (1.5%) in their walls (C-2) and followed by those formulated with C-3 containing 2.94–3.08% than the higher GA contents whereas could ascertain, the least values were reported for those prepared from C-1. It could have increased the emulsion viscosity, resulting in a slower rate of water diffusion from the droplet surface during drying. The higher moisture content can be articulated to the higher water-holding capacity of proteins (WPC) in an amorphous state. Tahir et al.28 corroborated this increased moisture content in microcapsules to the hydrophilic groups present in WPC and GA and increased viscosity of the feed solution which could have enhanced the moisture adsorption and slowed drying during spray drying of emulsions encapsulating walnut oil when encapsulating using MD and GA combinations. Premi and Sharma25 also found that encapsulated drumstick oil powder formulated with MD and GA in the ratio of 25:75 (1.64%) had more Moisture than those prepared with MD: GA ratios of 75:25 (1.44%).
Sonication treatment also significantly(p < 0.05) affected the microcapsules’ moisture content, and increased sonication duration reduced the moisture content. At C-1, microcapsules formed with no sonication treatment contained 2.19% moisture, which dropped to 1.95% on increasing the sonication time to 6 min. Similar trends were also found for C-2 and C-3, where the moisture contents dropped from 3.08 to 2.94% and 3.60–3.04%, respectively. The drop in Moisture can be explained by the increased surface hydrophobicity of protein portion (WPC) in the wall materials. With increased exposure to ultrasound, the unfolding of proteins could have resulted in structural changes. Zisu et al.29 also observed such an effect and noticed increased surface hydrophobicity of proteins in reconstituted WPC solutions when exposed to ultrasonic treatment for up to 5 min. A decrease in droplet size with increased exposure time could have also provided a higher surface area for moisture evaporation.
Conversely, the water activity microcapsules ranged from 0.38 to 0.60 (Table 1). The higher water activity was observed for microcapsules obtained with C-3 (0.44–0.60), containing a higher proportion of MD and GA, followed by those formulated with C-2 (0.51 to 0.58) and least for C-1 (0.38 to 0.44). A similar fashion was observed for the moisture content, depicting a strong correlation (moisture/aw = 0.821). Increased sonication exposure has not been shown to affect aw of microcapsules significantly. Nonetheless, appropriate levels were detected in the microcapsules, s, indicating successful water evaporation during spray drying. Foods having an aw of 0.6 or less are considered microbiologically safe30.
The microcapsules bulk density (BD) was observed to vary considerably (p < 0.05) and ranged between 0.356 and 0.576 g/mL (Table 1). Comparatively higher values were observed for microcapsules obtained from C-3, followed by those formulated with C-1, and the lowest for C-2. The trend in the variability in the BD values was related to the MD and GA concentrations, as at higher concentrations of MD, higher BD values were recorded. In contrast, the opposite effect of GA was observed on the BD. Many studies have incorporated WPC as a drying aid in spray dried apple juice powder which led to significantly lowered BD and TD as compared to powder prepared with MD. They attributed this decrease to the higher viscosity of WPC containing feed resulted in larger and more porous structure of microcapsules31. Likewise, Premi and Sharma25 followed a similar trend in the decreased BD with an increased GA proportion in the wall materials and stated that this might be due to the higher viscosity of MD: GA emulsion, leading to small droplet size production. Similarly, increased sonication treatment time also significantly influenced (p < 0.05) the BD of powders. AtC-2 and C-3, decreased values were reported with increased sonication times. The cavitation effect could have broken down the emulsion droplet into smaller and more uniform structures resulting into more porous, loosely bound particles. Tapped density (TD) was observed to vary from 0.492 to 0.635 g/mL (Table 1) and established the role of three-wall material composition. A significant and positive correlation could be seen between the powders’ BD and TD (R2 = 0.625, p < 0.001). Sonication time did not influence the microcapsules’ TD, but at C-1, minor variations could be seen. Powders’ flowing properties are usually judged by their Carr’s Index (% compressibility) and Hausner ratio (HR). Results of the current work revealed significant variations in powder’s HR and CI values. The microcapsules’ HR values were found to vary from 1.08 to 1.55, whereas CI values were 7.38 to 34.48 (Table 1). These values suggest a wide variation in the flowing properties, as they depict powders’ excellent to awful flow properties. However, most powdered microcapsules were observed to possess optimum flowing properties, indicating the suitability of the variable combination of different wall materials—sonication treatment showed a negative effect on HR values.
The powders in the present study were prepared through single-stage spray drying and showed no clumping or agglomeration. Food powders’ rehydration can be complicated; quantitative approaches may not capture this complexity. The microcapsules’ dispersibility ranged from 60.93 to 90.22% (Table 1), representing significant differences. Microcapsules from C-2 exhibited higher dispersibility rates (82.17 to 90.22%), followed by C-1 (79.00 to 85.20%) and C-3 (78.32 to 60.93%). Higher dispersibility rates at C-2 can be attributed to the higher proportion of WPC and lower proportion of MD and GA. WPC has more hydrophilic characteristics and hydrophilic sites (–OH), which could have increased the particles’ solubility by supporting the molecular interaction between microcapsule and water.
Similarly, Stoll et al.32 also reported a similar trend in the increased solubility rates of anthocyanin microcapsules. Increased sonication times significantly decreased the microcapsules’ dispersibility. The increased surface hydrophobicity could support this effect as more hydrophobic protein groups are exposed to the surface after ultrasonication. Microcapsules’ wettability time varied significantly (p < 0.05) and ranged from 1.53 min to 20.76 min. Microcapsules obtained with C-3 required the highest wettability time. This impact might be related to the lesser content of WPC and a higher proportion of GA along with the hydrophobic interaction of protein content on microparticles’ surface, which promotes protein-protein association. A study encapsulating fingered citron extract found that formulations containing WPC + MD + GA delivered superior wettability compared to MD or GA alone in the matrix. The blend was found to provide balanced surface hydrophilicity and microstructure and accelerated wetting times without affecting encapsulating efficiency32.
From the perusal of Fig. S1, the obtained microcapsules exhibited a cream to off-white colour. Significant differences (p < 0.05) were observed in WI and YI of microcapsules, ranging from 72.81 to 78.95 and 34.76 to 46.58, respectively (Table 1). This depicts the effect of wall material composition and ultrasonic emulsification exposure on their colour attributes. Microcapsules from P1, P2, and P3 exhibited the highest YI, which is related to their lower encapsulation rates and higher surface oil owing to no ultrasonication. A similar trend was observed for WI in all three wall material combinations. Increased sonication time significantly improved the appearance of microcapsules as whiter appearances were demonstrated with a significant rise in the WI.
Encapsulation efficiency (EE)
In the present study, emulsions were spray-dried to form oil capsules and possess an EE of 18.27–92.43% (Table 1). Significant variability (p < 0.05) was seen in EE as a function of compositional makeup and sonication times. The highest encapsulation rates (37.82–92.43%) were observed for microcapsules obtained from C-3, followed by those prepared from C-2 (34.72–90.67%) and C-1 (18.27–86.77%). Such a difference in the EE can be explained by the higher viscosities of emulsions, which could have promoted drying faster. An increased proportion of WPC in C-2 was observed to affect the encapsulation negatively and was related to its poor kinetic stability. Moreover, it has been observed that the insolubility of excess amounts of WPC could have restricted the molecular movement in emulsions and with other molecules. Premi and Sharma25 also found comparable results of higher EE of microcapsules obtained with MD: GA (82.67–91.01%) as compared to MD: WPC (66.23–69.96%). Increased sonication times significantly (p < 0.05) affected the encapsulation rates. Increasing the treatment time from 0 min to 6 min resulted in better oil encapsulation rates, regardless of the emulsion’ composition. With all three wall material compositions, the emulsions’ droplet size and EE were observed to have an inverse relationship with a negative correlation (−0.836), as verified in Table 1. This evidence agrees with the effect observed by Karrar et al. 33 Tontul and Topuz34, who also observed significantly higher microencapsulation rates when the sonication time increased from 40 sto 120 s. They attributed this effect to the higher energy released in the system, leading to decreased oil droplet sizes and higher encapsulation rates. They further stated that more than seven times longer than ultrasonication homogenization is required to achieve these sized microcapsules.
Morphology
Figure 3 illustrates the SEM micrographs of best-formulated microcapsules showing the highest microencapsulation efficiencies from the three different compositions. No effect of the wall material composition was observed. All three types of microcapsules possessed spherical shapes with varied sizes (5–15 μm), typical of spray-dried powders. They were also found to have uniform morphology with a smooth surface without cracks. The wall material structure was devoid of porosity, depicting the uniform containment of oil within the wall structure. However, some particles had few indentations on the surface with little concavity, which indicates low drying temperatures and better protection of the core compounds. Dents can also be caused by excessive solid content in the formulations, uneven drying and subsequent chilling, as reported in several earlier studies. In a similar study, Alcantara et al.15 observed depressions on the surface of microcapsules. They linked them to particle shrinkage during drying and cooling or droplet collapse during the initial drying stages. The coexistence of some smooth-shaped and hard crust particles was also observed, demonstrating their faster evaporation.
In-vitro release kinetics
Oil microcapsules were incubated in distinct digesting stages, replicating the fasting stomach and minor intestine conditions to examine microcapsules’ stability and oil release profile in various portions of the GI tract. The percentage of oil released from each composition was assessed from the best-selected microencapsulated powder samples. As depicted in Fig. 4, the percentage release of oil increased gradually with time. During gastric digestion, the food is first digested by gastric pepsin at a low pH of 2.0 and then reaches the intestines at pH 6.8. Food and a higher pH activate pancreatic enzymes to aid in the digestion and absorption of nutritional components. As a result, the impact of chronological exposure to SGF and SIF on the percentage of oil produced from various encapsulated formulations was tested to mimic gastric digesting conditions. Under simulated gastric conditions for 2 h, significantly higher oil rates were observed for P7 and P11 samples, corresponding to 9.36% and 9.61%, respectively. The least was observed for P9 (6.75%), which could be due to their differential wall material combination composition. The higher resistance of the microcapsules in P11 microcapsules can be attributed to the presence of a higher proportion of β-lactoglobulin in WPC, which could have restricted the action of pepsin owing to its complex conformations and globular structure. Moreover, MD and GA are are also secluded and encapsulated by a thick layer resistant to gastric digestion. The entire release was under 10%, indicating the efficient robustness of capsules under harsh gastric conditions.
(A) In-vitro release studies of oil form microcapsules under simulated gastro-intestinal conditions (B) Storage stability: Peroxide value of microcapsules loaded with ɷ−3 fatty acid. (*alphabets a, b,c and d indicate significant differences among different samples at a particular day of storage; alphabets A, B, C and D indicate significant differences among same samples at different storage days). **Sample code P7, P11 and P9 present the microcapsule obtained by the spray drying of the corresponding ɷ−3 fatty acid nanoemulsions E7, E11 and E9.
Higher oil percent release was observed on sequential exposure of encapsulated powders to SGF + SIF conditions compared to SGF alone. For all three samples, a sudden increase in the percent oil release rates was observed in the 30 min of intestinal incubation. This corresponded to oil release rates of 32.94%, 27.90%, and 33.80% for P7, P11, and P9 microcapsules, respectively, showing significant variations in their release rates. Such sudden increased oil release rates might be owing to enhanced microcapsule disintegration in pancreatin (amylase and trypsin) containing SGF + SIF, which hydrolyzes both proteins and carbohydrates, resulting in capsule structural changes (big pore creation) and subsequent oil release. At the end of sequential gastric and intestinal digestion, the total oil release rates were raised to 45.45%, 39.70%, and 55.45% for samples P7, P9, and P11, respectively, demonstrating significant differences. In a similar study, the increased proportion of WPC in a binary wall material mixture (WPC: MD) from 3:2 to 5:0 affected the release of polyphenols negatively, as significantly reduced release rates of polyphenols were observed when incubated for two hours under the simulated intestinal conditions35. In line with that, Eratte et al. 222 reported a similar release pattern of encapsulated tuna oil, with approximately 10% and 40% release rates in the gastric and intestinal stages, respectively, from a complex coacervate case material of whey protein isolate-GA. Flaxseed oil was released from microcapsules fitting well into the Korsmeyer-Peppas model (rP7 = 0.989, rP11 = 0.990, and rP9 = 0.995). The exponent of the model (nP7 = 1.27, nP11 = 1.43, and nP9 = 1.43) indicated the release by the swelling mechanism followed by matrix erosion. This model is also known as Super Case II Transport (n > 0.85). Kanha et al.36 also reported comparable results and observed an n-value closer to 1.45 for the release of anthocyanins from the spray-dried microcapsules formulated with chitosan and CMC coacervates in SIF. The oil rate constant (k) for different microcapsules was 0.05, 0.022, and 0.22 min−1, respectively.
Storage stability (peroxide value, rancimat test: accelerated oxidative stability)
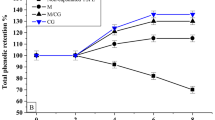
Initially (day 0), free flaxseed oil was observed to have the lowest peroxide value of 2.1 meq O2/kg of oil (Fig. IV b.) In contrast, the microcapsules had a higher peroxide value than 2.85 meq O2/kg of oil with no significant differences (p > 0.05) among all three microcapsules. Higher initial day PV of microcapsules could be attributed to the exposure of microcapsules to emulsifying and spray drying processes as well as the interaction of various formulations used for encapsulation, which could have affected the stability of flaxseed oil in it. The lower PV of flaxseed oil could be attributed to the absence of self-oxidation byproducts, indicating that auto-oxidation had not begun or was in its initial stages. whey encapsulates had greater peroxide values in the initial storage days than in the oil blank37. they also indicated that if a wall material with strong film-forming capabilities was utilized, auto-oxidation of encapsulated and non-encapsulated core oil occurred during drying, which might catalyze additional oxidation during storage38. They also stated that the quick-formed shell may have enhanced the resistance to captured moisture evaporation, resulting in a fast rise in particle temperature during drying. As a result, particle-free oil exposure at elevated temperatures was extended, resulting in peroxide levels exceeding those of unprocessed crude fish oil in the early storage stages. Other writers have also reported that particle ballooning during drying causes air to become trapped inside the particle, resulting in oxidation processes39. Advancements in storage days resulted in increased PV for all samples. Flaxseed oil samples were observed with a significant rise in PV, with a value of 5.01 meq O2/kg oil on the 14th day of storage and 15.18 meq O2/kg oil at the end of the storage. A significant rise in PV was also observed for microcapsules but was comparatively much lesser than the free samples, with values ranging from 2.94 to 3.4 meq O2/kg. Among the microcapsules, the P7 reported the highest rise on day 14, corresponding to 3.4 meq O2/kg oil. A similar trend was observed on weeks 2, 3, 4, and 5 for the same sample, followed by P9, and the comparatively most minor PV was observed for the E11thus, which exhibited excellent stability.
Figure 5 represents the oxidative stability of microencapsulated oil vis-a-vis control. The observed IP value of neat flaxseed oil is 1.4 h, which is comparable to the value reported for sunflower oil (1.3 h) and linseed oil (2.04 h) under similar conditions40. The typical induction period of 0.25–2.0 h has been reported for various polyunsaturated oils as per the Application Bulletin on evaluating their oxidative stability by the Rancimat method. The conductivity curves of the selected microencapsulated samples clearly show the protective effect of different wall materials on the oxidative stability of oils, as higher IP values were recorded for all samples. A maximum IP value of 11.8 h was observed for sample P11 containing WPC: MD: GA when present in a ratio of 1.5:0.5:0.2, contributing to 8.42 times better stability than bulk oil. This highly protective effect can be explained by the WPC’s antioxidant activity (due to lactoferrin), which protects the labile components in dispersed systems39.
Oxidative stability: Rancimat test of various microcapsules loaded with ɷ−3 fatty acid under accelerated storage conditions and made from spray drying nanoemulsions from each wall material combination (WPC: MD: GA) and sonication time; (A) P7 (1:1:0.15; 4 min), (B) P11 (1.5:0.5:0.10; 4 min) and (C) P9 (0.5:1.5:0.20; 4 min).
Similarly, in their work, Gallardo et al.40 also observed a significantly higher IP value (9.5 h) for a ternary blend of GA/MD/WPI when compared with a binary mixture of GA/MD (3.8 h) and GA alone (8 h). Following that, sample P9 also showed an increased IP value of 10.2 h. A decreased proportion of WPC, MD, and high GA are believed to contribute significantly to the extended IP values41. However, the stability period was short in both cases and lasted less than 25 h. Sample P7 also showed an increased IP value (2.2 h) compared with neat oil, but it was much less than other encapsulated samples. High MD levels could have been attributed to decreased stability.
Conclusion
Ultrasonication treatment significantly affected emulsions’ particle size and PDI. The emulsions’ viscosity imparted shear-thinning behaviour and varies with wall material and sonication duration. The dried microcapsules showed more than 90%EE. The flow characteristics were found to be affected by the wall material combination. The microcapsules’ wettability was reduced, and their dispersibility was elevated due to high WPC levels in the wall formulation. The morphology revealed that microcapsules have a spherical form with some unevenly shaped capsules, dents on the surface, and mild aggregation. The microcapsules obtained from P11 (WPC: MD: GA::1.5:0.5:0.10) exhibited higher oxidative stability with an increased induction period and lower PV after eight weeks. Furthermore, in simulated gastrointestinal conditions, the flaxseed oil is released more considerably (56%), rendering the developed microcapsules a stable and potent carrier of ALA and thus can be designated as an ideal food fortificant.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Ajith, T. A. & Jayakumar, T. G. Omega-3 fatty acids in coronary heart disease: recent updates and future perspectives. Clin. Exp. Pharmacol. Physiol. 46, 11–18 (2019).
Simopoulos, A. P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 8, 128 (2016).
Simopoulos, A. P. (ed) Healthy Agriculture, Healthy Nutrition, Healthy PeopleVol. 102 (Karger Medical and Scientific, 2011).
Brostow, D. P. et al. Omega-3 fatty acids and incident type 2 diabetes: the Singapore Chinese health study. Am. J. Clin. Nutr. 94, 520–526 (2011).
Orchard, T. S. et al. D. Fatty acid consumption and risk of fracture in the women’s health initiative. Am. J. Clin. Nutr. 92, 1452–1460 (2010).
Sharma, N., Kaur, G. & Khatkar, S. K. Optimization of emulsification conditions for designing ultrasound assisted Curcumin loaded nanoemulsion: characterization, antioxidant assay and release kinetics. LWT-Food Sci. Technol. 141, 110962 (2021).
Akhtar, M. & Dickinson, E. Whey protein–maltodextrin conjugates as emulsifying agents: an alternative to gum Arabic. Food Hydrocoll. 21, 607–616 (2007).
Charve, J. & Reineccius, G. A. Encapsulation performance of proteins and traditional materials for spray dried flavors. J. Agri Food Chem. 57, 2486–2492 (2009).
Shao, P., Feng, J., Sun, P. & Ritzoulis, C. Improved emulsion stability and Resveratrol encapsulation by Whey protein/gum Arabic interaction at oil-water interface. Int. J. Biol. Macromol. 133, 466–472 (2019).
Silva, E. K., Rosa, M. T. M. C. & Meireles, M. A. A. Ultrasound assisted formation of emulsions stabilized by biopolymers. Curr Opin Food Sci 5,50–59 Sukumar, A., Gurumoorthi, P., & Athmaselvi, K. A. (2023). Effect of ultrasonication on emulsion formulation, encapsulation efficiency, and oxidative stability of spray dried chia seed oil. Journal of Food Science and Technology, 60(6), 1761–1771. (2015).
Sukumar, A., Gurumoorthi, P. & Athmaselvi, K. A. Effect of ultrasonication on emulsion formulation, encapsulation efficiency, and oxidative stability of spray dried Chia seed oil. J. Food Sci. Technol. 60, 1761–1771 (2023).
Carneiro, H. C., Tonon, R. V., Grosso, C. R. & Hubinger, M. D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J Food Engg. 115, 443–451 (2013).
Chen, W. et al. Rice Bran protein-based nanoemulsion carrier for improving stability and bioavailability of Quercetin. Food Hydrocoll. 108, 106042 (2020).
Khatkar, A. B., Kaur, A., Khatkar, S. K. & Mehta, N. Optimization of processing time, amplitude and concentration for ultrasound-assisted modification of Whey protein using response surface methodology. J. Food Sci. Technol. 55 (6), 2298–2309 (2018).
Mehta, N. et al. Ultrasound-assisted extraction and the encapsulation of bioactive components for food applications. Foods 11 (19), 2973 (2022).
Quispe-Condori, S., Saldaña, M. D. & Temelli, F. Microencapsulation of flax oil with Zein using spray and freeze drying. LWT-Food Sci. Technol. 44, 1880–1887 (2011).
Turchiuli, C., Munguia, M. J., Sanchez, M. H., Ferre, H. C. & Dumoulin, E. Use of different supports for oil encapsulation in powder by spray drying. Powder Technol. 255, 103–108 (2014). 2014.
Thiengnoi, P., Suphantharika, M. & Wongkongkatep, P. Influence of binder type and concentration on physical properties of agglomerated, spray-dried, and high oil loaded microcapsules. J. Food Agri. Environ. 10, 141–150 (2012). (2012).
Teo, A., Lam, Y., Lee, S. J. & Goh, K. K. Spray drying of Whey protein stabilized nanoemulsions containing different wall materials–maltodextrin or Trehalose. LWT-Food Sci. Technol. 136, 110344 (2021).
Alcântara, M. A. et al. Influence of the emulsion homogenization method on the stability of Chia oil microencapsulated by spray drying. Powder Technol. 354, 877–885 (2019).
Minekus, M. et al. A. A standardized static in vitro digestion method suitable for food- an international consensus. Food Func. 5, 1113–1124 (2014).
Eratte, D., Dowling, K., Barrow, C. J. & Adhikari, B. P. -vitro digestion of probiotic bacteria and omega-3 oil co-microencapsulated in Whey protein isolate-gum Arabic complex coacervates. Food Chem. 227, 129–136 (2017).
Shanta, N. C. & Decker, E. A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 77, 421–424 (1994).
Hebishy, E., Zamora, A., Buffa, M., Blasco-Moreno, A. & Trujillo, A. J. Characterization of whey protein oil-in-water emulsions with different oil concentrations stabilized by ultra-high pressure homogenization. Proc. 5, 6. (2017).
Premi, M. & Sharma, H. K. Effect of different combinations of maltodextrin, gum Arabic and Whey protein concentrate on the encapsulation behavior and oxidative stability of spray dried drumstick (Moringa oleifera) oil. Int. J. Biol. Macromol. 105, 1232–1240 (2017).
Özbek, Z. A. & Ergönül, P. G. Optimization of wall material composition of freeze–dried pumpkin seed oil microcapsules: interaction effects of Whey protein, maltodextrin, and gum Arabic by D–optimal mixture design approach. Food Hydrocoll. 107, 105909 (2020).
Qayum, A. et al. Characterization and comparison of alpha-lactalbumin pre-and post-emulsion. J. Food Engg. 269, 109743 (2020).
Tahir, A., Ahmad, R. S., Khan, M. K., Imran, M. & Hailu, G. G. Optimization of production parameters for fabrication of gum arabic/whey Protein-Based walnut oil loaded nanoparticles and their characterization. ACS Omega. 9 (21), 22839–22850 (2024).
Zisu, B. et al. Ashok kumar, M. Effect of ultrasound on the physical and functional properties of reconstituted Whey protein powders. J. Dairy. Res. 78 (2), 226–232 (2011).
Quek, S. Y., Chok, N. K. & Swedlund, P. The physicochemical properties of spray-dried watermelon powders. Chem. Eng. Process: Process. Intensif. 46 (5), 386–392 (2007).
Sarabandi, K. H., Peighambardoust, S. H., Mahoonak, S., Samaei, S. P. & A. R., & Effect of different carriers on microstructure and physical characteristics of spray dried Apple juice concentrate. J. Food Sci. Technol. 55 (8), 3098–3109 (2018).
Stoll, L., Costa, T. M. H., Jablonski, A., Flôres, S. H. & de Oliveira Rios, A. Microencapsulation of anthocyanins with different wall materials and its application in active biodegradable films. Food Bioproc Technol. 9(1), 172–181 (2016).
Karrar, E. et al. Effect of maltodextrin combination with gum Arabic and Whey protein isolate on the microencapsulation of gurum seed oil using a spray-drying method. Int. J. Biol. Macromol. 171, 208–216 (2021).
Tontul, I. & Topuz, A. Influence of emulsion composition and ultrasonication time on flaxseed oil powder properties. Powder Technol. 264, 54–60 (2014).
Yadav, K., Bajaj, R. K., Mandal, S. & Mann, B. Encapsulation of grape seed extract phenolics using Whey protein concentrate, maltodextrin and gum Arabica blends. J. Food Sci. Technol. 57 (2), 426–434 (2020).
Kanha, N., Regenstein, J. M., Surawang, S., Pitchakarn, P. & Laokuldilok, T. Properties and kinetics of the in vitro release of anthocyanin-rich microcapsules produced through spray and freeze-drying complex coacervated double emulsions. Food Chem. 340, 127950 (2021).
Wang, Q., Lv, S., Lu, J., Jiang, S. & Lin, L. Characterization, stability, and in vitro release evaluation of carboxymethyl Chitosan coated liposomes containing fish oil. J. Food Sci. 80, C1460–C1467 (2015).
Drusch, S. & Berg, S. Extractable oil in microcapsules prepared by spray-drying: localisation, determination and impact on oxidative stability. Food Chem. 109, 17–24 (2008).
Keogh, M. K. et al. Stability to oxidation of spray-driedfish oil powder microencapsulated using milk ingredients. J. Food Sci. 66, 217–224 (2001).
Gallardo, G. et al. Microencapsulation of linseed oil by spray drying for functional food application. Food Res. Int. 52, 473–482 (2013).
Cervato, G., Cazzola, B. & Cestaro, B. Studies on the antioxidant activity of milk caesins. Int. J. Food Sci. Nutr. 50, 291–296 (1999).
Acknowledgements
We would like to thank Punjab Agricultural University for providing us with a necessity facility to carry out a research trial.
Funding
No funding available.
Author information
Authors and Affiliations
Contributions
Conceptualization: N.S, G.K, PT, SK, AS and SS; methodology, data collection and original data analysis: N.S, and SS.; data presentation, writing: N.S, G.K, PT, SK, AS; writing, reviewing and editing: N.S, G.K, PT, SK, AS and S.S.; funding acquisition: N.S, G.K, PT, SK, AS and SS;. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sharma, N., Kaur, G., Khatkar, S.K. et al. Effect of ultrasound on the encapsulation potential of a ternary blend of wall materials for protection of flaxseed oil. Sci Rep 15, 35288 (2025). https://doi.org/10.1038/s41598-025-19008-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-19008-4