Abstract
The central nervous system (CNS) requires specialized blood vessels to support neural function within specific microenvironments. During neurovascular development, endothelial Wnt/β-catenin signaling is required for BBB development within the brain parenchyma, whereas fenestrated blood vessels that lack BBB properties do not require Wnt/β-catenin signaling. Here, we used zebrafish to further characterize this phenotypic heterogeneity of the CNS vasculature. Using transgenic reporters of Wnt/β-catenin transcriptional activity, we found an inverse correlation between activated Wnt/β-catenin signaling in endothelial cells (ECs) versus non-ECs within these distinct microenvironments. Our results indicated that the level of Wnt/β-catenin signaling in non-ECs may regulate Wnt/β-catenin activity in adjacent ECs. To further test this concept, we generated a transgenic Tet-On inducible system to drive constitutively active β-catenin expression in neural progenitor cells (NPCs). We found that dose-dependent activation of Wnt/β-catenin in NPCs caused severe deficiency in CNS angiogenesis and BBB development. Additionally, we discovered a significant increase in the proliferation of microglia and infiltration of peripheral neutrophils indicative of a stereotypical neuroinflammatory response. In conclusion, our results demonstrate the importance of proper Wnt/β-catenin signaling within specific CNS microenvironments and highlights the potentially deleterious consequences of aberrant Wnt activation.
Similar content being viewed by others
Introduction
During embryonic development, angiogenesis is tightly regulated to ensure proper oxygen and nutrient supply to growing tissues1,2. To stimulate the angiogenic process, the tissue microenvironment, composed of various cell types with unique characteristics, secretes specific molecules such as vascular endothelial growth factor (VEGF), which controls endothelial cell (EC) sprouting, migration, proliferation, and tube formation3,4,5. In order to regulate tissue-specific functions, these newly formed blood vessels require extracellular cues that either promote or inhibit essential properties of the vasculature6,7. This phenotypic heterogeneity is particularly pronounced in the central nervous system (CNS) due to the presence of multiple unique microenvironments. For example, ECs that form blood vessels within the brain parenchyma possess blood–brain barrier (BBB) properties that include low rates of transcytosis, the expression of specific transporters and receptors, and the formation of tight junctions8,9,10. These BBB properties ensure precise control over the brain microenvironment by maintaining homeostasis and protecting the brain from potentially harmful substances. In contrast, blood vessels located within circumventricular organs (CVOs) and choroid plexuses form fenestrations and lack BBB properties11,12. These fenestrated vessels monitor blood composition, control physiological processes, and regulate immune surveillance by facilitating the exchange of information between the bloodstream and the brain. Despite our understanding of blood vessel heterogeneity, relatively little is known about the precise mechanisms within local CNS microenvironments that regulate vascular phenotypes during neurovascular development and disease.
To promote functional diversity of the CNS vasculature, ECs require the activation of signal transduction pathways initiated by the expression and release of specific signaling molecules. For example, neural progenitor cells (NPCs) secrete Wnt7a/b ligands that bind to an endothelial Wnt receptor complex comprised of Frizzled-4, Lrp5/6, Gpr124, and Reck13,14,15,16,17,18,19. The subsequent activation of endothelial Wnt/β-catenin signaling promotes Wnt target gene expression, VEGF-dependent tip cell migration, and the acquisition of BBB properties20,21,22,23. In contrast, endothelial Wnt/β-catenin signaling does not appear to play a role in the formation of fenestrated vessels associated with CVOs, the choroid plexuses, or the peripheral vasculature. Instead, recent studies have shown that fenestrated endothelium within the CNS requires a unique combination of Vegf ligands24,25. Furthermore, these fenestrated vessels are associated with active Wnt/β-catenin signaling within the adjacent non-ECs26,27. Thus, CNS vascular phenotypes appear to be correlated with the status of Wnt/β-catenin signaling within local microenvironments.
Wnt/β-catenin signaling also plays a prominent role in neurological conditions that influence vascular phenotypes28,29,30. For example, Wnt-medulloblastoma, driven by activating mutations in β-catenin31, form highly abnormal vessels that possess fenestrations and lack BBB properties32. As a consequence, this subtype of medulloblastoma responds remarkably well to chemotherapy presumably due to increased systemic exposure. With this in mind, therapeutic strategies designed to target the Wnt/β-catenin pathway are being investigated in the treatment of non-Wnt subtypes of medulloblastoma and other brain tumors with less favorable prognoses33,34. BBB dysfunction and breakdown are also hallmarks of several neurological disorders, including stroke, epilepsy, multiple sclerosis, and neurodegenerative diseases35,36,37. This disruption of BBB function is often associated with neuroinflammation, which may further contribute to the underlying pathology38,39,40 Thus, new therapies specifically developed to activate endothelial Wnt/β-catenin may help to repair and restore BBB function41,42. While these Wnt-specific therapies offer promise for treating a variety of neurological disorders, the potential for off-target effects by either activating or inhibiting Wnt/β-catenin signaling in the CNS must be carefully considered to avoid potentially harmful outcomes.
In this study, we examine transcriptional activation of Wnt/β-catenin within CNS microenvironments during neurovascular development. Here, we utilized zebrafish to visualize the in vivo distribution of Wnt/β-catenin signaling in ECs versus non-ECs using a variety of transgenic reporter lines. We discovered an inverse correlation between activated Wnt/β-catenin in brain ECs versus non-ECs associated with local vascular phenotypes. To examine the effects of aberrant activation of Wnt/β-catenin signaling, we generated an inducible transgenic system to control the cell-type specific expression of constitutively active β-catenin. Using this model, we showed that dose-dependent activation of β-catenin in NPCs causes a severe deficiency in BBB development and stimulates a stereotypical neuroinflammatory response throughout the CNS. Our results demonstrate the unique requirements for tightly regulated and localized Wnt/β-catenin signaling in establishing CNS vascular heterogeneity and highlight the potentially detrimental effects of aberrant Wnt/β-catenin signaling.
Results
Active Wnt/β-catenin signaling within the CNS microenvironment correlates with local vascular phenotypes
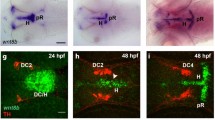
To establish the relationship between active Wnt/β-catenin in ECs versus non-ECs during neurovascular development, we examined brain regions with active Wnt/β-catenin signaling using a T-cell factor (TCF) transcriptional reporter. Here, we utilized the zebrafish lines Tg(7xTCF-Xla.Siam:EGFP)ia4, herein TCF:EGFP, and nuclear localized Tg(7xTCF-Xla.Siam:NLS-mCherry)ia5, herein TCF:NLS-mCherry, that reliably reflect endogenous regions of Wnt/β-catenin activity43. To examine the distribution of TCF-positive cells throughout the brain, we first bred TCF:EGFP to the endothelial reporter line Tg(kdrl:HRAS-mCherry)s896, herein kdrl:mCherry, and imaged live larvae at 3 days post fertilization (dpf). We found active Wnt/β-catenin signal primarily expressed in non-ECs located on the dorsal surface of the forebrain and midbrain and in ECs within the brain parenchyma (Fig. 1a and Supplementary Video 1). Furthermore, colocalization analysis revealed that the majority of active Wnt/β-catenin signal in the hindbrain was localized to the vasculature while the dorsal forebrain exhibited relatively low levels of active Wnt/β-catenin signaling in blood vessels (Supplementary Fig. 1a, b and Supplementary Videos 2,3). To quantify Wnt/β-catenin activity outside of the vasculature, we measured the percent of TCF:EGFP signal from non-ECs located on the dorsal surface or from non-ECs within the hindbrain. Our data indicate that the majority of TCF-positive cells on the dorsal surface are outside of the vessels, whereas few TCF-positive cells were found adjacent to the hindbrain vasculature (Fig. 1b). These observations are consistent with previous studies showing active Wnt/β-catenin signaling in non-ECs associated with the vasculature of CVOs24,26,27.
Inverse correlation between Wnt/β-catenin signaling in endothelial cells and adjacent non-endothelial cells. (a) Dorsal and lateral views of TCF: EGFP; kdrl:mCherry larvae at 3 dpf showing localization of Wnt/β-catenin signaling with respect to vasculature. Dotted outlines demarcate dorsal (white) and hindbrain (yellow) regions used for quantification. (b) Percentage of TCF: EGFP fluorescence signal localized outside of vessel boundaries in the dorsal surface vessels and the hindbrain. p-value was calculated using the parametric two-tailed T-test. (c) Dorsal views of plvap:EGFP, TCF:NLS-mCherry larvae and representative cropped dorsal surface vessel and hindbrain regions of interest used for quantification. (d) Percentage of endothelial cells in larvae from (c) that are TCF+ in dorsal and hindbrain regions of interest. (e) Percentage of endothelial cells in larvae from (c) which had ≥ 1 TCF+ non-endothelial cell within 20 μm in dorsal surface and hindbrain regions of interest. p-values of categorical data in (d, e) were calculated using the Fishers Exact Test. Data are shown as mean ± standard deviation. (f) Contingency square representation of all endothelial cells analyzed in (d, e) used to calculate phi coefficient.
To characterize the proximity of TCF-positive nuclei with respect to the brain vasculature, we bred TCF:NLS-mCherry to the plasmalemma vesicle associated protein (plvap) reporter line Tg(plvapb:EGFP)sj3, herein plvap:EGFP, and imaged live larvae at 3 dpf. As previously described, the plvap:EGFP transgenic line initially labels all brain vasculature during early development, and then the expression becomes restricted to fenestrated endothelium on the dorsal surface of the brain and subsides in brain parenchymal vessels during BBB maturation44. We therefore predicted that the surface vessels that form fenestrated vessels would show low levels of Wnt/β-catenin activation and that hindbrain vessels that form BBB vessels would show high levels of Wnt/β-catenin activation. As expected, we observed nuclear Wnt/β-catenin activation primarily localized to non-ECs on the dorsal surface and in blood vessels within the brain parenchyma (Fig. 1c). To further examine active Wnt/β-catenin signaling in blood vessels with barrier properties, we bred TCF-EGFP to the BBB reporter line Tg(glut1b:mCherry)sj1, herein glut1:mCherry, and found robust colocalization in the hindbrain vasculature (Supplemental Fig. 1c). To quantify our observations, we calculated the percentage of TCF-positive ECs on the dorsal surface and within the hindbrain. Again, we found a low percentage of TCF-positive ECs on the dorsal surface and a high percentage of TCF-positive ECs within the hindbrain (Fig. 1d).
Our data suggested that the status of active Wnt/β-catenin signaling in ECs is inversely correlated to the status of active Wnt/β-catenin signaling in non-ECs within the brain microenvironment. Therefore, we next measured the proximity of blood vessels located on the dorsal surface or within the hindbrain to the nearest TCF-positive nuclei from non-ECs. For these calculations, we quantified the percentage of plvap-positive nuclei from ECs located within 20 µm of TCF-positive nuclei from non-ECs. Consistent with our observations, we found a high percentage of TCF-positive nuclei from non-ECs in close proximity to TCF-negative vessels located on the dorsal surface, whereas very few TCF-positive nuclei from non-ECs were in close proximity to the hindbrain vessels (Fig. 1e). We also found a similar association in the trunk of the larvae where TCF-positive nuclei from non-ECs located within the somites were in close proximity to plvap-positive, TCF-negative ECs within the intersegmental vessels (Supplemental Fig. 1d). To measure the association between TCF-positive ECs and neighboring TCF-positive non-ECs, we calculated the mean square contingency coefficient using data from both the dorsal surface and hindbrain and found a phi coefficient (ɸ) of -0.62, indicating a strong inverse relationship (Fig. 1f). These results indicate that the status of Wnt/β-catenin activity in non-ECs regulates Wnt/β-catenin activity in ECs within the brain microenvironment.
Transgenic doxycycline-inducible activation of Wnt/β-catenin signaling in neural progenitor cells (NPCs)
To examine the vascular effects of activating Wnt/β-catenin signaling in non-ECs, we generated an inducible transgenic model. Here, we used constitutively active β-catenin (herein β-cat*), where all GSK3 phosphorylation sites in the N-terminal domain (NTD) are mutated to alanine residues to stabilize β-catenin by preventing phosphorylation-mediated degradation45. To facilitate visualization of the transgene, we fused EGFP in frame to the C-terminus to generate the β-cat*-EGFP DNA construct (Fig. 2a). To examine functionality of the fusion protein, we made β-cat*-EGFP mRNA and injected single-cell embryos. As shown in Fig. 2b, injection of β-cat*-EGFP mRNA resulted in EGFP-positive, dorsalized embryos (no tail), consistent with early ectopic activation of Wnt/β-catenin signaling.
Generation of Dox-inducible transgenic lines to activate Wnt/β-catenin signaling in NPCs. (a) Schematic representation of endogenous β-catenin (β-catenin WT) and engineered constitutively active β-catenin fused to EGFP (β-cat*-EGFP). Serine and threonine residues S33, S37, T41, and S45 (yellow) were mutated to alanine (green), and EGFP was added in frame to the C terminus. (b) β-cat*-EGFP mRNA was synthesized and injected into single-cell embryos. At 24 hpf, injected embryos are dorsalized (no tail) and express EGFP. (c) Schematic of DNA constructs used to make the Tg(gfap:rtTA, cmlc2:EGFP) driver line and Tg(TRE:β-cat*-EGFP, cryaa:EGFP) responder line. Driver and responder lines are distinguished by green hearts (cmlc2:EGFP) or green lenses (cryaa:EGFP), respectively. (d) Representative lateral images of 3 dpf transgenic gfap;rtTA, TRE:β-cat*-EGFP larvae, or wild type siblings (Con.), treated with indicated Dox concentrations. (e) Quantification of EGFP fluorescence intensity within the CNS of larvae shown in (d) (n = 10 for all conditions). (f) Quantification of whole animal cross sectional area of larvae shown in (d) (n = 10 for all conditions). p-values were calculated for comparisons of all groups using the parametric one-way analysis of variance (ANOVA) with Tukey HSD post hoc test (no label = not significant). p-values directly above a bar indicate significant difference to all other groups by at least the level shown. Data are shown as mean ± standard deviation.
As constitutively active β-catenin disrupts normal axis specification, we developed a Tet-On inducible system to control the dose-dependent expression of β-cat*-EGFP during early development46. To induce transgene expression throughout the developing CNS, we used the glial fibrillary acidic protein (gfap) promoter upstream of the reverse tetracycline transactivator (rtTA)47. Previous studies in zebrafish have shown that gfap-positive radial glial cells represent the major NPCs in the developing nervous system and are closely associated with the brain vasculature48,49,50. Here, we generated the transgenic driver line Tg(gfap:rtTA, cmlc2:EGFP)uw113, herein gfap:rtTA, and the transgenic responder line Tg(TRE:β-cat*-EGFP, cryaa:EGFP)uw114, herein TRE:β-cat*-EGFP (Fig. 2c). To facilitate transgene identification, the transgenesis markers, cmlc2:EGFP and cryaa:EGFP, were included in the driver and responder lines, respectively. Thus, the resulting double transgenic gfap:rtTA, TRE:β-cat*-EGFP embryos induce dose-dependent expression of β-cat*-EGFP in gfap-positive NPCs only in the presence of doxycycline (Dox).
To examine functionality of the gfap:rtTA, TRE:β-cat*-EGFP transgenics, we performed a dose-dependency experiment by exposing embryos at 6 h post fertilization (hpf) to a range of Dox (0 μg/ml to 10 μg/ml). As shown in Fig. 2d, Dox caused a dose-dependent increase of β-cat*-EGFP expression within the CNS as demonstrated by EGFP signal specifically within the brain and spinal cord at 3 dpf. The level of β-cat*-EGFP expression was quantified by measuring the relative fluorescence intensity in the CNS at the different Dox concentrations (Fig. 2e). Dox showed minimal morphological effects except for a slightly curved tail phenotype at the highest Dox concentration (10 μg/ml) and a small decrease in cross sectional area by 3 dpf at higher Dox concentrations (5 μg/ml and 10 μg/ml). We also noted that Dox caused a statistically significant dose-dependent increase in mortality in the gfap:rtTA, TRE:β-cat*-EGFP transgenics, but not in wild-type controls (Supplementary Fig. 2). Therefore, we limited our studies to early neurovascular development to avoid complications potentially associated with other health factors.
Induction of constitutively active β-catenin in NPCs promotes Wnt/β-catenin transcriptional activation
To investigate the activation of Wnt/β-catenin signaling in the Dox-inducible transgenic system, we bred gfap:rtTA, TRE:β-cat*-EGFP to the Wnt/β-catenin transcriptional reporter line TCF:NLS-mCherry. We found that Dox-dependent expression of β-cat*-EGFP resulted in a comparable increase in TCF:NLS-mCherry signal at 3 dpf (Fig. 3a). Furthermore, the β-cat*-EGFP and TCF:NLS-mCherry signals colocalized throughout the CNS (Fig. 3a, right panels). Similar to the β-cat*-EGFP signal shown in Fig. 2e, quantification of the TCF:NLS-mCherry relative fluorescence intensity also showed Dox dose-dependency in the CNS (Fig. 3b). We also quantified TCF-positive signal outside of the CNS as TCF:NLS-mCherry is expressed in many tissues during embryonic development. We found a small, non-CNS reduction of TCF-positive signal throughout the larvae that was not dose dependent, indicating no significant effects of β-cat*-EGFP outside of the CNS (Fig. 3c).
Expression of β-cat*-EGFP in NPCs promotes Wnt/β-catenin transcriptional activation. (a) Representative images of gfap:rtTA, TRE:β-cat*-EGFP, TCF:NLS-mCherry larvae or wild type siblings (Con.) at 3 dpf, treated with indicated Dox concentrations. (b, c) Quantification of mCherry fluorescence intensity within the CNS (b) and outside of the CNS (c) of larvae shown in (a) (n = 10 for all conditions). p-values were calculated for comparisons of all groups using the parametric one-way analysis of variance (ANOVA) with Tukey HSD post hoc test (no label = not significant). p-values directly above a bar indicate significant difference to all other groups by at least the level shown. Data are shown as mean ± standard deviation. (d) Representative lateral images of whole-mount in situ hybridization against axin2 transcript in gfap:rtTA, TRE:β-cat*-EGFP larvae at 3 dpf treated with indicated Dox concentrations (representative from n > 10 for all treatments). (e) Quantification of axin2 expression by qPCR. p-value was calculated using the parametric two-tailed t-test. Data are shown as mean ± standard deviation.
As Wnt/β-catenin signaling promotes the induction of Wnt-target genes, we tested the effects of β-cat*-EGFP by performing whole-mount in situ hybridization (WISH) and quantitative PCR (qPCR). Here, we examined the transcriptional activation of axin2, a negative regulator and direct target of Wnt/β-catenin signaling51. By performing WISH, we found increased expression of axin2 transcript within the brain and spinal cord of Dox-treated larvae (Fig. 3d). Furthermore, we found upregulation of axin2 transcript in response to Dox using qPCR (Fig. 3e). Therefore, we conclude that CNS expression of β-cat*-EGFP by NPCs promotes the transcriptional activation of Wnt-target genes as demonstrated by both increased TCF:NLS-mCherry signal and expression of axin2 transcript.
Activation of Wnt/β-catenin signaling in NPCs disrupts neurovascular development
To support the functional requirements of the CNS, the vasculature acquires specific properties by responding to the needs of the local microenvironment. Given that we observed an association of Wnt/β-catenin signaling in non-ECs with the adjacent vascular phenotypes, we asked whether the activation of Wnt/β-catenin in NPCs impacts the acquisition of BBB properties of vessels within the brain parenchyma. For these experiments, we bred gfap:rtTA, TRE:β-cat*-EGFP to glut1:mCherry, a transgenic reporter of Wnt-dependent barriergenesis in brain ECs21,44. We dosed embryos with a range of Dox (0–10 μg/ml) beginning at 6 hpf, and then imaged the brain vasculature at 3 dpf. As shown in Fig. 4a, increasing Dox concentrations reduced glut1:mCherry signal within the brain vasculature as the level of β-cat*-EGFP signal increased within the brain parenchyma. In control samples, gfap:rtTA, TRE:β-cat*-EGFP, glut1:mCherry larvae with no Dox and glut1:mCherry larvae with 10 μg/ml Dox (without the gfap:rtTA, TRE:β-cat*-EGFP transgenes) showed no differences in glut1:mCherry expression (Fig. 4a, top and bottom panels). To quantify the effects of β-cat*-EGFP expression in NPCs, we measured the volume of glut1:mCherry-positive vessels at the different Dox concentrations. We found a statistically significant dose-dependent decrease in glut1:mCherry volume (Fig. 4b). We further quantified the expression of glut1b transcript by qPCR and confirmed a significant decrease in response to β-cat*-EGFP expression in NPCs (Fig. 4c). These data suggest that activation of Wnt/β-catenin signaling in non-ECs within the brain parenchyma disrupts the Wnt-dependent acquisition of barrier properties in brain ECs.
Activation of Wnt/β-catenin signaling in NPCs disrupts neurovascular development. (a) Representative dorsal images of gfap:rtTA, TRE:β-cat*-EGFP, glut1:mCherry larvae, or wild type siblings (Con.), treated with indicated Dox concentrations. (b) Quantification of mCherry fluorescence volume of larvae shown in (a). (c) Quantification of glut1b expression by qPCR. p-value was calculated using the parametric two-tailed t-test. Data are shown as mean ± standard deviation. (d) Representative dorsal images of gfap:rtTA, TRE:β-cat*-EGFP, kdrl:mCherry larvae, or wild type siblings (Con.), treated with indicated Dox concentrations. (e) Quantification of mCherry fluorescence volume of larvae shown in (d). p-values were calculated for comparisons of all groups using the parametric one-way analysis of variance (ANOVA) with Tukey HSD post hoc test (no label = not significant). Data are shown as mean ± standard deviation. (f) Normalization allowed for comparison of phenotypic dose dependency. Note the steeper slope of glut1:mCherry compared to kdrl:mCherry. p-values were calculated comparing glut1b:mCherry volume and kdrl:mCherry volume at each dosage using the non-parametric two-tailed Mann–Whitney Test. Data are shown as normalized mean ± standard deviation with smooth curve fit.
Given the reduced glut1:mCherry expression in response to activated Wnt/β-catenin in NPCs, we reasoned that the decrease in glut1:mCherry signal could be attributed to defective CNS angiogenesis. For these experiments, we bred gfap:rtTA, TRE:β-cat*-EGFP to the endothelial reporter line kdrl:mCherry. We again dosed embryos with a range of Dox (0–10 μg/ml) beginning at 6 hpf, and then imaged the brain vasculature at 3 dpf. As shown in Fig. 4d, increasing Dox concentrations reduced the brain vasculature the brain parenchyma. In control samples, gfap:rtTA, TRE:β-cat*-EGFP, kdrl:mCherry larvae with no Dox and kdrl:mCherry larvae with 10 μg/ml Dox (without the gfap:rtTA, TRE:β-cat*-EGFP transgenes) showed no differences in the brain vasculature (Fig. 4c, top and bottom panels). In contrast to the dose-dependent decrease in the volume of glut1:mCherry-positive vessels (Fig. 4b), here quantification revealed a statistically significant decrease in vascular volume only at the highest concentrations of Dox (Fig. 4e). We also injected a fluorescent tracer (Dextran, Cascade blue, 3000 MW) to visualize the brain vasculature (Supplementary Fig. 3). We found that the tracer recapitulated the expression pattern of kdrl:mCherry, indicating that β-catenin overexpression does not impact transgene expression. These experiments also showed a potential increase in tracer extravasation in response to activated Wnt/β-catenin in NPCs, although these experiments are difficult to interpret as previous studies demonstrated that the zebrafish BBB does not become functional to tracer leakage until 5 dpf52. Our results suggest a differential response of ECs to increased activation of Wnt/β-catenin signaling in NPCs, where CNS angiogenesis is less affected in comparison to the acquisition of barrier properties (Fig. 4f). These effects may provide new insights into the mechanisms that regulate vascular phenotypes within CNS microenvironments.
Decreased endothelial tip cell migration causes CNS angiogenesis defects
To determine whether the reduction in brain vasculature was due to the regression of blood vessels or the blocking of initiation of CNS angiogenesis, we performed time-lapse confocal microscopy. In zebrafish, angiogenesis in the hindbrain begins at approximately 30 hpf when nascent endothelial tip cells located on the primordial hindbrain channels (PHBCs) extend filopodia and then migrate dorsally into the brain parenchyma53,54. As previously described, tip cells are characterized by their position, their long and dynamic filopodia, and their migratory behavior3,55. Here, we treated gfap:rtTA, TRE:β-cat*-EGFP, kdrl:mCherry larvae with no Dox or 10 µg/ml Dox at 6 hpf and then imaged filopodia and tip cell migration from 30 to 46 hpf. Figure 5 shows still frames presented at 4-h intervals from the time-lapse videos (Supplementary Videos 4 and 5). In untreated larvae, we found stereotypical characteristics of tip cells that extended filopodia from the PHBCs followed by the dorsal migration of tip cells into the brain parenchyma (Fig. 5, top panels and Supplementary Video 4). In contrast, Dox-treated embryos showed a significant reduction in filopodia and tip cell migration from the PHBCs (Fig. 5, bottom panels and Supplementary Video 5). These results are consistent with defects during the early stages of CNS angiogenesis and barriergenesis that are reminiscent of endothelial Wnt pathway mutants in mice and zebrafish15,17,18,19,44.
Activated Wnt/β-catenin signaling in NPCs decreases tip cell migration. Lateral still frames from time-lapse confocal microscopy of gfap:rtTA, TRE:β-cat*-EGFP larvae treated with indicated Dox concentrations. Snapshots are at 4-h intervals over 16 h of acquisition beginning at the onset of CNS angiogenesis (30 hpf). Magnified frames (4 × Zoom) highlight the hindbrain region bounded ventrally by the primordial hindbrain channels. Stereotypical tip cells are indicated by white asterisks. Stereotypical filipodia are indicated by red arrowheads. See supplementary videos 4, 5 for more detail.
Canonical Wnt pathway activators and inhibitors are transcriptionally regulated by Wnt/β-catenin activation in NPCs
Given that BBB development requires endothelial Wnt/β-catenin signaling, we reasoned that constitutively activate β-catenin in NPCs may interfere with Wnt/β-catenin signaling in brain ECs during neurovascular development. Under normal conditions, NPCs secrete Wnt7a and Wnt7b ligands that bind to an endothelial Wnt receptor complex that promotes endothelial Wnt/β-catenin signaling, which is essential for CNS angiogenesis and barriergenesis20,56. In contrast, disease conditions such as Wnt-medulloblastoma disrupt endothelial Wnt/β-catenin signaling through the secretion of Wnt pathway antagonists, such as Wnt Inhibitor Factor 1 (WIF1) and Dickkopf 1 (DKK1), that reduce BBB properties in tumor vasculature32. Thus, we predicted that activation of Wnt/β-catenin signaling in NPCs may either decrease Wnt agonist expression, increase Wnt antagonist expression, or both.
To examine the expression of Wnt pathway agonists and antagonists, we performed qPCR. Here, we treated gfap:rtTA, TRE:β-cat*-EGFP embryos with no Dox or 10 µg/ml Dox at 6 hpf, extracted RNA at 3 dpf, and then examined the normalized gene expression between samples. First, we analyzed the transcriptional expression of Wnt7 agonists. Unlike the mammalian genome which contains two Wnt7 genes, Wnt7a and Wnt7b, the zebrafish genome contains two paralogs for each gene, wnt7aa, wnt7ab, wnt7ba, and wnt7bb, which are expressed in neural progenitor zones during brain development57,58. Here, we found that activated Wnt/β-catenin in NPCs caused a significant decrease in the expression of both wnt7aa and wnt7ab, but no significant changes for either wnt7ba or wnt7bb (Fig. 6a). Interestingly, recent studies have shown that zebrafish wnt7aa mutants cause severe defects in CNS angiogenesis24,42, consistent with the disrupted brain vascular phenotype in our transgenic model.
Activation of Wnt/β-catenin signaling in NPCs regulates the transcription of Wnt pathway activators and inhibitors. (a, b) Quantification of select wnt agonists (a) and wnt antagonists (b) by qPCR. Normalized expression levels were calculated using the delta delta Ct method using β-actin as a housekeeping gene. p-values were calculated between treated and control groups for each transcript using two-tailed t-tests. Data are shown as mean ± standard deviation.
Next, we analyzed the transcriptional expression of Wnt antagonists, wif1, dkk1a, and dkk1b. Functionally, Wif1 sequesters Wnt ligands in the extracellular space, while Dkk1 competitively binds to the Wnt receptor Lrp5/659,60. Thus, both classes of proteins function as potent Wnt pathway antagonists and serve as negative feedback regulators of Wnt/β-catenin signaling. As such, upregulation of these antagonists could potentially inhibit Wnt/β-catenin signaling in brain ECs, thereby disrupting BBB development. For this analysis, we applied the same experimental paradigm used to quantify the Wnt7 transcripts. As shown in Fig. 6b, we found a significant increase (~ fourfold) in wif1 expression, no change in dkk1a expression, and a significant increase in dkk1b expression. Given that Wnt-medulloblastomas produce high levels of Wnt inhibitors in a negative feedback loop to silence activating β-catenin mutations32, our results suggest a similar mechanism in which the activation of Wnt/β-catenin signaling in NPCs upregulates the expression of Wnt antagonists and disrupts endothelial Wnt/β-catenin signaling during neurovascular development.
Activation of Wnt/β-catenin signaling in NPCs elicits a neuroinflammatory response
We next examined the effects of activated Wnt/β-catenin signaling in NPCs on microglia, the resident immune cells of the CNS. Following the initial stages of CNS angiogenesis, yolk-derived macrophages begin to populate the brain parenchyma at approximately 35 hpf and then transform into early microglia around 55–60 hpf61. We reasoned that alterations to the developmental microenvironment within the CNS could potentially impact microglia by either disrupting colonization or promoting an inflammatory response. To control for the effects of abnormal vascularization of the brain, we also examined microglia in gpr124 mutants which are deficient in Wnt-dependent CNS angiogenesis44. We found no obvious differences in the numbers or distribution of microglia between wildtype and gpr124 mutants, indicating that brain vasculature is not required for microglia colonization (Supplementary Fig. 4). These results are consistent with other studies indicating that microglia colonization of the zebrafish brain is independent of vascular circulation62,63.
For these experiments, we bred gfap:rtTA, TRE:β-cat*-EGFP to Tg(mfap4.1:Tomato-CAAX)xt6, herein mfap:Tomato, a membrane-bound transgenic reporter for microglia/macrophages64. As the mfap:Tomato transgenic line does not distinguish between microglia and macrophages, we use mfap-positive (mfap +) to designate these cell types. We dosed embryos with a range of Dox (0–10 μg/ml) beginning at 6 hpf and then imaged the larvae at 3 dpf. As shown in Fig. 7a, untreated larvae (0 μg/ml Dox; top panels) and Dox-treated control (mfap:Tomato only) larvae (10 μg/ml; bottom panels) exhibited a characteristic distribution of mfap + cells throughout whole larva and within the CNS as shown in the hindbrain (Hb) and spinal cord (Sc). In contrast, increasing concentrations of Dox resulted in a dose-dependent expansion in the number of mfap + cells in the CNS (Fig. 7a). We quantified the number of mfap + cells in the CNS as well as the total number of mfap + cells. Consistent with a neuroinflammatory response, we found a statistically significant increase in the number of mfap + cells in the CNS at the higher concentrations of Dox (5 and 10 μg/ml), whereas the total number of mfap + cells was not significantly different between treatment groups (Fig. 7b, c). While these results do not distinguish between the proliferation of microglia in the CNS, the infiltration of peripheral macrophages into the CNS, or a combination of both possibilities, our data support the conclusion that activated Wnt/β-catenin signaling in NPCs promotes a stereotypical neuroinflammatory response.
Activation of Wnt/β-catenin signaling in NPCs causes neuroinflammation. (a) Representative images of gfap:rtTA, TRE:β-cat*-EGFP, mfap:tdTomato larvae or wild type siblings (Con.) at 3 dpf, treated with indicated Dox concentrations. Magnified frames (5 × Zoom) highlight microglia/macrophages in the hindbrain (Hb) and spinal cord (Sc). The CNS is outlined by yellow dotted boxes. (b, c) Quantification of mfap+ cells within the CNS (b) and in the whole larva (c) of larvae shown in (a) (n = 6 for all conditions). p-values were calculated for comparisons of all groups using the parametric one-way analysis of variance (ANOVA) with Tukey HSD post hoc test (no label = not significant). Data are shown as mean ± standard deviation. (d) Representative lateral images of gfap:rtTA, TRE:β-cat*-EGFP, mpx:mCherry larvae or wild type siblings (Con.) at 3 dpf, treated with indicated Dox concentrations. Magnified frames (5 × Zoom) highlight neutrophils in the hindbrain (Hb) and spinal cord (Sc). The CNS is outlined by yellow dotted boxes. (e, f) Quantification of mpx+ cells within the CNS (e) and in the whole larva (f) of larvae shown in (d) (n = 6 for all conditions). p-values were calculated for comparisons of all groups using the parametric one-way analysis of variance (ANOVA) with Tukey HSD post hoc test (no label = not significant). Data are shown as mean ± standard deviation.
To further characterize neuroinflammation in this model, we examined the infiltration of neutrophils into the CNS. Under normal physiological conditions, neutrophils, the most abundant leukocyte in circulation, are absent from the CNS due to the presence of the BBB65,66. However, in response to proinflammatory stimuli originating from microglia and astrocytes in the CNS, neutrophils infiltrate neural tissues and may further exacerbate neuropathological conditions67. For these experiments, we bred gfap:rtTA, TRE:β-cat*-EGFP to Tg(mpx:mCherry)uwm7, herein mpx:mCherry, a transgenic reporter line to visualize neutrophils68. Embryos were dosed with a range of Dox (0–10 μg/ml) beginning at 6 hpf and then imaged by confocal microscopy at 3 dpf. As shown in Fig. 7d, untreated larvae (0 μg/ml Dox; top panels) and Dox-treated control (mpx:mCherry only) larvae (10 μg/ml; bottom panels) exhibited a stereotypical distribution of neutrophils throughout whole larvawith very few mpx + cells within the CNS. In contrast, increasing concentrations of Dox resulted in a dose-dependent transmigration of mpx + cells into the CNS (Fig. 7d). To determine the extent of neutrophil infiltration, the number of mpx + cells in the CNS as well as the total number of mpx + cells in whole larvae were quantified. Similar to the expansion of microglia/macrophages described above, we found a statistically significant increase in the number of mpx + cells in the CNS, whereas the total number of mpx + cells in whole larvae was similar between treatment groups (Fig. 7e, f).
As the neuroinflammatory response is often mediated by key proinflammatory cytokines69,70,71, we next examined the transcriptional expression of interleukin 1 beta (il1b), interleukin 6 (il6), tumor necrosis factor alpha (tnfa), and interferon gamma 1 (ifng1). For these experiments, mRNA levels for il1b, il6, tnfa, and ifng1 were quantified by qPCR. However, our results indicated that none of these transcripts were upregulated in response to activated β-catenin in NPCs (Supplementary Fig. 5a). Given these results, the experiment was replicated with our recently published transgenic model of Il-1β induced systemic inflammation72. Using this positive control, we found transcriptional upregulation of il1b, il6, and tnfa, but not ifng1 (Supplementary Fig. 5b). We also examined the transcriptional expression of the inflammation-associated transcripts matrix metalloproteinase 9 (mmp9), nitric oxide synthase 2a (nos2a), immunoresponsive gene 1, like (irg1l), and haptogloblin (hp). As shown for the proinflammatory cytokines, none of these inflammation-associated transcripts were upregulated in response to activated β-catenin in NPCs, but all transcripts were significantly upregulated in our transgenic model of Il-1β induced systemic inflammation (Supplementary Fig. 5c, d). Therefore, we reason that the activation of Wnt/β-catenin signaling in NPCs during embryogenesis creates a deleterious microenvironment within the CNS that disrupts neurovascular development and stimulates neuroinflammatory conditions independent of transcriptional upregulation of known neuroinflammatory mediators.
Discussion
Our study examines the functional and dysfunctional impact of Wnt/β-catenin signaling within CNS microenvironments during neurovascular development. Using a combination of transgenic zebrafish lines to visualize blood vessels and transcriptional reporters of Wnt-dependent activity, we determined that the level of Wnt signaling in non-ECs inversely correlates with the level of Wnt signaling in ECs. As endothelial Wnt/β-catenin signaling is required for the acquisition of BBB properties, we hypothesized that activating Wnt/β-catenin in adjacent non-ECs within the brain parenchyma would disrupt this developmental process. Therefore, we created and validated a new transgenic Tet-On system in zebrafish to drive expression of constitutively active β-catenin in neural progenitor cells (NPCs). Here, we found that the transcriptional expression of glut1:mCherry, a Wnt-dependent marker of the BBB, is dramatically reduced in response to Wnt activation in NPCs. We also found defects in CNS angiogenesis due to a significant reduction in endothelial tip cell migration. Mechanistically, we determined that the transcriptional levels of Wnt pathway agonists were downregulated while Wnt pathway antagonists were upregulated, consistent with a decrease in endothelial Wnt/β-catenin signaling. Finally, we discovered that aberrant activation of Wnt/β-catenin in NPCs causes a pronounced neuroinflammatory response as shown by increased numbers of microglia/macrophages and neutrophils in the CNS.
To identify regions of active Wnt/β-catenin signaling throughout the developing CNS, we used transcriptional reporters of Wnt activation. Previous studies have demonstrated that endothelial Wnt/β-catenin signaling regulates neurovascular development. For example, it has been well established that endothelial Wnt signaling is essential for CNS angiogenesis and the acquisition of blood–brain barrier properties15,17,18. Transcriptional activation of endothelial Wnt/β-catenin in brain parenchymal vessels has also been demonstrated in zebrafish transgenic reporter lines21,24,43. In contrast, transcriptionally active Wnt/β-catenin is low in fenestrated ECs associated with CVOs and the choroid plexuses24,26,27. In fact, recent studies have shown that the development of these fenestrated vessels requires a unique combination of Vegf ligands24,25. Intriguingly, transgenic expression of the constitutively active form of β-catenin in ECs induces BBB properties in the vessels associated with CVOs26,27 further highlighting the vital role of endothelial Wnt signaling in this process. However, the relationship between active Wnt/β-catenin signaling in ECs versus non-ECs within these CNS microenvironments had not been described during neurovascular development.
In our current study, we observed an inverse relationship between the level of Wnt activation in non-ECs in comparison to ECs within these distinct microenvironments. Consistent with previous studies, high levels of endothelial Wnt activation was found within the brain parenchyma and low levels of endothelial Wnt activation was found on the dorsal surface. Conversely, the level of Wnt activation in non-ECs showed the opposite levels of endothelial Wnt activation within these regions. Furthermore, the close proximity between these different cell types suggested a potential role of non-ECs in the activation of endothelial Wnt signaling. Based upon these results, we hypothesized that the level of active Wnt signaling in non-ECs could regulate Wnt/β-catenin activity in ECs within specific brain microenvironments. This proposed mechanism is consistent with paracrine signaling between neighboring cells to create positive and negative feedback loops in the regulation of Wnt/β-catenin signaling73.
We therefore assessed whether aberrant Wnt activation in non-ECs influences endothelial Wnt signaling during CNS development. To test this idea, we developed and validated a transgenic Tet-On system to drive doxycycline-dependent expression of constitutively active β-catenin in NPCs. Here, we chose the zebrafish gfap promoter to induce active β-catenin for three important reasons. First, previous studies have shown that the gfap promoter becomes transcriptionally active at approximately 12–15 hpf47, well before the beginning stages of CNS angiogenesis at 30 hpf53,54. Second, gfap-positive radial glial cells represent the primary NPCs in the developing CNS49 and these non-ECs are closely associated with the brain vasculature50,74. Third, NPCs are known to secrete Wnt and Vegf ligands required for neurovascular development75,76. Thus, this inducible transgenic system provides an ideal model to test the developmental consequences of activated β-catenin in NPCs.
Several studies in mice have established a vital role for endothelial Wnt/β-catenin in CNS angiogenesis and BBB development15,17,18. In zebrafish, we previously generated the endothelial Wnt-dependent transgenic reporter line, glut1b:mCherry, to visualize the simultaneous induction of CNS angiogenesis and barriergenesis (i.e. the acquisition of BBB properties)44. In our current study, we found that dose-dependent activation of β-catenin in NPCs reduced the level of glut1b:mCherry-postive vessels in the brain parenchyma, indicating the possibility for an inhibitory feedback loop. Indeed this result was reminiscent of paracrine signals found in Wnt-medulloblastoma, which secrete Wnt-inhibition factors that block the acquisition of BBB properties within the tumor vasculature32. Consistent with this observation, we found transcriptional upregulation of Wnt-inhibitory factors, wif1 and dkk1b, and downregulation of Wnt-activation factors, wnt7aa and wnt7ab. Thus, our model of Wnt-activated NPCs phenocopies some abnormal vascular features of Wnt-medulloblastoma by expressing paracrine signals that inhibit BBB formation.
Neuroinflammation is another striking feature that was discovered in response to the activation of Wnt in NPCs. Under steady state conditions, the BBB limits the transmigration of peripheral immune cells into the brain parenchyma. However, in response to CNS injuries, infections, and neurological disorders, proinflammatory signals promote the activation of resident microglia, disrupt BBB function, and stimulate the infiltration of peripheral immune cells into the brain parenchyma38,39,66. In our model, we found both an increase in CNS microglia and an infiltration of peripheral neutrophils, albeit the transgenic mfap:Tomato reporter does not distinguish between the proliferation of microglia and/or the infiltration of peripheral macrophages. In an attempt to identify contributing factors that underlie neuroinflammation in our model, we examined the transcriptional expression of proinflammatory cytokines (il1b, il6, tnfa, ifng1) and known inflammation-associated genes (mmp9, nos2a, irg1l, hp). While many of these transcripts were significantly upregulated in our model of Il-1β induced systemic inflammation, we did not observe transcriptional upregulation in our model of activated β-catenin in NPCs, indicating that alternative mechanisms may initiate the neuroinflammatory response. Indeed, neuroinflammation can occur in the absence of transcriptional regulation through the action of preformed mediators, posttranslational modifications, and other signaling pathways. These mechanisms rely on the rapid release, activation, or modification of pre-existing molecules and cellular machinery that bypass the need for new gene transcription. For example, signals from damage-associated molecular patterns (DAMPs), lipid mediators, and preformed cytokines and chemokines provide mechanisms that allow the CNS to respond rapidly before activating transcription-dependent processes77,78,79.
Based upon these observations, we propose that neuroinflammation in our model is likely due to glial cell dysfunction in response to aberrant Wnt activation in NPCs. Numerous studies have shown that dysfunctional glial cells can contribute to neuroinflammation through several mechanisms, including reactive astrocytosis, cytokine release, and glutamate dysregulation80. This abnormal glial activation is also implicated in various neurological disorders including neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease81,82,83. Interactions between glial cells (i.e. astrocytes and microglia) via cytokine and chemokine activation may also contribute to and amplify the inflammatory response84. For example, Liddelow et al. demonstrated that activated microglia express cytokines that induce A1 reactive astrocytes found in neuroinflammatory and neurodegenerative diseases85. Thus, we propose that a similar mechanism, whereby dysfunctional glial cells disrupt neurovascular development and create a disordered CNS microenvironment, may cause the neuroinflammatory phenotype.
During zebrafish development, microglia, the resident immune cells of the CNS, begin to colonize the brain parenchyma at approximately 35 hpf61 and this process occurs independent of vascular circulation62,63. In contrast, neutrophils are not normally found at significant levels in the CNS at any developmental stage due to “immune privilege” provided by a functional BBB65. As aberrant Wnt activation in NPCs caused disruption of BBB development in our model, the lack of normal brain vasculature could potentially contribute to the neuroinflammatory response. Yet, we also discovered that gpr124 mutants, which are deficient in Wnt-dependent CNS angiogenesis and barriergenesis44, have normal levels of microglia/macrophages, indicating that the lack of BBB properties alone does not elicit a neuroinflammatory response. Additionally, we observed immune cell infiltration into the spinal cord. Unlike the brain, microglia do not normally colonize the spinal cord until much later in development, occurring between 15 dpf and 1.5 months62,86, consistent with the timing of spinal cord vascularization beginning at 12–14 dpf87. Thus, the neuroinflammatory response found in the brain and spinal cord may not be related to abnormal BBB development.
These results also raise important questions about the relationship between neuroinflammation and BBB dysfunction in the context of CNS pathologies. For example, neurological diseases that promote or exacerbate a neuroinflammatory response are often associated with prolonged expression of proinflammatory cytokines69,70,71 and the subsequent breakdown of the BBB38,88,89,90. These effects are most frequently associated with chronic neurological diseases that compromise BBB function, enable the entry of peripheral immune cells and inflammatory mediators, exacerbate the neuroinflammatory response, and contribute to disease-related neuropathology. While most studies highlight BBB dysfunction as the causal mechanism, neuroinflammation also occurs in the absence of BBB impairment. For example, intrinsic mechanisms with the CNS, such as microglial activation and inflammasome activation, can stimulate local inflammatory conditions in the presence of an intact BBB79,91. These processes are particularly relevant during the early stages of neurological disease that, without effective treatments, may advance to chronic neuroinflammation, BBB breakdown, and disease progression92,93. Thus, we propose that therapeutic strategies targeting the prevention and resolution of neuroinflammation may provide new and effective treatments for a variety of neurological diseases associated with BBB dysfunction.
In conclusion, our study emphasizes the importance of proper Wnt/β-catenin signaling within specific CNS microenvironments and highlights the potentially deleterious consequences of aberrant Wnt activation. Furthermore, our results may have important implications regarding the therapeutic targeting of Wnt/β-catenin signaling. The Wnt pathway provides an attractive target developing new therapeutics as Wnt dysregulation is associated with many CNS disorders, including stroke94,95, brain tumors34,96, and neurodegenerative disorders36,37. As BBB dysfunction contributes to the pathology of these diseases, new approaches designed to restore BBB function by activating endothelial Wnt/β-catenin, while preventing harmful off-target effects, may hold therapeutic promise41,42. Our study underscores the importance of specificity in targeting Wnt/β-catenin signaling to avoid exacerbating the underlying neuropathology in these diseases by inadvertently promoting Wnt-dependent tumorigenesis or eliciting a neuroinflammatory response.
Methods
Zebrafish husbandry and transgenic lines
Zebrafish were maintained and bred using standard practices97. Embryos and larvae were maintained at 28.5 °C in egg water (0.03% Instant Ocean in reverse osmosis water). For imaging, 0.003% phenylthiourea (PTU) was used to inhibit melanin production. The AB strain was acquired from the Zebrafish International Resource Center. Transgenic lines Tg(glut1b:mCherry)sj1, Tg(plvapb:EGFP)sj3, Tg(ubb:IVS2GVEcR, cmlc2:EGFP), herein ubb:Gal4-EcR, and Tg(uas:GSP-Il1βmat, cmlc2:mCherry), herein uas:Il1βmat were previously generated in our lab44,72. Tg(kdrl:HRAS-mCherry)s896 was provided by Dr. Jan Huisken, Morgridge Institute for Research98. Transgenic lines Tg(7xTCF-Xla.Siam:EGFP)ia4 and Tg(7xTCF-Xla.Siam:NLS-mCherry)ia5 were provided by Dr. Junsu Kang, UW-Madison43. Transgenic lines Tg(mpx:mCherry)uwm7 and Tg(mfap4.1:Tomato-CAAX)xt6 were provided by Dr. Anna Huttenlocher, UW-Madison64,99. Experiments were performed on embryos and larvae that were heterozygous for all transgenes present. All experiments were performed in accordance with the University of Wisconsin-Madison Institutional Animal Care and Use Committee (protocol number M005020) and ARRIVE guidelines.
Microinjection of β-cat*-EGFP mRNA
We generated a DNA construct with activated β-catenin (β-cat*)45 fused in-frame with EGFP. To demonstrate functionality of the β-cat*-EGFP fusion protein, we generated the pCS2 + :β-cat*-EGFP construct by exchanging EGFP for mCherry from the construct pCS2 + :β-cat*-mCherry kindly provided by Wilson Clements. We made β-cat*-EGFP mRNA using the mMessage mMachine RNA transcription kit and microinjected approximately 50 pg of mRNA into single-cell embryos. Representative embryos were imaged at 24 hpf using a Nikon SMZ18 epifluorescence stereomicroscope equipped with a Nikon DS-Fi2 color camera. Images were processed using Nikon NIS-Elements software.
Generation of doxycycline-inducible transgenic lines Tg(gfap:rtTA; cmlc2:EGFP) uw113 and Tg(TRE:β-cat*-EGFP; cryaa:EGFP) uw114
Construction of plasmids
The TetOn system was used to drive the inducible expression of constitutively active β-catenin (β-cat*) in neural progenitor cells. Plasmids for transgenesis were generated using a combination of PCR, Gateway cloning, and vectors from the Tol2kit (p5E-MCS, pME-MCS, p3E:pA, and pDestTol2CG2)100. To construct gfap:rtTA, cmlc2:EGFP, the zebrafish gfap promoter was released from the gfap:EGFP vector47 and inserted into the 5’ entry clone p5E-MCS using the restriction enzymes XhoI and SalI to make p5E-gfap. The plasmid pME-rtTA was generated by inserting the rtTA2S-M2 from the pTet-On-Advance vector (Clontech) into the entry clone pME-MCS using EcoRI and BamHI restriction sites. The resulting p5E:gfap and pME:rtTA constructs were recombined with p3E:pA and the pDestTol2CG2 destination vector using LR Clonase II Plus (Invitrogen) to generate the final pDestTol2CG2 gfap:rtTA, cmlc2:EGFP transgenic construct.
To prepare the TRE:β-cat*-EGFP, cryaa:EGFP construct, p5E-TRE was made by inserting the tight TRE promoter from the pTRE-Tight vector (Clontech) into the entry clone p5E-MCS using the restriction enzymes XhoI and BamHI. The entry clone pME:β-cat*-EGFP vector was constructed using pCS2 + :β-cat*-mCherry (kindly provided by Wilson Clements, St. Jude Children’s Research Hospital) as a template to PCR amplify Xenopus constitutively active β-catenin (β-cat*) and transfer it into pME-MCS using the restriction enzymes KpnI and SalI45. EGFP was then amplified from pME:EGFP and inserted in-frame using the restriction enzymes SalI and SacI to make the final construct. The vectors p5E:TRE, pME:βcat*-EGFP, p3E:pA, and the destination vector pDestTol2pACryGFP (Addgene plasmid #64,022) were recombined using LR Clonase II Plus (Invitrogen) to make the pDestTol2pACryGFP TRE:βcat*-EGFP; cryaa:EGFP construct.
Microinjection of plasmids
To generate the transgenic lines Tg(gfap:rtTA, cmlc2:EGFP) and Tg(TRE:βcat*-EGFP, cryaa:EGFP), the respective Tol2 vectors were injected into single-cell embryos (50–100 pg total plasmid DNA) together with 20 pg of in vitro transcribed Tol2 transposase mRNA in a final volume of 1–2 nanoliters. Embryos with strong transient expression of the transgenesis markers were raised to adulthood and screened for germline transmission of the transgenes.
Doxycycline (Dox)-induced transgene expression
To induce expression of β-cat*-EGFP, double transgenic gfap:rtTA ; TRE:β-cat*-EGFP embryos were treated continuously with 1 to 10 μg/mL doxycycline (Dox), beginning at approximately 6 hpf. Dox (RPI) was prepared as 10 mg/mL stocks in water, stored at -20 °C, and thawed before application to egg water/PTU at the desired concentrations. During Dox treatment, embryos were protected from light by wrapping in aluminum foil and fresh Dox was applied on a daily basis for the duration of the experiment.
Confocal laser scanning microscopy
Zebrafish embryos and larvae were anesthetized in 0.02% tricaine and imbedded in 1.2% low melting point agarose (Invitrogen) in egg water with 0.003% PTU in a 35 mm glass-bottom dish, number 1.5 (MatTek). 0.02% Tricaine (MS-222) was added to embedded samples for time-lapse imaging. Confocal microscopy was performed using a Nikon Eclipse Ti microscope equipped with a Nikon A1R. All dorsal and lateral images of the brain are 2D maximum intensity projections of 3D z-stacks generated using NIS-Elements (Nikon) software. For clear presentation of the hindbrain vasculature, the lateral dorsal aorta (located beneath the hindbrain vasculature in a dorsal view) were cropped out of the z-stack of dorsal images before 2D compression101. All lateral images of whole larvae are large images (3 × 1 mm) stitched together with a 15% overlap. Time-lapse imaging was completed using resonant scanning to acquire z-stacks at 20-min intervals for 16 hours. Any image manipulation such as color, brightness, or contrast was equally applied to all images within an experiment and does not affect interpretation of data.
Quantification of fluorescence signal
Extravascular expression of TCF:EGFP
Expression of TCF:EGFP in the brain was quantified as the percentage of EGFP fluorescence outside of vasculature in specific regions of the brain. Dorsal confocal z-stacks of TCF:EGFP, kdrl:mCherry larvae were cropped to include only regions of interest using NIS Nikon Elements Software. Here, the dorsal surface was defined as a roughly 150 μm lateral × 100 μm anteroposterior × 100 μm dorsoventral box centered around the transition from the prosencephalic artery (PrA) to the anterior cerebral vein (ACeV). The hindbrain was defined as the region bounded ventrally by the basilar artery (BA), dorsally by the dorsal surface of the brain laterally by the primordial hindbrain channels (PHBCs), anteriorly by the middle cerebral vein (MCeV), and posteriorly by the dorsal longitudinal anastomotic vessel (DLAV). A binary mask of the vasculature in each region was created in FIJI (ImageJ) by thresholding the mcherry channel and adding the binary z-stack to a 3D ROI Manager102. EGFP fluorescence was quantified by first thresholding the EGFP channel, then total volume of EGFP fluorescence signal was quantified using the “Quantif 3D” command in the 3D ROI Manager. Next, the corresponding vascular 3D ROI (described above) was overlaid onto the TCF:EGFP images. The signal inside the vascular ROI was cleared, and the EGFP signal was quantified again using the “Quantif 3D” command in the 3D ROI Manager. This data was then presented as percentage of total EGFP signal outside of vasculature.
TCF:EGFP expression in endothelial versus non-endothelial cells
To calculate the inverse correlation between Wnt/β-catenin signaling in endothelial cells and adjacent non-endothelial cells, we utilized the nuclear localizing Wnt/β-catenin reporter TCF:NLS-mCherry line crossed to our endothelial cell labeling plvap:EGFP. Dorsal confocal z-stacks of relevant larvae were cropped to include only regions of interest using NIS-Elements Software (Nikon). Here, dorsal surface vessels were defined as the prosencephalic artery (PrA), anterior cerebral vein (ACeV), mesencephalic vein (MsV), metencephalic artery (MtA), middle cerebral vein (MceV), dorsal longitudinal vein (DLV), and the posterior cerebral vein (PCeV). The hindbrain was defined as above. Endothelial cells closest to a predetermined, evenly distributed sample layout were identified via plvap:EGFP expression (n = 14 in dorsal surface vessels, and n = 12 in hindbrain vessels). The Wnt/β-catenin signaling status of selected endothelial cells was determined using a thresholded mCherry signal. Using volume rendering, the distance between a selected endothelial cell and the closest TCF:NLS-mCherry+ cell was determined in NIS-Elements Software (Nikon). Data was then presented as the average percentage of endothelial cells with the relevant trait. To calculate the phi coefficient as a measure of association, data from dorsal surface vessels and hindbrain endothelial cells were combined.
Fluorescence intensity of β-cat*-EGFP and TCF:NLS-mCherry
To validate the functionality of β-cat*-EGFP to activate transcriptional activity of Wnt/β-catenin signaling, the relative fluorescence intensities (RFI) of β-cat*-EGFP and TCF:NLS-mCherry were quantified inside and outside the CNS. ROIs were drawn by hand around the entire larvaand the CNS of lateral confocal z-stacks of gfap:rtTA, β-cat*-EGFP, TCF:NLS-mCherry larvae. To determine the RFI of each signal within the CNS, all fluorescence outside the CNS ROI was cleared, and the remaining signal was quantified using the “Quantif 3D” command in the 3D ROI Manager. In a similar fashion, the RFI of each signal outside the CNS was determined by clearing the outside of the whole larvaROI and the inside of the CNS ROI and again using the “Quantif 3D” command in the 3D ROI Manager. Finally, RFI within a background ROI was subtracted from results and data were presented at background corrected RFIs within or outside the CNS.
Fluorescence tracing and volume of vasculature
Fluorescence signal intensity across an image was shown using the tracing tool on NIS-Elements Software (Nikon). The volume of kdrl and glut1b expression was quantified from dorsal confocal z-stacks of appropriate transgenic larvae, cropped to include only the brain. A binary mask of the fluorescence signal was created in FIJI (ImageJ) by thresholding and adding the binary z-stack to a 3D ROI Manager102. Total volume of fluorescence signal was quantified using the “Quantif 3D” command in the 3D ROI Manager.
Cross sectional area of whole larvae
Area was quantified from the whole larvaROI (described above) using the “area” measurement tool in FIJI (ImageJ).
Microglia/macrophage and neutrophil numbers
To quantify the number of immune cells in the whole animal and CNS, ROIs were drawn by hand around the entire larvaand the CNS of lateral confocal z-stacks of appropriate transgenic larvae. Images were then binarized with thresholding in FIJI (ImageJ). To prevent overlap of cells causing reduced counts, images were run through a watershed step using Process < Binary < Watershed, which automatically adds a 1-pixel line between adjacent, overlapping cells. In a repeated process, the outsides of ROIs were cleared and Analyze < 3d Object Counter was used with min size filter set to 10, to obtain total cell number within each ROI.
Statistical analysis
Fishers Exact tests were performed to compare categorical data between two groups. Two-tailed T-tests were performed to compare continuous means between two groups. One-way analysis of variance (ANOVA) with Tukey HSD post hoc tests were performed to compare continuous means between multiple groups. All statistical tests were completed using the Real Statistics Resource Pack Excel add-in (www.real-statistics.com). All statistical tests are named in the figure legends where they are presented.
Survival analysis
Embryos were treated with Dox at the indicated concentrations beginning at 6 hpf. Dox was replaced daily with freshly prepared solutions. The number of surviving larvae was monitored daily until 7 dpf. Kaplan–Meier survival statistics were calculated using the Real Statistics Resource Pack Excel add-in (www.real-statistics.com).
Fluorescent tracer injections
Larvae were injected with fluorescent tracer to examine blood vessel functionality in response to activated Wnt/β-catenin in NPCs. Dextran, Cascade Blue, 3000 MW (Invitrogen) was prepared as a stock concentration of 10 mg/ml in dH2O. Larvae at 3 dpf were anesthetized in 0.02% tricaine, positioned in an agarose injection mold, and microinjected with approximately 2 nl (5 mg/ml) into the pericardial cavity. At 30 min post-injection, larvae were embedded laterally in 1.0% low-melting point agarose and imaged by confocal microscopy.
Whole mount in situ hybridization (WISH)
Synthesis of RNA probes
DNA primers were designed to PCR amplify a 796 bp fragment of axin2 cDNA (NCBI Ref Seq: NM_131561.1). The T3 and T7 RNA polymerase promoter sequences (underlined below) were included on the forward and reverse primers, respectively.
For: 5’-AATTAACCCTCACTAAAGGGTGCACTGCTCCTTACATTCG-3’ and Rev: 5’-TAATACGACTCACTATAGGGCTCGAACACAGCACCACACT-3’.
PCR amplified bands were gel purified and then Dig-labelled RNA probes were synthesized using the DIG RNA labeling kit (Roche) with either T3 RNA polymerase or T7 RNA polymerase (Promega) to produce sense and anti-sense probes, respectively.
Hybridization, staining, and imaging
Transgenic gfap:rtTA, TRE:β-cat*-EGFP embryos were either untreated or treated with 10 μg/ml Dox. Larvae at 3 dpf were anesthetized in 0.02% tricaine, fixed in 4% paraformaldehyde (PFA)/1X PBS at 4ºC overnight, washed in 1X PBS, dehydrated through a methanol series, and stored at -20ºC in methanol until use. Hybridization was performed according to Thisse and Thisse with slight modifications described below103. Briefly, fixed larvae were rehydrated through a methanol/1X PBSTwTx (1X PBS, 0.1% Tween 20, and 0.1% Triton X-100) series, followed by permeabilization with 10 μg/ml proteinase K in 1X PBSTwTx, post fixation in 4% PFA in 1X PBSTwTx, and washout with 1X PBSTwTx. Hybridization of Dig-labeled probes was performed for 2 days. Stringency washes were performed as described. Anti-digoxigenin-AP Fab Fragments (Sigma) and BM Purple AP Substrate were used to develop chromogenic precipitate overnight. Larvae were stored in 70% glycerol until they were imaged in 100% glycerol on a Nikon SMZ18 epifluorescence using a Nikon DS-Fi2 color camera.
RNA extraction, cDNA synthesis, and quantitative PCR
Transgenic gfap:rtTA, TRE:β-cat*-EGFPlarvae at 3 dpf (treated with 0 or 10 μg/ml Dox beginning at 6 hpf) were anesthetized in 0.04% Tricaine (MS-222), washed with fresh egg water, and collected in triplicate (n = 25 per replicate). For control experiments, ubb:Gal4-EcR, uas:Il1βmat larvae at 3 dpf were untreated or treated with 10 μM tebufenozide (Teb) for 12 h to stimulate Il-1β induced systemic inflammation72. Total RNA was extracted using TRIzol Reagent (Invitrogen) and treated with ezDNase (Invitrogen) following the manufacturer’s protocol. Two micrograms of total RNA were reverse transcribed using Superscript IV First-Strand Synthesis System (Invitrogen) with Oligo(dT)20 primers according to the manufacturer’s protocol. Quantitative PCR (qPCR) was performed using PowerUp SYBR Green Master Mix using a QuantStudio 3 (Thermo Fisher Scientific) according to the manufacturer’s protocols. All samples were run in triplicate and the data were calculated and analyzed according to the 2-ΔΔCt method. The following DNA primers were used for qPCR:
actin Fwd: 5’-CCCTCCATTGTTGGACGAC-3’
actin Rev: 5’-AGGATACCTCTCTTGCTCTGG-3’
axin2 Fwd: 5’-CAAGCCTTCAGAGAGACAAGAG-3’
axin2 Rev: 5’-GACAGCCTGACATCTTCTTAGG-3’
dkk1a Fwd: 5’-TGAGACGTGTCTGAGGTCTT-3’
dkk1a Rev: 5’-TGCAAACTTGTCCCTCTGTC-3’
dkk1b Fwd: 5’-AAAGGTCTGGAGGGAGAGAA-3’
dkk1b Rev: 5’-TTTAGGACAGGCTTGCAGATT-3’
glut1b Fwd: 5’-CACTGTCCTCCTGCTGATATTC-3’
glut1b Rev: 5’-GAAGCTGGCTGTGATCTCTT-3’
ifng1 Fwd: 5’-CCAGCCTCTCCTTTGTAGCT-3’
ifng1 Rev: 5’-AATGCACACCCCATCTTCCT-3’
il1b Fwd: 5’-CATGAGAAAGCAGAGGAACTTAAC-3’
il1b Rev: 5’-CCGTCTGTCTCCCACAAA-3’
il6 Fwd: 5’-GCACTCCTCTCCTCAAACCT-3’
il6 Rev: 5’-CCATCTCTCCGTCTCTCACC-3’
hp Fwd: 5’-GCTGGCCGTAATCTGTTTGT-3’
hp Rev: 5’-TGTGTTCTGGAAGCCTGGAT-3’
irg1l Fwd: 5’-TGTGAAGCTTTCTCCAACGC-3’
irg1l Rev: 5’-CGGTTAGAAAGTCAAGCCCG-3’
mmp9 Fwd: 5’-CTGAGGGTGCAATGTGTCAC-3’
mmp9 Rev: 5’-TGGTGCTTCATTGCTGTTCC-3’
nos2a Fwd: 5’-GCAACTACAAGGATGGCTCG-3’
nos2a Rev: 5’-ATGAAATCCTCTGCCTGGCT-3’
tnfa Fwd: 5’-GCAGTGATGCGCTTTTCTGA-3’
tnfa Rev: 5’-TAGAAGTGCTGTGGTCGTGT-3’
wif1 Fwd: 5’-GAGTCCAGGCGTGTGTATTT-3’
wif1 Rev: 5’-GAAACACGTCCCTCCATTCA-3’
wnt7aa Fwd: 5’-AGCCCATGGACACAGATTTAG-3’
wnt7aa Rev: 5’-AGTCTTGTTGCAGATCCTTCC-3’
wnt7ab Fwd: 5’-GAACATGCGTCTGGAGTGTAA-3’
wnt7ab Rev: 5’-GATGTATCCCAGCTCTCGAAAC-3’
wnt7ba Fwd: 5’-CATCAACGAGTGCCAGTATCA-3’
wnt7ba Rev: 5’-GCAGTGATGGCGTAGGTAAA-3’
wnt7bb Fwd: 5’-GTGGATGCTCGGGAGATTAAA-3’
wnt7bb Rev: 5’-TCCAGCTTCATTCGCTCTTC-3’
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 6, 389–395. https://doi.org/10.1038/74651 (2000).
Risau, W. Mechanisms of angiogenesis. Nature 386, 671–674. https://doi.org/10.1038/386671a0 (1997).
Blanco, R. & Gerhardt, H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect Med. 3, a006569. https://doi.org/10.1101/cshperspect.a006569 (2013).
Gerhardt, H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis 4, 241–246. https://doi.org/10.4161/org.4.4.7414 (2008).
Ferrara, N., Gerber, H. P. & LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 9, 669–676. https://doi.org/10.1038/nm0603-669 (2003).
Aird, W. C. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res. 100, 174–190. https://doi.org/10.1161/01.RES.0000255690.03436.ae (2007).
Aird, W. C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 100, 158–173. https://doi.org/10.1161/01.RES.0000255691.76142.4a (2007).
Engelhardt, B. & Liebner, S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. 355, 687–699. https://doi.org/10.1007/s00441-014-1811-2 (2014).
Hagan, N. & Ben-Zvi, A. The molecular, cellular, and morphological components of blood–brain barrier development during embryogenesis. Semin. Cell Dev. Biol. 38, 7–15. https://doi.org/10.1016/j.semcdb.2014.12.006 (2015).
Chow, B. W. & Gu, C. The molecular constituents of the blood–brain barrier. Trends Neurosci. 38, 598–608. https://doi.org/10.1016/j.tins.2015.08.003 (2015).
Ben-Zvi, A. & Liebner, S. Developmental regulation of barrier- and non-barrier blood vessels in the CNS. J. Intern Med. 292, 31–46. https://doi.org/10.1111/joim.13263 (2022).
Miyata, S. New aspects in fenestrated capillary and tissue dynamics in the sensory circumventricular organs of adult brains. Front Neurosci. 9, 390. https://doi.org/10.3389/fnins.2015.00390 (2015).
Anderson, K. D. et al. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc. Natl. Acad. Sci. U. S. A. 108, 2807–2812. https://doi.org/10.1073/pnas.1019761108 (2011).
Cullen, M. et al. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc. Natl. Acad. Sci. U. S. A. 108, 5759–5764. https://doi.org/10.1073/pnas.1017192108 (2011).
Daneman, R. et al. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 106, 641–646. https://doi.org/10.1073/pnas.0805165106 (2009).
Kuhnert, F. et al. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science 330, 985–989. https://doi.org/10.1126/science.1196554 (2010).
Liebner, S. et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 183, 409–417. https://doi.org/10.1083/jcb.200806024 (2008).
Stenman, J. M. et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322, 1247–1250. https://doi.org/10.1126/science.1164594 (2008).
Ulrich, F. et al. Reck enables cerebrovascular development by promoting canonical Wnt signaling. Development 143, 147–159. https://doi.org/10.1242/dev.123059 (2016).
Cho, C., Smallwood, P. M. & Nathans, J. Reck and Gpr124 are essential receptor cofactors for Wnt7a/Wnt7b-specific signaling in Mammalian CNS angiogenesis and blood-brain barrier regulation. Neuron 95, 1056. https://doi.org/10.1016/j.neuron.2017.07.031 (2017).
Fetsko, A. R., Sebo, D. J. & Taylor, M. R. Brain endothelial cells acquire blood-brain barrier properties in the absence of Vegf-dependent CNS angiogenesis. Dev. Biol. 494, 46–59. https://doi.org/10.1016/j.ydbio.2022.11.007 (2023).
Zhou, Y. & Nathans, J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev. Cell 31, 248–256. https://doi.org/10.1016/j.devcel.2014.08.018 (2014).
Zhou, Y. et al. Canonical WNT signaling components in vascular development and barrier formation. J. Clin. Invest. 124, 3825–3846. https://doi.org/10.1172/jci76431 (2014).
Parab, S. et al. Local angiogenic interplay of Vegfc/d and Vegfa controls brain region-specific emergence of fenestrated capillaries. Elife https://doi.org/10.7554/eLife.86066 (2023).
Parab, S., Quick, R. E. & Matsuoka, R. L. Endothelial cell-type-specific molecular requirements for angiogenesis drive fenestrated vessel development in the brain. Elife https://doi.org/10.7554/eLife.64295 (2021).
Benz, F. et al. Low wnt/beta-catenin signaling determines leaky vessels in the subfornical organ and affects water homeostasis in mice. Elife https://doi.org/10.7554/eLife.43818 (2019).
Wang, Y. et al. Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. Elife https://doi.org/10.7554/eLife.43257 (2019).
Chang, J. et al. Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat. Med. 23, 450. https://doi.org/10.1038/nm.4309 (2017).
Lengfeld, J. E. et al. Endothelial Wnt/beta-catenin signaling reduces immune cell infiltration in multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 114, E1168–E1177. https://doi.org/10.1073/pnas.1609905114 (2017).
Reis, M. et al. Endothelial Wnt/beta-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J. Exp. Med. 209, 1611–1627. https://doi.org/10.1084/jem.20111580 (2012).
Northcott, P. A. et al. The whole-genome landscape of medulloblastoma subtypes. Nature 547, 311–317. https://doi.org/10.1038/nature22973 (2017).
Phoenix, T. N. et al. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell 29, 508–522. https://doi.org/10.1016/j.ccell.2016.03.002 (2016).
Manfreda, L., Rampazzo, E. & Persano, L. Wnt signaling in brain tumors: A challenging therapeutic target. Biology (Basel) https://doi.org/10.3390/biology12050729 (2023).
Manoranjan, B. et al. Wnt activation as a therapeutic strategy in medulloblastoma. Nat. Commun. 11, 4323. https://doi.org/10.1038/s41467-020-17953-4 (2020).
Obermeier, B., Daneman, R. & Ransohoff, R. M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 19, 1584–1596. https://doi.org/10.1038/nm.3407 (2013).
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R. & Zlokovic, B. V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 99, 21–78. https://doi.org/10.1152/physrev.00050.2017 (2019).
Zhao, Z., Nelson, A. R., Betsholtz, C. & Zlokovic, B. V. Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064–1078. https://doi.org/10.1016/j.cell.2015.10.067 (2015).
Lopes Pinheiro, M. A. et al. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim. Biophys. Acta 1862, 461–471. https://doi.org/10.1016/j.bbadis.2015.10.018 (2016).
Mapunda, J. A., Tibar, H., Regragui, W. & Engelhardt, B. How does the immune system enter the brain?. Front Immunol. 13, 805657. https://doi.org/10.3389/fimmu.2022.805657 (2022).
Profaci, C. P., Munji, R. N., Pulido, R. S. & Daneman, R. The blood–brain barrier in health and disease: Important unanswered questions. J. Exp. Med. https://doi.org/10.1084/jem.20190062 (2020).
Ding, J. et al. Therapeutic blood-brain barrier modulation and stroke treatment by a bioengineered FZD(4)-selective WNT surrogate in mice. Nat. Commun. 14, 2947. https://doi.org/10.1038/s41467-023-37689-1 (2023).
Martin, M. et al. Engineered Wnt ligands enable blood-brain barrier repair in neurological disorders. Science 375, eabm4459. https://doi.org/10.1126/science.abm4459 (2022).
Moro, E. et al. In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Dev. Biol. 366, 327–340. https://doi.org/10.1016/j.ydbio.2012.03.023 (2012).
Umans, R. A. et al. CNS angiogenesis and barriergenesis occur simultaneously. Dev. Biol. 425, 101–108. https://doi.org/10.1016/j.ydbio.2017.03.017 (2017).
Yost, C. et al. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 10, 1443–1454. https://doi.org/10.1101/gad.10.12.1443 (1996).
Gossen, M. et al. Transcriptional activation by tetracyclines in mammalian cells. Science 268, 1766–1769 (1995).
Bernardos, R. L. & Raymond, P. A. GFAP transgenic zebrafish. Gene Expr Patterns 6, 1007–1013. https://doi.org/10.1016/j.modgep.2006.04.006 (2006).
Jeong, J. Y. et al. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res. Bull 75, 619–628. https://doi.org/10.1016/j.brainresbull.2007.10.043 (2008).
Johnson, K. et al. Gfap-positive radial glial cells are an essential progenitor population for later-born neurons and glia in the zebrafish spinal cord. Glia 64, 1170–1189. https://doi.org/10.1002/glia.22990 (2016).
Umans, R. A. et al. Using Zebrafish to Elucidate Glial-vascular interactions during CNS development. Front Cell Dev. Biol. 9, 654338. https://doi.org/10.3389/fcell.2021.654338 (2021).
Jho, E. H. et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell Biol. 22, 1172–1183. https://doi.org/10.1128/MCB.22.4.1172-1183.2002 (2002).
O’Brown, N. M., Megason, S. G. & Gu, C. Suppression of transcytosis regulates zebrafish blood-brain barrier function. Elife https://doi.org/10.7554/eLife.47326 (2019).
Fujita, M. et al. Assembly and patterning of the vascular network of the vertebrate hindbrain. Development 138, 1705–1715. https://doi.org/10.1242/dev.058776 (2011).
Ulrich, F., Ma, L. H., Baker, R. G. & Torres-Vazquez, J. Neurovascular development in the embryonic zebrafish hindbrain. Dev. Biol. 357, 134–151. https://doi.org/10.1016/j.ydbio.2011.06.037 (2011).
Gerhardt, H. et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177. https://doi.org/10.1083/jcb.200302047 (2003).
Posokhova, E. et al. GPR124 functions as a WNT7-specific coactivator of canonical beta-catenin signaling. Cell Rep. 10, 123–130. https://doi.org/10.1016/j.celrep.2014.12.020 (2015).
Beretta, C. A., Brinkmann, I. & Carl, M. All four zebrafish Wnt7 genes are expressed during early brain development. Gene Exp. Patterns 11, 277–284. https://doi.org/10.1016/j.gep.2011.01.004 (2011).
Duncan, R. N., Panahi, S., Piotrowski, T. & Dorsky, R. I. Identification of Wnt genes expressed in neural progenitor zones during Zebrafish Brain development. PLoS ONE 10, e0145810. https://doi.org/10.1371/journal.pone.0145810 (2015).
Glinka, A. et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357–362. https://doi.org/10.1038/34848 (1998).
Hsieh, J. C. et al. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 398, 431–436. https://doi.org/10.1038/18899 (1999).
Herbomel, P., Thisse, B. & Thisse, C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev. Biol. 238, 274–288. https://doi.org/10.1006/dbio.2001.0393 (2001).
Xu, J., Wang, T., Wu, Y., Jin, W. & Wen, Z. Microglia colonization of developing Zebrafish Midbrain is promoted by apoptotic neuron and lysophosphatidylcholine. Dev. Cell 38, 214–222. https://doi.org/10.1016/j.devcel.2016.06.018 (2016).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. https://doi.org/10.1126/science.1194637 (2010).
Walton, E. M., Cronan, M. R., Beerman, R. W. & Tobin, D. M. The macrophage-specific promoter mfap4 allows live, long-term analysis of macrophage behavior during mycobacterial infection in zebrafish. PLoS ONE 10, e0138949. https://doi.org/10.1371/journal.pone.0138949 (2015).
Engelhardt, B., Vajkoczy, P. & Weller, R. O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 18, 123–131. https://doi.org/10.1038/ni.3666 (2017).
Marchetti, L. & Engelhardt, B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc. Biol. 2, H1–H18. https://doi.org/10.1530/VB-19-0033 (2020).
Salken, I., Provencio, J. J. & Coulibaly, A. P. A potential therapeutic target: The role of neutrophils in the central nervous system. Brain Behav. Immun. Health 33, 100688. https://doi.org/10.1016/j.bbih.2023.100688 (2023).
Yoo, S. K. et al. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev. Cell 18, 226–236. https://doi.org/10.1016/j.devcel.2009.11.015 (2010).
Becher, B., Spath, S. & Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 17, 49–59. https://doi.org/10.1038/nri.2016.123 (2017).
Ferrari, C. C. et al. Reversible demyelination, blood-brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am. J. Pathol. 165, 1827–1837. https://doi.org/10.1016/S0002-9440(10)63438-4 (2004).
Kwon, H. S. & Koh, S. H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl. Neurodegener 9, 42. https://doi.org/10.1186/s40035-020-00221-2 (2020).
Lanham, K. A., Nedden, M. L., Wise, V. E. & Taylor, M. R. Genetically inducible and reversible zebrafish model of systemic inflammation. Biol. Open https://doi.org/10.1242/bio.058559 (2022).
Liu, J. et al. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target Ther. 7, 3. https://doi.org/10.1038/s41392-021-00762-6 (2022).
Quinonez-Silvero, C., Hubner, K. & Herzog, W. Development of the brain vasculature and the blood-brain barrier in zebrafish. Dev. Biol. 457, 181–190. https://doi.org/10.1016/j.ydbio.2019.03.005 (2020).
Ma, S., Kwon, H. J., Johng, H., Zang, K. & Huang, Z. Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling. PLoS Biol. 11, e1001469. https://doi.org/10.1371/journal.pbio.1001469 (2013).
Matsuoka, R. L. et al. Radial glia regulate vascular patterning around the developing spinal cord. Elife https://doi.org/10.7554/eLife.20253 (2016).
Lima, I. V., Bastos, L. F., Limborco-Filho, M., Fiebich, B. L. & de Oliveira, A. C. Role of prostaglandins in neuroinflammatory and neurodegenerative diseases. Mediators Inflamm. 2012, 946813. https://doi.org/10.1155/2012/946813 (2012).
Roh, J. S. & Sohn, D. H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 18, e27. https://doi.org/10.4110/in.2018.18.e27 (2018).
Voet, S., Srinivasan, S., Lamkanfi, M. & van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. https://doi.org/10.15252/emmm.201810248 (2019).
Lee, H. G., Lee, J. H., Flausino, L. E. & Quintana, F. J. Neuroinflammation: An astrocyte perspective. Sci. Transl. Med. 15, eadi7828. https://doi.org/10.1126/scitranslmed.adi7828 (2023).
Iovino, L., Tremblay, M. E. & Civiero, L. Glutamate-induced excitotoxicity in Parkinson’s disease: The role of glial cells. J. Pharmacol. Sci. 144, 151–164. https://doi.org/10.1016/j.jphs.2020.07.011 (2020).
Marogianni, C. et al. Neurodegeneration and inflammation-an interesting interplay in Parkinson’s disease. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21228421 (2020).
Ransohoff, R. M. How neuroinflammation contributes to neurodegeneration. Science 353, 777–783. https://doi.org/10.1126/science.aag2590 (2016).
Linnerbauer, M., Wheeler, M. A. & Quintana, F. J. Astrocyte crosstalk in CNS inflammation. Neuron 108, 608–622. https://doi.org/10.1016/j.neuron.2020.08.012 (2020).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. https://doi.org/10.1038/nature21029 (2017).
Xu, J. et al. Temporal-spatial resolution fate mapping reveals distinct origins for embryonic and adult Microglia in Zebrafish. Dev. Cell 34, 632–641. https://doi.org/10.1016/j.devcel.2015.08.018 (2015).
Wild, R. et al. Neuronal sFlt1 and Vegfaa determine venous sprouting and spinal cord vascularization. Nat. Commun. 8, 13991. https://doi.org/10.1038/ncomms13991 (2017).
Hawkins, B. T. & Davis, T. P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 57, 173–185. https://doi.org/10.1124/pr.57.2.4 (2005).
Zlokovic, B. V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201. https://doi.org/10.1016/j.neuron.2008.01.003 (2008).
Engelhardt, B. & Ransohoff, R. M. Capture, crawl, cross: The T cell code to breach the blood-brain barriers. Trends Immunol. 33, 579–589. https://doi.org/10.1016/j.it.2012.07.004 (2012).
Nikolopoulos, D. et al. Microglia activation in the presence of intact blood-brain barrier and disruption of hippocampal neurogenesis via IL-6 and IL-18 mediate early diffuse neuropsychiatric lupus. Ann. Rheum Dis. 82, 646–657. https://doi.org/10.1136/ard-2022-223506 (2023).
Zhang, W., Xiao, D., Mao, Q. & Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target Ther. 8, 267. https://doi.org/10.1038/s41392-023-01486-5 (2023).
Zusso, M., Stokes, L., Moro, S. & Giusti, P. Editorial: neuroinflammation and its resolution: From molecular mechanisms to therapeutic perspectives. Front Pharmacol. 11, 480. https://doi.org/10.3389/fphar.2020.00480 (2020).
Mo, Z. et al. Activation of Wnt/Beta-catenin signaling pathway as a promising therapeutic candidate for cerebral ischemia/reperfusion injury. Front Pharmacol 13, 914537. https://doi.org/10.3389/fphar.2022.914537 (2022).
Xu, D. et al. Effect of Wnt signaling pathway on neurogenesis after cerebral ischemia and its therapeutic potential. Brain Res. Bull 164, 1–13. https://doi.org/10.1016/j.brainresbull.2020.07.005 (2020).
Lee, Y., Lee, J. K., Ahn, S. H., Lee, J. & Nam, D. H. WNT signaling in glioblastoma and therapeutic opportunities. Lab Invest. 96, 137–150. https://doi.org/10.1038/labinvest.2015.140 (2016).
Westerfield, M. The Zebrafish Book (University of Oregon Press, 1995).
Chi, N. C. et al. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 22, 734–739. https://doi.org/10.1101/gad.1629408 (2008).
Mathias, J. R. et al. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 80, 1281–1288. https://doi.org/10.1189/jlb.0506346 (2006).
Kwan, K. M. et al. The Tol2kit: A multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099. https://doi.org/10.1002/dvdy.21343 (2007).
Isogai, S., Horiguchi, M. & Weinstein, B. M. The vascular anatomy of the developing zebrafish: An atlas of embryonic and early larval development. Dev. Biol. 230, 278–301. https://doi.org/10.1006/dbio.2000.9995 (2001).
Ollion, J., Cochennec, J., Loll, F., Escude, C. & Boudier, T. TANGO: A generic tool for high-throughput 3D image analysis for studying nuclear organization. Bioinformatics 29, 1840–1841. https://doi.org/10.1093/bioinformatics/btt276 (2013).
Thisse, C. & Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69. https://doi.org/10.1038/nprot.2007.514 (2008).
Acknowledgements
We thank Dr. Junsu Kang (UW-Madison) for providing the Tg(7xtcf-Xla.Siam:EGFP)ia4 and Tg(7xTCF-Xla.Sia:NLS-mCherry)ia5 transgenic lines, Dr. Jan Huisken (Morgridge Institute for Research and UW-Madison) for providing the Tg(kdrl:HRAS-mCherry)s896 transgenic line, Dr. Anna Huttenlocher (UW-Madison) for providing the Tg(mpx:mCherry)uwm7 and Tg(mfap4.1:tdTomato-CAAX)xt6 transgenic lines. We also wish to thank Randal Kopielski for assistance with zebrafish husbandry and animal facility care and maintenance. This project was funded by NIH RO1NS116043.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.R.T.; methodology, D.J.S., I.A., A.R.F., A.A.T., and M.R.T.; validation, D.J.S., I.A., A.R.F., and M.R.T.; formal analysis, D.J.S., and M.R.T.; investigation, D.J.S., I.A., A.R.F., A.A.T., and M.R.T.; resources, M.R.T.; writing—original draft, D.J.S., and M.R.T.; writing—review & editing, D.J.S., and M.R.T.; visualization, D.J.S., and M.R.T.; supervision, M.R.T.; funding acquisition, M.R.T.
Corresponding author
Ethics declarations
Competing interests
The author(s) declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Supplementary Video 2.
Supplementary Video 3.
Supplementary Video 4.
Supplementary Video 5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sebo, D.J., Ali, I., Fetsko, A.R. et al. Activation of Wnt/β-catenin in neural progenitor cells regulates blood–brain barrier development and promotes neuroinflammation. Sci Rep 15, 3496 (2025). https://doi.org/10.1038/s41598-025-85784-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85784-8
Keywords
This article is cited by
-
Lamin A/C regulates cerebellar granule cell maturation
Cell Biology and Toxicology (2025)