Abstract
To evaluate if egg consumption is associated with sleep quality and psychological health (depression, anxiety, and stress) in women with type 2 diabetes. A cross-sectional study was conducted on women with type 2 diabetes (n = 230). Weight, height, waist circumference (WC), and blood pressure were measured. A Food Frequency Questionnaire (FFQ) was used to obtain dietary intake data and estimate total egg consumption, which was presented in tertiles. The Pittsburgh Sleep Quality Index (PSQI) and Depression, Anxiety, and Stress Scale (DASS-21) were used to evaluate sleep and mental health outcomes, respectively. Dietary intake of carbohydrates, sodium, saturated fatty acids, and cholesterol was greater in the highest tertile of egg consumption (P < 0.05). Dietary intake of fat, monounsaturated, and polyunsaturated fatty acids was lower in the highest tertile of egg consumption (P < 0.05). WC was greater in the highest tertile of egg consumption in the crude model (p = 0.03), however, there was no evidence of this association in the adjusted model. There was no evidence of an association between egg consumption and the odds of poor psychological health or sleep quality in unadjusted or adjusted models. There was no association between egg consumption and poor sleep or mental disorders. Future studies are needed to confirm these findings and to identify the mechanism of action.
Similar content being viewed by others
Introduction
Individuals with diabetes usually experience various complications resulting in a reduced quality of life. The complications may include cardiovascular disease, retinopathy, nephropathy, sleep problems, depression, anxiety, or other psychological disorders1. Many studies have evaluated the macro- or micro-vascular complications of diabetes, but few explore the impact on sleep quality or psychological disorders.
Research has found a correlation between having diabetes and depression, irritation, and anxiety especially among the female gender2,3. A meta-analysis revealed that the mortality rate of individuals with diabetes who experienced depression was 1.5 times higher than those without depression4. Sleep disorders, abnormal sleep timing, sleep apnea, and lower amounts of plasma melatonin have been found in patients with type 2 diabetes compared with healthy controls5,6. Abnormal sleep duration and sleep apnea were independently associated with higher HbA1c in individuals with type 2 diabetes7. Increased insulin resistance, inflammation, and oxidative stress lead to hypoxia, which has been suggested as the mechanism by which individuals with diabetes experience sleep apnea and sleep disturbances8. It is well-established that improving glycemic control can result in more favorable diabetes outcomes and reduce complications. Proper nutrition and a healthy diet are effective interventions for improving glycemic control9,10.
Many people consume eggs during the week, either independently or in combination with other foods. Eggs are a great source of many essential nutrients including, but not limited to, protein, lutein, folate, vitamin A, vitamin B12, and selenium. Evidence evaluating the relationship between egg consumption and cardiovascular factors is rare and controversial11,12. Two meta-analyses on the association between egg and CVD presented controversial results. Shin et al. revealed that egg consumption is not associated with the risk and mortality of CVDs11, while Li et al. presented a dose-response positive association between egg and the risk of CVDs12. Moreover, Shi et al., revealed that egg consumption is positively associated with the risk of diabetes among the Chinese population, especially in women13. A cohort study suggested that higher daily egg consumption is directly associated with the risk of type 2 diabetes in both genders14. Shin et al., showed that egg consumption may be associated with an increasing incidence of type 2 diabetes among the general population and increased CVD comorbidity among patients with diabetes11. The association between egg and the incidence of diabetes may be related to high cholesterol intake that can increase fasting plasma glucose and induce hyperinsulinemia15. Although relevant information on the association between egg and psychological as well as sleep health is scarce and inconsistent, a cross-sectional study reported that eating eggs more than once a week might improve Iraninan depressive symptoms16. Also, egg consumption was related to a lower risk of depressive symptoms in a cohort study among Chinese elderly17. To our knowledge, there have been no studies that evaluate the association between egg consumption and sleep quality or psychological disorders among diabetic patients. The objective of the present study was to evaluate if egg consumption is associated with sleep quality and psychological health (depression, anxiety, and stress) in women with type 2 diabetes.
Methods
We conducted a cross-sectional study on 230 women with type 2 diabetes in Tehran, Iran (α = 0.05, β = 0.2, r = 0.2, which was mentioned in our previous study)18. Women were recruited into the study through referral to diabetes research or health centers. Women were asked to participate in the study if they met the inclusion criteria, which were that they were not following a specific diet and were not diagnosed with any additional chronic diseases (apart from type II diabetes). All participants provided written consent and were aware that participation in the study was voluntary.
Assessment of anthropometric measures
Weight (kg) was collected by weighing the participant using a calibrated digital scale (SECA, 803, Germany). Height (cm) was determined while participants were standing using an unstretched tape measure to the nearest 0.1 mm. Body mass index (BMI) was determined by dividing weight by height (kg/m2). Waist circumference (WC) was measured using an unstretched tape measure with an accuracy of 0.1 cm at the narrowest point of the waist in light clothing. All the anthropometric indices were measured once only.
Assessment of dietary intake
A validated and reliable semi-quantitative food frequency questionnaire (FFQ) consisting of 168 food items was used to obtain dietary intake data from participants19. All participants reported the amount and frequency of consumption of each food item on a daily, weekly, or monthly basis over the past year. A registered dietitian (RD) provided instructions to participants on how to complete the FFQ. All the reported portion sizes of foods were converted to grams per day (g/day). Moreover, individuals who reported total energy intake of < 800 and > 4200 kcal/day were excluded20. Nutrient analysis was performed using the Nutritionist IV software (Version 7.0; N-Squared Computing, Salem, OR, USA), which is adapted for Iranian foods. Chicken egg consumption was evaluated using this FFQ and was reported in tertiles of consumption among participants in the present study. The participants were remembered to consider their egg consumption if they consumed it in mixed dishes. A standard portion size was used to evaluate the amount of egg and other foods intake. Then, the portion sizes were transformed to grams/day by using household measurements.
Assessment of sleep
Pittsburgh Sleep Quality Index (PSQI) is a validated self-reported sleep assessment tool21,22. The tool measures an individual’s sleep patterns and quality over the past month. The PSQI consists of 19 questions that are combined to form seven component scores including: (i) subjective sleep quality, (ii) sleep latency, (iii) sleep duration, (IV) habitual sleep efficiency, (V) sleep disturbances, (VI) use of sleep medication, and (VII) daytime dysfunction. Each score has a range of 0 (no difficulty) to 3 (severe difficulty) points. These seven scores can be combined to create a total score (0–21). A score of > 5 suggests poor sleep quality, and as the score increases the degree of poor sleep quality worsens.
Assessment of stress, anxiety, and depression
Depression, Anxiety, and Stress Scale (DASS-21) is a valid and reliable self-reported questionnaire that contains 21 items that are presented in three separate components to measure symptoms of depression, anxiety, and stress over the previous week23,24. Each component includes 7 questions with a rating score from 0 (does not apply to me) to three (applies to me very much). The recommended cut-offs for evaluating the DASS-21 vary based on the component score (i.e. depression, anxiety, or stress) and are categorized as normal, mild, moderate, severe, and extremely severe. For depression, a total score of ≤ 9 is considered normal and a score of > 9 indicates some degree of depression. For anxiety, a total score of ≤ 7 is considered normal and a score of > 7 indicates some degree of anxiety. For stress, a total score of ≤ 14 is considered normal and a score of > 14 indicates some degree of stress. For each component, as the total score increases, the severity of that domain increases.
The PSQI questionnaire and the DASS-21 were collected at the same time as the FFQ.
Assessment of other variables
Sociodemographic information including the participant’s age, and the participant’s and partners (if applicable) education level, employment, and income was assessed by a questionnaire. Physical activity levels were recorded over a week and were reported as metabolic equivalent hours per week (MET.h/wk) by self-reporting records of participants25. Blood pressure was measured two times with the participant in a sitting position after 5 min of resting using a sphygmomanometer; the average of the two measurements was calculated and reported. Biochemical markers including fasting blood sugar (FBS), 2-hour postprandial blood sugar (2hpp), hemoglobin A1C (Hb A1C), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglyceride (TG) were collected from the participant’s medical profile within 1 month of participation. Moreover, the consumption of various supplements such as pills, powder, or beverages that contain protein, carbohydrates, vitamins, and minerals was recorded in the general questionnaire.
Statistical analysis
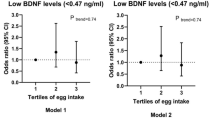
Participant characteristics were compared by analysis of variance (ANOVA) or chi-square tests through the dietary scores for continuous and categorical variables, respectively, and reported as mean ± standard deviation (SD) or sample percentage (%). Dietary intake was adjusted for energy intake using the residual method. Multiple ANCOVA or binary logistic models were used to evaluate the association between egg consumption and sleep quality or psychological status. A binomial outcome was used to evaluate sleep quality by comparing poor sleep quality (total PSQI score > 5) to better sleep quality issues (total PSQI score ≤ 5) reported using odds ratios (ORs). Model 1 was adjusted for age, socio-economic status, physical activity, energy intake, sleep latency, and sleep duration. Model 2 was adjusted for all covariates in model 1 with the addition of years post-menopause, supplement intake, vitamin B12 and D intake, MUFA and PUFA intake, sugar intake, and BMI. P trend was calculated by logistic regression considering categorical variables. The statistical package for social sciences (SPSS) software (version 16; SPSS Inc, Chicago, IL) was used for all statistical analyses, and statistical significance was considered as P < 0.05.
Results
In the final analysis, 230 women were included. The baseline characteristics of participants are presented in Table 1. There was a significant positive association between tertiles of egg consumption and WC (P = 0.029), duration of postmenopause (P = 0.029), physical activity (P = 0.001), and supplement use (P = 0.009).
Dietary intakes across tertiles of egg consumption among participants are presented in Table 2. The mean intake of carbohydrates (341.88 vs. 330.18 g/day), sodium (3473 vs. 3200 mg/day), saturated fatty acids (SFAs; 21.28 vs. 19.26 mg/day), and cholesterol (225.33 vs. 113.53 mg/day) were greater in the highest vs. lowest tertile of egg consumption (P < 0.05). The mean intake of total fat (79.74 vs. 85.39 g/day), polyunsaturated fatty acids (PUFAs; 16.81 vs. 20.44 mg/day), monounsaturated fatty acids (MUFAs; 26.70 vs. 29.59 mg/day), and vitamin A (1143 vs.1371 mcg/day) were lower in the highest vs. lowest tertile of egg consumption (P < 0.05).
Cardiovascular-related factors including anthropometric measures, biochemical tests, and blood pressure among tertiles of egg consumption are presented in Table 3. WC was greater in the highest tertile of egg consumption in the crude model (p = 0.03), however, there was no evidence of this association in the adjusted models.
Table 4 presents the association between tertiles of egg consumption and the odds of poor psychological health or poor sleep quality. There was no evidence of an association between egg consumption and poor psychological health or the sleep quality score in the crude or adjusted models.
Discussion
To the best of our knowledge, this is the first cross-sectional study to investigate if egg consumption is associated with sleep quality, depression, anxiety, and stress among women with type 2 diabetes. In the present study, no significant associations were observed between egg intake and sleep quality or psychological health (depression, anxiety, and stress) in unadjusted or adjusted models.
Sleep
Previous studies have investigated the association between the bioactive and nutritional components found in eggs and sleep disorders. Eggs are rich in vitamins and other nutrients like tryptophan which may be associated with an improved sleep quality26,27. Increased sleep duration and efficiency and decreased sleep latency following the consumption of tryptophan is reported28. Furthermore, there is evidence that tryptophan depletion is associated with a reduction in sleep quality29. A suggested mechanism is that tryptophan competes with other large neutral amino acids (e.g., valine, leucine, isoleucine, tyrosine, and phenylalanine) to cross the blood-brain barrier, where it is converted to serotonin, the precursor to the sleep-promoting hormone, melatonin30. A potential reason for our findings is that previous studies were among patients with psychological problems or in healthy adults; however, our study was in women with diabetes. Tryptophan metabolism is triggered by inflammation and/or degeneration in diabetes causing increased kynurenine metabolites. Kynurenine metabolism is hypothesized to be a key mechanism that links inflammation with sleep disturbance27. Clinical and experimental data suggest that the increased metabolism of tryptophan in diabetes, resulting from up-regulation of the tryptophan – kynurenine pathway, may influence findings and conclusions compared with previous evidence31,32. Additionally, other research (reviews and clinical trials) has explored the positive association of other egg components (e.g., B vitamins, omega 3 fatty acids) on sleep duration, and sleep quality33,34. An important consideration for the differences between previous findings and the results from the present study is that previous evidence has focused on evaluating the intake of a single nutrient or component. The nutrients and components that were evaluated in isolation were instead interacting in eggs which may explain the contrary findings in the present study. Contrary to our results, a longitudinal cohort35 study has investigated the association between egg consumption and sleep quality in in older adults. This study suggested that daily egg consumption was associated with increased good sleep quality compared with those who rarely consumed or did not consume eggs. A potential reason for the contrasting findings is the difference in study designs and the lack of control for several important confounders. Furthermore, mentioned study observed a large number of heterogeneous populations which included healthy people or individuals with other diseases or comorbidities; however, the present study was conducted in women with type 2 diabetes. Additionally, the tools and questionnaires used to evaluate the outcomes vary among studies making it difficult to draw comparisons and conclusions.
Depression, anxiety, and distress
Studies reported that Omega-3 fatty acids (PUFAs) and monounsaturated fatty acids (MUFAs) consumption can improve symptoms of depression, anxiety sensitivity, and intolerance of uncertainty, along with dimensions of emotional regulation. Proposed mechanisms of antidepressant and cognitive health beneficial effects of eggs can be confirmed by the role of PUFAs and MUFAs on reduction of inflammation, effect on 5-HT and dopamine, impact upon protein kinase C (PKC), and effects on serotonergic and dopaminergic neurotransmission. Accordingly, the residual effects of these nutrients (MUFAs and PUFAs) may distort or mask the effects of other variables on psychological state, therefore, we considered them as confounding variables and are adjusted in our study17,36,37.
In the present study, no associations were observed between egg intake and depression. In line with our findings, formerly published studies have reported no association between egg consumption and depression38,39. Evidence also suggests that higher dietary intake of egg components like tryptophan is associated with a lower prevalence of depression40, additionally, B vitamins deficiencies and low consumption of them were associated with greater risk for depression34,41.
With regard to anxiety, previous studies investigated conflicting results about the associations between egg components including tryptophan42, vitamin D43,44, B vitamins41,45, and omega-3 fatty acids46 and anxiety.
We found no significant association between egg intake and distress in women with diabetes. However, several studies have evaluated the influence of high doses of nutrients found in eggs like B vitamins on lower risk for psychological distress41.
The present study considered total egg consumption rather than individual nutrients, which is more representative of practical consumption patterns in the population. These individual nutrient (tryptophan, vitamin D, B vitamins) levels found in egg may not be high enough to influence mental health17.
Other variables
The present study found that dietary intake of carbohydrates, sodium, SFAs, and cholesterol was greater in the highest tertile of egg consumption; and fat, MUFAs, and PUFAs intakes were lower in the highest tertile of egg consumption. Furthermore, WC was greater in the highest tertile of egg consumption in the crude model, but not in the adjusted model. It is possible that the higher intake of carbohydrates, sodium, SFAs, and cholesterol and a lower intake of PUFAs mediated the association between higher egg consumption and greater WC. Nicklas47 also found in a cohort study that consuming eggs in 18,987 adults aged ≥ 19 years was significantly associated with a higher BMI and WC. Moreover, in previous studies, higher egg consumption has been associated with an increased risk of heart disease and related comorbidities among patients with diabetes47,48.
Our results show no relationship between egg consumption and blood lipid parameter (TC, HDL-c, LDL-c, or TG) levels in women with diabetes, our results are consistent with previous publications which indicate that, although eggs are known as one of the main sources of cholesterol, other bioactive components of the eggs such as phospholipids, amino acids (i.e., glycine, methionine, cysteine) can improve the body’s response to the egg cholesterol49,50.
A strength of the current study is that egg consumption from mixed foods was considered, which increased the validity of our results. Our findings should be interpreted with consideration of the limitations. First, the cross-sectional design limited our ability to evaluate a causal relationship between egg consumption and mental health or sleep quality. Our study used the FFQ for dietary intake assessments which may lead to misclassification and recall bias. Although the PSQI and DASS-21 are validated questionnaires, they rely on participants to self-report symptoms rather than a qualified psychiatrist or professional to evaluate symptoms. With other studies in other populations. Moreover, we considered only the consumption of egg, not egg products. Furthermore, egg consumption was limited to chicken eggs in this study, while chicken eggs are differentiated from duck, goose, or other fowl’s egg. According to size and nutritional content differences by type of fowl and various types of egg consumption in different cultures39, we suggest considering this concern for controlling the residual effects in future studies.
In conclusion, we found no evidence of an association between egg consumption and psychological health or sleep quality in women with type 2 diabetes. Future research exploring this association is warranted.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Sajjadi, A., Bakhshani, N., Baghban-Haghighi, M., Samadi, R. & Lashkaripoor, K. Prevalence of psychiatric disorders in patients with diabetes type 2. Zahedan J. Res. Med. Sci. 14 (1), 82–85 (2012).
Al-Ayed, M., Moosa, S. R., Robert, A. A. & Al Dawish, M. Anxiety, depression and their associated risk factors among patients with diabetic foot ulcer: a two center cross-sectional study in Jordan and Saudi Arabia. Diabetes Metabolic Syndrome. 15 (1), 237–242 (2020).
Pashaki, M. S. et al. The prevalence of comorbid depression in patients with diabetes: a meta-analysis of observational studies. Diabetes Metabolic Syndrome. 13 (6), 3113–3119 (2019).
Park, M., Katon, W. J. & Wolf, F. M. Depression and risk of mortality in individuals with diabetes: a meta-analysis and systematic review. Gen. Hosp. Psychiatry. 35 (3), 217–225 (2013).
Mäntele, S. et al. Daily rhythms of plasma melatonin, but not plasma leptin or leptin mRNA, vary between lean, obese and type 2 diabetic men. PloS One. 7 (5), e37123 (2012).
Reutrakul, S. & Van Cauter, E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann. N. Y. Acad. Sci. 1311, 151–173 (2014).
Tan, X. & Benedict, C. Sleep characteristics and HbA1c in patients with type 2 diabetes on glucose-lowering medication. ;8(1). (2020).
Lavie, L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia–revisited–the bad ugly and good: implications to the heart and brain. Sleep Med. Rev. 20, 27–45 (2015).
Gaster, B. & Hirsch, I. B. The effects of Improved Glycemic Control on complications in type 2 diabetes. Arch. Intern. Med. 158 (2), 134–140 (1998).
Janmohammadi, P. et al. Is there any association between dietary patterns, food security status and psychiatric disorders among Iranian earthquake victims? BMJ Mil Health. 167 (3), 153–157 (2021).
Shin, J. Y., Xun, P., Nakamura, Y. & He, K. Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis. Am. J. Clin. Nutr. 98 (1), 146–159 (2013).
Li, Y., Zhou, C., Zhou, X. & Li, L. Egg consumption and risk of cardiovascular diseases and diabetes: a meta-analysis. Atherosclerosis 229 (2), 524–530 (2013).
Shi, Z., Yuan, B., Zhang, C., Zhou, M. & Holmboe-Ottesen, G. Egg consumption and the risk of diabetes in adults. Jiangsu China Nutr. 27 (2), 194–198 (2011).
Djoussé, L., Gaziano, J. M., Buring, J. E. & Lee, I-M. Egg consumption and risk of type 2 diabetes in men and women. Diabetes care. 32 (2), 295–300 (2009).
Basciano, H. et al. Metabolic effects of dietary cholesterol in an animal model of insulin resistance and hepatic steatosis. Am. J. Physiology-Endocrinology Metabolism. 297 (2), E462–E73 (2009).
Sangsefidi, Z. S., Mirzaei, M. & Hosseinzadeh, M. The relation between dietary intakes and psychological disorders in Iranian adults: a population-based study. BMC Psychiatry. 20 (1), 257 (2020).
Li, F. et al. Egg consumption reduces the risk of depressive symptoms in the elderly: findings from a 6-year cohort study. BMC Psychiatry. 23 (1), 44 (2023).
Daneshzad, E. et al. Association of dietary acid load and plant-based diet index with sleep, stress, anxiety and depression in diabetic women. Br. J. Nutr. 123 (8), 901–912 (2020).
Azadbakht, L. & Esmaillzadeh, A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J. Nutr. 139 (2), 335–339 (2009).
Daneshzad, E., Keshavarz, S. A., Qorbani, M., Larijani, B. & Azadbakht, L. Association between a low-carbohydrate diet and sleep status, depression, anxiety, and stress score. J. Sci. Food. Agric. 100 (7), 2946–2952 (2020).
Emami Zeydi, A. et al. Sleep quality and its correlation with serum C-reactive protein level in hemodialysis patients. Saudi journal of kidney diseases and transplantation: an official publication of the Saudi Center for Organ Transplantation. Saudi Arabia. 25 (4), 750–755 (2014).
Farrahi Moghaddam, J., Nakhaee, N., Sheibani, V., Garrusi, B. & Amirkafi, A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep. Breath. = Schlaf Atmung. 16 (1), 79–82 (2012).
Samani, S. Validity and reliability of the short form of depression anxiety stress scales. J. Social Sci. Humanit. Shiraz Univ. 26 (3), 65–77 (2008).
S E. Examine the relationship between religion restraint and death anxiety among students and seminarians of Qom city. Relig. Health ;3(1):55–68. (2010).
Ainsworth, B. E. et al. Compendium of physical activities: an update of activity codes and MET intensities. Med. Sci. Sports. Exerc. 32 (9 Suppl), S498–504 (2000).
Réhault-Godbert, S., Guyot, N. & Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 11 (3), 684 (2019).
Cho, H. J. et al. Sleep disturbance and kynurenine metabolism in depression. J. Psychosom. Res. 99, 1–7 (2017).
Bravo, R. et al. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age (Dordr). 35 (4), 1277–1285 (2013).
Duan, K. M. et al. The role of tryptophan metabolism in postpartum depression. Metab. Brain Dis. 33 (3), 647–660 (2018).
Bhatti, T. et al. Effects of a tryptophan-free amino acid drink challenge on normal human sleep electroencephalogram and mood. Biol. Psychiatry. 43 (1), 52–59 (1998).
Gürcü, S. et al. Neopterin and biopterin levels and tryptophan degradation in patients with diabetes. Sci. Rep. 10 (1), 17025 (2020).
Oxenkrug, G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol. Neurobiol. 48 (2), 294–301 (2013).
Aspy, D. J., Madden, N. A. & Delfabbro, P. Effects of vitamin B6 (pyridoxine) and a B Complex Preparation on dreaming and Sleep. Percept. Mot Skills. 125 (3), 451–462 (2018).
Mikkelsen, K., Stojanovska, L. & Apostolopoulos, V. The effects of vitamin B in Depression. Curr. Med. Chem. 23 (38), 4317–4337 (2016).
Fan, H., Lee, Y. H., Chang, Y. C. & Shelley, M. Associations of dietary habits and sleep in older adults: a 9-year follow-up cohort study. Eur. Geriatr. Med. 12 (1), 123–131 (2021).
Fernandes, M. F., Mutch, D. M. & Leri, F. The relationship between fatty acids and different depression-related brain regions, and their potential role as biomarkers of response to antidepressants. Nutrients ;9(3). (2017).
Freeman, M. P. Omega-3 fatty acids and perinatal depression: a review of the literature and recommendations for future research. Prostaglandins Leukot. Essent. Fat. Acids. 75 (4–5), 291–297 (2006).
Bishop, N. J. & Zuniga, K. E. Egg Consumption, Multi-domain Cognitive Performance, and short-term cognitive change in a Representative Sample of older U.S. adults. J. Am. Coll. Nutr. 38 (6), 537–546 (2019).
Cherian, G., Holsonbake, T. B. & Goeger, M. P. Fatty acid composition and egg components of specialty eggs. Poult. Sci. 81 (1), 30–33 (2002).
Suga, H., Asakura, K., Kobayashi, S., Nojima, M. & Sasaki, S. Association between habitual tryptophan intake and depressive symptoms in young and middle-aged women. J. Affect. Disord. 231, 44–50 (2018).
Poudel-Tandukar, K. & Dietary, B. Vitamins and depression in persons with human immunodeficiency virus infection: the positive living with HIV (POLH) study. J. Nutr. Sci. Vitaminol. 62 (6), 388–396 (2016).
Lindseth, G., Helland, B. & Caspers, J. The effects of dietary tryptophan on affective disorders. Arch. Psychiatr Nurs. 29 (2), 102–107 (2015).
Fallah, M., Askari, G. & Asemi, Z. Is vitamin D Status Associated with Depression, anxiety and sleep quality in pregnancy: a systematic review. Adv. Biomed. Res. 9, 32 (2020).
Bičíková, M. et al. Vitamin D in anxiety and affective disorders. Physiol. Res. 64 (Suppl 2), S101–S103 (2015).
Shor-Posner, G. et al. Impact of vitamin B6 status on psychological distress in a longitudinal study of HIV-1 infection. Int. J. Psychiatry Med. 24 (3), 209–222 (1994).
Polokowski, A. R., Shakil, H., Carmichael, C. L. & Reigada, L. C. Omega-3 fatty acids and anxiety: a systematic review of the possible mechanisms at play. Nutr. Neurosci. 23 (7), 494–504 (2020).
Nicklas, T. A., O’Neil, C. E. & Fulgoni, V. L. Differing statistical approaches affect the relation between egg consumption, adiposity, and cardiovascular risk factors in adults. J. Nutr. 145 (1), 3 (2015).
Qin, C. et al. Associations of egg consumption with cardiovascular disease in a cohort study of 0.5 million Chinese adults. Heart 104 (21), 1756–1763 (2018).
Mesas, A. E. et al. Egg Consumption and blood lipid parameters according to the Presence of Chronic Metabolic disorders: the EVIDENT II study. J. Clin. Endocrinol. Metab. 107 (3), e963–e72 (2022).
Magriplis, E. et al. Frequency and quantity of Egg Intake is not Associated with Dyslipidemia: the Hellenic National Nutrition and Health Survey (HNNHS). Nutrients 11 (5), 1 (2019).
Acknowledgements
We would like to express our gratitude to Dr. Nasli, secretary of the Diabetes Research Center of Tehran University of Medical Sciences in Tehran, Iran, and all staff in this center.
Funding
The present study was supported by the Tehran University of Medical Sciences. (Grant number: 34260). The funder had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
ED and LA conceptualized the study design and designed the study methodology. ED and MQ performed statistical analyses. ED, PJ, and VB prepared the first draft of the manuscript. LA was responsible for editing and approval of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods in the present study were performed following the guidelines and regulations of the Declaration of Helsinki. All subjects declared their willingness to participate in the study and provided written informed consent. Furthermore, the present study was approved by the ethical committee of the Tehran University of Medical Sciences (Ethical Code: 96-01-161-34260).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Daneshzad, E., Janmohammadi, P., Basirat, V. et al. Egg consumption, sleep, and mental health status among women with type II diabetes. Sci Rep 15, 1368 (2025). https://doi.org/10.1038/s41598-025-85347-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85347-x