Abstract
Malaria continues to pose a significant global health challenge, with its persistent transmission creating major difficulties for healthcare systems worldwide. Tackling this problem calls for innovative and effective methods to enhance understanding and control of the disease. In this work, we proposed a fractional-order mathematical model to study the dynamics of malaria transmission, integrating essential control measures such as treatment of humans and management of mosquito populations. The model employed three different types of non-integer order differential operators: the Caputo operator, the Caputo–Fabrizio operator with exponential decay, and the Atangana–Baleanu operator with an extended Mittag–Leffler kernel. Using fixed-point theory, we proved the existence and uniqueness of solutions for the proposed model. Numerical simulations are carried out to assess the impact of varying fractional orders on the progression of the disease. The results revealed that increasing the fractional order slows down the spread of malaria, reduces the peak number of infections, and prolongs the duration of outbreaks highlighting the memory-dependent nature of fractional systems. Our findings demonstrated that fractional-order models offer a more accurate and flexible approach to capturing the complex dynamics of malaria transmission. The study underscores the importance of integrating both therapeutic interventions and vector control strategies in reducing disease burden. Based on the findings of this study, we recommended the integration of fractional order modeling into malaria control strategies, as it captures the memory effects and long-term dynamics of disease transmission more accurately than classical models. Public health programs should adopt combined intervention approaches incorporating both effective treatment and vector control measures to significantly reduce infection rates. Furthermore, control efforts should be sustained over time, as fractional models reveal that short-term interventions may not be sufficient in curbing prolonged outbreaks. Policymakers are encouraged to use insights from these models to design adaptive, data-driven strategies that enhance the efficiency and sustainability of malaria control programs.
Similar content being viewed by others
Introduction
Malaria remains one of the world’s deadliest infectious diseases, caused by Plasmodium parasites and transmitted through the bites of female Anopheles mosquitoes1. Despite decades of global efforts, malaria continues to pose a major public health threat, particularly in sub-Saharan Africa where conditions support year-round transmission2. In 2023, there were an estimated 263 million malaria cases and approximately 597,000 deaths globally3. Most of these deaths occurred among children under five, highlighting the vulnerability of this age group3. The human impact of malaria goes beyond statistics. Millions of families face repeated illness, loss of income, and the emotional toll of preventable deaths4. Social determinants such as poverty, poor housing, inadequate access to healthcare, and lack of education increase exposure and reduce the chances of early treatment5. In many rural regions, communities still lack basic tools such as insecticide-treated bed nets and rapid diagnostic tests6. Nonetheless, progress has been made. Since 2000, global malaria control initiatives have averted over 2.2 billion cases and 12.7 million deaths7. However, progress has stalled in recent years due to emerging resistance to artemisinin-based therapies and insecticides, as well as underfunded health programs8. Between 2022 and 2023 alone, malaria cases rose by 11 million, demonstrating a worrying reversal of previous gains3.
Encouragingly, scientific breakthroughs are offering renewed hope. Two WHO-approved malaria vaccines RTS,S/AS01 and R21/Matrix-M have been introduced into immunization programs across multiple African countries9. These vaccines have been shown to significantly reduce severe malaria cases in young children10. Additionally, interventions like seasonal chemoprevention, improved diagnostic tools, and next-generation insecticide-treated nets are strengthening malaria control11. Despite these advances, substantial challenges remain. Artemisinin resistance is spreading in East Africa, jeopardizing the efficacy of front-line treatments12. Climate change is expanding mosquito habitats, leading to increased transmission in previously low-risk areas13. Furthermore, the global funding gap continues to limit the scale-up of life-saving interventions only about $4 billion was available in 2023, far short of the $8.1 billion required annually10. To achieve the 2030 malaria targets, sustained investment, innovative tools, and community-based strategies will be essential.
Malaria remains a major public health concern in Nigeria, accounting for one of the highest burdens of the disease globally. In 2021, Nigeria alone contributed 26.6% of global malaria cases and 31.3% of deaths, highlighting the country’s critical role in the global malaria fight14. The disease is endemic throughout Nigeria, with seasonal variations in transmission intensity influenced by ecological zones and rainfall patterns15. Despite the availability of control strategies such as insecticide-treated nets (ITNs), indoor residual spraying (IRS), and artemisinin-based combination therapies (ACTs), malaria continues to exert significant socio-economic pressure on households and the healthcare system16. Several factors hinder effective control, including drug resistance, poor health-seeking behavior, and inadequate funding for malaria programs17. Moreover, gaps in healthcare infrastructure and limited access to diagnostic tools in rural communities exacerbate the challenge18. Malaria prevalence remains highest among children under five and pregnant women, who face the greatest risk of severe disease and death19. Climate variability and environmental changes have also been shown to influence vector population and disease transmission20. Additionally, weak community participation and low usage of preventive measures like ITNs have undermined progress21. Although progress has been made through various donor-supported initiatives, sustaining long-term control requires domestic investment and policy commitment22. Recent modeling studies emphasize the need for integrated interventions, including environmental management and vaccination, to accelerate progress toward malaria elimination in Nigeria23,24.
Yusuf et al.25 developed a fractional-order model to investigate the transmission dynamics of malaria using Caputo derivatives. The study incorporated key compartments such as susceptible, exposed, infectious, and recovered classes for both human and mosquito populations. The model was analyzed for existence, uniqueness, and stability of solutions. Numerical simulations demonstrated that the fractional-order system captured memory effects more realistically, showing slower convergence to equilibrium compared to the classical integer-order model.
Ngonhala et al.26 formulated a fractional-order malaria model that integrated environmental effects, including seasonal variation in mosquito biting rates. They applied the Atangana-Baleanu fractional derivative in the Caputo sense to account for the memory and hereditary properties of the disease. Their results indicated that fractional derivatives provided a more accurate fit to real data than classical models, and that incorporating environmental variability led to improved understanding of malaria outbreaks in endemic regions. Omame et al.27 proposed a fractional-order malaria model incorporating treatment, prevention, and relapse mechanisms. The model used the Caputo fractional operator and included control strategies such as bed net use and drug administration. Stability analysis was conducted using the Mittag–Leffler function, and numerical simulations showed that lower fractional orders led to prolonged disease persistence, emphasizing the importance of early intervention. Kumar and Agrawal28 analyzed a fractional malaria model using a generalized Mittag–Leffler kernel to reflect the memory effect of disease transmission. They introduced a new numerical method to solve the system and compared the results with real epidemiological data. Their findings showed that the model provided a better fit and richer dynamics compared to the classical models, particularly in representing long-term behavior. Abdelrazec et al.29 developed a fractional-order model focusing on the combined impact of vaccination and vector control strategies. They used Caputo-Fabrizio derivatives to eliminate singularities and observed smoother trajectories. Their simulation results suggested that fractional models with memory kernels provided more realistic predictions, and they recommended such models for guiding public health policies in malaria-endemic countries.
Sweilam et al.30 focused on optimal control of malaria using a single fractional operator, emphasizing the cost-effectiveness of intervention strategies. The recent work extended this by comparing Caputo, Caputo–Fabrizio, and Atangana–Baleanu operators, which improved upon Sweilam et al.’s model by linking different kernel structures to distinct epidemiological memory processes, thereby enhancing interpretability and practical applicability. Boulkroune et al.31 applied adaptive fuzzy control techniques to achieve fixed-time synchronization of fractional-order chaotic systems, demonstrating robustness and efficiency in engineering control problems. In contrast, the recent malaria study focused on comparing Caputo, Caputo–Fabrizio, and Atangana–Baleanu operators, thereby improving upon works like Boulkroune et al. by linking the choice of fractional kernel to specific biological memory processes. While the fuzzy control study advanced synchronization performance in chaotic systems, the recent work advanced interpretability in epidemiological modeling, offering practical insights into how memory effects shape intervention outcomes in malaria control. Zouarin32 developed a neural network-based adaptive control for chemotherapy, ensuring stability through Lyapunov methods. The recent malaria study improved upon this by using fractional-order models with Caputo, Caputo–Fabrizio, and Atangana–Baleanu derivatives to better capture complex dynamics and prove global stability. While Zouari focused on cancer treatment, the malaria study applied advanced mathematical methods to epidemiological modeling. Gassem et al.33 introduced a generalized fractional derivative framework using power non-local kernels, unifying Caputo–Fabrizio, Atangana–Baleanu, and Hattaf derivatives, focusing on theoretical modeling of nonlinear fractional systems. The recent malaria study improved on this by applying fractional-order modeling to real-world malaria transmission, using Caputo, Caputo–Fabrizio, and Atangana–Baleanu operators. It showed how varying fractional orders affected disease progression, proved existence and uniqueness of solutions, and provided insights for treatment and vector control strategies. This study bridged the gap between theoretical fractional calculus and practical epidemiological applications.
Khan et al.34 showed how memory effects improve epidemic modeling. The new malaria study went further by comparing multiple operators, incorporating treatment and mosquito control strategies, and linking results to public health policies, making it more comprehensive and practically relevant. Khan et al.35 used a single modified-ABC fractional derivative to establish theoretical results for smoking behavior, focusing on existence, uniqueness, and stability. The new malaria study advanced further by comparing multiple fractional operators, integrating treatment and mosquito control, and linking results directly to public health applications, making it more comprehensive and policy-relevant. Alzabut et al.36 focused on a discrete-time fractional system using a Caputo-type operator, where existence, uniqueness, and synchronization were proven and validated with numerical results. The new malaria study also established existence and uniqueness through fixed-point theory but advanced further by comparing multiple fractional operators, integrating treatment and mosquito control, and demonstrating through simulations how fractional orders affected malaria outbreaks, making it more comprehensive and policy-relevant.
Khan et al.37 developed a fractal fractional model for tuberculosis and proved the existence of solutions, showing that such models can capture the persistence and long-term effects of the disease more accurately than classical approaches. Similarly, Eiman et al.38 studied a rotavirus infection model using a piecewise modified ABC fractional derivative, demonstrating how different operators influence disease dynamics and control strategies. In another application, Shah39 proposed a fractal-fractional model for multiple sclerosis, a chronic disease, to highlight how fractional operators account for long-term memory effects inherent in progressive illnesses. Abidemi et al.40 developed non-fractional and fractional-order models to examine Lassa fever transmission, including nosocomial infections. The authors demonstrated that increasing the fractional order slowed disease spread, lowered peak infections, and prolonged outbreaks. They highlighted the importance of memory effects and suggested that fractional-order models could improve predictions and public health strategies.
Abidemi et al.41 proposed a nonlinear model of Lassa fever transmission with vertical transmission and nonlinear incidence rates. The study showed that vertical transmission and hygiene practices significantly influenced outbreak dynamics. The model identified the basic reproduction number and offered insights for targeted interventions to reduce transmission.
Shyamsunder et al.42 introduced a fractional-order model to investigate vaccination effects on COVID-19 dynamics. It incorporated memory effects and different vaccination stages. Simulations revealed that accounting for fractional-order memory improved prediction accuracy and demonstrated that vaccination strategies could more effectively control outbreaks. Abidemi et al.43 assessed Lassa fever dynamics with a focus on environmental sanitation using an optimal control approach. The study found that combined interventions, particularly sanitation and rodent control, were most effective and cost-efficient in reducing disease burden. The results provided guidance for designing practical, resource-efficient control strategies. Nisar et al.44 focused on typhoid fever and showed that mass vaccination effectively reduced disease spread. Abboubakar et al.45 studied typhoid fever in Cameroon, incorporating vaccination and calibrating models with real clinical data, identifying key factors influencing transmission. Nabi et al.46 modeled COVID-19, demonstrating that fractional derivatives captured memory effects and provided more accurate forecasts than classical integer-order models.
The choice of fractional operator carries biological significance in malaria modeling. The Caputo-Fabrizio operator, with its exponential decay kernel, is well suited for short-term processes such as the rapid decline of mosquito infectivity or the temporary impact of interventions. The Atangana-Baleanu operator, based on the Mittag–Leffler kernel, better captures long-range memory effects like waning immunity, relapse cycles, and seasonal vector dynamics. In contrast, the Caputo operator, with its power-law kernel, reflects cumulative and persistent effects characteristic of repeated exposures in endemic settings. This comparison underscores that different operators correspond to distinct epidemiological processes, enhancing both the interpretability and practical relevance of fractional-order models.
The model assumed constant total human and mosquito populations, homogeneous mixing between individuals, and no seasonal variation in mosquito dynamics. While these assumptions were necessary to focus on the influence of fractional operators and establish theoretical results, they simplify the biological complexity of malaria transmission. In reality, human and vector populations fluctuate, contact patterns are heterogeneous, and mosquito abundance is strongly affected by environmental and seasonal factors. Future research will aim to relax these assumptions by incorporating variable populations, seasonality, and spatial heterogeneity, thereby enhancing the biological realism and applicability of the model.
While the introduction of fractional orders increases model flexibility by capturing long-term dependencies and memory effects, it also raises practical challenges in real-world applications. Parameter estimation can become more difficult when working with limited or noisy epidemiological data, as fractional parameters often require numerical fitting rather than direct biological measurement. Although fractional-order models are computationally more demanding than classical integer-order models, modern numerical schemes make them tractable for simulation. Importantly, a carefully calibrated integer-order model may perform comparably in short-term predictions of intervention outcomes, particularly when data are sparse. The main strength of fractional models lies in their ability to represent delayed effects, long-term persistence, and cumulative memory processes, which are often underestimated by classical models. Thus, fractional models should be regarded as complementary tools: while classical models are useful for operational planning with limited data, fractional models provide deeper insight into the memory-driven dynamics of malaria when high-quality longitudinal data are available.
The fractional-order nature of the model allows it to capture memory-dependent effects inherent in malaria transmission. Specifically, the Caputo, Caputo–Fabrizio, and Atangana–Baleanu operators enable the model to account for the influence of past states on current dynamics. This feature reflects biological processes such as delayed immunity development in humans, time-lagged mosquito life cycles, and environmental factors that affect vector population persistence. Although these mechanisms are not explicitly modeled as separate compartments, the memory property of fractional derivatives effectively represents their cumulative impact on the progression and spread of malaria, providing a more realistic and biologically interpretable modeling framework.
This study is novel in its comparative use of three distinct fractional-order differential operators Caputo, Caputo-Fabrizio, and Atangana-Baleanu to model malaria transmission dynamics. Unlike previous models that often rely on a single operator or classical integer-order systems, this work uniquely explores how different memory kernels affect the trajectory of disease spread. Additionally, the integration of key control strategies such as treatment and mosquito management within a fractional-order framework provides a more flexible and realistic tool for analyzing long-term disease dynamics. The use of fixed-point theory to establish existence and uniqueness further adds mathematical rigor to the modeling approach. Traditional malaria models typically assume memory less transmission dynamics and instantaneous intervention effects, limiting their ability to capture the delayed and cumulative impacts of control measures. This study fills that gap by incorporating memory-dependent behavior using fractional calculus, thereby offering a more accurate representation of real-world malaria progression. Moreover, while some previous studies have used fractional models, few have analyzed and compared multiple fractional operators in the context of malaria with integrated intervention strategies. This study addresses that deficiency and provides new insights for long-term, adaptive public health planning.
The aim of this study is to develop and analyze a fractional-order mathematical model that captures the transmission dynamics of malaria while integrating key intervention strategies such as treatment and vector control. The objectives of the study are to formulate a novel malaria model using three different fractional derivatives Caputo, Caputo-Fabrizio, and Atangana-Baleanu operators to explore the role of memory and hereditary effects in disease progression. The study further seeks to establish the existence and uniqueness of the model’s solution using fixed-point theory, to perform numerical simulations assessing the impact of varying fractional orders on malaria dynamics, and to evaluate how combined control measures influence infection rates and outbreak duration.
Model formulation
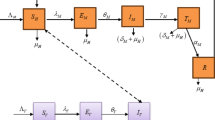
A deterministic compartmental model has been formulated to represent the transmission dynamics of malaria47. In this model, the human population is divided into five separate compartments: susceptible individuals, humans exposed to malaria, infectious humans, humans undergoing treatment for malaria, and humans who have recovered from the disease. The mosquito population is similarly classified into three compartments: susceptible mosquitoes, exposed mosquitoes, and infectious mosquitoes. The recruitment level of humans into the susceptible is \(\Lambda_{H}\) and \(\beta_{M}\) denotes the effective contact rate. \(\theta_{M}\) is the infectious rate of exposed humans and \(\alpha_{M}\) represents rate of recovery rate. The rate at which a recovered individuals move to susceptible class is \(\omega_{M}\) where \(\mu_{H}\) is the natural death rate. \(\delta_{M}\) denotes the disease-induced death rate . The proportion of individuals using insecticide-treated bed nets, which helps to lower malaria transmission, is represented by \(\phi\) . The frequency at which mosquitoes carrying malaria bite humans over a given time period is denoted by \(m\). Furthermore, the rate at which new Anopheles mosquitoes capable of transmitting malaria enter the population is given by \(\Lambda_{V}\) , and \(\beta_{V}\) represents the effective contact rate, which quantifies the likelihood of a human becoming infected from a single bite by an infected mosquito. The infectious rate of exposed mosquitoes is denoted by \(\theta_{V}\).The natural death rate of the mosquitoes is denoted \(\mu_{V}\) and \(\delta_{V}\) represents mosquitoes mortality due to quest for blood meal.
Table 1, presents a comprehensive summary of the variables and parameters utilized in the model, highlighting their roles within the analysis48. Each variable is described alongside its definition. The model parameters, including constants and coefficients, define the framework’s relationships and dynamics47,49. This table serves as a crucial reference, enabling readers to understand the model’s structure and application while promoting transparency and reproducibility.
Model equations
where:
\(\lambda_{M} = \frac{{\left( {1 - \phi } \right)m\beta_{M} I_{V} }}{{N_{H} }}\), \(\lambda_{V} = \frac{{m\beta_{V} I_{M} }}{{N_{H} }}\).
Figure 1a shows a schematic diagram of the malaria transmission model, offering a visual representation of how individuals move through different stages of the disease47,49. It highlights key groups such as susceptible, exposed, infectious, and recovered individuals, as well as any other relevant stages involved in the transmission process50. The arrows between these groups indicate how people transition from one stage to another through events such as being bitten by an infected mosquito, becoming infectious after exposure, recovering from the illness, or, in some cases, dying from malaria.
Assumptions in the model formulation
-
1.
The model assumes that both humans and mosquitoes interact randomly and evenly. In other words, every person is just as likely to be bitten by a mosquito as anyone else, and all mosquitoes have the same chance of biting an infected human47,51. This means factors like where people live, how they behave, or their socio-economic status which can affect exposure are not considered. Everyone is treated as equally at risk.
-
2.
Malaria transmission is assumed to happen at a steady rate over time. That means the model doesn’t take into account environmental or seasonal changes like weather patterns or mosquito breeding seasons that can impact how actively mosquitoes spread the disease37,47. It also assumes mosquitoes, such as those from the Anopheles species, are evenly spread throughout the study area, with no variation from one location to another.
-
3.
Throughout the study period, the total number of humans and mosquitoes is assumed to stay the same. This means any changes due to births, natural deaths, or people moving in and out of the area are not considered38,47. While the model might account for deaths caused specifically by malaria, it ignores broader demographic shifts or migrations that could affect transmission dynamics.
-
4.
This model focuses only on malaria and doesn’t account for other diseases that people might have at the same time39,47. Individuals are categorized as either infected with malaria or not52. It also assumes no one in the population is vaccinated, so all susceptible people can become infected if exposed to the parasite.
-
5.
Once someone is infected, they’re assumed to go through a straightforward progression: incubation, illness, treatment (if available), and then either recovery or death47,53. The model doesn’t factor in differences in how people’s bodies respond, possible treatment failures, or drug resistance. Treatment is assumed to be available to everyone and effective for all.
-
6.
People who recover from malaria might gain some short-term immunity, but it doesn’t last forever. In areas where malaria is common, it’s very possible for them to get sick again if they’re bitten by an infected mosquito later on47,54. This mirrors real-world situations where repeated exposure is common due to the temporary nature of post-infection immunity.
-
7.
. Mosquitoes that become infected with the malaria parasite stay infectious until they die. They don’t recover or lose the ability to transmit the disease38,47. The model also doesn’t consider any special mosquito control strategies like genetic modifications or mosquito-targeted vaccines39,47.
-
8.
The number of mosquitoes in the environment is assumed to remain steady over time. The model doesn’t include the effects of seasonal changes or mosquito control efforts—like spraying insecticides, draining breeding grounds, or changes due to weather patterns like rainfall47,49.
-
9.
It is assumed that everyone in the human population has the same access to malaria diagnosis and treatment services55. Real-world differences like limited healthcare facilities in remote areas or economic barriers are not reflected in the model, even though such inequalities can significantly affect how well people are diagnosed and treated.
Preliminaries
Definition 1
The Liouville-Caputo fractional order derivative of order θ ∈ [0,1) is defined as follows:25,56.
Definition 2
The Caputo-Fabrizio (CF) derivative, which features a non-singular kernel for a function of fractional order, is defined as follows:29.
where \(N\left( \theta \right) = N\left( 0 \right) = N\left( 1 \right)\). Secondly, \(x \in Q{\prime} \left( {0,T} \right),T > 0\).
Definition 3
For the function fff, the fractional order integral of order α\alphaα is defined as follows:
Definition 4
The Atangana-Baleanu (AB) fractional order operator of order θ in the Caputo sense is defined as follows56.
where \(\gamma_{\theta }\) is a Mittag–Leffler function and is defined as:
also \(AB\left( \theta \right) = 1 - \theta + \frac{\theta }{{\left| {\overline{{{\mkern 1mu} \left( \theta \right){\mkern 1mu} }} } \right.}}\) represents the normalization function.
The fractional order integral discussion related to the Atagana-Baleanu derivative is defined as:
Fractional malaria mathematical model
We extend the integer model described previously by substituting the classical derivative with fractional derivatives28,56. The general fractional form is expressed as follows:
with initial conditions:
\(S_{H0} (t) = S_{H} (0)\), \(E_{M0} (t) = E_{M} (0)\), \(I_{M0} (t) = I_{M} (0)\), \(T_{M0} (t) = T_{M} (0)\), \(R_{0} (t) = R(0)\), \(S_{V0} (t) = S_{V} (0)\), \(E_{V0} (t) = E_{V} (0)\), \(I_{V0} (t) = I_{V} (0)\).
The model is re-modeled by replacing the classical derivative \((D_{t} )\) using \((_{0}^{C} D_{t}^{\theta } )\), \((_{0}^{CF} D_{t}^{\theta } )\) and \((_{0}^{ABC} D_{t}^{\theta } )\) which represents the Caputo derivative with fractional order \(\theta\), Caputo-Fabrizio derivative with fractional order \(\theta\) and Atangana-Baleanu derivative with fractional order \(\theta\) respectively29,56.
The modified malaria model with Caputo derivative of fractional order $\theta$ with power law is presented as:
The modified malaria model with Caputo-Fabrizio derivative
The adjusted malaria model utilizing the Caputo-Fabrizio derivative of fractional order θ, characterized by an exponential kernel law, is expressed as follows29:
The modified malaria model with Atangana-Baleanu derivative
The revised malaria model incorporating the Atangana-Baleanu derivative with the generalized Mittag–Leffler function is expressed as follows56:
Numerical method for the Caputo derivative
We introduce briefly the predictor–corrector type numerical algorithm to solve the malaria model with Caputo derivative of fractional order \((\theta )\).
The general form is25:
This equation is equivalent to the Volterra integral equation29:
Setting \(P = \frac{T}{N}\), \(t_{r} = rp\), \((r = 0,1,2,3,...,R)\).
We discretize the equation as follows:
where \(x_{p} (t_{r + 1} )\) is the predicted value evaluated using the Adams–Bashforth method:
where:
and
Predictor–corrector numerical method for the malaria model with Caputo fractional derivative
From the fractional malaria model with Caputo derivative, we employ the predictor–corrector numerical method to solve the fractional operator. The model is discretized as follows25:
The predicted values \(S_{H,r + 1}^{V} ,E_{M,r + 1}^{V} ,I_{M,r + 1}^{V} ,T_{M,r + 1}^{V} ,R_{r + 1}^{V} ,S_{V,r + 1}^{V} ,E_{V,r + 1}^{V} ,I_{V,r + 1}^{V}\) are computed as:
The system can be written in the form:
Existence and uniqueness of solutions for the modified malaria model with Caputo-Fabrizio derivative
In this section, we employ the fixed point theorem to investigate the existence and uniqueness of solution for the modified malaria model with Caputo-Fabrizio derivative29.
The model is transformed into an integral equation given as:
The kernels are defined as follows:
Using the arbitrary integral, we obtain:
Theorem 1
The kernels \(Y_{1} ,Y_{2} ,Y_{3} ,Y_{4} ,Y_{5} ,Y_{6} ,Y_{7}\) and \(Y_{8}\) fulfill the Lipschitz and contraction condition provided that the inequality given below is satisfied:
where \(g_{i}\) represents the bounds for each state variable.
Proof
Starting with kernel \(Y_{1}\) and assuming that kernel \(Y_{1}\) has \(S_{H}\) and \(S_{H}^{*}\) as functions, then we can say:
Let
Considering that:
are bounded functions where \(g_{1} ,g_{2} , \ldots ,g_{8}\) are non-negative constants. We therefore have:
This means kernel \(Y_{1}\) satisfies the Lipschitz condition. For the contraction condition, \(0 \le \rho_{1} < 1\) settles the case28,29.
Similarly, we can write expressions for the remaining kernels:
Introducing the recursive formula gives:
where
\(S_{{H_{0} }} = S_{H} (0)\), \(E_{{M_{0} }} = E_{M} (0)\), \(I_{{M_{0} }} = I_{M} (0)\), \(T_{{M_{0} }} = T_{M} (0)\), \(R_{0} = R(0)\), \(S_{{V_{0} }} = S_{V} (0)\), \(E_{{V_{0} }} = E_{V} (0)\), \(I_{{V_{0} }} = I_{V} (0)\) are the initial conditions28.
Taking the difference between successive terms:
Expressing the model variables in terms of infinite series:
Taking the norm of the difference equation for \(S_{H}\):
Applying the triangle inequality:
Using the Lipschitz condition which is satisfied from theorem (1), we now rewrite the corresponding equation as:
Therefore we have:
The following results are obtained following the same procedure used in Eq. (29):
Theorem 2
The fractional order malaria model has a coupled solution that exists, if there exist some \(t_{0}\) whenever:
for \(i = 1,2,...,8\).
Proof
The model variables \(S_{H} (t)\), \(E_{M} (t)\), \(I_{M} (t)\), \(T_{M} (t)\), \(R(t)\), \(S_{V} (t)\), \(E_{V} (t)\), and \(I_{V} (t)\) are taken to be bounded functions for which the Lipschitz condition is fulfilled by their kernels.
We therefore obtain the following results by employing Eqs. (29) and (30) recursively29,56:
We assume the following so as to show that Eq. (31) is a unique solution of the Caputo-Fabrizio derivative malaria model29,56:
such that:
Then:
When \(t = t_{0}\), we have:
Taking the limit of Eq. (35) as \(r \to \infty\), then we have \(\left| {A_{r} (t)} \right|^{r}\) leading to zero29.
In the same way, we can show that
\(\left| {B_{r} (t)} \right| \to 0\), \(\left| {C_{r} (t)} \right| \to 0\), \(\left| {D_{r} (t)} \right| \to 0\), \(\left| {F_{r} (t)} \right| \to 0\), \(\left| {G_{r} (t)} \right| \to 0\), \(\left| {H_{r} (t)} \right| \to 0\), \(\left| {J_{r} (t)} \right| \to 0\).
We therefore conclude that the solution to our malaria model exists and this is proved.
Theorem 3
The fractional malaria model with Caputo-Fabrizio derivative has a unique solution whenever:
for \(i = 1,2,...,8\).
Proof
Assuming that the fractional malaria model has another solution of the form \(S_{H}^{*} (t)\), \(E_{M}^{*} (t)\), \(I_{M}^{*} (t)\), \(T_{M}^{*} (t)\), \(R^{*} (t)\), \(S_{V}^{*} (t)\), \(E_{V}^{*} (t)\), and \(I_{V}^{*} (t)\), therefore:
Taking the norm of the above Eq. (31), we have:
Since the Lipschitz condition is satisfied by the kernel, then we have:
Equation (33) can also be expressed as:
But:
Therefore:
This implies the model solution is proved to be unique. The remaining components \(E_{M} (t)\), \(I_{M} (t)\), \(T_{M} (t)\), \(R(t)\), \(S_{V} (t)\), \(E_{V} (t)\), and \(I_{V} (t)\) results can be obtained following the same procedure25,29,56.
General Caputo-Fabrizio numerical method
For the general Caputo-Fabrizio fractional differential equation:
Using the fundamental theorem of calculus:
For the discrete case, we have:
Using the Adams–Bashforth approximation for the integral29:
This gives us the two-step Adams–Bashforth-Moulton formula:
Application to malaria model
Let us define the right-hand side functions for our malaria model:
Caputo-Fabrizio malaria model numerical scheme
Applying the two-step Adams–Bashforth-Moulton method to each compartment29:
Adams-type predictor corrector numerical method with Atagana-Baleanu-derivative fractional order model
In this section, we employ the Adams-type predictor corrector numerical method used to obtain an approximate solution of our Malaria model29,56.
The malaria model in the sense of Atangana-Baleanu derivative with generalized Mittag–Leffler function is56:
This can be written as:
Applying the Atangana-Baleanu fractional integral on both sides56:
To investigate the above fractional order integral numerically, we therefore approximate the fractional order integral numerically56. Employing the Adams-type predictor–corrector method presented in for Atagana-Baleanu fractional integral, we now have
by letting \(P = \frac{T}{N},t_{r} = rp,\left( {r = 0,1,2,3,...R} \right)\).
We therefore consider the solution in the interval of [0,T]56.
Therefore the corrector formula for the Atagana-Baleanu fractional-integral version is presented as29:
where
Similarly, the predictor \(x^{V} \left( {t_{r + 1} } \right)\) is expressed as given below:
where
We therefore present our malaria model using the Adam-type predictor–corrector numerical method:
where \(S_{Hr + 1}^{V} ,E_{Mr + 1}^{V} ,I_{Mr + 1}^{V} ,T_{Mr + 1}^{V} ,R_{r + 1}^{V} ,S_{Vr + 1}^{V} ,E_{Vr + 1}^{V} ,I_{Vr + 1}^{V}\) are the predictors given as:
Fitting data for malaria models
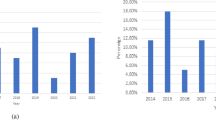
In this tudy, the dataset includes yearly records from 2014 to 2021, showing the number of confirmed cases reported each year57. The data are clearly organized in the table below, providing an overview of the trends in malaria incidence over the given period.
Table 2 displays the number of malaria cases reported in Nigeria between 2010 and 2022, based on data from the World Health Organization (WHO)48,57. It offers a yearly breakdown of reported cases, highlighting the trends and changes in malaria incidence across the years58.
The fitted malaria cases closely aligned with the observed data from 2010 to 2022, with only minimal deviations (RMSE ≈ 670 cases) and an R2 value of 0.9997, indicating that the fractional-order model reliably captured the underlying dynamics of malaria transmission. Epidemiologically, this strong fit demonstrated the model’s ability to reflect the gradual and persistent increase in malaria cases during the study period, a trend driven by mosquito–human interactions, treatment effects, and environmental influences49,59,60. The incorporation of fractional operators allowed the model to account for memory-dependent processes such as mosquito life cycles, incubation delays, and the gradual development of host immunity, which are critical features of malaria epidemiology often overlooked in classical models47,61. By closely reproducing real-world case data, the model not only confirmed its mathematical soundness but also highlighted its practical value for public health. The strong alignment between fitted and observed cases underscored its potential for forecasting future trends and evaluating the long-term impact of interventions49. This enhances its relevance as a tool for policymakers seeking adaptive, data-driven strategies to reduce malaria burden and achieve sustainable control.
The Fig. 1c and d showed a consistent and gradual rise in reported malaria cases in Nigeria between 2010 and 2022, increasing from approximately 350,000 cases in 2010 to nearly 480,000 cases by 2022. Epidemiologically, this trend highlights that malaria remains a persistent and pressing public health concern, reinforcing Nigeria’s position as the country with the highest global malaria burden. The upward trajectory may be attributed to multiple interwoven factors, including sustained transmission in malaria-endemic zones due to favorable climatic conditions, rising levels of insecticide resistance among mosquito vectors, and growing evidence of antimalarial drug resistance10. Additionally, gaps in healthcare access and socioeconomic inequalities continue to hinder effective prevention and treatment. Improved surveillance systems may also partly explain the increase, as better reporting and case detection capture more malaria cases over time. The significance of this chart lies in its clear demonstration that malaria control in Nigeria has not achieved the expected decline in incidence, raising concerns about the long-term feasibility of elimination goals13. Interventions such as the scale-up of insecticide-treated nets (ITNs), indoor residual spraying, seasonal malaria chemoprevention (SMC) for children under five, and the use of artemisinin-based combination therapies (ACTs) have been widely implemented, yet their impact is offset by resistance, logistical challenges, and limited community uptake. This suggests that malaria control in Nigeria requires renewed efforts, stronger community engagement, improved access to healthcare, and innovations such as next-generation insecticides, vaccines, and integrated vector management.
Table 3 presents the parameter values used in the numerical simulations, along with their corresponding units. These values were selected based on available data and relevant literature to accurately reflect the dynamics of the system49,58,63. The table serves as a transparent reference point, allowing other researchers to replicate or extend the study64. Most parameters were sourced from published studies, WHO reports, and regional malaria statistics50. For parameters without direct data, the least squares fitting method was employed to align the model with observed malaria cases, ensuring both accuracy and biological relevance65. The fitting process was conducted within realistic parameter ranges, and sensitivity analysis was used to confirm the significance of key variables..
Discussion
Figure 2 displays the phase plane relationship between exposed and infected humans, showing curved trajectories that form characteristic loops for different \(\beta_{M}\) values (0.2, 0.3, 0.4, 0.5). The curves exhibit increasing amplitude and complexity as \(\beta_{M}\) increases, with the highest contact rate (\(\beta_{M} = 0.5 = 0.5\) = 0.5) producing the most expansive trajectory reaching peaks of approximately 650 infected humans and 700 exposed humans. The trajectories demonstrate oscillatory behavior, suggesting cyclical epidemic patterns rather than steady-state equilibria. Epidemiologically, this phase plane analysis reveals the dynamic relationship between malaria exposure and active infection in the population. The expanding loops with higher \(\beta_{M}\) values indicate that increased contact rate between susceptible humans and infected mosquitoes lead to more volatile epidemic cycles with higher peak infections. The oscillatory nature suggests that malaria transmission in this model exhibits boom-bust cycles, where periods of high transmission are followed by temporary reductions due to depletion of susceptible populations, before rebuilding toward subsequent epidemic waves. Figure 3 presents the relationship between recovered and infected humans, showing bell-shaped curves that peak at different heights depending on the mortality parameter \(\alpha_{M}\) (0.05, 0.1, 0.15, 0.2). Lower mortality rates pro]duce higher peaks, with \(\alpha_{M} = 0.05\) = 0.05 reaching approximately 680 recovered individuals, while higher mortality rates show progressively lower peaks. All curves demonstrate a characteristic rise-and-fall pattern, suggesting epidemic waves with eventual decline phases.
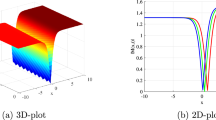
The epidemiological significance of this figure lies in understanding how disease-induced mortality affects population recovery dynamics. Lower mortality rates (\(\alpha_{M} = 0.05\)) allow more individuals to survive the infection and contribute to the recovered population, creating larger immune pools. Higher mortality rates reduce the recovered population size, potentially maintaining larger susceptible populations for future transmission cycles. This relationship is crucial for understanding herd immunity development and the long-term sustainability of malaria elimination efforts. Figure 4 shows a three-dimensional surface plot of susceptible humans over time and \(\beta_{M}\) values, displaying a complex landscape with multiple peaks and valleys. The surface demonstrates how susceptible populations fluctuate dramatically based on transmission intensity, with higher \(\beta_{M}\) values generally associated with lower baseline susceptible populations but more dramatic temporal fluctuations. The surface reaches maximum values around 2,500 individuals under certain parameter combinations. Epidemiologically, this three-dimensional visualization captures the complex interplay between transmission intensity and susceptible population dynamics over time. The varying heights of the surface indicate that optimal conditions for maintaining large susceptible populations occur at specific combinations of time and transmission rates. The dramatic fluctuations suggest that malaria transmission systems are inherently unstable, with susceptible populations being rapidly depleted during high transmission periods and slowly rebuilding during low transmission phases.
Figure 5 presents the temporal dynamics of infected humans across different \(\beta_{M}\) values, showing wave-like patterns that peak at different times and amplitudes. Lower \(\beta_{M}\) values (0.1, 0.2) produce smaller, later peaks, while higher values (0.3, 0.4) generate larger, earlier peaks reaching up to 700 infected individuals. The three-dimensional surface shows how infection dynamics vary continuously across the parameter space and time. The epidemiological interpretation reveals how transmission intensity fundamentally alters epidemic timing and magnitude. Higher contact rates accelerate epidemic development and increase peak infection levels, while lower rates produce more gradual epidemic curves. This relationship is critical for public health planning, as it demonstrates how transmission reduction interventions can both delay and reduce epidemic peaks, potentially allowing healthcare systems more time to respond and reducing overall disease burden. Figure 6 illustrates the dynamics of exposed humans over time and \(\beta_{M}\) values, showing surface patterns that complement the infected human dynamics. The surface demonstrates earlier peaks for exposure compared to infection, reflecting the natural progression from exposure to active disease. Maximum exposure levels reach approximately 1,600 individuals under optimal transmission conditions. Epidemiologically, this figure captures the leading indicator nature of exposure dynamics in malaria transmission. The exposed population serves as a reservoir of future infections, making it a critical metric for early warning systems. The relationship between \(\beta_{M}\) and exposure timing suggests that transmission interventions targeting the exposure phase (such as vector control or bed net usage) may be more effective when implemented before peak exposure periods.
Figure 7 displays the treated human population dynamics, showing relatively modest peaks compared to infection levels, with maximum treatment populations reaching approximately 200 individuals. The surface pattern suggests that treatment capacity utilization varies significantly with transmission intensity and timing, with some parameter combinations producing sustained treatment demand while others show more sporadic patterns. The epidemiological significance relates to healthcare system capacity and treatment accessibility. The relatively low treatment numbers compared to infection levels may indicate either limited treatment access or high treatment effectiveness leading to rapid population turnover. The temporal patterns suggest that treatment demand peaks lag behind infection peaks, creating potential opportunities for proactive treatment capacity planning based on transmission monitoring. Figure 8 presents the recovered human population dynamics, showing substantial peaks reaching up to 1,750 individuals under certain conditions. The surface demonstrates complex temporal patterns where recovery populations can either build steadily or show dramatic fluctuations depending on transmission parameters. The highest recovery levels occur under moderate transmission conditions rather than at the extremes. Epidemiologically, this figure illustrates the development of population immunity over time. The substantial recovered populations indicate that natural immunity acquisition plays a significant role in malaria dynamics. The non-linear relationship with transmission intensity suggests that moderate transmission levels may be most effective for building population immunity, while very high transmission may overwhelm recovery capacity through increased mortality or immune system compromise.
Figure 9 showed the susceptible vector population dynamics, displaying surface patterns that reach maximum values around 7,000 vectors. The surface demonstrates how vector populations respond to transmission dynamics, with complex temporal patterns that reflect both natural population cycles and disease-induced mortality effects. The epidemiological interpretation focuses on vector population management for malaria control. The large susceptible vector populations highlight the enormous reproductive potential of mosquito vectors and the challenges this presents for vector control programs. The temporal fluctuations suggest that vector control interventions may need to be timed strategically to coincide with periods of vector population vulnerability. Figure 10 illustrates exposed vector dynamics, showing surface patterns that complement the susceptible vector populations. The exposed vector population represents vectors that have acquired malaria parasites but are not yet infectious. Peak exposed vector populations reach approximately 4,000 individuals, indicating substantial parasite circulation within vector populations. Epidemiologically, this figure captures the latent infection dynamics within vector populations. The exposed vector population serves as a reservoir for future transmission potential, making it a critical target for intervention strategies. The temporal patterns suggest that there may be optimal timing for vector control interventions based on the natural cycles of vector exposure and infection development.
Figure 11 presents infected vector population dynamics, showing complex surface patterns with peaks reaching up to 3,500 infected vectors. The surface demonstrates how infected vector populations drive human transmission, with temporal patterns that directly influence human epidemic dynamics. The epidemiological significance lies in understanding the vector-driven nature of malaria transmission. The infected vector population directly determines transmission pressure on human populations, making it a key target for intervention strategies. The temporal patterns suggest that vector infection dynamics may be more stable than human infection dynamics, potentially providing more predictable targets for control interventions. Figure 12 displays a comprehensive surface plot showing the relationship between time and parameter \(\phi\) (bed net compliance rate), with infected humans as the outcome variable. The surface revealed how bed net usage dramatically affects infection levels, with higher compliance rates associated with substantially lower infection peaks. The maximum infection levels reach approximately 2,000 individuals under low compliance conditions.
Epidemiologically, this figure quantifies the protective effect of bed net usage on malaria transmission. The dramatic reduction in infections with increased compliance demonstrates the population-level benefits of vector control interventions. The relationship suggests that bed net programs may exhibit threshold effects, where moderate increases in compliance produce disproportionately large reductions in transmission. This finding supports the importance of achieving high coverage rates in bed net distribution programs. Figure 13 showed how mosquito mortality influences infection dynamics over time. As the natural death rate increases, the population of infected vectors declines more rapidly, and the infection curve flattens. Conversely, when the death rate is lower, vectors survive longer, allowing the infection to persist and reach higher levels. This highlights that vector longevity plays a crucial role in sustaining transmission: higher survival rates provide more opportunities for mosquitoes to acquire and transmit the pathogen. Figure 14 showed the relationship between time and the vector biting rate parameter (m), illustrating how vector behavior affects human infection levels. The surface demonstrates that higher biting rates lead to dramatically increased infection levels, with peaks reaching over 1,200 infected individuals. The temporal patterns show that increased biting rates not only increase infection levels but also accelerate epidemic development. The epidemiological interpretation emphasizes the critical role of vector behavior in malaria transmission dynamics. The dramatic increase in infections with higher biting rates underscores the importance of vector control strategies that target feeding behavior. The relationship also suggests that environmental or behavioral factors that influence mosquito biting rates, such as temperature, humidity, or human activity patterns, may significantly impact transmission dynamics and should be considered in malaria control planning.
The numerical results demonstrate that variations in the fractional order have a pronounced impact on malaria dynamics, particularly in terms of peak infection levels and outbreak duration. Lower fractional orders tend to prolong epidemic persistence and reduce infection peaks, reflecting stronger memory effects within the system. Although no sharp threshold was observed, qualitative changes in dynamics become more evident when the fractional order falls below approximately 0.7. Compared to traditional parameters such as transmission or recovery rates, the fractional order functions as a global modifier, influencing the time-scaling and persistence of disease dynamics rather than altering a single biological process. This distinction emphasizes the epidemiological value of fractional models, as they capture cumulative delays and long-term memory effects that cannot be adequately represented in classical integer-order frameworks.
The fractional nature of the model alters the expected impact and timing of interventions by accounting for memory effects in malaria transmission. Our findings suggest that under fractional dynamics, the benefits of treatment and vector control persist longer but emerge more gradually, reflecting the system’s retention of past infection states. This implies that interventions must be sustained over extended periods to achieve full effectiveness, whereas a classical model might underestimate the required duration or intensity of control measures. Consequently, fractional-order models provide a more realistic framework for designing adaptive malaria control strategies, emphasizing the importance of long-term and continuous interventions rather than short-term campaigns.
The results were described in terms of infection peaks, outbreak durations, and the overall progression of malaria for the Caputo, Caputo–Fabrizio, and Atangana–Baleanu operators. The Caputo operator showed a moderate reduction in the peak number of infections and a gradual decline in disease prevalence over time. The Caputo–Fabrizio operator, with its exponential decay kernel, demonstrated a faster decrease in infection rates, leading to shorter but sharper outbreaks compared to the Caputo operator. In contrast, the Atangana–Baleanu operator, featuring the extended Mittag–Leffler kernel, produced more prolonged outbreak dynamics with smoother progression, indicating a slower but sustained transmission pattern. These differences highlighted how the choice of fractional-order operator influenced both the intensity and timing of outbreaks. By narratively comparing these outcomes, the study provided a clearer understanding of how each operator affected the dynamics of malaria transmission and offered valuable insights for designing more effective and tailored intervention strategies.
Conclusion
To understand disease transmission within a specific population, this study examined a variable-order fractional model of malaria outbreaks. We enhanced the model by incorporating fractional derivatives, utilizing Caputo’s power law, Fabrizio’s exponential law, and the Atangana-Baleanu’s generalized Mittag–Leffler function.For improved system approximation, we employed three different kernels in the fractional-order derivative operators. A numerical method was developed to address alternative systems: the Caputo Fabrizio derivative was solved using a two-step Adams–Bashforth approach, the Atangana-Baleanu fractional derivative was tackled with an Adams-type predictor–corrector method, and the Caputo fractional derivative utilized an Adams–Bashforth-Moulton scheme. Furthermore, the application of fixed-point theory in our analysis indicated that the Caputo Fabrizio fractional-order system supports the existence of a unique solution. Our numerical simulations revealed that an increase in the fractional order (reflecting a memory effect) generally results in a slower disease progression. Our visual data assesses the effectiveness and reliability of the proposed methods. The influence of various arbitrary-order values was explored and represented in several figures.The findings underscore that treatment and vector control significantly affect malaria transmission dynamics. An increase in the treatment rate for infected individuals (γ_M) leads to a marked reduction in the total number of new cases. This effect is due to the swift removal of infected individuals from the population, which decreases interactions between infected people and susceptible vectors, thereby slowing parasite transmission. Similarly, effective vector control methods that quickly diminish the vector population before they can spread the infection can greatly lower the transmission rate. The graphs clearly illustrate that higher treatment and vector control rates correlate with lower infection peaks and a quicker reduction in new cases, emphasizing the importance of these interventions in managing outbreaks. These results highlight the vital role of timely and effective treatment and vector control strategies in reducing the impact of infectious diseases.
While the current study focused on deterministic fractional-order models, we recognize that parameter uncertainty and modeling assumptions may affect the robustness of the conclusions.. Future extensions of the model could incorporate stochastic effects, such as random fluctuations in mosquito populations or human exposure, as well as spatial heterogeneity to account for differences in vector density, human movement, and environmental conditions across regions. Integrating these features would enhance the realism, predictive power, and policy relevance of the model, supporting the development of adaptive, data-driven malaria control strategies.
Data availability
The data used in this study are presented and referenced in Table 3 of this manuscript. The source code used for simulation is available from the corresponding author on reasonable request .
References
Global Fund. Results Report 2024: Malaria investment impact and trends. The Global Fund. Retrieved from https://www.theglobalfund.org/media/15029/core_2024-results-malaria_report_en.pdf (2024).
World Health Organization. World malaria report 2024: Reinvigorated global efforts needed to curb rising malaria threat. Retrieved from https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (2024).
World Health Organization.. Malaria vaccines (RTS,S and R21). Retrieved from https://www.who.int/news-room/questions-and-answers/item/q-a-on-rts-s-malaria-vaccine (2024)
Brown, B. J. et al. Data-driven malaria prevalence prediction in large densely-populated urban holoendemic sub-Saharan West Africa: Harnessing machine learning approaches and 22 years of prospectively collected data. arXiv. https://doi.org/10.48550/arXiv.1906.07502 (2019).
Chutiyami, M. et al. Malaria vaccine efficacy, safety, and community perception in Africa: A scoping review of recent empirical studies. Infection 52(6), 1027–1042. https://doi.org/10.1007/s15010-024-02196-y (2024).
Isiko, I. et al. Determinants of malaria spread among under-five children in Nigeria: Results from a 2021 Nigerian malaria indicator cross-sectional survey. BMC Pediatr. 24, 646. https://doi.org/10.1186/s12887-024-05135-w (2024).
CDC. Malaria Vaccines – Public Health Strategy. Centers for Disease Control and Prevention. Retrieved from https://www.cdc.gov/malaria/php/public-health-strategy/malaria-vaccines.html (2024).
Le Monde. Lutte contre le paludisme : une nouvelle résistance aux traitements standards observée chez les enfants. Retrieved https://www.lemonde.fr/sciences/article/2024/11/20/traitements-standards-observee-chez-les-enfants_6404172_1650684.html (2025).
World Health Organization. African health leaders and global partners unite to confront rising threat of antimalarial drug resistance. WHO News. Retrieved https://www.who.int/news/item/20-05-2025-african-health-leaders-and-global-partners (2025).
Onah, S. I., Collins, O. C., Okoye, C. & Mbah, G. C. E. Dynamics and control measures for malaria using a mathematical epidemiological model. Electron. J. Math. Anal. Appl. 7(1), 65–73. https://doi.org/10.21608/ejmaa.2019.312741 (2019).
Nwele, D. E., Onyali, I. O., Iwueze, M. O., Elom, M. O. & Uguru, O. E. S. Malaria endemicity in the rural communities of Ebonyi State, Nigeria. Korean J. Parasitol. 60(3), 173–179. https://doi.org/10.3347/kjp.2022.60.3.173 (2022).
Lancet Microbe.. WHO world malaria report 2024. The Lancet Microbe. Retrieved from https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(25)00001-1/fulltext (2025)
Duru, E. C., Anyanwu, M. C. & Mbah, G. C. E. Mathematical analysis of a malaria model with vaccination, treatment, and vector control using sterile-insect technique. J. Math. Anal. Model. 6(1), 82–106. https://doi.org/10.48185/jmam.v6i1.1432 (2025).
Adelaja, O. I., Rudge, J. W. & Banerjee, A. Spatial and temporal trends of malaria in Nigeria: A sub-national analysis of surveillance data. BMJ Glob. Health 8(5), e011294. https://doi.org/10.1136/bmjgh-2022-011294 (2023).
Okeke, T. A. & Uzochukwu, B. S. C. Improving malaria treatment services in Nigeria: An evaluation of strategies for improving prompt access and reducing health inequities. Health Policy 90(1), 72–79. https://doi.org/10.1016/j.healthpol.2008.09.012 (2009).
Ajayi, I. O. & Falade, C. O. Pre-hospital treatment of febrile illness in children attending the general outpatient clinic, University College Hospital, Ibadan, Nigeria. Afr. J. Med. Med. Sci. 37(3), 265–271 (2008).
Duru, E. C. & Mbah, G. C. E. Approximate solution for a malaria model using the homotopy analysis method. Biometrical Lett. 62(1), 1–27. https://doi.org/10.2478/bile-2025-0001 (2025).
Duru, E. C., Anyanwu, M. C. & Mbah, G. C. E. A mathematical model to investigate the effect of misdiagnosis and wrong treatment in the co-circulation and co-infection of malaria and Zika virus disease. Bull. Biomath. 3(1), 79–110. https://doi.org/10.59292/bulletinbiomath.1711811 (2025).
Okeke, C. C., Umeokonkwo, C. D., Ifeadike, C. O. & Ubajaka, C. Improving malaria diagnosis and treatment in under-five children in Nigeria: A cross-sectional study of primary health care facilities. Malar. J. 15(1), 1–9. https://doi.org/10.1186/s12936-016-1209-5 (2016).
Samdi, L. M., Ajayi, J. A., Oguche, S. & Ayanlade, A. Climate change and malaria transmission dynamics in Nigeria: A review. Asian J. Epidemiol. 5(4), 115–123. https://doi.org/10.3923/aje.2012.115.123 (2012).
Mokuolu, O. A., Okoronko, U., Adedoyin, O. T. & Afolabi, B. B. Effectiveness of a community-based intervention to increase ITN use among children under five in Nigeria. Malar. J. 6, 145. https://doi.org/10.1186/1475-2875-6-145 (2007).
Uzochukwu, B. S. C., Onwujekwe, O. E., Uguru, N. P. & Okwuosa, C. The challenge of bridging the gap between evidence and policy in malaria control in Nigeria. Malaria Res. Treat. 2013, 708197. https://doi.org/10.1155/2013/708197 (2013).
Adepoju, K. A. & Olaniyan, O. Mathematical modelling and optimal control of malaria transmission dynamics. J. Taibah Univ. Sci. 14(1), 1–13. https://doi.org/10.1080/16583655.2019.1700972 (2020).
Musa, A. B. & Luka, Y. Modelling the impact of vector control and vaccination on malaria dynamics in Nigeria. J. Appl. Math. Bioinform. 12(2), 41–60. https://doi.org/10.47260/jamb/1224 (2022).
Yusuf, T. T., Benyah, F. & Okosun, K. O. A fractional-order model for the transmission dynamics of malaria with Caputo derivatives. Math. Model. Nat. Phenomena 17(6), 1–20. https://doi.org/10.1051/mmnp/2022023 (2022).
Ngonghala, C. N., Iboi, E. A. & Gumel, A. B. Mathematical assessment of the role of environmental interventions in malaria transmission dynamics using fractional calculus. Nonlinear Dyn. 106(1), 537–559. https://doi.org/10.1007/s11071-021-06522-1 (2021).
Omame, A., Okuonghae, D. & Okyere, E. A fractional-order malaria model with treatment and relapse: Analysis and optimal control. Chaos Solitons Fract. 169, 113225. https://doi.org/10.1016/j.chaos.2022.113225 (2023).
Kumar, D. & Agrawal, O. P. A fractional mathematical model of malaria with a generalized Mittag-Leffler kernel. Comput. Math. Methods Med. 2020, 3839240. https://doi.org/10.1155/2020/3839240 (2020).
Abdelrazec, A., Elaiw, A. M. & Mahdy, A. M. Fractional-order malaria model with vaccination and vector control using Caputo-Fabrizio derivative. Adv. Differ. Equ. 2024(1), 45. https://doi.org/10.1186/s13662-024-04321-y (2024).
Sweilam, N. H., Al-Mekhlafi, S. M. & Albalawi, A. O. Optimal control for a fractional order malaria transmission dynamics mathematical model. Alex. Eng. J. 59(3), 1677–1692. https://doi.org/10.1016/j.aej.2020.04.020 (2020).
Boulkroune, A., Zouari, F. & Boubellouta, A. Adaptive fuzzy control for practical fixed-time synchronization of fractional-order chaotic systems. Nonlinear Dyn. https://doi.org/10.1177/10775463251320258 (2025).
Zouari, F. Neural network-based adaptive backstepping dynamic surface control of drug dosage regimens in cancer treatment. Neurocomputing 358, 1–11. https://doi.org/10.1016/j.neucom.2019.05.045 (2019).
Gassem, F. et al. Nonlinear fractional evolution control modeling via power non-local kernels: A generalization of Caputo-Fabrizio, Atangana-Baleanu, and Hattaf derivatives. Fract. Fract. 9(2), 104. https://doi.org/10.3390/fractalfract9020104 (2025).
Khan, H. et al. A fractional order COVID-19 epidemic model with Mittag-Leffler kernel. Chaos Solitons Fract. 148, 111030. https://doi.org/10.1016/j.chaos.2021.111030 (2021).
Khan, H., Alzabut, J., Gómez-Aguilar, J. F. & Alkhazan, A. Essential criteria for existence of solution of a modified-ABC fractional order smoking model. Ain Shams Eng. J. 15(5), 102646. https://doi.org/10.1016/j.asej.2024.102646 (2024).
Alzabut, J., Dhineshbabu, R., Selvam, A. G. M., Gómez-Aguilar, J. F. & Khan, H. Existence, uniqueness and synchronization of a fractional tumor growth model in discrete time with numerical results. Results Phys. 54, 107030. https://doi.org/10.1016/j.rinp.2023.107030 (2023).
Khan, A., Shah, K., Abdeljawad, T. & Amacha, I. Fractal fractional model for tuberculosis: Existence and numerical solutions. Sci. Rep. 14, 12211. https://doi.org/10.1038/s41598-024-62386-4(Nature) (2024).
Eiman, Shah, K., Sarwar, M. & Abdeljawad, T. On rotavirus infectious disease model using piecewise modified ABC fractional order derivative. Netw. Heterogeneous Media 19(1), 214–234. https://doi.org/10.3934/nhm.2024010 (2024).
Shah, K., Abdalla, B., Abdeljawad, T. & Alqudah, M. A. A fractal–fractional order model to study multiple sclerosis: A chronic disease. Fractals 32(2), 2440010. https://doi.org/10.1142/S0218348X24400103 (2024).
Abidemi, A. & Owolabi, K. M. Unravelling the dynamics of Lassa fever transmission with nosocomial infections via non-fractional and fractional mathematical models. Eur. Phys. J. Plus 139(2), 108. https://doi.org/10.1140/epjp/s13360-024-04910-z (2024).
Abidemi, A., Owolabi, K. M. & Pindza, E. Modelling the transmission dynamics of Lassa fever with nonlinear incidence rate and vertical transmission. Phys. A 597, 127259. https://doi.org/10.1016/j.physa.2022.127259 (2022).
Shyamsunder, S. et al. A new fractional mathematical model to study the impact of vaccination on COVID-19 outbreaks. Decis. Anal. J. 6, 100156. https://doi.org/10.1016/j.dajour.2022.100156 (2022).
Abidemi, A., Owolabi, K. M. & Pindza, E. Assessing the dynamics of Lassa fever with impact of environmental sanitation: Optimal control and cost-effectiveness analysis. Model. Earth Syst. Environ. 9, 2259–2284. https://doi.org/10.1007/s40808-022-01624-y (2022).
Nisar, K. S., Abboubakar, H. & Regonne, R. K. Fractional dynamics of typhoid fever transmission models with mass vaccination perspectives. Fract. Fract. 5(4), 149. https://doi.org/10.3390/fractalfract5040149 (2021).
Abboubakar, H., Kombou, L. K., Koko, A. D., Fouda, H. P. E. & Kumar, A. Projections and fractional dynamics of the typhoid fever: A case study of Mbandjock in the Centre Region of Cameroon. Chaos Solitons Fract. 150, 111129. https://doi.org/10.1016/j.chaos.2021.111129 (2021).
Nabi, K. N., Abboubakar, H. & Kumar, P. Forecasting of COVID-19 pandemic: From integer derivatives to fractional derivatives. Chaos Solitons Fract. 141, 110283. https://doi.org/10.1016/j.chaos.2020.110283 (2020).
Agbata, B. C. et al. Fractional-order model of malaria incorporating treatment and prevention strategies. Sci. Rep. 15, 29290. https://doi.org/10.1038/s41598-025-14280-w (2025).
Omeje, D., Acheneje, G., Odiba, P. & Bolarinwa, B. Modelling the transmission dynamics of the coinfection of Malaria and Tuberculosis with optimal control strategies cost effectiveness analysis. Res. Sq. Preprint. https://doi.org/10.21203/rs.3rs-5312505/v1 (2024).
Agbata, B. C. et al. Mathematical analysis of the transmission dynamics of malaria and tuberculosis co-infection with control strategies. Eng. Rep. 7(6), e70210. https://doi.org/10.1002/eng2.70210 (2025).
Bolarinwa, B., Onoja, T., Agbata, B. C., Omede, B. I. & Odionyenma, U. B. Dynamical analysis of HIV-TB coinfection in the presence of treatment for TB. Bull. Biomath. 2(1), 21–56. https://doi.org/10.59292/bulletinbiomath.2024002 (2024).
Diekmann, O., Heesterbeek, J. A. P. & Metz, J. A. J. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 28(4), 365–382. https://doi.org/10.1007/BF00178324 (1990).
Méndez, F. A. & Trussell, J. W. Healthcare access and malaria control: Evaluating the effectiveness of interventions in low-income settings. Glob. Health. 16, 103. https://doi.org/10.1186/s12992-020-00621-x (2020).
Okell, L. C., Drakeley, C. J., Ghani, A. C., Bousema, T. & Sutherland, C. J. Reduction of transmission from malaria patients by artemisinin combination therapies: A pooled analysis of six randomized trials. Malar. J. 13, 428. https://doi.org/10.1186/1475-2875-13-428 (2014).
Agbata, C. B., Meco, Z. M., Agbebaku, D. F., Dervishi, R. & Ezike, M. G. C. Fractional-order analysis of malaria and tuberculosis co-dynamics: A Laplace Adomian decomposition approach. Edelweiss Appl. Sci. Technol. 9(4), 1675–1714. https://doi.org/10.55214/25768484.v9i4.6353 (2025).
Akowe, E. et al. A novel malaria mathematical model: Integrating vector and non-vector transmission pathways. BMC Infect. Dis. 25, 322. https://doi.org/10.1186/s12879-025-10653-8 (2025).
Atangana, A. Mathematical model of survival of fractional calculus, critics and their impact: How singular is our world?. Adv. Differ. Equ. 2021(403), 403. https://doi.org/10.1186/s13662-021-03494-7 (2021).
World Health Organization. World malaria report https://www.who.int/publications/i/item/9789240068487 (World Health Organization, 2023).
Agbata, B. C. et al. A mathematical model of COVID-19 transmission dynamics with effects of awareness and vaccination program. J. Ghana Sci. Assoc. 21(2), 59–61 (2023).
Ahman, Q. O., Omale, D., Asogwa, C. C., Nnaji, D. U. & Mbah, G. C. E. Transmission dynamics of Ebola Virus Disease with vaccine, condom use, quarantine, isolation, and treatment drug. Afr. J. Infect. Dis. 15(1), 10–23 (2021).
Vanden Driessche, P. & Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 180(1–2), 29–48 (2002).
Mbah, G. C. E. et al. Mathematical modelling approach of the study of Ebola Virus Disease transmission dynamics in a developing country. Afr. J. Infect. Dis. 17(1), 10–26. https://doi.org/10.21010/Ajidv17i1.2 (2023).
Alzahrani, A. K. & Khan, M. A. The co-dynamics of malaria and Tuberculosis with optimal control strategies. Filomat 36(6), 1789–1818. https://doi.org/10.2298/FIL2206789A (2022).
Odeh, J. O. et al. A mathematical model for the control of chlamydia disease with treatment strategy. J. Math. Acumen Res. 7(1), 1–20 (2024).
Agbata, B. C. et al. Numerical solution of fractional order model of measles disease with double dose vaccination. DUJOPAS 10(3B), 202–217. https://doi.org/10.4314/dujopas.v10i3b.19 (2024).
Acheneje, G. O. et al. Approximate solution of the fractional order mathematical model on the transmission dynamics of the co-infection of COVID-19 and Monkeypox using the Laplace-Adomian decomposition method. Int. J. Math. Stat. Stud. 12(3), 17–51 (2024).
Author information
Authors and Affiliations
Contributions
Agbata, Benedict Celestine, Simulation, Conceptualization, Model formulation, Methodology, Writing Original draft preparation, Analysis, Investigation. Simulations. Mbah, Godwin Christopher Ezike, Literature review, Analysis, Investigation, Model formulation, Methodology, writing introduction, Editing, Simulations, Analysis, Supervision. RaimondaDervishi, Model formulation, Methodology, Conceptualization Methodology, Writing Original draft preparation, Model formulation, Analysis. Mehmet Gümüş, Editing, Literature review, writing introduction. Writing Original draft preparation, Writing Original draft preparation, Analysis, Investigation. Simulations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Agbata, B.C., Dervishi, R., Gümüş, M. et al. Analysis of fractional-order model for the transmission dynamics of malaria via Caputo–Fabrizio and Atangana–Baleanu operators. Sci Rep 15, 36359 (2025). https://doi.org/10.1038/s41598-025-20191-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20191-7