Abstract
This study aimed to evaluate the outcomes of patients with advanced renal cell carcinoma (aRCC) who were histologically diagnosed with non-clear cell carcinoma (nccRCC) and received combination immunotherapy. Oncological outcomes were compared between patients with non-clear cell RCC (nccRCC group) and those with clear cell RCC (cc-RCC group) who received either immune checkpoint inhibitor (ICI) + ICI combination therapy (ICI + ICI) or ICI + tyrosine kinase inhibitor (TKI) therapy (ICI + TKI). This retrospective study analyzed patients with aRCC who received combination immunotherapy as first-line treatment at 12 institutions between August 2018 and November 2023. The primary endpoint was the comparison of overall survival (OS) between the nccRCC and cc-RCC groups. The secondary endpoints included progression-free survival (PFS) and objective response rate (ORR) in both groups. The median follow–up periods for the nccRCC and cc-RCC groups were 17.7 and 20.4 months, respectively. The median OS was significantly shorter in the nccRCC group compared to the cc-RCC group (27.8 months vs. 62.8 months; p < 0.001). The ORRs in the nccRCC and cc-RCC groups were 52.4% and 63.6%, respectively. Among patients treated with ICI + ICI, the median OS was significantly shorter in the nccRCC group compared to the cc-RCC group (23.5 months vs. 62.8 months; p < 0.001). In the current era of ICI treatment, patients with nccRCC exhibit poorer oncological outcomes than those with cc-RCC. Indications should be evaluated thoroughly before selecting ICI + ICI treatment combinations for these patients.
Similar content being viewed by others
Introduction
Renal cell carcinoma (RCC) accounts for approximately 2% of malignant neoplasms worldwide, and its incidence has exhibited a consistent upward trend over the past several decades1,2,3,4. The 5-year cancer-specific survival (CSS) rate for patients diagnosed with early-stage RCC is reported to be ~ 90%5,6. However, approximately one-third of patients with RCC already have metastatic disease at the time of their initial diagnosis, and 20–50% of patients who undergo definitive surgery for RCC develop locoregional or distant metastases7. The 5–year CSS rate for patients with advanced RCC (aRCC) has been documented to be particularly unfavorable, ranging between 12 and 14%5,6,8.
The advent of immune checkpoint inhibitors (ICIs) has resulted in significant changes in treatment strategies for aRCC9. Phase III randomized controlled trials for aRCC have included ICI + ICI combination therapies such as nivolumab + ipilimumab (NIVO + IPI) and ICI + tyrosine kinase inhibitor (TKI) therapies including avelumab + axitinib (AVE + AXI), pembrolizumab + axitinib (PEM + AXI), nivolumab + cabozantinib (NIVO + CABO), and pembrolizumab + lenvatinib (PEM + LEN) have been shown to significantly improve oncological outcomes compared to sunitinib (SUN), the standard of care prior to the ICI era10,11,12,13,14. These trials predominantly focused on clear cell renal cell carcinoma (cc-RCC), which constitutes 70–85% of RCC cases15,16,17. The available data on the therapeutic efficacy for non-clear cell renal cell carcinoma (nccRCC), which exhibits distinct pathological characteristics and occurs less frequently, remain limited. Consequently, there is a paucity of recommended systemic therapies for nccRCC, and the management of these histological features is mainly consistent with that of cc-RCC. Recent reports have emerged regarding the efficacy and safety of combination immunotherapies for nccRCC18,19,20,21,22,23,24, with both PEM + LEN and NIVO + CABO being included as preferred regimens in the National Comprehensive Cancer Network guidelines25. However, clinical evidence regarding the efficacy and safety of ICI + ICI and ICI + TKI for the treatment of nccRCC remains limited. Moreover, comparative reports that directly assess the efficacy and safety of ICI + ICI or ICI + TKI as treatment modalities for nccRCC and cc-RCC are lacking.
This study aimed to ascertain the efficacy and safety of these treatment approaches among patients with nccRCC, among a broader cohort of those with aRCC who received combination immunotherapy, and to compare them with those in patients with cc-RCC. A comparative analysis was also conducted of the oncological outcomes of patients diagnosed with nccRCC and cc-RCC who received ICI + ICI or ICI + TKI regimens.
Materials and methods
Patients
This study was approved by the Institutional Review Board of Gifu University (approval number: 2023–147) and was conducted after obtaining implementation approval at each participating institution. All methods were carried out in accordance with relevant guidelines and regulations and in accordance with the World Medical Association Declaration of Helsinki. Informed consent was not required because of the retrospective nature of the study. Moreover, in accordance with the Japanese Ethics Committee and its ethical guidelines, written informed consent was not obtained because retrospective and observational studies using existing materials and other data have already been published. Instead, we used an opt-out approach and provided patients with the opportunity to decline participation. More information on this study, which is available only in Japanese, can be found at https://rinri.med.gifu-u.ac.jp/esct/publish_document.aspx? ID=2772 (accessed September 19, 2023).
In this retrospective multi-institutional study, patients with aRCC who were treated with ICI combination therapies at 12 Japanese institutions between August 2018 and November 2023 were registered. We defined aRCC as simultaneous metastasis at the time of RCC diagnosis, recurrence after surgery, or locally advanced cancer that was unresectable for curative purposes. The enrolled patients were stratified into favorable, intermediate, or poor risk groups, according to the International Metastatic RCC Database Consortium (IMDC) risk classification26 The following clinicopathological data were collected: age, sex, IMDC risk classification, histological type of the primary tumor, surgical history, therapeutic regimen of ICI combination therapy, and metastatic sites. For patients who did not undergo nephrectomy, the pathological diagnosis was primarily confirmed by image-guided needle biopsy. The decision to perform nephrectomy and its timing were determined at each institution based on the patient’s performance status, the location and number of metastases, and other clinical factors.
ICI combination therapy
In the NIVO + IPI combination immunotherapy regimen, patients received intravenous administrations of nivolumab at a dose of 3 mg/kg, and ipilimumab at a dose of 1 mg/kg every three weeks, prior to September 2018. The induction phase consisted of four courses of NIVO + IPI, followed by nivolumab monotherapy at a dose of 3 mg/kg every two weeks as the Maintenance phase. Beginning in October 2018, nivolumab was administered to the patients at a dose of 240 mg. For the AVE + AXI regimen, AVE was administered at a dose of 10 mg/kg body weight every two weeks, and AXI was administered orally at a dose of 5 mg twice daily. For the PEM + AXI treatment group, PEM was administered intravenously at a dose of 200 mg every three weeks or 400 mg every six weeks, and AXI was administered orally at a dose of 5 mg twice per day. For the NIVO + CABO protocol, NIVO was administered intravenously at 240 mg every two weeks or 480 mg every four weeks, whereas CABO was administered orally at 40 mg once daily. In the PEM + LEN cohort, PEM was administered intravenously at 200 mg every three weeks or 400 mg every six weeks, and LEN was administered orally at 20 mg once a day.
The treatment regimens and doses were determined for each institution, and the patients continued their treatments until disease progression (as assessed by imaging studies) or the emergence of intolerably severe treatment-related adverse events (TRAEs).
Patient evaluation
The baseline evaluation included a comprehensive medical history and physical examination as well as chest, abdominal, and pelvic computed tomography (CT) and magnetic resonance imaging (MRI) scans. Tumor staging was performed according to the American Joint Committee on Cancer Staging Manual27.
All patients underwent CT or MRI every 1–3 months until disease progression according to radiological evaluation or treatment discontinuation because of TRAEs. The best overall response (BOR) was documented as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD), using the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines version 1.128. The objective response rate (ORR) was defined as the proportion of patients with a confirmed best response of CR or PR according to the RECIST criteria. The disease control rate (DCR) was defined as the proportion of patients who exhibited CR, PR, or SD according to the RECIST classification.
Safety
TRAEs were defined as those occurring within ≥ 100 days of the last administration of ICI + ICI or ICI + TKI, according to the Common Terminology Criteria for Adverse Events ver. 5.0 of the National Cancer Institute29.
Statistical analysis
The primary endpoint of the present study was overall survival (OS) in patients with aRCC who were treated with combination therapy based on ICI. The secondary endpoints included progression-free survival (PFS), ORR, and DCR in those with nccRCC or cc-RCC who received ICI + ICI or ICI + TKI. The date of ICI combination therapy administration was used as the starting point for the OS and PFS estimates. OS was defined as the time from the initiation of ICI combination therapy to death from any cause. PFS was defined as the time between the initiation of ICI combination therapy and disease progression. The Kaplan–Meier method was used to evaluate OS and PFS, whereas the log-rank test was used to compare subgroups. The two groups—patients with nccRCC and those with cc-RCC —were compared using Fisher’s exact test for categorical variables. JMP 14 software (SAS Institute Inc., Cary, NC, USA) was used to analyze the data. All tests were two-sided, and statistical significance was set at p < 0.05.
Results
Patients
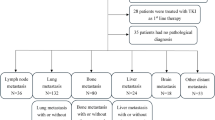
A total of 186 patients diagnosed with aRCC were enrolled, including 165 with cc-RCC and 21 with nccRCC. Their demographic data are presented in Table 1. The median patient age was 66.5 years (interquartile range [IQR], 57.0–73.0), and males accounted for 74.2% of the total. Compared to the other risk groups, 116 patients (62.4%) with intermediate risk, according to the IMDC risk classification, were most prevalent. Overall, 170 patients (91.4%) had at least one metastasis, with the lungs being the most common site (64.0%). Nephrectomy was performed before ICI combination therapy in 103 patients (55.4%), and deferred cytoreductive nephrectomy was performed in 13 (15.7%). The median follow-up period for the enrolled patients was 20.0 months (IQR, 9.3–33.8 months).
The first-line treatment modalities for the nccRCC and cc-RCC groups are listed in Table 2. NIVO + IPI was administered to 99 patients (53.2%), with the highest number of patients with nccRCC and cc-RCC receiving this treatment.
Efficacy and oncological outcomes
The median OSs were 27.8 months in the nccRCC group and 62.6 months in the cc-RCC group (p < 0.001; Fig. 1a). Although there was no statistically significant difference in PFS, the median PFSs were 5.3 months in the nccRCC group and 32.8 months in the cc-RCC group (Fig. 1b).
(a) Overall survival (OS) of patients with advanced renal cell carcinoma (aRCC) who received combination immunotherapy. The median OSs in the clear cell renal cell carcinoma (cc-RCC) and non-clear cell renal cell carcinoma (nccRCC) groups are 62.8 months and 27.8 months, respectively. The OS in the nccRCC group is significantly shorter than that in the cc-RCC group (p < 0.001). (b) Progression-free survival (PFS) in patients with aRCC who received combination immunotherapy. The median PFSs in the cc-RCC and nccRCC groups are 32.3 and 5.3 months, respectively. Although no statistically significant difference in PFS is observed, the median PFS is shorter in the nccRCC group than in the cc-RCC group. (p = 0.113).
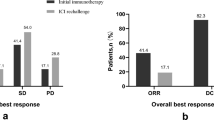
The treatment effects of ICI combination therapies in patients with nccRCC and cc-RCC are shown in Table 3. The best overall responses to ICI + ICI and ICI + TKI were similar in both groups. ORR and DCR were more favorable in the cc-RCC group than in the nccRCC group in patients treated with ICI + TKI, whereas both groups showed similar treatment efficacy in those treated with ICI + ICI. In the nccRCC group, the ORRs for patients treated with AVE + AXI, PEM + AXI, NIVO + CABO, and PEM + LEN were 50.0%, 50.0%, 100%, and 50%, respectively, while DCRs of 50%, 100%, 100%, and 75%. In contrast, the ORRs in the ccRCC group were 65.0%, 65.0%, 84.6%, and 79.2%, respectively, and the DCRs were 95%, 100%, 100%, and 91.7%, respectively. The ORR and DCR by histologic subtype were as follows: 60% and 73.3% for papillary RCC, 50% and 100% for chromophobe RCC, 0% and 100% for acquired cystic disease-associated RCC, 0% and 0% for MiT family translocation RCC, and 100% and 100% for fumarate hydratase deficient RCC.
In patients treated with ICI + ICI, the median OS in the nccRCC group was significantly shorter than that in the cc-RCC group (p < 0.001; Fig. 2a), whereas PFS tended to be shorter in the nccRCC group, although there was no statistically significant difference between the two (Fig. 2b). A comparative analysis of patients treated with ICI + TKI revealed no statistically significant differences in OS and PFS between the nccRCC and cc-RCC groups (Fig. 2c, d). As shown in Table 4, ORR, OS, and PFS results for the nccRCC and ccRCC groups are stratified by the presence or absence of cytoreductive nephrectomy (CN). While no significant differences in oncological outcomes were observed between CN-treated and CN-untreated patients in the ccRCC group, patients in the nccRCC group who did not undergo CN had a noticeably lower ORR and significantly shorter median OS and PFS. Additionally, no substantial differences were observed in ORR, OS, or PFS between the nccRCC and cc-RCC groups based on IMDC risk classification.
(a) Overall survival (OS) of patients with advanced renal cell carcinoma (aRCC) treated with immune checkpoint inhibitor (ICI) + ICI combination therapy. The median OSs in the clear cell renal cell carcinoma (cc-RCC) and non-clear cell renal cell carcinoma (nccRCC) groups are 62.8 and 23.5 months, respectively. The OS in the nccRCC group is significantly shorter than that in the cc-RCC group (p < 0.001). (b) Progression-free survival (PFS) in patients with aRCC who are treated with ICI + ICI combination therapy. The median PFSs in the cc-RCC and nccRCC groups are 23.0 and 11.5 months, respectively. There is no significant difference in PFS between the cc-RCC and nccRCC groups (p = 0.072). (c) OS in patients with aRCC treated with ICI + tyrosine kinase inhibitor (TKI) therapy. The median OS is not achieved in either the cc-RCC or nccRCC groups, and there is no significant difference between them (p = 0.551). (d) PFS in patients with aRCC who are treated with ICI + TKI therapy. The median PFS is not achieved in either the cc-RCC or nccRCC groups, and there is no significant difference between them (p = 0.821).
Safety
The TRAEs are listed in Table 5. The incidence rates of all-grade TRAEs in the nccRCC group treated with AVE + AXI, PEM + AXI, NIVO + CABO, and PEM + LEN were 100% in each group, while the incidence rates of grade ≥ 3 TRAEs were 0%, 50.0%, 0%, and 75.0%, respectively. In contrast, within the ccRCC cohort, the incidence rates of all-grade TRAEs were 80.0%, 90.0%, 92.3%, and 100%, respectively, while the incidence of grade ≥ 3 TRAEs were observed in 5.0%, 40.0%, 38.5%, and 26.7% of patients, respectively. In the nccRCC group, three patients (27.3%) treated with ICI + ICI experienced treatment discontinuation due to TRAEs; however, none of the patients treated with ICI + TKI required treatment discontinuation. Conversely, 36 patients (40.9%) who received ICI + ICI and 22 (28.6%) who were treated with ICI + TKI in the cc-RCC group discontinued treatment because of TRAEs. None of the patients in this study died because of TRAEs.
Discussion
The results of phase III clinical trials, such as the CheckMate 214, JAVELIN Renal 101, KEYNOTE–426, CheckMate 9ER, and CLEAR studies, showed that ICI + TKI combination therapies, including NIVO + IPI, AVE + AXI, PEM + AXI, NIVO + CABO, and PEM + LEN, significantly improved the oncological outcomes of aRCC compared to SUN10,11,12,13,14. In clinical practice, multiple reports have demonstrated the safety and efficacy of ICI combination therapies for treating RCC30,31,32,33,34. However, clinical trials have been conducted in patients with cc-RCC, which accounts for ~ 70–80% of cases, and various real-world clinical studies have also been conducted, primarily in patients with cc-RCC15,16,17. Recently, the prospective randomized phase II SUNNIFORECAST trial directly compared NIVO + IPI to standard therapy in patients with nccRCC35. The study found a significantly higher 12-month OS and ORR for the immunotherapy group, supporting the conclusion that dual checkpoint inhibitor therapy is a clinically beneficial option for nccRCC35. Additionally, cc-RCC is associated with alterations in the Von Hippel-Lindau gene, resulting in increased vascular endothelial growth factor expression and hyperactivation of the mammalian target of rapamycin pathway36. In contrast, given the limited number of cases of nccRCC and the presence of various subtypes, it has been suggested that the efficacy of ICI combination therapy may exhibit significant variability contingent on the biological characteristics of each subtype37. Therefore, an independent evaluation of therapeutic modalities and their outcomes for nccRCC is warranted to examine why the oncologic outcomes of nccRCC tend to be worse than those of cc-RCC37.
In this study, the oncological outcomes of patients with aRCC were compared according to the respective types of ICI combination therapy they received18,19,20,21,24,38,39,40. Several studies have demonstrated the effectiveness of ICI + ICI for aRCC18,39,40. A study of 18 patients with nccRCC who received NIVO + IPI found that the median duration of administration was 2.4 months (range, 0.7–12.3 months)38. A total of 33.3% of patients exhibited an ORR, with a median duration of 4.3 months and a median PFS of 7.1 months in the overall patient population38. As demonstrated in the CheckMate 920 study, a multicenter phase 3b/4 trial of NIVO + IPI, the efficacy in advanced nccRCC was demonstrated in patients with previously untreated aRCC with clinical characteristics that were excluded from the phase III trial39. A total of 52 patients were enrolled in the study, with a minimum follow-up period of 24.1 months18. The median OS and PFS were 21.2 and 3.7 months, respectively, and the ORR was lower in patients with nccRCC than in those with cc-RCC18.
As demonstrated in previous studies, ICI + TKI has been shown to have a higher response rate than ICI + ICI in patients with nccRCC10,11,12,13,14. A significant prolongation of PFS in papillary RCC, a subtype of nccRCC, has been reported for cabozantinib, a multi-kinase inhibitor that inactivates MET, compared to SUN40. In a phase II trial of NIVO + CABO for nccRCC, the ORR was 54%, and the median OS and PFS were 28 and 11 months, respectively, with a median follow-up period of 34 months22. In a phase II trial of LEN + PEM for nccRCC, the ORR was 49%, and the OS and PFS at 12 months were 82% and 63%, respectively24. Moreover, no statistically significant differences were observed in the median OS and PFS between the nccRCC and cc-RCC groups (p = 0.551 and p = 0.821, respectively)24. The findings of the present study indicate that combination therapy involving ICI and TKI for patients with nccRCC may yield outcomes comparable to those observed in patients with cc-RCC with respect to both ORR and oncological outcomes.
Table 6 provides a comprehensive overview of extant studies that evaluated the efficacy and safety of combined ICI therapies in Japanese patients diagnosed with nccRCC18,19,20,21. In the context of oncological outcomes in patients with aRCC treated with NIVO + IPI, OS was found to be significantly shorter in those with nccRCC than in those with cc-RCC (median 20.8 months vs. not reached; p = 0.04); however, no significant difference was observed in PFS (median 6.3 months vs. 10.8 months; p = 0.21)18. Teishima et al.19 reported an ORR of 33.3% in 36 patients with nccRCC who were treated with combination immunotherapy. In a retrospective study of the clinical efficacy of ICI combination therapy in 44 patients with nccRCC, the ORR was 36.4% with a median follow-up of 15.2 months, and the median OS and PFS were reported to be 23.9 months and 8.8 months, respectively20. Our study aimed to elucidate the potential disparities in oncological outcomes between patients diagnosed with nccRCC or cc-RCC undergoing treatment for aRCC in conjunction with ICI therapy. The median OS was significantly shorter in the nccRCC group than that in the cc-RCC group. Conversely, the median PFS was not significantly different between the two groups; however, it tended to be shorter in the nccRCC group, as previously documented18,19,20,21. In addition, the proportion of patients with PD was higher in the nccRCC group than in the cc-RCC group. Consequently, it is imperative to establish a multidisciplinary treatment strategy that incorporates ICI combination therapies to enhance the prognosis of patients with nccRCC.
The incidence of TRAEs for ICI + ICI in patients with nccRCC has been reported to range between 55.0 and 73.1% for all grades and between 15.0 and 46.2% for grades 3–518,19,20,38. In contrast, the incidence of TRAEs with ICI + TKI in these patients has been reported to range between 70% and 94% for all grades and between 30% and 55% for grades 3–520,22,23,24. In this study, the safety profile was consistent with that reported in previous studies.
It is important to note that this study was subject to a few limitations. First, this was a retrospective observational study, and the total number of enrolled patients and nccRCC cases was relatively small. Therefore, statistical power is limited, and results may not be generalizable to the general population; thus, they should be interpreted with caution. To validate these findings, larger prospective studies are needed. Furthermore, the pathological diagnosis in patients who did not undergo nephrectomy was based on needle biopsy specimens, potentially introducing bias in the histological diagnosis. Second, given the multicenter nature of the study, the possibility of inherent bias in the results obtained owing to variations in diagnosis and treatment approaches across the different participating centers merits consideration. Additionally, discrepancies in pathological diagnostic procedures and treatment protocols across facilities may have contributed to variations in data interpretation. Third, as the number of ICI combination therapies for aRCC has increased, the treatment of choice has gradually shifted and may continue to evolve in real-world clinical practices. Fourth, given the inclusion of various histological types with disparate genetic backgrounds and molecular characteristics in nccRCC, it is imperative to consider each histological type individually when establishing customized treatments for nccRCC. Moreover, given the current focus of major clinical trials on ccRCC, it is essential to exercise caution when extrapolating efficacy and safety results for ccRCC to the more heterogeneous population of nccRCC. Therefore, the implementation of clinical trials specifically designed for nccRCC is crucial. A recent Phase II trial supports the rationale for personalized treatment approaches, demonstrating that the combination therapy of sintilimab and axitinib achieved high response rates and sustained disease control in patients with advanced renal cell carcinoma lacking fumarate hydratase, a rare non-clear cell subtype41. Consequently, determining treatment strategies based on genomic information may become clinically significant in the future. Finally, the discrepancy in therapeutic efficacy between ICI + ICI and ICI + TKI in patients with nccRCC remains to be elucidated19,20,21, underscoring the necessity for further investigations through large prospective multicenter studies that include more substantial cohorts of patients with nccRCC.
Conclusions
In this study, patients with nccRCC who received ICI combination therapy, particularly NIVO + IPI, demonstrated significantly shorter OS than those with cc-RCC. Therefore, the oncological outcomes of patients with nccRCC treated with ICI combination therapy are less favorable than those of patients with cc-RCC. Consequently, the choice of treatment modality for these patients should be carefully considered based on specific indications.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. https://doi.org/10.3322/caac.21660 (2021).
Padala, S. A. et al. Epidemiology of renal cell carcinoma. World J. Oncol. 11 (3), 79–87. https://doi.org/10.14740/wjon1279 (2020).
Siegel, R. L. et al. Cancer statistics, 2021. CA Cancer J. Clin. 71 (1), 7–33. https://doi.org/10.3322/caac.21654 (2021).
Sasaki, T., Higashi, T. & Inoue, T. Urological cancer statistics on incidence from 1975 to 2019 and mortality from 1958 to 2022 in Japan. Int. J. Clin. Oncol. 29 (8), 1088–1095. https://doi.org/10.1007/s10147-024-02575-3 (2024).
https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts figures/cancer-facts-figures-2022.htmlReturn to ref 2 in article.
Ljungberg, B. et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur. Urol. 82 (4), 399–410. https://doi.org/10.1016/j.eururo.2022.03.006 (2022).
Gupta, K. et al. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat. Rev. 34 (3), 193–205. https://doi.org/10.1016/j.ctrv.2007.12.001 (2008).
Motzer, R. J. et al. NCCN guidelines insights: kidney cancer, version 2.2020. J. Natl. Compr. Canc Netw. 17 (11), 1278–1285. https://doi.org/10.6004/jnccn.2019.0054 (2019).
Thompson, R. H. et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 66 (7), 3381–3385. https://doi.org/10.1158/0008-5472.CAN-05-4303 (2006).
Tannir, N. M. et al. Nivolumab plus ipilimumab versus Sunitinib for first-line treatment of advanced renal cell carcinoma: extended 8-year follow-up results of efficacy and safety from the phase III checkmate 214 trial. Ann. Oncol. 35 (11), 1026–1038. https://doi.org/10.1016/j.annonc.2024.07.727 (2024).
Choueiri, T. K. et al. Updated efficacy results from the JAVELIN renal 101 trial: first-line avelumab plus axitinib versus Sunitinib in patients with advanced renal cell carcinoma. Ann. Oncol. 31 (8), 1030–1039. https://doi.org/10.1016/j.annonc.2020.04.010 (2020).
Powles, T. et al. Pembrolizumab plus axitinib versus Sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 21 (12), 1563–1573. https://doi.org/10.1016/S1470-2045(20)30436-8 (2020).
Powles, T. et al. Nivolumab plus Cabozantinib versus Sunitinib for first-line treatment of advanced renal cell carcinoma: extended follow-up from the phase III randomised checkmate 9ER trial. ESMO Open. 9 (5), 102994. https://doi.org/10.1016/j.esmoop.2024.102994 (2024).
Motzer, R. J. et al. Characterization of responses to lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma at the final prespecified survival analysis of the phase 3 CLEAR study. Eur. Urol. 86 (1), 4–9. https://doi.org/10.1016/j.eururo.2024.03.015 (2024).
Mai, K. T. et al. G renal cell carcinoma with mixed features of papillary and clear cell cytomorphology: a fluorescent in situ hybridization study. Virchows Arch. 456 (1), 77–84. https://doi.org/10.1007/s00428-009-0871-2 (2010).
Sims, J. N. et al. Racial disparities and preventive measures to renal cell carcinoma. Int. J. Environ. Res. Public. Health. 15 (6), 1089. https://doi.org/10.3390/ijerph15061089 (2018).
Albiges, L., Molinie, V. & Escudier, B. Non-clear cell renal cell carcinoma: does the mammalian target of Rapamycin represent a rational therapeutic target? Oncologist 17 (8), 1051–1062. https://doi.org/10.1634/theoncologist.2012-0038 (2012).
Izumi, K. et al. Clinical outcomes of nivolumab plus ipilimumab in patients with metastatic non-clear cell renal cell carcinoma: Real-world data from a Japanese multicenter retrospective study. Int. J. Urol. 30 (9), 714–721. https://doi.org/10.1111/iju.15128 (2023).
Teishima, J. et al. Therapeutic outcome of combination therapy using immune-checkpoint inhibitors and tyrosine kinase inhibitors for metastatic non-clear-cell renal cell carcinoma. Can. Urol. Assoc. J. 18 (5), E162–E166. https://doi.org/10.5489/cuaj.8548 (2024).
Yoshimura, A. et al. Clinical outcomes of first-line combination therapy with immune checkpoint inhibitor for metastatic non-clear cell renal cell carcinoma: a multi-institutional retrospective study in Japan. Int. J. Clin. Oncol. 29 (12), 1916–1924. https://doi.org/10.1007/s10147-024-02612-1 (2024).
Toyoda, S. et al. Clinical outcomes and prognostic factors in metastatic nonclear cell renal cell carcinoma treated with immuno-oncology combination therapy. Jpn J. Clin. Oncol. 54 (12), 1336–1342. https://doi.org/10.1093/jjco/hyae108 (2024).
Fitzgerald, K. N. et al. Cabozantinib plus nivolumab in patients with Non-Clear cell renal cell carcinoma: updated results from a phase 2 trial. Eur. Urol. 86 (2), 90–94. https://doi.org/10.1016/j.eururo.2024.04.025 (2024).
Hilser, T. et al. Cabozantinib plus nivolumab in adult patients with advanced or metastatic renal cell carcinoma: A retrospective, Non-Interventional study in a Real-World cohort/guardians project. Cancers (Basel). 16 (17), 2998. https://doi.org/10.3390/cancers16172998 (2024).
Albiges, L. et al. Pembrolizumab plus lenvatinib as first-line therapy for advanced non-clear-cell renal cell carcinoma (KEYNOTE-B61): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 24 (8), 881–891. https://doi.org/10.1016/S1470-2045(23)00276-0 (2023).
National Comprehensive Cancer Network. Kidney Cancer Guidelines Version 1.2026 [Internet]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
Heng, D. Y. et al. External validation and comparison with other models of the international metastatic Renal-Cell carcinoma database consortium prognostic model: a population-based study. Lancet Oncol. 14 (2), 141–148. https://doi.org/10.1016/S1470-2045(12)70559-4 (2013).
Edge, S. B. & Compton, C. C. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 17 (6), 1471–1474. https://doi.org/10.1245/s10434-010-0985-4 (2010).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer. 45 (2), 228–247. https://doi.org/10.1016/j.ejca.2008.10.026 (2009).
https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
Iinuma, K. et al. Efficacy and safety of nivolumab and ipilimumab for advanced or metastatic renal cell carcinoma: A multicenter retrospective cohort study. Curr. Oncol. 28 (2), 1402–1411. https://doi.org/10.3390/curroncol28020133 (2021).
Taniguchi, T. et al. Real-World oncological outcomes of nivolumab plus ipilimumab in advanced or metastatic renal cell carcinoma: A multicenter, retrospective cohort study in Japan. Curr. Oncol. 31 (12), 7914–7923. https://doi.org/10.3390/curroncol31120583 (2024).
Doshi, G. K. et al. Real-World outcomes in patients with metastatic renal cell carcinoma treated with First-Line nivolumab plus ipilimumab in the united States. JCO Clin. Cancer Inf. e2400132. https://doi.org/10.1200/CCI.24.00132 (2024).
Iinuma, K. et al. The efficacy and safety of immune checkpoint inhibitor and tyrosine kinase inhibitor combination therapy for advanced or metastatic renal cell carcinoma: A multicenter retrospective Real-World cohort study. Cancers (Basel). 15 (3), 947. https://doi.org/10.3390/cancers15030947 (2023).
Hara, T. et al. Efficacy and safety of lenvatinib and pembrolizumab as first-line treatment for advanced renal cell carcinoma patients: real-world experience in Japan. Int. J. Clin. Oncol. 29 (12), 1931–1936. https://doi.org/10.1007/s10147-024-02633-w (2024).
Bergmann, L. et al. Prospective randomized phase-II trial of ipilimumab/nivolumab versus standard of care in non-clear cell renal cell cancer - results of the SUNNIFORECAST trial. Ann. Oncol. 36 (7), 796–806. https://doi.org/10.1016/j.annonc.2025.03.016 (2025).
Kim, H. et al. Loss of von Hippel-Lindau (VHL) tumor suppressor gene function: VHL-HIF pathway and advances in treatments for metastatic renal cell carcinoma (RCC). Int. J. Mol. Sci. 22 (18), 9795. https://doi.org/10.3390/ijms22189795 (2021).
Barthélémy, P. et al. Non-clear cell renal carcinomas: review of new molecular insights and recent clinical data. Cancer Treat. Rev. 97, 102191. https://doi.org/10.1016/j.ctrv.2021.102191 (2021).
Gupta, R. et al. Clinical activity of ipilimumab plus nivolumab in patients with metastatic Non-Clear cell renal cell carcinoma. Clin. Genitourin. Cancer. 18 (6), 429–435. https://doi.org/10.1016/j.clgc.2019.11.012 (2020).
Tykodi, S. S. et al. Safety and efficacy of nivolumab plus ipilimumab in patients with advanced non-clear cell renal cell carcinoma: results from the phase 3b/4 checkmate 920 trial. J. Immunother Cancer. 10 (2), e003844. https://doi.org/10.1136/jitc-2021-003844 (2022).
Pal, S. K. et al. A comparison of Sunitinib with cabozantinib, crizotinib, and Savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet 397 (10275), 695–703. https://doi.org/10.1016/S0140-6736(21)00152-5 (2021).
Zhang, X. et al. Sintilimab plus axitinib for advanced fumarate Hydratase-Deficient renal cell carcinoma: A phase 2 nonrandomized clinical trial. JAMA Oncol. e252497 https://doi.org/10.1001/jamaoncol.2025.2497 (2025).
Funding
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (22K16816).
Author information
Authors and Affiliations
Contributions
Koji Iinuma: Data analysis, manuscript writing/editing, data collection and management; Yuta Sano: data collection and management; Kohei Nishikawa: data collection and management; Tomoki Taniguchi: data collection and management; Aika Matsuyama: data collection and management; Kaori Ozawa: data collection and management; Takashi Ishida: data collection and management; Kosuke Tochigi: data collection and management; Masataka Tamura: data collection and management; Yasuaki Kubota: data collection and management; Shusuke Akamatsu: data collection and management, review; Takahiro Inoue: data collection and management; Takuya Koie: Protocol/project development, data management, manuscript writing/editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Iinuma, K., Sano, Y., Nishikawa, K. et al. Efficacy and safety of combination immunotherapy for treating advanced non-clear cell renal cell carcinoma: A multicenter retrospective study in Japan. Sci Rep 15, 35526 (2025). https://doi.org/10.1038/s41598-025-19523-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19523-4