Abstract
Introduction
Curcumin has demonstrated significant potential as a chemoprotective agent by inducing death in malignant cells while exhibiting selective cytotoxicity toward normal cells. However, curcumin has limited bioavailability which hindered its full potential. Thus, 2,6-bis-(4-hydroxyl-3-methoxybenzylidene) cyclohexanone (BHMC), a curcuminoid derivative is produced by eliminating unstable β-diketone component, transformed it with double bonds while retaining the phenolic hydroxyl group. Of note, BHMC triggers greater cytotoxic effect via inducing higher oxidative stress damage through reactive oxygen species (ROS)-mediated pathway. Increased of ROS cause the redox buffering system to collapse, resulting in lipid peroxidation and disintegration of the mitochondrial membrane potential, which eventually causes cell death in malignant cells.
Methodology
Total antioxidant activity was determined using Ferric Reducing Antioxidant Power (FRAP) assay biochemically and on HepG2 cells. Intracellular ROS was then measured using 2′,7′-Dichlorodihydrofluorescein diacetate (DCFDA) assay which was confirmed by determined the level of intracellular glutathione. The expression of Keap1 and Nrf2 was further analysed using immunocytochemistry.
Result and Discussion
BHMC exhibits antioxidant properties by demonstrating greater total antioxidant activity both biochemically and in HepG2 cells. Further analysis shows that BHMC significantly reduces intracellular ROS levels in HepG2 cells at low concentrations of 15 µM after 18 h and 10 µM after 24 h compared to untreated. However, at a higher concentration of 20 µM, BHMC induces oxidative stress like curcumin by lowering the ratio of reduced glutathione to glutathione disulfide (GSH/GSSG) and upregulating Nrf2 expression. The effects of BHMC are dose-dependent, with the compound acting as either an antioxidant or pro-oxidant depending on the concentrations.
Conclusion
BHMC exhibits potent antioxidant activity by reducing harmful reactive oxygen species and boosting protective glutathione levels in cells at low doses, but at higher doses, it may induce oxidative stress. These findings suggest BHMC’s dose-dependent role in balancing antioxidant and pro-oxidant effects, highlighting its potential for further therapeutic exploration.
Similar content being viewed by others
Introduction
Reactive oxygen species (ROS), also known as reactive oxygen metabolites (ROMs) or intermediates (ROIs), are highly reactive oxygen-containing molecules critical for cellular signaling, proliferation, and homeostasis1,2,3. Generated endogenously through mitochondrial respiration, enzymatic reactions (e.g., nitric oxide synthase, NADPH oxidases, xanthine oxidase), and endoplasmic reticulum protein folding, ROS also arise from exogenous sources like xenobiotic metabolism and radiation. ROS encompass free radicals, such as superoxide (O₂•⁻) and hydroxyl radicals (HO•), and non-radical oxidants, like hydrogen peroxide (H₂O₂)4.
Excessive ROS production surpassing antioxidant defenses results in oxidative stress, causing damage to lipids, proteins, and DNA, mitochondrial dysfunction, and cell death5. In cancer, ROS exhibit a dual role: high levels can induce apoptosis, while sub-lethal levels promote tumor proliferation, angiogenesis, and metastasis6,7. Cancer cells adapt to elevated basal ROS through metabolic reprogramming and enhanced antioxidant capacity, enabling survival under oxidative stress8. Thus, therapeutic strategies targeting ROS either elevating them to cytotoxic levels or reducing them to inhibit tumor progression are of significant interest9.
Curcumin, a polyphenolic compound from Curcuma longa, displays both antioxidant and pro-oxidant properties depending on the cellular context. Its anticancer effects include suppressing proliferation, invasion, angiogenesis, and chemoresistance, while inducing apoptosis through modulation of ROS, Nuclear factor erythroid 2-related factor 2 (Nrf2), nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathways10,11. However, curcumin’s clinical utility is hindered by poor bioavailability, low water solubility, rapid metabolism, and systemic elimination12,13. To address these limitations, structural modifications have been explored to develop synthetic analogues with improved pharmacokinetics14,15.
2,6-Bis(4-hydroxy-3-methoxybenzylidene) cyclohexanone (BHMC), a synthetic curcumin analogue, was designed to enhance bioavailability while preserving therapeutic properties16. Its structural modifications include replacing the unstable β-diketone moiety with a cyclohexanone structure and conjugated double bonds, while retaining phenolic hydroxyl groups critical for antioxidant and anti-inflammatory activities17. These changes also enhance cytotoxic selectivity18, with BHMC demonstrating 3–5-fold greater cytotoxicity against HepG2 hepatocellular carcinoma cells compared to curcumin, accompanied by pronounced cell shrinkage and reduced viable cell counts19.
Structural modifications in BHMC were also reported influence its redox potential. The substitution of curcumin’s β-diketone linker with a cyclohexanone structure eliminates the central methylene group, impairing hydride transfer and β-alkoxyl radical generation, both of which are important for phenoxy radical formation and antioxidant activity. Electrochemical analyses show that BHMC exhibits a higher redox potential than curcumin, with an oxidation peak at + 0.87 V (vs. Ag/AgCl at pH 7.4), compared to curcumin’s + 0.66 V. This higher redox potential indicates reduced ease of oxidation, and thus, lower antioxidant capacity. Similar findings are reported for other analogues, such as dimethyl curcumin, where blocking phenolic -OH groups or altering the diketone structure elevates redox potential and reduces electron transfer efficiency20,21.
While BHMC’s cytotoxic and pro-apoptotic effects have been documented in cancer cell lines such as MCF-7, MDA-MB-231, and HepG2, prior studies primarily focused on apoptosis, cell cycle arrest, and signaling alterations, with limited exploration of its antioxidant capacity. Given cancer cells’ heightened vulnerability to ROS-mediated cytotoxicity due to elevated basal oxidative stress22,23, and considering curcumin’s dual role as a pro-oxidant (elevating ROS to trigger apoptosis) or antioxidant (scavenging reactive species and activating detoxifying enzymes like glutathione-S-transferase)24,25, BHMC’s ROS-modulatory potential warrants investigation.
Considering BHMC’s structural advantages over curcumin and the central role of ROS in cancer progression and therapy, the present study aims to investigate the antioxidant and ROS-modulatory effects of BHMC in HepG2 hepatocellular carcinoma cells, relative to curcumin. Focus is also placed on its capacity to modulate intracellular glutathione (GSH) levels and influence the Nrf2/Keap1 signalling pathway, which governs cellular antioxidant defences and redox homeostasis.
Materials and methods
Chemicals and reagents
Dulbecco’s Modified Eagle Medium (DMEM) (4.5 g/L glucose) supplemented with L-glutamine, sodium pyruvate, and a penicillin–streptomycin mixed solution, along with 0.25% trypsin/EDTA containing phenol red, and dimethyl sulfoxide (DMSO) were obtained from Nacalai Tesque (Kyoto, Japan). Foetal bovine serum (FBS) was purchased from Tico Europe (Amstelveen, Netherlands). Triton X-100, hydrochloric acid, and phosphate-buffered saline (PBS) tablets were sourced from Oxoid and Thermo Fisher Scientific (Massachusetts, USA). Sodium hydroxide and 99.6% denatured absolute ethanol were supplied by Systerm Chemicals (Selangor, Malaysia). Hydrogen peroxide (H₂O₂), L-ascorbic acid (vitamin C), and glacial acetic acid were purchased from HmbG Chemicals (Kuala Lumpur, Malaysia). The glutathione assay kit was obtained from Elabscience (Texas, USA). Paraformaldehyde, sodium acetate anhydrous, iron (II) sulfate heptahydrate, tris(2-pyridyl)-s-triazine (TPTZ), iron (III) chloride hexahydrate, goat serum, Tween-20, and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (Missouri, USA). 2′,7′-Dichlorofluorescein diacetate (DCFDA) was obtained from Tocris Bioscience (Bristol, United Kingdom). Primary antibodies against Keap1 and Nrf2 were sourced from Santa Cruz Biotechnology (Texas, USA), and the DayLight 488-conjugated secondary antibody was obtained from GeneTex (California, USA).
Preparation of compounds
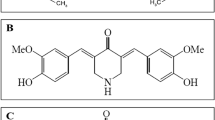
Curcumin (CAS: 458-37-7; ≥98% purity) was purchased from Nacalai Tesque (Kyoto, Japan) (Fig. 1A). BHMC was synthesised as described by Razak et al.26 and confirmed to be 99.9% pure by high-performance liquid chromatography (HPLC) analysis (Fig. 1B). The compound was kindly provided by Associate Professor Dr. Lam Kok Wai, Faculty of Pharmacy, Universiti Kebangsaan Malaysia (UKM). Both curcumin and BHMC were initially dissolved in 100% DMSO to prepare 50 µM stock solutions, which were then diluted to the desired concentrations for the assays. The final DMSO concentration was maintained at 0.1% in all experiments.
Cell line
The human hepatocellular carcinoma cell line HepG2 (HB-8065) was obtained from the American Type Culture Collection (ATCC, Virginia, USA). These adherent cells, originally derived from the liver of a 15-year-old Caucasian male, have a doubling time of approximately 48 h. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin and maintained at 37 °C in a humidified atmosphere of 5% CO₂ using an Eppendorf Galaxy 170R/S incubator (Hamburg, Germany). Experiments were initiated when cultures reached 80–90% confluency.
Total antioxidant activity
Biochemical FRAP assay
The FRAP assay was conducted as described by Benzie and Strain27 and Noorfaiz et al.28, with minor modifications. In a 96-well plate, 50 µL of BHMC, curcumin (0.78–200 µM), L-ascorbic acid (positive control), or FeSO₄·7 H₂O standards (100–1000 µM) were added in triplicate. Subsequently, 150 µL of freshly prepared FRAP reagent (300 mM acetate buffer, 10 mM TPTZ, and 20 mM FeCl₃·6 H₂O; 10:1:1, v/v) prewarmed to 37 °C was added. Absorbance was read at 595 nm using a BioTek ELx808 microplate reader, and FRAP values were determined from the Fe²⁺ standard curve.
FRAP assay in HepG2 cells
The cellular FRAP assay was performed as described by Hasiah et al.29 and Noorfaiz et al.28, with minor modifications. HepG2 cells (4 × 10⁴ cells/well) were seeded in triplicate in 96-well plates with 100 µL complete growth medium and incubated for adhesion. Cells were then treated with BHMC, curcumin, or L-ascorbic acid (6.25–50 µM), 0.1% DMSO (negative control), or left untreated. After 24, 48, or 72 h, cells were sonicated for 30 s, followed by addition of 150 µL prewarmed FRAP reagent. Absorbance at 595 nm was recorded using a BioTek ELx808 microplate reader and FRAP values (µM) were calculated from the ferrous (Fe²⁺) standard curve.
Measurement of intracellular ROS production
Intracellular ROS levels were measured using the DCFDA cellular ROS detection assay as described by Alexander et al.30 with minor modifications. Briefly, HepG2 cells (4 × 10⁴ cells/well) were seeded in triplicate in 96-well black plates with 100 µL complete growth medium and incubated for 24 h. Cells were then treated with BHMC (10, 15 and 20 µM), curcumin (25, 50 µM), H₂O₂ (200 µM), cisplatin (15 µM), 0.1% DMSO, or left untreated. BHMC and curcumin concentrations were selected based on previous findings31. After 18–24 h, cells were incubated with 25 µM DCFDA for 1 h to allow intracellular esterases to hydrolyse the probe to non-fluorescent DCF, which is then oxidised to fluorescent 2′,7′-dichlorofluorescein. Fluorescence was measured at 495/529 nm (excitation/emission) using a Tecan Infinite 200 Pro microplate reader (Männedorf, Switzerland).
Measurement of glutathione level
Total glutathione (T-GSH), reduced glutathione (GSH), and oxidised glutathione (GSSG) levels were quantified using commercial kits (Elabscience, USA) according to the manufacturer’s instructions and Alexander et al.30 with minor modifications. HepG2 cells (1 × 10⁶ cells/well) were seeded in triplicate in 6-well plates containing 3 mL complete medium and treated with BHMC (10, 15, 20 µM), curcumin (25, 50 µM), 0.1% DMSO, or left untreated. After 18–24 h, T-GSH and GSSG were measured.
For T-GSH, samples and standards were loaded into 96-well plates, followed by addition of assay cocktail (sodium dihydrogen phosphate, glutathione reductase, and 5,5′-dithiobis-(2-nitrobenzoic acid)) and incubation at 25 °C for 5 min. NADH-Na₂ was then added, and plates were incubated for 25 min at 25 °C. For GSSG, samples and standards were first treated with diethanolamine and ammonium sulfate, incubated for 1 h at 25 °C, and processed following the T-GSH protocol. Absorbance was recorded at 405 nm using a Tecan Infinite F50 microplate reader (Männedorf, Switzerland). Glutathione concentrations were derived from standard curves, and GSH was calculated using the formula provided in the manufacturer’s instructions.
Immunocytochemistry
Immunocytochemistry was performed to evaluate Keap1 and Nrf2 protein expression as described by Liang et al.32 and Mohamed et al.33 with minor modifications. HepG2 cells (1 × 10⁶) were seeded on coverslips in 6-well plates containing 3 mL complete medium and treated with BHMC (10, 15, 20 µM), curcumin (25, 50 µM), cisplatin (15 µM), 0.1% DMSO, or left untreated. Cells were fixed with paraformaldehyde-based fixation buffer for 30 min at room temperature (RT), permeabilised with Triton X-100 buffer for 15 min, and blocked with BSA/goat serum/Tween-20 buffer for 30 min at RT. Primary antibodies against Keap1 and Nrf2 (1:200) were applied overnight at 4 °C, followed by DyLight 488-conjugated secondary antibody (1:200) incubation for 2 h at RT in the dark. Nuclei were counterstained with Hoechst for 15 min at RT. Coverslips were mounted in PBS, imaged using a fluorescence microscope, and subsequently analysed with ImageJ.
Statistical analysis
Data were analysed using GraphPad Prism 8 (GraphPad Software). One-way ANOVA followed by Dunnett’s post hoc test was used to compare BHMC and curcumin treatments with the untreated control. Statistical significance was set at p < 0.05.
Discussion
Effect of BHMC and Curcumin on total antioxidant capacity assessed by the FRAP assay
BHMC is one of the curcumin’s analogues. The β-diketone moiety was substituted with a monocyclic ketone featuring an α,β-unsaturated bis-enone system, resulting in the synthesis of BHMC16,31. This modification led to enhanced biological benefits, including anti-inflammatory, antioxidant, and anti-tumour properties19,34. The structural alteration of curcumin aimed to improve its effectiveness as an anticancer agent by addressing issues related to instability and interference with some modalities in in vitro studies. Consequently, this modified structure is likely to contribute to the increased cytotoxicity of BHMC which aligns with earlier in vitro studies observed several cancer cell lines (19, 26, 31].
Based on our preliminary data as reported by Mohd Shafiee et al.31, BHMC at 24 h exerted the IC50 value of 16.73 µM compared to curcumin with IC50 value of 46.03 µM in HepG2 cells. BHMC was also reported to have cytotoxic selective effect towards Hs27 cells with IC50 value of 34.32 µM at 24 h and the selectivity index of more than 231,35. Thus, using the concentrations obtained from the preliminary data, further analysis was done to look at the underlying molecular mechanism in regulating ROS triggered by BHMC.
ROS are oxygen-containing molecules produced by cellular metabolism. Although it is crucial for cell growth and survival, at relatively higher level, it can cause oxidative stress that lead to the cancer development36,37. Through variety of mechanisms, antioxidants can scavenge free radicals and counteract the undesired effects of oxidative stress9. In this study, biochemical FRAP assay was first performed to determine the total antioxidant activity of BHMC and curcumin in the absence of cells. FRAP values reflect the antioxidant capacity of compounds based on their ability to reduce iron (III)-tris(2-pyridyl)-s-triazine (Fe³⁺-TPTZ) to iron (II)-tris(2-pyridyl)-s-triazine (Fe²⁺-TPTZ), generating an intense blue colour with absorbance measured at 595 nm38.
As shown in Fig. 2, all compounds including L-ascorbic acid exhibited antioxidant activity in concentration-dependent manner as the antioxidant activity increase with the concentration when tested for 24 h. This was supported by Borra et al.39 that reported after incubation with FRAP reagent, curcumin and vitamin C showed an increase in antioxidant activity along with an increase in concentration. Vitamin C is selected as a positive control due to its well-established antioxidant activity by readily donates one or two electron to potentially harmful oxidizing radicals generated from cellular metabolism and exposure to xenobiotics, toxins or pollutants40,41. Thereby showcasing its potent antioxidant properties and functioning as a reducing agent. Similarly, the findings also demonstrated that BHMC significantly exerting its antioxidant properties compared to untreated with p < 0.01. This is due to the preservation of the phenolic hydroxyl group from curcumin during the synthesis of BHMC which is crucial for its antioxidant property while substituting the β-diketone moiety with a cyclohexanone structure enhances stability and bioavailability, resulting in a superior curcumin analogue, BHMC20,21. However, curcumin exhibited a slightly stronger antioxidant effect than BHMC across all tested concentrations, as indicated by its marginally higher FRAP values.
The ability of curcumin to exert great antioxidant activity in reducing iron (III) to iron (II) has been reported in numerous studies. Curcumin exerted its antioxidant effects via modulating a variety of cells signalling pathways, such as oxidative stress, and by preventing the development of cancer37. This has been further confirmed with numerous in vitro and ex vivo antioxidant assays, including the FRAP assay and the AAPH-induced haemolysis in erythrocyte assay that curcumin possessed high antioxidant activity39. These abilities include free radical scavenging, reducing power, as well as erythrocyte lipid per oxidation inhibition. To further confirmed the antioxidant activity of BHMC and curcumin on cells, FRAP assay was performed on HepG2 treated BHMC or curcumin (Fig. 3). Based on the result obtained from the biochemical FRAP assay, concentration of treatments from 3.13, 6.25, 12.5, 25 and 50 µM were selected for FRAP assay on HepG2 cells. This is due to the significant different observed as low as 3.13 µM. Figure 4.5 shows the total antioxidant activity of BHMC, curcumin and vitamin C on HepG2 cells after 24 h of incubation.
The effect of BHMC, curcumin and vitamin C at various concentrations on total antioxidant activity tested for 24 h. Data are presented as mean ± S.E.M. and represent of three independent experiments. Statistically significant differences are indicated with *p < 0.05; **p < 0.01 of various concentration within same compounds by using One-way ANOVA followed by Dunnett’s post hoc tests compared to untreated group.
The effect of BHMC, curcumin and vitamin C at various concentrations on total antioxidant activity in HepG2 cells after (A) 24 h, (B) 48 h and (C) 72 h. Data are presented as mean ± S.E.M. and represent of three independent experiments. Statistically significant differences are indicated with *p < 0.05; **p < 0.01 of various concentration within same compounds by using One-way ANOVA followed by Dunnett’s post hoc tests compared untreated group.
Both BHMC and curcumin shown to significantly (p < 0.01) exert its antioxidant activity in a concentration dependent manner, which higher concentration has higher antioxidant activity. Although the antioxidant activity of BHMC was slightly higher than curcumin at lower concentration (3.13–25 µM), the increased were not significant until the concentration reached 25 µM compared to control. Interestingly, control group without any treatment exhibited small antioxidant activity. Thus, this shown that HepG2 cells has its own endogenous antioxidant activity. This finding was also supported by Hasiah et al.29 and Noorfaiz et al.28 that reported untreated group of HepG2, A431 and 3T3 cells exhibit FRAP value due to its endogenous antioxidant activity.
Our finding also showed that compared to BHMC and curcumin, vitamin C was discovered to have lower antioxidant activity at certain concentrations. This was in contrast to a study conducted by Noorfaiz et al.28 which demonstrated that vitamin C greatly outperforms the lawsone effect in exerting its antioxidant activity on the 3T3 and A431 cells. Although there is yet any exact explanation on the underlying mechanism, Munteanu and Apetrei42 reported that it may be due to the possibility that a single timepoint may not accurately represent the overall reaction, different antioxidants require varied detection times. Besides, the redox statuses of cancer cells varied based on the type and severity of the tumour, leading to varying susceptibility to the oxidative damage43.
These findings suggest that the FRAP assay alone does not provide sufficient evidence to conclude that BHMC possesses stronger antioxidant activity than curcumin. The FRAP assay specifically measures reducing power, based on the ability to convert ferric-tripyridyltriazine into its ferrous form. However, as noted by Heckman et al.44 this method does not fully reflect the overall antioxidant potential of a compound, particularly when other mechanisms are involved. Furthermore, FRAP cannot detect antioxidants that act through radical-quenching pathways45. Since different assays capture different mechanisms, multiple antioxidant assays are necessary to accurately confirm antioxidant activity38. Therefore, to further evaluate whether BHMC or curcumin modulates oxidative stress in HepG2 cells, intracellular ROS and glutathione levels were assessed.
Effect of BHMC and Curcumin on intracellular ROS and glutathione level
To comprehensively evaluate the antioxidant potential of BHMC, a curcumin analogue, multiple assays are essential due to their distinct mechanisms. Like curcumin, BHMC may exhibit dual antioxidant and pro-oxidant properties in cancer cells, influenced by factors such as concentration and exposure duration46. This study assessed intracellular reactive oxygen species (ROS) and glutathione levels in HepG2 liver cancer cells treated with BHMC or curcumin, revealing BHMC’s enhanced potency and concentration-dependent effects.
The DCFDA assay, used to measure intracellular ROS, demonstrated that BHMC significantly reduced ROS levels in HepG2 cells in a concentration-dependent manner, effective at 15 µM after 18 h (p < 0.05) and 10 µM after 24 h (p < 0.01) compared to controls (Fig. 5). In contrast, curcumin required a higher concentration (25 µM, p < 0.01) to achieve comparable ROS reduction. These findings suggest BHMC’s superior efficacy in mitigating oxidative stress, likely due to its structural modifications enhancing stability and bioavailability compared to curcumin31. However, the DCFDA assay’s inability to differentiate specific ROS types limits mechanistic insights into which species (e.g., superoxide, hydrogen peroxide) are primarily affected47.
Cancer cells, including HepG2, exhibit elevated basal ROS levels due to heightened metabolic activity, mitochondrial dysfunction, and oncogene activation (e.g., C-myc, K-ras, BRCA1), rendering them more susceptible to oxidative stress-induced cytotoxicity compared to normal cells22,23. While ROS overproduction can trigger cell death, both low and high ROS levels may induce cytotoxic effects, highlighting the delicate redox balance in cancer cells23. BHMC’s ability to reduce ROS at lower concentrations aligns with its potential to modulate this balance, possibly enhancing its cytotoxic selectivity, as previously reported with a selectivity index > 2 in Hs27 fibroblasts35.
Effect of BHMC and curcumin on intracellular ROS levels in HepG2 cells after 18- and 24-hours treatment. Intracellular ROS levels were quantified using the DCFDA cellular ROS detection assay according to the manufacturer’s instructions. Data are expressed as mean ± S.E.M. from three independent experiments. Statistical significance was assessed using one-way ANOVA, followed by Dunnett’s post hoc test for multiple comparisons with the untreated control group. Significant differences at 18 and 24 h of incubation are indicated as *p < 0.05 and **p < 0.01.
Glutathione, a critical endogenous antioxidant, plays a pivotal role in maintaining redox homeostasis alongside the thioredoxin system48,49. These antioxidants can protect normal cells by scavenging ROS or activating cytoprotective enzymes, thereby reducing oxidative damage and apoptosis, and can also prevent the activation of chemoresistance pathways triggered by oxidative stress.
As shown in Fig. 5(A), BHMC significantly increased total glutathione (T-GSH) levels in HepG2 cells in a concentration-dependent manner (p < 0.05 at 18 h; p < 0.01 at 24 h across all concentrations), consistently outperforming curcumin, which showed slight fluctuations at 24 h (p < 0.01). Notably, in Figure (B), BHMC at 20 µM after 24 h significantly elevated oxidized glutathione (GSSG) levels (p < 0.01), while lower concentrations favoured reduced glutathione (GSH) induction (p < 0.01). Conversely, curcumin increased GSSG at both 25 and 50 µM after 18 h (p < 0.01) but reduced GSH levels, indicating a stronger pro-oxidant shift (Fig. 5(C)).
The GSH: GSSG ratio, a key indicator of oxidative stress, further elucidated these dynamics. As shown in Fig. 6, BHMC at 10 and 15 µM significantly increased the ratio after 18 and 24 h (p < 0.05 and p < 0.01, respectively), reflecting reduced oxidative stress. However, at 20 µM after 24 h, the ratio decreased (p < 0.05), indicating oxidative stress induction. Curcumin consistently lowered the ratio at 25 and 50 µM (p < 0.01), suggesting a predominant pro-oxidant effect at these concentrations. These findings align with curcumin’s reported dual role, acting as an antioxidant at lower doses and a pro-oxidant at higher doses or longer exposures50. It is important to note that, under normal conditions, the ratio exceeds 100:1 but can drop to 10:1 or even 1:1 under oxidative stress51. BHMC’s ability to shift from antioxidant to pro-oxidant activity at a lower concentration threshold (20 µM vs. curcumin’s 25 µM) underscores its enhanced potency.
The observed increase in GSSG at higher BHMC concentrations suggests that excessive ROS production overwhelms the glutathione peroxidase (GPx) system, which converts GSH to GSSG to neutralize ROS, forming stable compounds52. Under oxidative stress, disrupted GSH homeostasis leads to GSSG accumulation, GSH depletion, and potential export, a hallmark of pathological states like cancer53. The elevated T-GSH levels in cisplatin-treated HepG2 cells, consistent with cisplatin-resistant cell lines54, further support the role of glutathione in countering oxidative stress-induced apoptosis, potentially contributing to chemoresistance55.
Although no prior studies have directly investigated BHMC’s effects on glutathione synthesis, its structural similarity to curcumin, which upregulates T-GSH via glutamate-cysteine ligase activation56, suggests a plausible mechanism. The GSH: GSSG ratio’s utility as an apoptosis marker57 and therapeutic target in cancers like AML58 highlights BHMC’s potential to modulate redox-sensitive pathways for therapeutic benefit. However, the exact mechanisms, including possible Keap1/Nrf2 pathway involvement, require further exploration.
Therefore, BHMC exhibits a concentration- and time-dependent dual role in HepG2 cells, reducing oxidative stress at lower concentrations (10–15 µM) via ROS suppression and.
Effect of BHMC and curcumin on (A) Total Glutathione level (T-GSH), (B) Oxidised Glutathione level (GSSG) and (C) Reduced Glutathione level (GSH) in HepG2 cells at 18- and 24 h of treatments. Glutathione levels were quantified using commercial kits according to the manufacturer’s instructions. Data are presented as mean ± S.E.M. and represent of three independent experiments. Statistically significant differences are indicated with *p < 0.05; **p < 0.01 of treatment groups by using One-way ANOVA followed by Dunnett’s post hoc tests compared to untreated group.
GSH: GSSG ratio in HepG2 cells treated with BHMC and curcumin at 18 and 24 h. The ratios were quantified using commercial kits according to the manufacturer’s instructions. Data are presented as mean ± S.E.M. and represent of three independent experiments. Statistically significant differences are indicated with *p < 0.05; **p < 0.01 of treatment groups by using One-way ANOVA followed by Dunnett’s post hoc tests compared to untreated group.
GSH induction, while promoting oxidative damage at 20 µM after 24 h (p < 0.01). Its superior potency compared to curcumin, particularly in T-GSH synthesis and ROS modulation, positions BHMC as a promising curcumin analogue. Future studies should investigate the Keap1/Nrf2 pathway’s role in these effects and employ additional antioxidant assays to fully characterize BHMC’s therapeutic potential.
Effect of BHMC and Curcumin on Nrf2 and Keap1 protein expression
To investigate the molecular effects of BHMC and curcumin on the Keap1/Nrf2 pathway, their impact on Keap1 and Nrf2 protein expression in HepG2 liver cancer cells was assessed using immunocytochemistry and quantified with ImageJ software (Supplementary Fig. S1). Nrf2, a central regulator of the antioxidant defence system, maintains redox homeostasis by activating antioxidant response element (ARE)-driven genes, such as glutathione, superoxide dismutase, and heme oxygenase-1 (HO-1), in response to oxidative stress and xenobiotics59. Keap1 and Nrf2 expression were evaluated at 18 and 24 h, alongside measurements of reactive oxygen species (ROS), total glutathione (T-GSH), and GSH: GSSG ratios.
As shown in Fig. 7, untreated HepG2 cells displayed weak Nrf2 protein expression at 18 and 24 h, quantified by low fluorescence intensity via ImageJ. This minimal expression contrasts sharply with the strong Nrf2 signal in H₂O₂-treated cells (positive control), indicating robust pathway activation under oxidative stress. The low basal Nrf2 in untreated cells is attributed to efficient Keap1-mediated ubiquitination and proteasomal degradation, given Nrf2’s short half-life of approximately 20 min60. This aligns with the modest endogenous antioxidant activity observed in untreated HepG2 cells in the FRAP assay, suggesting limited basal Nrf2-driven antioxidant defences, such as glutathione synthesis. H₂O₂ was selected as a positive control due to its role as a ROS species that triggers redox-sensitive signalling, elevating ROS levels and upregulating antioxidant proteins like Keap1 and Nrf261.
In BHMC-treated HepG2 cells, Nrf2 expression showed a modest increase at 10 and 15 µM compared to controls, with fluorescence intensity indicating a dose-dependent trend. At 20 µM BHMC, Nrf2 expression was significantly elevated at 18 h (p < 0.05) and further increased at 24 h (p < 0.01) relative to untreated cells. Conversely, curcumin at 50 µM induced a strong Nrf2 signal at both 18 and 24 h (p < 0.01), reflecting potent antioxidant response activation. At 25 µM, curcumin significantly increased Nrf2 expression at 18 h (p < 0.01), but this effect slightly declined by 24 h (p < 0.05), suggesting time-dependent attenuation. Prior studies report curcumin-induced Nrf2 expression detectable within 4 h, peaking around 16 h62. The current study’s focus on 18 and 24-hour time points, aligned with ROS and glutathione assays, may have missed earlier signalling dynamics, highlighting the need for future assessments at 6 and 12 h.
In cancer cells with functional Keap1, such as HepG2, Nrf2 upregulation typically results from Keap1 functional inhibition rather than reduced Keap1 protein levels. Figure 8 confirms that Keap1 expression remained detectable across most treated groups, despite elevated Nrf2 levels in cells treated with 20 µM BHMC or 25 and 50 µM curcumin. This is consistent with findings in MCF-7 cells, where oxidative stressors like H₂O₂ increase Nrf2 protein without significantly altering Keap1 levels63. The primary mechanism involves oxidative or electrophilic disruption of Keap1-Nrf2 binding, preventing Nrf2 degradation and enabling its nuclear accumulation.
Activation of the Keap1/Nrf2/ARE pathway is critical for glutathione synthesis in response to ROS, as evidenced by BHMC’s and curcumin’s effects on T-GSH and GSH: GSSG ratios. BHMC likely modulates glutathione synthesis, potentially via glutamate-cysteine ligase (GCL) activation, as reported for curcumin56. The GSH: GSSG ratio data further link oxidative stress levels to Nrf2 expression: curcumin at 50 µM, associated with lower ratios (higher oxidative stress), induced the strongest Nrf2 signal at both time points, while BHMC at 20 µM for 24 h showed a lower ratio and robust Nrf2 expression. In contrast, BHMC at 10 and 15 µM yielded higher GSH: GSSG ratios, indicating reduced oxidative stress and lower Nrf2 activation. These trends align with Shahcheraghi et al.59, who noted that curcumin derivatives enhance Nrf2 and ARE-driven genes like HO-1, with prolonged exposure amplifying phase II detoxifying enzymes.
No prior studies have reported BHMC’s effects on Nrf2/Keap1 in cancer cells, making these findings novel. The concentration- and time-dependent modulation by BHMC with antioxidant at 10–15 µM and pro-oxidant at 20 µM effects were in parallels with curcumin’s dual effects but at lower thresholds, suggesting enhanced potency. The persistence of Keap1 expression across treatments supports functional inhibition as the primary driver of Nrf2 upregulation. However, BHMC’s modulation of glutathione levels may involve additional pathways beyond Keap1/Nrf2/ARE, warranting further mechanistic studies.
In conclusion, BHMC and curcumin significantly enhance Nrf2 expression in HepG2 cells in a dose- and time-dependent manner, with BHMC showing notable induction at 20 µM and curcumin at 50 µM, without substantial changes in Keap1 protein levels. These findings underscore BHMC’s potential as a curcumin analogue in regulating redox homeostasis and highlight the need for earlier time-point analyses and broader pathway investigations.
Effect of BHMC and curcumin on Nrf2 expression after treatment for (A) 18 h and (B) 24 h in HepG2 cells. The relative fluorescence intensity was measure using Image J software. Data are expressed as mean ± S.E.M. from three independent experiments. Statistical significance was determined using one-way ANOVA, followed by Dunnett’s post hoc test for multiple comparisons with the untreated control group. Significant difference at 18 and 24 h of incubation are indicated as *p < 0.05 and **p < 0.01.
Effect of BHMC and curcumin on Keap1 expression after treatment for (A) 18 h and (B) 24 h in HepG2 cells. The relative fluorescence intensity was measure using Image J software. Data are expressed as mean ± S.E.M. from three independent experiments. Statistical significance was determined using one-way ANOVA, followed by Dunnett’s post hoc test for multiple comparisons with the untreated control group. Significant difference at 18 and 24 h of incubation are indicated as *p < 0.05 and **p < 0.01.
Conclusion
Our previous research demonstrated that BHMC exhibits greater cytotoxicity against hepatocellular carcinoma HepG2 cells than curcumin in a concentration- and time-dependent manner. In the present study, BHMC appears to exert a direct influence on glutathione levels and modulate ROS-mediated mechanisms, contributing to its higher cytotoxic potential compared to curcumin. This was further supported by assessments of total antioxidant activity, intracellular ROS levels, glutathione content, and Keap1/Nrf2 expression, all of which play key roles in ROS-mediated pathways. Notably, this is the first study to propose potential molecular targets underlying the BHMC mechanism, particularly involving the ROS-mediated pathway in HepG2 liver cancer cells. These findings advance our understanding of BHMC’s molecular mechanisms and support its potential as a versatile therapeutic agent with applications in cancer treatment and oxidative stress-related conditions.
Summary of key findings
-
Both BHMC and curcumin exhibited dose-dependent effects, with curcumin showing slightly greater activity in the chemical FRAP assay, while BHMC demonstrated higher activity at certain concentrations in the cellular assay.
-
At lower doses, BHMC reduced ROS and increased glutathione levels, whereas higher doses induced oxidative stress.
-
Nrf2 activation reflected the oxidative stress status, with both compounds eliciting stronger pathway activation at higher, stress-inducing concentrations.
-
Overall, the results indicate that BHMC, like curcumin, possesses dual antioxidant and pro-oxidant properties, influenced by dose and exposure time, and may modulate oxidative stress partly via Nrf2-mediated mechanisms.
Significance of the findings
The present findings highlight the nuanced bioactivity of BHMC compared to its parent compound, curcumin. Both compounds displayed clear dose-dependent effects, underscoring the importance of determining optimal therapeutic concentrations for desired biological outcomes. While curcumin exhibited slightly higher antioxidant capacity in chemical FRAP assays, BHMC demonstrated greater activity at certain concentrations in cellular assays, suggesting that cellular context, uptake, and metabolism significantly influence functional activity.
The biphasic nature of BHMC acting as an antioxidant at lower doses by reducing ROS and enhancing glutathione levels yet inducing oxidative stress at higher doses reflects a dual functional potential similar to curcumin. This property is of particular interest, as low-dose antioxidant effects may protect normal cells from oxidative damage, whereas high-dose pro-oxidant effects could be strategically exploited for targeting cancer cells or other pathological states characterized by elevated oxidative stress.
Nrf2 activation was also observed in parallel with changes in oxidative stress, reinforces its role as a central mediator of BHMC’s effects. As a master regulator of cytoprotective and detoxification pathways, Nrf2-mediated modulation of oxidative stress highlights BHMC’s potential for versatile therapeutic applications.
Overall, the dual antioxidant/pro-oxidant potential of BHMC, modulated by dose and exposure time, provides opportunities for context-specific applications—ranging from disease prevention to targeted therapy. These findings warrant further investigation into BHMC’s pharmacodynamics, bioavailability, and safety profile, as well as exploration of synergistic effects with other Nrf2-modulating agents.
Implications of the findings
This study advances the field by providing novel insight into the redox-modulating properties of BHMC, a curcumin analogue, and its underlying mechanisms. By integrating biochemical and cellular assays, we reveal a distinct dose-dependent profile in which BHMC functions as an antioxidant at lower concentrations but shifts toward pro-oxidant activity at higher doses. This biphasic behaviour not only refines current understanding of curcumin-derived compounds but also underscores the importance of precise dosing to harness their therapeutic potential. Mechanistically, the observed alignment between oxidative stress modulation and Nrf2 activation positions BHMC within a clinically relevant cytoprotective pathway, highlighting its capacity to both mitigate oxidative injury and, in specific contexts, exploit oxidative stress for therapeutic gain. Moreover, the divergence between chemical assay results and cellular responses reinforces the need for physiologically relevant models in antioxidant drug discovery. Collectively, these findings expand the conceptual and practical framework for developing multifunctional redox-active agents.
Comparison of current findings with existing findings on BHMC
Based on a comprehensive review of existing literature on BHMC (2,6-bis(4-hydroxy-3-methoxybenzylidene)cyclohexanone), a curcumin analogue, prior studies primarily focus on its synthesis, cytotoxicity, apoptosis induction, cell cycle arrest, anti-inflammatory effects, and in vivo anti-tumor activity in models like breast cancer (e.g., MCF-7, MDA-MB-231, 4T1 cells) and liver cancer (HepG2 cells). Key existing data includes:
-
Enhanced cytotoxicity compared to curcumin, with lower IC50 values (e.g., ~ 16–21 µM in HepG2 at 24 h vs. curcumin’s ~ 46 µM), selective toxicity (e.g., selectivity index > 2 in Hs27 fibroblasts), and 3–7 times higher toxicity at lower concentrations in HepG2.
-
Induction of apoptosis and cell cycle arrest (e.g., S-phase in HepG2), activation of pathways like p38 MAPK, and downregulation of invasion-related proteins (e.g., RhoA, MMP-2, MMP-9).
-
Preliminary evidence of ROS modulation in HepG2 but limited to general intracellular effects without detailed concentration/time-dependent analysis or links to glutathione/Nrf2.
-
No prior reports on BHMC’s effects on total glutathione synthesis, GSH: GSSG ratios, or Nrf2/Keap1 protein expression in any cancer model, including HepG2. Antioxidant assays like FRAP are mentioned in conference proceedings but lack cellular comparisons or mechanistic depth.
The provided data extends these by exploring underlying molecular mechanisms related to oxidative stress regulation in HepG2 cells, revealing several novel insights. These represent advancements beyond cytotoxicity/apoptosis, emphasizing BHMC’s dual antioxidant/pro-oxidant roles and potential pathway modulations. Below is a structured comparison:
Aspect | Existing data on BHMC | Novel findings from provided data |
|---|---|---|
Antioxidant Capacity (FRAP Assay) | Limited to basic cell-free antioxidant activity; one proceeding notes higher cellular FRAP values at specific concentrations (6.25-25 µM) in HepG2, but without direct curcumin comparison or mechanistic explanation. | - BHMC exhibits concentration-dependent antioxidant activity in both cell-free and cellular FRAP assays, with significant effects (p < 0.01) from 3.13 µM. - In cell-free: Curcumin slightly superior across concentrations. - In HepG2 cells: BHMC slightly higher than curcumin at lower concentrations (3.13-25 µM), though not always significant until 25 µM; both outperform vitamin C at certain doses, contrasting prior vitamin C superiority in other cells. - Highlights preservation of phenolic hydroxyl groups for stability/bioavailability, addressing curcumin’s limitations. |
Intracellular ROS Levels | Preliminary mentions of ROS modulation in HepG2 (e.g., via NF-κB/GLUT1 inhibition), but no detailed concentration/time-dependent data or comparison to curcumin’s thresholds. | - BHMC reduces ROS in a concentration-dependent manner, effective at lower thresholds (15 µM at 18 h, p < 0.05; 10 µM at 24 h, p < 0.01) than curcumin (25 µM, p < 0.01). - Supports BHMC’s enhanced potency in countering cancer cell oxidative stress, aligning with higher basal ROS in HepG2 due to metabolic dysfunction. |
Total Glutathione (T-GSH) Levels | No prior studies directly examine BHMC’s effects on T-GSH synthesis or levels in any cell type. | - BHMC increases T-GSH in a concentration-dependent manner (p < 0.05 at 18 h; p < 0.01 at 24 h across all tested concentrations), consistently higher than curcumin. - Mimics curcumin’s parent effects but suggests superior induction; plausible link to glutamate-cysteine ligase activation, though mechanism requires further study. |
GSH: GSSG Ratio and Dual Role | Absent in literature; no data on oxidative stress markers like GSH: GSSG or BHMC’s pro-oxidant potential. | - BHMC modulates ratio in concentration/time-dependent way: Increases (reduced stress) at 10–15 µM (p < 0.05 − 0.01); decreases (increased stress) at 20 µM after 24 h (p < 0.05). - Demonstrates dual antioxidant (low conc.) and pro-oxidant (high conc./longer exposure) roles, similar to curcumin but at lower effective concentrations; ratio drops indicate potential apoptosis trigger. |
BHMC’s Efects on Nrf2/Keap1 | No prior reports on BHMC modulating Nrf2/Keap1 in any cancer cell model, including HepG2. BHMC studies emphasize cytotoxicity and apoptosis in breast cancer cells, without Nrf2/Keap1 involvement. | - First evidence of BHMC modulating Nrf2/Keap1 expression in HepG2 cells (or any cancer cells). BHMC induces concentration-dependent Nrf2 upregulation: slight increase at 10–15 µM (antioxidant-like), prominent at 20 µM at 18 h, and significant at 24 h (p < 0.01). Keap1 remains detectable, supporting functional inhibition over protein reduction. Correlates with oxidative stress: Higher stress (low GSH: GSSG) triggers Nrf2 upregulation for homeostasis. Suggests BHMC may activate Keap1/Nrf2/ARE for glutathione synthesis or act independently; recommends earlier time points (e.g., 6–12 h) for peak detection, addressing protein half-life issues |
These novel findings provide the first evidence of BHMC’s detailed oxidative stress modulation in HepG2, emphasizing its superiority over curcumin in potency and selectivity at lower doses. They highlight the need for multiple assays (beyond FRAP) to confirm antioxidant effects and suggest therapeutic potential in targeting ROS-sensitive pathways, though limitations like single-timepoint assays and assay specificity are noted. Future work could validate mechanisms (e.g., via Keap1/Nrf2 knockdown) and extend to in vivo models.
Limitations and recommendations for future work
This study has several limitations that warrant consideration for future research. Although previous studies have reported the cytotoxicity of BHMC in various cancer cell models, significant evidence gaps remain. While our findings show that BHMC exhibits greater cytotoxic effects than curcumin, the influence of its structural modifications on oxidative stress modulation is not yet fully understood. Furthermore, limited data are available on the underlying mechanisms by which BHMC affects glutathione synthesis, intracellular ROS levels, and the activation of downstream antioxidant genes regulated by Nrf2.
Additional antioxidant assays such as lipid peroxidation, superoxide dismutase (SOD) activity, catalase (CAT) activity, and mitochondrial membrane potential should be performed. Likewise, molecular analyses including caspase activity assays, RT-qPCR, ELISA, and western blotting could help elucidate the precise molecular mechanisms through which BHMC modulates the ROS pathway and how this relates to its cytotoxic effects. To comprehensively map the ROS-related molecular mechanisms of BHMC, future investigations should assess additional markers such as SOD, CAT, lipid peroxidation, mitochondrial membrane potential, and caspase activities.
Future research will focus on in vivo studies and detailed mechanistic investigations to validate these in vitro results and further evaluate the therapeutic potential of BHMC. By examining how BHMC modulates ROS levels and regulates related genes and proteins, these studies aim to strengthen the understanding of its role in promoting apoptosis in liver cancer cells.
Data availability
All data generated or analysed during this study are included in this published article. No additional data are available.
References
Li, Y. R. & Trush, M. Defining ROS in biology and medicine. Reactive Oxygen Species. 1, 9–21. https://doi.org/10.20455/ros.2016.803 (2016).
Juan, C. A., de la Lastra, J. M. P., Plou, F. J. & Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (dna, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 22 (9), 4642. https://doi.org/10.3390/ijms22094642 (2021).
Mansoor, S. et al. Reactive oxygen species in plants: from source to sink. Antioxidants 11 (2), 225. https://doi.org/10.3390/ANTIOX11020225 (2022).
Liu, G. Y. et al. 3,3′-OH Curcumin causes apoptosis in HepG2 cells through ROS-mediated pathway. Eur. J. Med. Chem. 112, 157–163. https://doi.org/10.1016/j.ejmech.2016.02.019 (2016).
Jakubczyk, K., Drużga, A., Katarzyna, J. & Skonieczna-żydecka, K. Antioxidant potential of curcumin—a meta-analysis of randomized clinical trials. Antioxidants 9 (11), 1–13. https://doi.org/10.3390/antiox9111092 (2020).
Uy, B., McGlashan, S. R. & Shaikh, S. B. Measurement of reactive oxygen species in the culture media using acridan lumigen PS-3 assay. J. Biomol. Techniques. 22, 95–107 (2011).
Weinberg, F., Ramnath, N. & Nagrath, D. Reactive oxygen species in the tumor microenvironment: an overview. Cancers 11 (8), 1191. https://doi.org/10.3390/CANCERS11081191 (2019).
Shah, M. A. & Rogoff, H. A. Implications of reactive oxygen species on cancer formation and its treatment. Semin. Oncol. 48 (3), 238–245. https://doi.org/10.1053/j.seminoncol.2021.05.002 (2021).
Alisi, I. O., Uzairu, A., Abechi, S. E. & Idris, S. O. Evaluation of the antioxidant properties of Curcumin derivatives by genetic function algorithm. J. Adv. Res. 12, 47–54. https://doi.org/10.1016/j.jare.2018.03.003 (2018).
Prasad, S., Gupta, S. C., Tyagi, A. K. & Aggarwal, B. B. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol. Adv. 32 (6), 1053–1064. https://doi.org/10.1016/j.biotechadv.2014.04.004 (2014).
Sohn, S. I. et al. Biomedical applications and bioavailability of curcumin—an updated overview. Pharmaceutics 13 (12), 13122102. https://doi.org/10.3390/pharmaceutics13122102 (2021).
Feng, T., Wei, Y., Lee, R. J. & Zhao, L. Liposomal Curcumin and its application in cancer. Int. J. Nanomed. 12, 6027–6044. https://doi.org/10.2147/IJN.S132434 (2017).
Tomeh, M., Hadianamrei, R. & Zhao, X. A. Review of Curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 20 (5), 1033. https://doi.org/10.3390/ijms20051033 (2019).
Gupta, S. C., Patchva, S. & Aggarwal, B. B. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 15 (1), 195–218. https://doi.org/10.1208/s12248-012-9432-8 (2013).
Lopresti, A. L. The problem of Curcumin and its bioavailability: could its Gastrointestinal influence contribute to its overall health-enhancing effects? Adv. Nutr. 9 (1), 41–50. https://doi.org/10.1093/advances/nmx011 (2018).
Tham, C. L. et al. A. A synthetic curcuminoid derivative inhibits nitric oxide and Proinflammatory cytokine synthesis. Eur. J. Pharmacol. 628 (1–3), 247–254. https://doi.org/10.1016/j.ejphar.2009.11.053 (2010).
Tham, C. L., Yeoh, S. Y., Ong, C. H., Harith, H. H. & Israf, D. A. A Synthetic Curcuminoid Analogue, 2,6-Bis-4-(Hydroxyl-3-Methoxybenzylidine)-Cyclohexanone (BHMC) Ameliorates Acute Airway Inflammation of Allergic Asthma in Ovalbumin-Sensitized Mice. Mediators of Inflammation. 1, 9725903; (2021). https://doi.org/10.1155/2021/9725903
Ali, N. M. et al. Synthetic Curcumin derivative DK1 possessed G2/M arrest and induced apoptosis through accumulation of intracellular ROS in MCF-7 breast cancer cells. Cancer Cell Int. 17 (1), 1–12. https://doi.org/10.1186/s12935-017-0400-3 (2017).
Syed Alwi, S. S., Zahari, S., Haron, A. S. & Alexander, H. R. Cytotoxic effect of 2,6-bis(4-Hydroxy-3-Methoxybenzylidene) cyclohexanone (BHMC) and Curcumin on human liver cancer cells, HepG2. Malaysian J. Med. Health Sci. 15, 44–50 (2019).
Amalraj, A., Pius, A., Gopi, S. & Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives - A review. J. Tradit Complement. Med. 15, 205–233. https://doi.org/10.1016/j.jtcme.2016.05.005 (2016).
Chiorcea-Paquim, A. M. Electrochemical sensing of curcumin: A review. Antioxid. (Basel). 22 (2029). https://doi.org/10.3390/antiox12122029 (2023).
Martin-Cordero, C., Leon-Gonzalez, J., Manuel Calderon-Montano, A., Burgos-Moron, J., Lopez-Lazaro, M. & E. & Pro-Oxidant natural products as anticancer agents. Curr. Drug Targets. 13 (8), 1006–1028. https://doi.org/10.2174/138945012802009044 (2012).
Larasati, Y. A., Yoneda-kato, N., Nakamae, I. & Yokoyama, T. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Scientific Reports. 8, ; (2039). https://doi.org/10.1038/s41598-018-20179-6 (2018).
Li, P. et al. Curcumin inhibits MHCC97H liver cancer cells by activating ROS / TLR-4 / Caspase signaling pathway. Asian Pac. J. Cancer Prev. 15, 2329. https://doi.org/10.7314/apjcp.2014.15.5.2329 (2014).
Ojo, O. A. et al. Anticancer properties of Curcumin against colorectal cancer: A review. Front. Oncol. 12, 881641. https://doi.org/10.3389/fonc.2022.881641 (2022).
Razak, N. A. et al. In vivo anti-tumor effect of Curcumin derivative (2 E,6 E)-2,6-bis(4-hydroxy-3-methoxybenzylidene)cyclohexanone (BHMC) on 4T1 breast cancer cells. RSC Adv. 7, 36185–36192. https://doi.org/10.1039/c7ra06580a (2017).
Benzie, I. F. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 239, 70–76. https://doi.org/10.1006/ABIO.1996.0292 (1996).
Noorfaiz, M. N. M., Abdullah, S. S., Hamid, A., Mohammad Latif, H., Md Tohid, S. F. & M. A. & Antioxidant activity of Lawsone and prediction of its activation property on superoxide dismutase. Int. J. Pharmacol. 18 (5), 1058–1070. https://doi.org/10.3923/IJP.2022.1058.1070 (2022).
Hasiah, A. H. et al. Cytotoxic and antioxidant effects of methoxylated Stilbene analogues on HepG2 hepatoma and Chang liver cells: implications for structure activity relationship. Hum. Exp. Toxicol. 30 (2), 138–144. https://doi.org/10.1177/0960327110368739 (2011).
Alexander, H. R., Alwi, S., Yazan, S. S., Ansar, L. S. Z., Ong, Y. S. & F. H. & Migration and proliferation effects of thymoquinone-Loaded nanostructured lipid carrier (TQ-NLC) and thymoquinone (TQ) on In vitro wound healing models. Evidence-Based Complement. Altern. Med. 1, 9725738. https://doi.org/10.1155/2019/9725738 (2019).
Mohd Shafiee, M. A. et al. Proliferation and migration effects of 2, 6-bis- (4- hydroxyl-3-methoxybenzylidine) cyclohexanone (BHMC) on human liver cancer, HepG2 cells. Malaysian J. Med. Health Sci. 20, 174–185. https://doi.org/10.47836/mjmhs.20.3.24 (2024).
Liang, F. et al. Isoflavone Biochanin A, a novel nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element activator, protects against oxidative damage in HepG2 cells. BioFactors 45 (4), 563–574. https://doi.org/10.1002/biof.1514 (2019).
Mohamed, Z. A. et al. A. Neuroprotective effects of 7-Geranyloxycinnamic acid from melicope Lunu Ankenda leaves. Molecules 25, 3724. https://doi.org/10.3390/molecules25163724 (2020).
Nakhjiri, M. et al. Asymmetrical 2,6-bis(benzylidene)cyclohexanones: synthesis, cytotoxic activity and QSAR study. Eur. J. Med. Chem. 50, 113–123. https://doi.org/10.1016/j.ejmech.2012.01.045 (2012).
Mohd Shafiee, M. A., Muhamad Asri, M. A. & Syed Alwi, S. S. Review on the in vitro cytotoxicity assessment in accordance to the international organization for standardization (ISO). Malaysian J. Med. Health Sci. 17 (2), 261–269 (2021).
Adewumi Akanji, M., Fatinukun, D., Emmanuel Rotimi, H. & Lawrence Afolabi, D. B. & Stephen Adeyemi, O. The Two Sides of Dietary Antioxidants in Cancer Therapy. Antioxidants - Benefits, Sources, Mechanisms of Action. IntechOpen. (2021). https://doi.org/10.5772/intechopen.94988
Gupta, N. et al. Free radicals as a Double-Edged sword: the cancer preventive and therapeutic roles of Curcumin. Molecules 25 (22), 5390. https://doi.org/10.3390/MOLECULES25225390 (2020).
Sadeer, N. B., Montesano, D., Albrizio, S., Zengin, G. & Mahomoodally, M. F. The versatility of antioxidant assays in food science and Safety-Chemistry, applications, strengths, and limitations. Antioxid. (Basel Switzerland). 9 (8). https://doi.org/10.3390/ANTIOX9080709 (2020).
Borra, S. K. et al. Antioxidant and free radical scavenging activity of Curcumin determined by using different in vitro and ex vivo models. J. Med. Plants Res. 7 (36), 2680–2690. https://doi.org/10.5897/JMPR2013.5094 (2013).
Du, J., Cullen, J. J. & Buettner, G. R. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta Rev. Cancer. 1826, 443–457. https://doi.org/10.1016/j.bbcan.2012.06.003 (2012).
Carr, A. C. & Maggini, S. Vitamin C and immune function. Nutrients 9, 1211. https://doi.org/10.3390/nu9111211 (2017).
Munteanu, I. G. & Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 22, 3380. https://doi.org/10.3390/ijms22073380 (2021).
Balkan, B. M., Meral, Ö., Kismali, G. & Sel, T. Antioxidant enzyme activities in ascorbic acid and selenium applied hepatocellular carcinoma cells. J. Turkish Chem. Soc. Sect. A: Chem. 7 (2), 581–588. https://doi.org/10.18596/jotcsa.724117 (2020).
Heckmann, M. et al. Identification of Oxidative-Stress-Reducing plant extracts from a novel extract Library—Comparative analysis of Cell-Free and Cell-Based In vitro assays to quantitate antioxidant activity. Antioxidants 13, 297. https://doi.org/10.3390/antiox13030297 (2024).
Malta, L. G. & Liu, R. H. Analyses of Total Phenolics, Total Flavonoids, and Total Antioxidant Activities in Foods and Dietary Supplements. Encyclopedia of Agriculture and Food Systems. Academic Press. Preprint at (2014). https://www.sciencedirect.com/science/article/pii/B9780444525123000589
Jung, K. H. et al. Effects of Curcumin on cancer cell mitochondrial function and potential monitoring with 18F-FDG uptake. Oncol. Rep. 35 (2), 861–868. https://doi.org/10.3892/or.2015.4460 (2016).
Murphy, M. P. et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metabolism. 4 (6), 651–662. https://doi.org/10.1038/s42255-022-00591 (2022).
Rizzo, A. M. et al. Endogenous antioxidants and radical scavengers. Adv. Exp. Med. Biol. 698, 52–67. https://doi.org/10.1007/978-1-4419-7347-4_5 (2010).
Dai, F. et al. Insights into the importance for designing curcumin-inspired anticancer agents by a prooxidant strategy: the case of diarylpentanoids. Free Radic. Biol. Med. 85, 127–137. https://doi.org/10.1016/j.freeradbiomed.2015.04.017 (2015).
Etemad, L. et al. Protective effects of nanomicelle Curcumin on the phosphineinduced oxidative stress and apoptosis in human liver HepG2 cells. Nanomed. J. 9 (2), 156–163. https://doi.org/10.22038/NMJ.2022.63370.1662 (2022).
Zitka, O. et al. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 4 (6), 1247. https://doi.org/10.3892/OL.2012.931 (2012).
Alkazemi, D., Rahman, A. & Habra, B. Alterations in glutathione redox homeostasis among adolescents with obesity and anemia. Sci. Rep. 11, 3034. https://doi.org/10.1038/s41598-021-82579-5 (2021).
Nuhu, F., Gordon, A., Sturmey, R., Seymour, A. M. & Bhandari, S. Measurement of glutathione as a tool for oxidative stress studies by high performance liquid chromatography. Molecules 25 (18), 4196. https://doi.org/10.3390/molecules25184196 (2020).
Jamali, B. et al. Intracellular GSH alterations and its relationship to level of resistance following exposure to cisplatin in cancer cells. Iran. J. Pharm. Res. 14 (2), 513–519 (2015).
Becit, M., Aydın Dilsiz, S. & Başaran, N. Interaction of Curcumin on cisplatin cytotoxicity in HeLa and HepG2 carcinoma cells. İstanbul J. Pharm. 50 (3), 202–210. https://doi.org/10.26650/istanbuljpharm.2020.0039 (2020).
Zheng, S., Yumei, F. & Chen, A. De Novo synthesis of glutathione is a prerequisite for Curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic. Biol. Med. 43, 444–453. https://doi.org/10.1016/j.freeradbiomed.2007.04.016 (2007).
Borghei, Y. S. & Hosseinkhani, S. Colorimetric assay of apoptosis through in-situ biosynthesized gold nanoparticles inside living breast cancer cells. Talanta 208, 120463. https://doi.org/10.1016/j.talanta.2019.120463 (2020).
Abbas, G., Cui, M., Wang, D., Li, M. & Zhang, X. E. Construction of genetically encoded biosensors to monitor subcellular Compartment-Specific glutathione response to chemotherapeutic drugs in acute myeloid leukemia cells. Anal. Chem. 95, 2838–2847. https://doi.org/10.1021/acs.analchem.2c04255 (2023).
Shahcheraghi, S. H. et al. Nrf2 regulation by curcumin: molecular aspects for therapeutic prospects. Molecules 27, 167. https://doi.org/10.3390/molecules27010167 (2022).
Wu, A. G. et al. Targeting Nrf2-Mediated oxidative stress response in traumatic brain injury: therapeutic perspectives of phytochemicals. Oxidative Med. Cell. Longev. 1, 1015791. https://doi.org/10.1155/2022/1015791 (2022).
Kim, E. N., Lee, H. S. & Jeong, G. S. Cudratricusxanthone o inhibits h2o2-induced cell damage by activating nrf2/ho-1 pathway in human chondrocytes. Antioxidants 9, 788. https://doi.org/10.3390/antiox9090788 (2020).
González-Reyes, S., Guzmán-Beltrán, S., Medina-Campos, O. N. & Pedraza-Chaverri, J. Curcumin pretreatment induces Nrf2 and an antioxidant response and prevents hemin-induced toxicity in primary cultures of cerebellar granule neurons of rats. Oxidative Med. Cell. Longev. 1, 801418. https://doi.org/10.1155/2013/801418 (2013).
Tan, J-Q., Li, P-C., Li, Q. L., Tangm, J-T. & Xue, H-K. Protective effect of Procyanidin B2 on hydrogen peroxide (H2O2)-induced oxidative damage in MCF-7 cells. Appl. Biol. Chem. 63, 58. https://doi.org/10.1186/s13765-020-00545-7 (2020).
Acknowledgements
The authors express their sincere appreciation to Nurul Munirah Manan, Nor Aishah Norsabarudin, Zulkhairi Zainol, and Hasnijah Alias @ Yaakub for their invaluable help in facilitating the use of laboratory equipment. Additionally, the authors would like to acknowledge Dr. Hasiah Ab Hamid, Wan Mohd Ikhtiaruddin Wan Abdul Aziz, Henna Roshini Alexander, Noor Izzah Abd Rahman, Hani Syahirah Zulkefle and Shazleen Sofea Abdullah for their insightful guidance throughout the project execution.
Funding
This study was supported by the Universiti Putra Malaysia under Geran Putra Berimpak (Grant No. UPM/800-3/3/1/GPB/2019/9682400).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.A.M.S., and S.S.S.A.; methodology, M.A.M.S., S.S.S.A., and A.N.; validation, S.S.S.A., A.N., and Z.O.; formal analysis, M.A.M.S., and S.S.S.A.; investigation, M.A.M.S., and S.S.S.A; resources, S.S.S.A., A.N., and Z.O.; data curation, M.A.M.S., and S.S.S.A.; writing—original draft preparation, M.A.M.S.; writing—review and editing, M.A.M.S., S.S.S.A., A.N., Z.O., and N.N.M.D; visualization, M.A.M.S., and N.N.M.D; supervision, S.S.S.A., A.N., and Z.O.; funding acquisition, S.S.S.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Not applicable..
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mohd Shafiee, M., Syed Alwi, S., Othman, Z. et al. Dual redox effects of 2,6-bis-(4-hydroxyl-3-methoxybenzylidene) cyclohexanone (BHMC) on human liver cancer cells, HepG2 via ROS, glutathione and Nrf2/Keap1 pathway. Sci Rep 15, 35731 (2025). https://doi.org/10.1038/s41598-025-19874-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19874-y