Abstract
Tissue inhibitor of matrix metalloproteinase 1 (TIMP1) is a multifaceted, cytokine-like bioactive molecule whose levels are elevated in a wide range of inflammatory diseases and are associated with prognosis. Additionally, TIMP1 may play a role in driving systemic inflammation. TIMP1 immunohistochemistry and TIMP1 serum concentrations were analyzed in a cohort of 776 colorectal cancer patients. TIMP1 histoscore by cell type (tumor cell, other) was quantified using digital image analysis. Serum TIMP1 levels were evaluated for correlations with tumor TIMP1 expression, and their associations with tumor characteristics, inflammation, and prognosis were investigated. High serum TIMP1 concentrations associated with shorter overall survival (multivariable HR 1.85, 95% CI 1.30–2.65). Serum TIMP1 levels positively correlated with markers of systemic inflammation and tumor necrosis percentage but not with TIMP1 expression in tumor tissue. High TIMP1 intensity in tumor stroma associated with longer cancer-specific and overall survival in univariable analysis but not in multivariable models. T cell densities in tumor tissue positively correlated with tumor stromal TIMP1 expression and negatively with tumor epithelial TIMP1 expression. Serum TIMP1 levels show promise as a prognostic marker for colorectal cancer and correlate with systemic inflammatory markers, but do not correlate with TIMP1 expression in tumor tissue.
Similar content being viewed by others
Introduction
Tissue inhibitor of metalloproteinases 1 (TIMP1) is a multifactorial molecule with both protease inhibitor and cytokine-like activities. As a member of the TIMP family, it inhibits matrix metalloproteinases (MMPs), enzymes that degrade extracellular matrix components such as collagen and elastin1. Emerging evidence highlights the cytokine functions of TIMP1, mediated through interactions with at least 18 partners, including membrane receptors2. Beyond its protease-inhibitory role, TIMP1 regulates angiogenesis, cell cycle, and differentiation, while also exhibiting anti-apoptotic activities3. Elevated circulating or tissue TIMP1 levels have been observed in numerous inflammatory and cancer diseases, and they have been associated with poor prognosis4,5,6,7,8.
Colorectal cancer (CRC) is the third most common cancer disease globally, with 1.9 million new cases in 20209. Novel serum and tissue biomarkers are needed for early detection and accurate prognostication of the disease, and high serum TIMP1 concentrations have been reported to predict worse prognosis2,6. In addition, high TIMP1 expression on tumor cells has also been associated with worse prognosis, as well as with changes in immune infiltration7,8,10. In cancer tissue, TIMP1 is produced by tumor cells, myofibroblasts and various immune cells. Its multifunctional roles include promoting tumor metastasis and cell proliferation, exhibiting anti-apoptotic effects, and modulating angiogenesis5,11,12. Additionally, TIMP1 can influence immune cell activities, potentially shaping tumor-associated immune reactions13. Larger-scale studies are required to further elucidate the biological roles of TIMP1 and confirm its utility as a prognostic indicator.
Few studies have comprehensively assessed TIMP1 expression in CRC tissue, especially its distribution in tumor epithelium and stroma8. Furthermore, no prior studies have examined the relationship between tumor TIMP1 expression and circulating TIMP1 levels. In this study, we analyzed TIMP1 expression in tumor epithelial and stromal cells, as well as TIMP1 serum levels, in a cohort of 776 CRC patients (606 with preoperative serum TIMP1 data and 757 with tissue TIMP1 data). We explored the correlation between serum and tumor TIMP1 levels and investigated their associations with tumor characteristics, inflammation, and prognosis. TIMP1 expression in tumor epithelial and stromal cells was analyzed with immunohistochemistry (IHC) combined with supervised machine learning based image analysis, facilitating consistent analysis across a large number of cases.
Methods
Study population
The study included all CRC patients who underwent tumor resection in Oulu University Hospital during 2006–2020 and provided informed consent for participation, including sample collection (N = 1011). A flowchart representing the patient selection process is shown in Fig. S1. The study cohort is being prospectively collected and has previously been described for the years 2006–2014 and now extends to 202014,15. Patients who had received preoperative chemotherapy or radiotherapy (N = 235) were excluded from further analyses, after which data of 776 patients could be utilized (of which 757 with tissue TIMP1 data and 606 with serum TIMP1 data). Both serum and tissue TIMP1 data were available for 591 patients. For survival analyses, the patients who died within 30 days of surgery (N = 5 for tissue data, N = 4 for serum data) were additionally excluded to account for probable surgery-related deaths.
Patients had follow-up data until December 31, 2021, with a median follow-up time of 5.6 years (IQR 3.7–9.3) for censored cases. Up to 10 years of follow-up data were used, as most CRC-related deaths occur within this timeframe16. Follow-up data were collected from clinical records and Statistics Finland. The primary endpoints were cancer-specific survival (CSS), defined as the time from surgery to death from the same cancer, and overall survival (OS), defined as the time from surgery to death from any cause. Clinical data, including preoperative American Association of Anesthesiologists (ASA) classification (estimate of overall disease burden and fitness for anesthesia) and comorbidities (diabetes, coronary artery disease, asthma, and COPD), were collected as a part of standard care, and recovered from medical records. All patients provided informed written consent to participate in the study. The REporting recommendations for tumor MARKer prognostic studies (REMARK) were taken into account in the study design17.
Histopathological analysis
Heamatoxylin & eosin (H&E)-stained tumor sections were used for histopathological evaluation. TNM stage was determined using the UICC/AJCC (Union for International Cancer Control/The American Joint Committee on Cancer) criteria and tumor grade using the World Health Organization criteria. Lymphovascular invasion and the percentage of tumor necrosis were visually estimated18,19.
Tissue microarray construction
Tissue microarrays (TMAs) were constructed from formalin-fixed paraffin-embedded (FFPE) tissue samples with either Galileo TMA CK4500 (Integrated Systems Engineering, Milan, Italy) or TMA Master II (3DHistech Ltd., Budapest, Hungary)20. Four 1.0-mm diameter cores were taken from each tumor: two from the center of the tumor (CT) and two from the invasive margin (IM).
Immunohistochemistry and image analysis
TMA blocks were sectioned at 3.5 μm and immunohistochemically stained for TIMP1 using an anti-TIMP1 antibody [EPR18352] (Abcam), diluted 1:4000 and incubated for 30 min at room temperature. Staining was performed on a BOND RX instrument (Leica Biosystems) with Bond Polymer Refine Detection Kit (Leica Biosystems, DS9800). Antigen retrieval was performed for 30 min at 100 °C using BOND Epitope Retrieval Solution 2 (Leica Biosystems, AR9640).
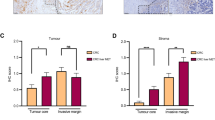
Digital image analysis was conducted using QuPath (version 0.4.3), an open source bioimage analysis software21. The previously validated machine-learning algorithms of QuPath were used to detect individual cells, calculate additional features (Haralick’s features and smoothed features) and train an object classifier to recognize tumor cells, stromal cells (including immune cells), and cells in necrotic regions (excluded from analysis) by annotating representative areas22. The cores with insufficient material were excluded. QuPath was used to measure the chromogen (3,3’-Diaminobenzide, DAB) intensity along with other features for each cell. The process of the automatic classification was supervised. Examples of TMA cores with TIMP1 staining and respective cell classifications can be seen in Fig. 1.
Examples of TIMP1 immunohistochemistry images and image analysis result images. (a–d) Examples of four tissue microarray (TMA) cores with increasing tumor TIMP1 staining intensity. (e–h) Cell detection and classification of the four TMA cores using QuPath. Dark red denotes cancer cells, yellow other cells, and bright red cells in necrotic areas (as e.g. in f), which were ignored from the analyses.
We processed the cell data using RStudio (version 2023.06.02) and R statistical programming (version 4.3.1, R core Team). For each cell, cytoplasmic DAB intensity was classified into four categories; DAB < 0.1 intensity units indicated negative (0), DAB 0.1–0.15 indicated weakly positive (1), DAB 0.15–0.3 indicated moderately positive (2) and DAB > 0.3 indicated strongly positive (3). The intensity values were used to calculate TIMP1 histoscores for each core, separately for cancer and stromal cells, using the formula: TIMP1 histoscore = 100 × (3 × percentage of strong positive cells + 2 × percentage of moderate positive cells + 1 × percentage of weak positive cells)23. For most downstream analyses, a single histoscore was used for each tumor, calculated as the average of histoscores from all individual cores. TIMP1 histoscores specific to the CT and IM were calculated as the averages of the respective cores and could be defined for 754 and 745 patients, respectively.
To assess the accuracy of the image analysis algorithm, we manually annotated cells on 30 TMA cores from consecutive tumors alongside performing automatic classification. Among cells manually annotated as tumor cells, the machine-learning algorithms correctly classified 86% correctly as tumor. Similarly, 88% of stromal cells were accurately recognized by the algorithm. To further evaluate the reliability of the results, histoscores were calculated for these 30 validation TMA cores using three methods: (1) automated classification by QuPath, (2) manual annotation-based evaluation using QuPath, and (3) fully manual visual assessment. The histoscores generated by the automated classifier showed strong correlations with those derived by manual annotations of tumor regions (r = 0.99 for epithelial cell histoscore and r = 0.93 for stromal cell histoscore). Additionally, the automated histoscores demonstrated a high correlation with visually evaluated histoscores for epithelial cells (r = 0.79) and a moderate correlation for stromal cells (r = 0.54).
TMA cores were stained for CD3 and CD8, and T cell densities were calculated, as described before20. BRAF and mismatch repair (MMR) status of the tumors were defined from the TMA cores by IHC20.
Blood and serum analysis
Preoperative venous blood samples were collected and centrifuged for serum extraction. Samples were stored at − 70 °C. Blood neutrophil and lymphocyte counts, and C-reactive protein (CRP) and albumin concentrations were measured in the laboratory of Oulu University Hospital. The analysis was successful for 730, 730, 722, and 766 cases, respectively12. The modified Glasgow prognostic Score (mGPS) was determined based on the serum CRP and albumin levels; mGPS 0 indicated no changes in CRP (≤ 10 mg/L), mGPS 1 indicated an increase of CRP (> 10 mg/L) without changes in albumin levels (≥ 35 g/L), and mGPS 2 indicated both an increase in CRP (> 10 mg/L) and decrease in albumin (< 35 g/L) levels. TIMP1 was analyzed using TIMP1 enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) from 606 patients between 2010 and 202024. Serum IL6 analyses were performed by Olink Target 96 Immuno-Oncology panel18, and the analysis was successful for 603 cases between 2010 and 2020.
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics for Windows (IBM Corp. version 29.0). Findings with two-sided p < 0.05 were considered statistically significant. The statistical significance of the associations between categorical and continuous variables were analyzed using Mann-Whitney U test for variables with two categories, and Kruskal-Wallis test for variables with three or more categories. Spearman correlation coefficients were used to assess the correlation between the manually derived estimates of TIMP1 expression and the results of the supervised machine-learning algorithm for the 30 validation TMA cores. Pearson correlation coefficients were used to assess the correlation between serum TIMP1 values, TIMP1 histoscores (cancer cells and stromal cells), T cell densities, tumor necrosis percentage, blood cell counts, and serum inflammatory markers. The normality of the data was assessed visually using histograms, and variables with positive skewness were normalized through logarithmic transformation. Linear regression models were used to adjust the correlation coefficients for the following covariates: age (< 65, ≥ 65), sex (male, female), tumor localization (colon, rectum), T class (T1-T2, T3-T4), N class (N0, N1-N2), M class (M0, M1), BRAF status (wild-type, mutant), MMR status (proficient, deficient), and tumor grade (low, high). The assumptions of linear regression model (linearity, normality, and homoscedasticity) were examined using a normal probability plot of residuals and a scatterplot of residuals versus predicted values.
Receiver operating characteristics (ROC) analysis was performed for TIMP1 serum values and TIMP1 histoscores. The area under the curve (AUC) from the ROC analysis was used to assess the capacity of the variables to predict 10-year CSS and OS. An optimal cut-off value was determined based on the longest distance from the diagonal reference line23. Kaplan-Meier curves were drawn to visualize survival in groups defined by the chosen cut-off values, and statistical significance was tested by using the log rank test. We used Cox proportional hazard regression models to estimate the hazard ratios (HRs) for TIMP1 serum values and TIMP1 histoscores in relation to CSS and OS. Multivariable Cox proportional hazards regression models were adjusted for sex, age (< 65, 65–75, > 75), year of operation (2006–2010, 2011–2015, 2016–2020), tumor location (proximal colon, distal colon, rectum), disease stage (I–II, III, IV), tumor grade (low-grade, high-grade), lymphovascular invasion (negative, positive), MMR status (proficient, deficient), and BRAF status (wild-type, mutant). The proportionality of hazards was examined by plotting the Shoenfeld residuals, which supported the proportionality during most of the follow-up period up to 10 years.
Results
Serum and tissue TIMP1 in relation to clinicopathological features
Serum TIMP1 concentrations were successfully measured for 606 patients, and TIMP1 immunohistochemistry was successful for 757 patients (Table 1). In immunohistochemical analysis, anti-TIMP1 antibody labeled tumor epithelial cells with varying intensities, necrosis strongly, and stroma (including immune cells) more diffusely with generally weaker intensity (Fig. 1). We separately measured TIMP1 expression in tumor epithelial cells and stromal cells using the histoscore method, with tumor epithelial and stromal histoscores showing positive correlation (r = 0.76, p < 0.001).
The association of TIMP1 serum levels and tissue expression with key clinicopathological features was assessed (Table 1). The median age of patients was 71 years (range: 30–92). High TIMP1 serum levels associated with MMR deficient status, high ASA classification, older age, elevated mGPS (all p < 0.001), proximal tumor location (p = 0.032), and BRAF mutation (p = 0.006). High TIMP1 histoscore in both tumor epithelial and stromal cells strongly associated with proximal tumor location, MMR deficient status, and BRAF mutation (all p < 0.001). Additionally, high TIMP1 histoscore in tumor cells associated with high mGPS (p < 0.001), while high TIMP1 histoscore in stromal cells associated with high mGPS (p = 0.009) and lower stage (p = 0.047). No associations were observed between TIMP1 levels (serum or tissue) and the analyzed comorbidities (Table S1).
TIMP1 immunohistochemistry in relation to inflammatory tumor microenvironment
To explore the potential role of TIMP1 in tumor-host interactions, we analyzed the correlations of TIMP1 expression in tumor tissue with T cell densities, tumor necrosis percentage, systemic inflammation markers, and blood cell counts (Table 2). TIMP1 histoscore in tumor and stromal cells showed distinct correlations with T cell densities in a consistent pattern across the whole tumor, CT, and IM levels (Table S2). TIMP1 histoscore in tumor cells negatively correlated with T cells located in the CT. For example, for stromal CD3+ cell density, the multivariable beta coefficient was − 0.16 (p < 0.001). Conversely, TIMP1 histoscore in stromal cells did not significantly correlate with T cell densities in the CT, but positively correlated with T cell densities in the IM and overall tumor regions. For example, overall CD8+ cell density had a multivariable beta 0.13 (p < 0.001).
Tumor necrosis percentage negatively correlated with TIMP1 histoscore in tumor cells (beta=-0.14, p < 0.001). Among systemic inflammatory markers and blood cell counts, serum albumin levels inversely correlated with TIMP1 histoscores in both tumor and stromal cells (p < 0.001 for both). TIMP1 histoscore in neither tumor nor stromal cells showed statistically significant correlation with serum TIMP1 levels.
TIMP1 serum values in relation to systemic inflammation
We also explored the correlations between serum TIMP1 concentrations and systemic inflammatory markers (Table 2). Serum TIMP1 levels positively correlated with blood neutrophil count (beta = 0.22, p < 0.001), but not with lymphocyte count. Positive correlations were also found between serum TIMP1 levels and markers of systemic inflammation, including neutrophil-to-lymphocyte ratio (NLR), CRP and IL6 (all p < 0.001). The strongest correlations were observed with CRP and IL6 (beta = 0.41 for both). Serum TIMP1 levels also showed a weak positive correlation with tumor necrosis percentage (beta = 0.091, p = 0.034).
Survival analyses
Finally, we analyzed the prognostic potential of tissue TIMP1 expression and serum TIMP1 levels. The results from ROC and Kaplan-Meier analyses are shown in Fig. 2. The AUC values of ROC curves varied between 0.535 and 0.591, indicating relatively weak discrimination among patients. Additional metrics for tissue and serum TIMP1 as prognostic biomarkers (such as true and false positive and negative rate and accuracy) are provided in Table S3.
Receiver operating characteristics (ROC) and Kaplan-Meier survival analyses. (a,b) ROC curves for TIMP1 histoscore in tumor and stromal cells for (a) cancer-specific survival (CSS) and (b) overall survival (OS). (c,d) ROC curves for TIMP1 serum concentrations for (c) CSS and (d) OS. (e,h) Associations of TIMP1 tumor cell histoscore with (e) CSS and (h) OS. (f, i) Associations of TIMP1 other cell histoscore with (f) CSS and (i) OS. (g,j) Associations of TIMP1 serum concentrations with (g) CSS and (j) OS.
Among patients with TIMP1 IHC data, there were 238 deaths (31.7%), including 133 cancer deaths, during the 10-year follow-up. ROC analyses showed that TIMP1 histoscore in stromal cells had higher AUC values than TIMP1 histoscore in tumor cells. Based on the ROC curves, cut-off values of 54.2 and 76.7 were chosen for stromal and tumor histoscores, respectively. Kaplan-Meier survival functions showed higher probabilities of CSS and OS for patients with higher TIMP1 histoscore in stromal cells (log rank p < 0.001 for both). In contrast, no differences in survival were observed between groups stratified by TIMP1 histoscore in tumor cells. Cox regression analyses (Table 3) showed a similar association between high TIMP1 histoscore in stromal cells and improved survival in univariable analysis (CSS: HR 0.53, 95% CI 0.37–0.75; OS: HR 0.69, 95% CI 0.53–0.90). However, these associations were not significant after adjustment for clinicopathological features in multivariable models (CSS: HR 0.91, 95% CI 0.62–1.35; OS: HR 0.99, 95% CI 0.74–1.34).
Among patients with TIMP1 serum data, there were 164 deaths (27.2%), including 90 cancer deaths, during the 10-year follow-up. ROC analysis suggested that TIMP1 serum levels had greater prognostic value for OS than CSS. A cut-off value of 339.7 µg/ml was chosen for survival analyses. Kaplan-Meier survival functions showed lower survival probabilities for patients with higher serum TIMP1 values (CSS log rank p = 0.003, OS log rank p < 0.001). Cox regression analyses (Table 3) showed an association between higher TIMP1 serum values and worse survival in univariable analysis (CSS: HR 2.05, 95% CI 1.27–3.30; OS: HR 2.66, 95% CI 1.90–3.73). In multivariable analysis adjusted for other established prognostic parameters such as disease stage, MMR status, and lymphovascular invasion, the association between high serum TIMP1 levels and worse OS remained statistically significant (HR 1.85, 95% CI 1.30–2.65).
Complete multivariable Cox regression models of CSS and OS according to tissue and serum TIMP1 alongside other covariates can be seen in Tables S4-S6.
Discussion
In this study, we examined the significance of both TIMP1 serum levels and TIMP1 tissue expression patterns in CRC. We discovered that TIMP1 expression in cancer tissue was correlated with T cell infiltration. Stromal TIMP1 correlated positively with overall T cell densities, while TIMP1 expression in cancer cells showed inverse correlation with T cell densities in the tumor center—suggesting divergent associations of TIMP1 with T cell densities depending on the location of TIMP1 expression and T cells in the tumor microenvironment. TIMP1 serum levels were correlated with the markers of systemic inflammation, and high TIMP1 serum values independently associated with shorter OS. These findings highlight the multifaceted role of TIMP1 in colorectal cancer that may depend on the site of action (tumor epithelium, tumor stroma, circulation).
We hypothesized that higher TIMP1 expression in stromal cells might be associated with a better disease outcome due to the protease inhibitory functions of TIMP11. Supporting this, we discovered that higher TIMP1 expression in the stroma associated with a decrease in lymphovascular invasion and better survival in univariable models. In the tumor microenvironment, immune cells contribute to TIMP1 secretion3. Therefore, the positive correlation of stromal TIMP1 expression with T cell densities in our study could be an indication of enhanced immune cell infiltration.
In contrast, TIMP1 expression in tumor cells showed a negative correlation T cell densities the tumor center, supporting the hypothesis that TIMP1 in carcinoma cells may act as a pro-tumor cytokine. Previous studies have highlighted the anti-apoptotic and growth-factor like features of TIMP1 and suggested that its expression in tumor cells may enable cancer to evade immune responses, and create an immunosuppressive microenvironment4. Our observation of an inverse correlation between tumor cell TIMP1 expression tumor necrosis percentage aligns with this view, as TIMP1 may protect hypoxic cancer cells from dying. However, increased TIMP1 expression in tumor cells was not associated with increased disease stage or lymphovascular invasion, suggesting that its influence may be more localized to microenvironmental interactions rather than overt tumor progression.
Systemic inflammation in cancer involves a complex, multifaceted processes that remain incompletely understood. TIMP1 can trigger monocyte and neutrophil activation and cytokine production12,25, and hence TIMP1 is a potential tumor-derived factor driving systemic inflammation in CRC. In this study, serum TIMP1 levels were elevated in systemic inflammation, as indicated by their correlations with markers such as NLR, CRP, and IL6. Necrotic regions within tumors showed intensive TIMP1 staining, and serum TIMP1 levels positively correlated with tumor necrosis percentage. Although this correlation was relatively weak, TIMP1 released from necrotic tumor regions may contribute to the activation of circulating immune cells during systemic inflammation. Instead, tumor stromal or epithelial TIMP1 expression were not associated with serum TIMP1 levels.
Oncogenic BRAF mutations, high-level MSI, and proximal location are features frequently associated with colorectal cancer originating from sessile serrated lesions26. Colorectal cancers developing via serrated pathway have variable prognosis. Microsatellite stable, BRAF -mutated cancers have poor prognosis in general, whereas MSI-H reverts this26,27,28. In this study, BRAF status and proximal tumor location associated with both high TIMP1 serum levels and TIMP1 expression in tumor tissue. An association between elevated TIMP1 concentrations and BRAF mutations has been found in thyroid cancer, but not in CRC29,30. Larger studies are required to assess whether the prognostic role of TIMP1 might differ according to BRAF mutation status.
As our main analysis, we evaluated the prognostic value of TIMP1 serum levels and tissue expression. Higher TIMP1 serum levels associated with increased mortality in our cohort, which is the largest—to our knowledge—evaluating the prognostic value of TIMP1 levels in CRC. These results align with previous studies29. Notably, while high serum TIMP1 levels were associated with older age, they independently predicted shorter OS in the multivariable model adjusted for age. Beyond age, high serum TIMP1 levels were strongly associated with higher ASA classification, reflecting age-related conditions that contribute to general health. High TIMP1 levels have been associated with many conditions such as heart diseases31 and inflammatory diseases3 that may contribute to mortality in CRC patients. However, we did not observe any association between serum TIMP1 levels and coronary artery disease, diabetes, asthma, or COPD among CRC patients. Nevertheless, the results indicate that TIMP1 could represent a useful serum marker to predict overall mortality of CRC patients.
In contrast to some previous studies that reported associations between elevated TIMP1 levels in tumor tissue and poor prognosis in CRC8,13, our results showed no association between tumor cell TIMP1 expression and survival. However, higher stromal TIMP1 histoscore was linked to longer survival in univariable survival analysis, though this association did not remain significant in multivariable Cox regression models, suggesting that it was outperformed by other known prognostic factors. The observed positive survival effect of stromal TIMP1 expression differs from prior studies8,13, which have not distinguished between TIMP1 expression in epithelial and stromal cells8. Our results suggest that stromal TIMP1 expression, specifically, may be associated with better outcome. We hypothesize that this could be attributed to the positive correlation of stromal TIMP1 with immune cell densities, or its role as MMP inhibitor.
Our study had limitations. First, TIMP1 concentrations and immune cell densities were defined using TMAs, which only represent small tumor areas. However, prior research has shown the utility of TMAs in assessing immune infiltrates20. Second, data on postoperative cancer treatments were not available. Therefore, the predictive value of TIMP1 serum levels and tissue expression requires further study. Third, our study could have benefited from a validation cohort, and larger studies are required to assess the significance of TIMP1 in more specific patient subgroups such as those with BRAF-mutated tumors. Fourth, the manual assessment of histoscores for testing the reliability of the automated image analysis algorithms was challenging, particularly for stromal regions with lower cell densities. The strengths of the study include a large study population that contains extensive clinicopathological, histological, and serum biomarker data. Our use of supervised machine-learning algorithms to estimate TIMP1 staining intensities enabled accurate and consistent analysis of a large cohort. Validation analyses showed that these algorithms reliably distinguished tumor epithelial and stromal regions and calculated histoscores.
In conclusion, our study examined the prognostic role of TIMP1 in CRC, as well as its associations with host responses. We found that high serum TIMP1 values independently predict worse overall survival of colorectal cancer patients, thus reinforcing the utility of serum TIMP1 as a prognostic marker. TIMP1 expression in tumor epithelial and stromal cells show divergent associations with T cell infiltrates in tumors and patient outcome, suggesting that TIMP1 may harbor compartment-dependent functions within the tumor microenvironment.
Data availability
Data generated and/or analyzed during this study are not publicly available. The sharing of data will require approval from relevant ethics committees and/or biobanks. Further information including the procedures to obtain and access data of Finnish Biobanks are described at https://finbb.fi/en/fingenious-service.
References
Cui, N., Hu, M. & Khalil, R. A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 147, 1–73 (2017).
Eckfeld, C., Häußler, D., Schoeps, B., Hermann, C. D. & Krüger, A. Functional disparities within the TIMP family in cancer: hints from molecular divergence. Cancer Metast. Rev. 38, 469–481 (2019).
Schoeps, B., Frädrich, J. & Krüger, A. Cut loose TIMP-1: an emerging cytokine in inflammation. Trends Cell Biol. 33, 413–426 (2023).
Qiu, X. et al. Unraveling TIMP1: a multifaceted biomarker in colorectal cancer. Front. Genet. 14, 1265137 (2023).
Łukaszewicz-zając, M. & Mroczko, B. Circulating biomarkers of colorectal cancer (CRC)—Their utility in diagnosis and prognosis. J. Clin. Med. 10, 2391 (2021).
Prokopchuk, O. et al. A novel tissue inhibitor of metalloproteinases-1/liver/cachexia score predicts prognosis of gastrointestinal cancer patients. J. Cachexia Sarcopenia Muscle 12, 378–392 (2021).
Ershov, P., Poyarkov, S., Konstantinova, Y., Veselovsky, E. & Makarova, A. Transcriptomic signatures in colorectal cancer progression. Curr. Mol. Med. 23, 239–249 (2023).
Qin, L. et al. Tissue inhibitor of metalloproteinase-1 (TIMP-1) as a prognostic biomarker in gastrointestinal cancer: A meta-analysis. PeerJ 9, e10859 (2021).
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Jensen, S. A., Vainer, B., Bartels, A., Brünner, N. & Sørensen, J. B. Expression of matrix metalloproteinase 9 (MMP-9) and tissue inhibitor of metalloproteinases 1 (TIMP-1) by colorectal cancer cells and adjacent stroma cells—associations with histopathology and patients outcome. Eur. J. Cancer 46, 3233–3242 (2010).
Jackson, H. W., Defamie, V., Waterhouse, V. & Khokha, R. TIMPs: versatile extracellular regulators in cancer. Nat. Rev. Cancer 17, 38–53 (2016).
Schoeps, B. et al. TIMP1 triggers neutrophil extracellular trap formation in pancreatic cancer. Cancer Res. 81, 3568–3579 (2021).
Nakai, N. et al. Cancer cell–induced tissue inhibitor of metalloproteinase–1 secretion by cancer–associated fibroblasts promotes cancer cell migration. Oncol. Rep. 47, 112 (2022).
Väyrynen, J. P. et al. Platelet count, aspirin use, and characteristics of host inflammatory responses in colorectal cancer. J. Transl. Med. 17, 199 (2019).
Väyrynen, J. P. et al. Preoperative anemia in colorectal cancer: relationships with tumor characteristics, systemic inflammation, and survival. Sci. Rep. 8, 1126 (2018).
Biller, L. H. & Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA 325, 669–685 (2021).
McShane, L. M. et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J. Natl. Cancer Inst. 97, 1180–1184 (2005).
Kastinen, M. et al. Immunological and prognostic significance of tumour necrosis in colorectal cancer. Br. J. Cancer 128, 2218–2226 (2023).
Väyrynen, S. A. et al. Clinical impact and network of determinants of tumour necrosis in colorectal cancer. Br. J. Cancer 114, 1334–1342 (2016).
Elomaa, H. et al. Prognostic significance of spatial and density analysis of T lymphocytes in colorectal cancer. Br. J. Cancer 127, 514–523 (2022).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Väyrynen, J. P. et al. Prognostic significance of immune cell populations identified by machine learning in colorectal cancer using routine hematoxylin and eosin–stained sections. Clin. Cancer Res. 26, 4326–4338 (2020).
Zlobec, I., Steele, R., Terracciano, L., Jass, J. R. & Lugli, A. Selecting immunohistochemical cut-off scores for novel biomarkers of progression and survival in colorectal cancer. J. Clin. Pathol. 60, 1112–1116 (2007).
Väyrynen, J. P. et al. Serum MMP-8 levels increase in colorectal cancer and correlate with disease course and inflammatory properties of primary tumors. Int. J. Cancer 131, E463–474 (2012).
Eckfeld, C. et al. TIMP-1 is a novel ligand of amyloid precursor protein and triggers a proinflammatory phenotype in human monocytes. J. Cell. Biol. 222, e202206095 (2023).
Mezzapesa, M. et al. Serrated colorectal lesions: an up-to-date review from histological pattern to molecular pathogenesis. Int. J. Mol. Sci. 23, 4461 (2022).
Nakanishi, Y., Diaz-Meco, M. T. & Moscat, J. Serrated colorectal cancer: the road less travelled? Trends Cancer 5, 742 (2019).
Phipps, A. I. et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology 148, 77–87 (2015).
Macedo, F. C. et al. A prospective cohort study of TIMP1 as prognostic biomarker in gastric and colon cancer. Chin. Clin. Oncol. 11, 43 (2022).
Smallridge, R. C. et al. RNA sequencing identifies multiple fusion transcripts, differentially expressed genes, and reduced expression of immune function genes in BRAF (V600E) mutant vs BRAF wild-type papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 99, E338–347 (2014).
Barton, P. J. R. et al. Increased expression of extracellular matrix regulators TIMP1 and MMP1 in deteriorating heart failure. J. Hear. Lung Transplant. 22, 738–744 (2003).
Acknowledgements
The study benefited from samples/data from Northern Finland Biobank Borealis (Oulu, Finland).
Funding
This study was funded by Cancer Foundation Finland (59-5619 to JPV), Finnish Medical Foundation (6021 to JPV, 8018 to AK), Sigrid Jusélius Foundation (230229 to JPV), Orion Research Foundation sr (to AK), and the State Research Funding (to MJM and JPV). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: A. Kehusmaa, A. Tuomisto. Data curation: A. Kehusmaa, A. Tuomisto, P. Sirniö, H. Karjalainen, M. Kastinen, V. V. Tapiainen, V. K. Äijälä, J. P. Väyrynen. Formal Analysis: A. Kehusmaa, A. Tuomisto. Funding acquisition: M. J. Mäkinen, J. P. Väyrynen. Investigation: A. Kehusmaa, A. Tuomisto, P. Sirniö, H. Karjalainen, M. Kastinen, V. V. Tapiainen, V. K. Äijälä, T. Tervahartiala, T. Sorsa, J. Rintala, S. Meriläinen, J. Saarnio, T. Rautio, M. J. Mäkinen, J. P. Väyrynen. Methodology: A. Kehusmaa, J. P. Väyrynen. Supervision: A. Tuomisto, J. P. Väyrynen. Visualization: A. Kehusmaa. Writing—original draft: A. Kehusmaa. Writing—review & editing: A. Kehusmaa, A. Tuomisto, P. Sirniö, H. Karjalainen, M. Kastinen, V. V. Tapiainen, V. K. Äijälä, T. Tervahartiala, T. Sorsa, J. Rintala, S. Meriläinen, J. Saarnio, T. Rautio, M. J. Mäkinen, J. P. Väyrynen
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was conducted under approval from the Regional medical research ethics committee of the Wellbeing services county of North Ostrobothnia (25/2002, 42/2005, 122/2009, 37/2020), Biobank Borealis (BB-2017_1012) and Fimea (FIMEA/2022/001941). The study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kehusmaa, A., Tuomisto, A., Sirniö, P. et al. Associations of serum and tissue TIMP1 with host response and survival in colorectal cancer. Sci Rep 15, 1440 (2025). https://doi.org/10.1038/s41598-025-85549-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85549-3
Keywords
This article is cited by
-
Urothelium marker UPK2 identifies aggressive colorectal cancers with distinct molecular and histological features
British Journal of Cancer (2025)