Abstract
Progestogens commonly used in the clinic include levonorgestrel, etonogestrel, medroxyprogesterone, hydroxyprogesterone, progesterone, desogestrel, and megestrol. Progestogens are widely used for contraception and the treatment of endometriosis, threatened abortion and other diseases. However, the correlation between progestogen use and depression is not clear. Therefore, this study used data from the FDA Adverse Event Reporting System (FAERS) database to assess the relationship between progestogen levels and depression. In this study, all data from the first quarter of 2004 to the third quarter of 2024 were extracted and imported into SAS 9.4 software for data cleaning and analysis. The reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN) and Multi-item Gamma Poisson Shrinker (MGPS) were used for Bayesian analysis and disproportionation analysis. Levonorgestrel, medroxyprogesterone, etonogestrel and desogestrel presented positive signals for depression, and medroxyprogesterone also presented positive signals for major depression. Although none of the progestogens presented positive signals for suicide or self-harm, medroxyprogesterone presented a positive signal for suicidal ideation. Conclusion Analysis of data from the FAERS database revealed that levonorgestrel, medroxyprogesterone, etonogestrel, and desogestrel were correlated with depression. These findings provide real-world evidence of the potential risk of progestogen-related depression.
Similar content being viewed by others
Introduction
Depression has one of the highest incidence rates among mental illnesses, and previous studies have shown that the incidence of depression in women is more than twice that in men1. Approximately 17% of women experience depression in their lifetime2. A considerable number of women fail to meet the clinical criteria for depression, yet exhibit symptoms such as diminished mood and reduced interest3. Depression and depressed mood have serious negative impacts on women’s mental health4.
Progestogens are the most commonly used contraceptive drugs for women; according to statistics, more than half of women of reproductive age in the United States choose to use progestogens for contraception5. In addition to their use for contraception, progestogens are widely used in the treatment of functional uterine bleeding, dysmenorrhea, endometriosis, threatened abortion and other obstetrical and gynaecological diseases6,7. When used for contraception, progestogens not only have satisfactory contraceptive effects but also provide some health benefits for women of reproductive age, but they also carry some risks9. Common adverse effects of progestogens include fluid retention, weight gain or loss, and acne10,11. In addition to these common adverse effects, progestogens may have emotional effects on women and may cause depression and anxiety12. Epidemiological studies have shown that women are twice as likely to experience depression as men are13, and the link between progestogen and depression has attracted much attention14.
Although numerous clinical investigations have explored the relationship between progestogens and depression, different studies have reached inconsistent conclusions. Progestogens are often used as contraceptives for postpartum women15. Moreover, postpartum women are at high risk for depression16, and these factors make it difficult to determine the correlation between progesterone and depression.
The FDA adverse events reporting system (FARES) is a database that collects information on self-reported adverse events (AEs). Self-reported adverse drug reaction records from 2004 to the present are an important data source for postmarketing adverse drug reaction signal mining research17,18. Therefore, this retrospective pharmacovigilance study aimed to analyse depression caused by progestogens that are commonly used through data from the FARES database, excavate potential pharmacovigilance signals, evaluate the safety of these progestogens, and provide references for rational clinical drug use.
Methods
Data source
The data were obtained from the FAERS database. The FAERS database has been releasing data packets on a quarterly basis since the first quarter of 2004. All the American Standard Code for Information Interchange (ASCII) data packets from the first quarter of 2004 to the third quarter of 2024 (83 packets) were downloaded and imported into SAS 9.4 software for data cleaning and analysis. The data released by the FAERS database every quarter includes seven different files: patient demographic and administrative information (DEMO), drug information (DRUG), adverse events (REAC), patient outcomes (OUTC), report sources (RPSR), therapy start and end dates for reported drugs (THER), and indications for drug administration (INDI). The extracted data includes age, sex, adverse event, type of reporter, reporting country, outcome of event, start date and event date.
Target drug population screening
Each patient in the database has a unique ‘primary suspect drug (PS)’. When determining the target drug population, only the drug that the patient first used was considered. Because progestogens are also used as gender-affirming therapy for male patients19, these patients has a higher risk of depression20. The gender information of the patients in the FAERS database was recorded in the DEMO. Gender is categorized into three types: Female, Male and Unknown. In order to eliminate the potential bias introduced by male patients, we conducted a gender-hierarchical analysis when calculating pharmacovigilance signals. The calculations for male patients and patients without specified gender were excluded, only calculations for female patients were retained. Data on clinical characteristics, outcomes, and onset times were exclusively included for female patients. The drug name and active ingredient (PROD_AI) in the FAERS database were standardized by the World Health Organization (WHO) DRUG dictionary, and the standardized names were used to screen target drugs.
Data processing
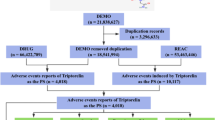
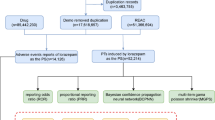
We selected the most recent FDA_DT items with the same CASEID. In cases where CASEID and FDA_DT are the same, we prioritized the higher PRIMARYID. Depression cases were obtained by searching using the Medical Dictionary for Regulatory Activities (MedDRA) (version 28.0). In this study, depression related AEs were defined by 7 preferred terms (PTs) : ‘depression’, ‘depression (suicidal)’, ‘major depression’, ‘menopausal depression’, ‘persistent depressive disorder’, ‘perinatal depression’, and ‘mixed anxiety and depressive disorder’. Pharmacovigilance signals were calculated after the combination of PTs. The study drugs included were progestogens (levonorgestrel, etonogestrel, medroxyprogesterone, hydroxyprogesterone, progesterone, desogestrel, megestrol) that are currently on the market. After screening the raw data, a total of 6,502 reports exhibiting depression related to progestogen were identified. The specific process is shown in Fig. 1. This study also examined the correlation between the utilization of progestogen and occurrences of suicide and self-harm. suicide and self-harm related AEs were defined by 7 PTs: ‘suicidal ideation’, ‘suicide attempt’, ‘self-injurious ideation’, ‘intentional self-injury’, ‘completed suicide’, ‘suicidal behaviour’, and ‘suicide threat’. Pharmacovigilance signals were calculated after the combination of PTs. Furthermore, this study conducted a separate calculation of the pharmacovigilance signal pertaining to progestogen-related suicidal ideation.
Statistical analysis
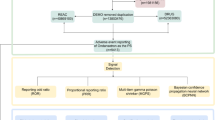
A two-by-two contingency table was constructed for disproportionation analysis and Bayesian analysis. The two-by-two contingency table comprises four components, namely a, b, c, and d. a, b, c, d represents reports of the suspected drug with the interested ADR, other drugs with the interested ADR, the suspected drug with other ADRs, and other drugs with other ADRs. The Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and Muti-item Gamma Poisson Shrinker (MGPS) are utilized to calculate ROR, PRR, Information Component (IC), and empirical Bayes geometric mean (EBGM) respectively. The ROR and PRR methods are classified under the category of disproportionation analysis, while the BCPNN and MGPS methods fall into the realm of Bayesian analysis. The calculation of ROR and PRR is straightforward, but it may lead to false positive results21.The calculation method of BCPNN is intricate and exhibits reduced sensitivity; however, it can yield effective outcomes in scenarios involving missing data22. The implementation of MGPS can effectively mitigate the influence of confounding factors23.The calculation formula and criteria follow: Table 1.
EBGM 05:the lower limit of the 90% one-sided CI of the EBGM.
Results
Descriptive analysis
The FAERS database included 6,502 cases of progestogen-related depression from the first quarter of 2004 through the third quarter of 2024. The demographic characteristics of the patients with progestogen-related depression are shown in Table 2. Patients aged 18–45 years composed the main reporting group. The number of reported cases showed an increasing trend before 2017, reaching a peak in 2017, and gradually decreased after 2017. Cases were reported mainly in North America and Europe, with consumers being the main reporters of progestogen-related depression. Levonorgestrel had the greatest number of cases, followed by etonogestrel and medroxyprogesterone.
Disproportionality analysis and bayesian analysis
The analysis results revealed that only a subset of progestogens were associated with depression. Levonorgestrel, medroxyprogesterone and desogestrel presented positive signals in all four algorithms. Hydroxyprogesterone, progesterone and megestrol did not show positive signals. The ROR, PRR and IC of etonogestrel presented positive signals, whereas the EBGM presented negative signals. The correlations between different progestogens and depression were ranked as follows: levonorgestrel (ROR = 2.55, 95% CI: 2.48–2.63) > medroxyprogesterone (ROR = 2.27, 95% CI: 2.07–2.49) > desogestrel (ROR = 2.13, 95% CI: 1.14–3.96) > etonogestrel (ROR = 1.65, 95% CI: 1.56–1.75) > progesterone (ROR = 0.95, 95% CI: 0.66–1.37) > hydroxyprogesterone (ROR = 0.85, 95% CI: 0.70–1.03) > megestrol (ROR = 0.16, 95% CI: 0.02–1.17) (Table 3). The relationships between the different progestogens and major depression were analysed. The results revealed that only medroxyprogesterone had positive signals in the four algorithms, and the other progestogens did not have positive signals (Table 4). The analysis of suicide and self-harm revealed no positive signals for any of the progestogens (Table 5). The study encompasses seven PTs that pertain to suicide and self-harm. The pharmacovigilance signals of these seven PTs were computed independently in this study. However, only medroxyprogesterone-associated suicidal ideation exhibited a positive pharmacovigilance signal (ROR = 1.81, 95% CI: 1.52–2.17; PRR = 1.82, 95% CI: 1.53–2.17; IC = 0.86, IC 025 = 0.59) (Table 6). The pharmacovigilance signals for the remaining PTs are presented in Supplementary Tables 1–6.
Outcomes due to depression
The data on outcomes related to depression were acquired from OUTC. The outcomes related to depression included life-threatening events, hospitalization, disability, death, congenital anomalies, the need for intervention to prevent permanent damage and other AEs. Among these outcomes, the number of adverse reactions associated with progesterone, desogestrel and megestrol was less than 30; thus, the reference value is significantly limited. Among drugs with more than 100 reported adverse reactions, hydroxyprogesterone had the lowest incidence of life-threatening events (0.9%), whereas the remaining three progestogens presented minimal variation. Levonorgestrel had the highest hospitalization rate (31.1%), whereas etonogestrel had the lowest hospitalization rate (9.8%). Although most progestogens have a low mortality rate attributed to depression, medroxyprogesterone had a mortality rate of 1.3%, surpassing that of other medications (Table 7).
Hierarchical analysis based on age subgroups
Levonorgestrel-related depression presented a positive signal across all four algorithms for individuals aged 18–44 years and 45–64 years. Etonogestrel-related depression presented positive signals in individuals under 18 years of age and those aged 18–44 years, as indicated by the ROR, PRR, and IC algorithms; however, the EBGM algorithm did not present any positive signals. Medroxyprogesterone-related depression presented a positive signal in all four algorithms for individuals under 18 years of age and those aged 18–44 years. In the 45–64-year age group, ROR and PRR algorithms presented positive signals, whereas the remaining three algorithms did not. The ROR, PRR, and IC algorithms presented positive signals for hydroxyprogesterone and progesterone in individuals older than 65 years; however, the EBGM algorithm did not present positive signals (Table 8).
Onset times of Depression
After the use of progestogens, the time of onset of depression is within 180 days. A lower proportion of depression occurred after 180 days of progestogen use. This suggests that when progestogens are used, the time of onset of depression is mainly within 180 days of treatment initiation. However, a small number of patients still develop depression after 5 years, with medroxyprogesterone having the highest rate (7.6%) (Table 9).
Discussion
This study revealed that depression was disproportionately associated with reports of AEs related to levonorgestrel, etonogestrel, medroxyprogesterone, and desogestrel use. Levonorgestrel and medroxyprogesterone presented positive signals for the PRR, ROR, IC025 and EBGM05, suggesting a strong correlation between the use of these drugs and depression. The ROR and PRR of etonogestrel presented positive signals, whereas the IC025 and EBGM05 presented negative signals. The PRR, ROR, IC025 and EBGM05 of hydroxyprogesterone, progesterone and megestrol were all negative. However, there are few records of hydroxyprogesterone, progesterone, megestrol and desogestrel in the FAERS database. Therefore, it is not possible to determine the associations between the use of these drugs and depression through the available data.
After levonorgestrel-releasing intrauterine device (LNG-IUD)-related adverse reactions were brought to the attention of the French media, a study of French women revealed that 38.8% of women using LNG-IUDs experienced symptoms of depression over a two-year period24. A retrospective review of levonorgestrel-related AEs recorded in the FAERS database between 2004 and 2015 suggested that the use of progestogen or LNG-IUDs may be associated with the risk of postpartum depression25. A study of the clinical efficacy of an LNG-IUD revealed that of the 678 women who used an LNG-IUD, 13 developed depression over a 5-year period26. A survey of LNG-IUD users also revealed an association between levonorgestrel use and depression, with more than 17,000 users taking part in the survey, 36% of whom experienced depression while using LNG-IUDs. Although the results of this questionnaire are not sufficient to prove a correlation between levonorgestrel use and depression, the incidence of depression in people using LNG-IUDs is as high as 36%, which still requires sufficient attention. Another partially randomized trial of 1,600 LNG-IUD users revealed that 5.4% of users developed depression or depressed mood27. In addition, several reports suggest that depression and mood swings are among the important reasons leading to the discontinuation of LNG-IUD use28,29,30,31,32.
However, some studies do not support a correlation between levonorgestrel use and depression. One study with a sample of 350 women reported no significant difference in depression scores between women using LNG-IUDs and women using copper-containing IUDs33. Another study of 120 premenopausal women using LNG-IUDs revealed no significant difference in depression scores after LNG-IUD use compared with baseline scores34. Our results revealed that out of more than 436,000 records of levonorgestrel-related AEs in the FAERS database, 4,517 included reports of depression. The ROR, PRR, BCPNN, and MGPS all showed positive signals between levonorgestrel use and depression, suggesting that there is a significant correlation between levonorgestrel use and depression. Although the available results on the correlation between levonorgestrel use and depression are still controversial, our study of many adverse reactions recorded in the FAERS database revealed a strong pharmacovigilance signal between levonorgestrel use and depression, so we believe that the occurrence of depression during treatment with levonorgestrel should receive attention.
Medroxyprogesterone is a progestogen that is administered by injection and orally. The current results on the association between medroxyprogesterone use and depression is a topic of controversy. A cohort study determined Center for Epidemiologic Studies Depression (CESD) scores for 80 women using medroxyprogesterone, with a CESD score over 16 indicating depression. The results of the study revealed that women using medroxyprogesterone had a mean CESD score of 15.6, which did not meet the diagnostic criteria for depression, but medroxyprogesterone still increased the risk of depression35. A randomized controlled study evaluating women using medroxyprogesterone via the Edinburgh Postnatal Depression Scale (EPDS) and Beck Depression Inventory (BDI) score revealed a low EPDS score after 1 month of medroxyprogesterone use and a low BDI score after 3 months of medroxyprogesterone use; these results revealed an increased risk of depression with short-term medroxyprogesterone use36. These findings are consistent with those of our study. However, some studies have shown that medroxyprogesterone may reduce the risk of depression. A study of perimenopausal and postmenopausal women revealed that short-term medroxyprogesterone use did not increase the risk of depression37. Importantly, our study revealed a strong pharmacovigilance signal between levonorgestrel and depression but no positive signal between levonorgestrel and major depression. Medroxyprogesterone presented positive signals with both depression and major depression. In addition, the results of the age-stratified analysis indicated that medroxyprogesterone exhibited a positive signal in individuals younger than 18 years. Therefore, clinicians should pay attention to the occurrence of depressive symptoms in individuals using medroxyprogesterone. More importantly, although none of the seven progestogens, including levonorgestrel and medroxyprogesterone, presented negative pharmacovigilance signals for suicide and self-harm, medroxyprogesterone presented positive pharmacovigilance signals for suicidal thoughts. Moreover, among the 436,000 AEs recorded for levonorgestrel, 18 successful suicides were reported, but among the 48,000 AEs recorded for medroxyprogesterone, 5 successful suicides were reported. Although our results did not reveal a positive pharmacovigilance signal between progestogen and suicide completion, medroxyprogesterone presented a positive pharmacovigilance signal for suicidal thoughts; therefore, clinicians should be vigilant for possible suicidal behaviour in individuals using medroxyprogesterone. In the FAERS database, 85.4% of patients used levonorgestrel as an IUD. Compared with IUDs, oral and injection results in patients receiving a larger dose. Therefore, we speculate that the greater risk of depression caused by medroxyprogesterone may be related to the dose of administration.
However, the mechanism by which progestogen causes depression has not been fully elucidated. A previous study revealed that LNG-IUD users have significantly increased responsiveness to psychosocial stress, which is closely related to the absorption of levonorgestrel released by LNG-IUDs into the blood. The entry of levonorgestrel into the blood circulation can affect the hypothalamic/pituitary axis, which can adversely affect mood38. A basic study in rats revealed that 17α-hydroxyprogesterone caproate inserted into progesterone receptors in the medial prefrontal cortex during development caused damage to the medial prefrontal cortex serotonergic nerve, thereby affecting 5-HT nerve-mediated behaviour39. Since impaired 5-HT transmission is considered one of the important mechanisms of depression40, this report suggests that progesterone use may be correlated with the occurrence of depression.
Admittedly, there are several limitations to our study. First, the FAERS database is a spontaneous reporting system for adverse reactions, and some reporters may lack information when providing information related to adverse reactions, which can lead to the loss of some valid data. Second, only information about adverse reactions is recorded in the FAERS database, and information about drug users who do not experience adverse reactions is not included; therefore, we could not calculate the exact incidence of adverse reactions. Disproportionation analysis and Bayesian analysis can be used to detect signals of new or unusual AEs for drugs, but cannot be used as a substitute for relative risk or ratio ratios. Third, since progestogen is used in combination with other drugs in some cases but we did not consider accompanying drugs, the next step will be to study drug interactions. Although our study has several limitations, the FAERS database recorded 6,502 AEs related to progesterone-induced depression, so our study can provide important reference information for the practical application of progestogens in clinical practice.
Conclusions
The results of this study showed that levonorgestrel, medroxyprogesterone and etonogestrel showed positive pharmacovigilance signals associated with depression. Medroxyprogesterone also showed a positive pharmacovigilance signal associated with suicidal ideation.We recommend that clinicians monitor for depression-related symptoms when using the aforementioned progestogen. At the same time, due to the small sample size, more clinical studies are needed to confirm the safety of progesterone, desogestrel and megestrol.
Data availability
All data is publicly available on the FDA website (https:// fis. fda. gov/ exten sions/ FPD- QDE- FAERS/ FPD- QDE- FAERS. html).
References
Young, E. & Korszun, A. Sex, trauma, stress hormones and depression. Mol. Psychiatry. 15 (1), 23–28 (2010).
Hasin, D. S. et al. Epidemiology of major depressive disorder: results from the national epidemiologic survey on Alcoholism and related conditions. Arch. Gen. Psychiatry. 62 (10), 1097–1110 (2005).
Wight, R. G., Sepulveda, J. E. & Aneshensel, C. S. Depressive symptoms: how do adolescents compare with adults? J. Adolesc. Health. 34 (4), 314–323 (2004).
Chandra, A. et al. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 23 ;(25):1–160. (2005).
Tayebeh Rakhshani, P. et al. The relationship between spiritual health, quality of life, stress, anxiety and depression in working women. Front. Public. Health 2024 Aug 29:121366230 .
Xu, S., Wang, X. & Zhang, Y. Et,al.Comparison the effects of progestin-primed ovarian stimulation (PPOS) protocol and GnRH-a long protocol in patients with normal ovarian reserve function. Gynecol. Endocrinol. 39 (1), 2217263 (2023).
Zhang, P. & Wang, G. Progesterone resistance in endometriosis: current evidence and putative mechanisms. Int. J. Mol. Sci. 24 (8), 6992 (2023).
Shao, F., Li, Y. & Zhao, Y. Progestin plus metformin improves outcomes in patients with endometrial hyperplasia and early endometrial cancer more than progestin alone: a meta-analysis. Front. Endocrinol. (Lausanne). 14, 1139858 (2023).
Shufelt, C. L. & Bairey Merz, C. N. Contraceptive hormone use and cardiovascular disease. J. Am. Coll. Cardiol. 53 (3), 221–231 (2009).
Jewson, M., Purohit, P. & Lumsden, M. A. Progesterone and abnormal uterine bleeding/menstrual disorders. Best Pract. Res. Clin. Obstet. Gynaecol. 69, 62–73 (2020).
Bosanac, S. S. et al. Progestins and acne vulgaris: a review. Dermatol. Online J. 24 (5), 13030 (2018).
Xiao, L. et al. Autism-like behavior of murine offspring induced by prenatal exposure to progestin is associated with gastrointestinal dysfunction due to claudin-1 suppression. FEBS J. 290 (13), 3369–3382 (2023).
Sassarini, D. J. Depress. Midlife Women Maturitas 2016 Dec. ; 94:149–154 .
Wium-Andersen, M. K. et al. Association of hormone therapy with Depression during Menopause in a cohort of Danish women. JAMA Netw. Open. 5 (11), e2239491 (2022).
Guillard, H. et al. Modeling the potential benefit of an over-the-counter progestin-only pill in preventing unintended pregnancies in the U.S. Contraception 117, 7–12 (2023).
Guay, É. et al. Rapid Improvement of Post-partum Depression with Subanesthetic Racemic Ketamine.J Clin Psychopharmacol. Mar-Apr 01 (2), 196–198 (2024).
Shu, Y. et al. Adverse events with risankizumab in the real world: postmarketing pharmacovigilance assessment of the FDA adverse event reporting system. Front. Immunol. 14, 1169735 (2023).
Bu, K. et al. Dysphagia Risk in patients prescribed Rivastigmine: a systematic analysis of FDA adverse event reporting system. J. Alzheimers Dis. 89 (2), 721–731 (2022).
Grindlay, K. et al. Interest in over-the-counter progestin-only pills among transgender, nonbinary, and gender-expansive individuals in the United States. Am. J. Obstet. Gynecol. 230 (6), 657e1–657e17 (2024).
Chumakov, E. M. et al. Anxiety and Depression among Transgender people: findings from a cross-sectional online survey in Russia. LGBT Health 2021 Aug-Sep ;8(6):412–419 .
Eugène, P. et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 11 (1), 3–10 (2002 Jan-Feb).
Bate, A. et al. A bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54 (4), 315–321 (1998).
Du Mouchel, W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting. System[J] Am. Stat. 53 (3), 177–190 (1999).
Claire Langlad, A. et al. Adverse events reported for levonorgestrel-releasing IUD Mirena® in France and impact of media coverage. Br. J. Clin. Pharmacol.85 (9), 2126–2133 (2019).
Horibe, M. et al. Contraceptives as possible risk factors for postpartum depression: a retrospective study of the food and drug administration adverse event reporting system, 2004–2015. Nurs. Open. 5 (2), 131–138 (2018).
Cox, M. & Tripp, J. Sarah Blacksell.Clinical performance of the levonorgestrel intrauterine system in routine use by the UK Family Planning and Reproductive Health Research Network: 5-year report. J. Fam Plann. Reprod. Health Care. 28 (2), 73–77 (2002).
Eisenberg, D. L. et al. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception 92 (1), 10–16 (2015).
Backman, T., Huhtala, S., Blom, T., Luoto, R. & Rauramo I,Koskenvuo, M. Length of use and symptoms associated with premature removal of the levonorgestrel intrauterine system: a nation-wide study of 17,360 users. BJOG.2000;107(3):335–339. (2000).
Cox, M., Tripp, J. & Blacksell, S. Clinical performance of the levonorgestrel intrauterine system in routine use by the UK Family Planning and Reproductive Health Research Network:5-year report. J. Fam Plann. Reprod. Health Care. 28 (2), 73–77 (2002).
Elovainio, M. et al. Depressive symptoms as predictors of discontinuation of treatment of menorrhagia by levonorgestrel-releasing intrauterine system. Int. J. Behav. Med. 14 (2), 70–75 (2007).
Daud, S. & Ewies, A. A. Levonorgestrel-releasing intrauterine system: why do some women dislike it? Gynecol. Endocrinol. 24 (12), 686–690 (2008).
Hall, K. S. et al. Contraception and mental health: a commentary on the evidence and principles for practice. Am. J. Obstet. Gynecol. 212 (6), 740–746 (2015).
Enzlin, P. et al. Sexual functioning in women using levonorgestrel-releasing intrauterine systems as compared to copper intrauterine devices. J. Sex. Med. 9 (4), 1065–1073 (2012).
Tazegul Pekin, A. et al. Depressive symptomatology and quality of life assessment among women using the levonorgestrel-releasing intrauterine system: an observational study. Arch. Gynecol. Obstet. 290 (3), 507–511 (2014).
Westhoff, C., Wieland, D. & Tiezzi, L. Depression in users of depo-medroxyprogesterone acetate. Contraception 51, 351–354 (1995).
Singata-Madliki, M., Hofmeyr, G. J. & Lawrie, T. A. The effect of depot medroxyprogesterone acetate on postnatal depression: a randomized controlled trial. J. Fam Plann. Reprod. Health Care 2016:1–6 .
Maria Pia Rogines-Velo & Heberle, A. E. Hadine Joffe. Effect of medroxyprogesterone on depressive symptoms in depressed and nondepressed perimenopausal and postmenopausal women after discontinuation of transdermal estradiol therapy.Menopause. ;19(4):471–475. (2012).
Aleknaviciute, J. et al. The levonorgestrel-releasing intrauterine device potentiates stress reactivity. Psychoneuroendocrinology 80, 39–45 (2017).
Fahrenkopf, A., Li, G. & Wood, R. I. Developmental exposure to the synthetic progestin, 17α-hydroxyprogesterone caproate, disrupts the mesocortical serotonin pathway and alters impulsive decision-making in rats. Dev. Neurobiol. 81 (6), 763–773 (2021).
Krupa, A. J., Wojtasik-Bakalarz, K. & Siwek, M. Vortioxetine-pharmacological properties and use in mood disorders. The current state of knowledge. Psychiatr Pol. 57 (6), 1109–1126 (2023).
Funding
Funding for this research came from project ZR2022QC167 supported by Shandong Provincial Natural Science Foundation and project XY20BS11 supported by Heze University doctoral Fundation.
Author information
Authors and Affiliations
Contributions
H.G was responsible for the design of the research, experimental data analysis, and manuscript preparation. X.Z and Y.H were responsible for the interpretation of data. H.W was responsible for designed and directed the research. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Author contributions
Hui Gao was responsible for the design of the research, experimental data analysis, and manuscript preparation.Xiaohan Zhai and Yan Hu were responsible for the interpretation of data. Hang Wu was responsible for designed and directed the research.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, H., Zhai, X., Hu, Y. et al. Pharmacovigilance study of the association between progestogen and depression based on the FDA adverse event reporting System (FAERS). Sci Rep 15, 1302 (2025). https://doi.org/10.1038/s41598-025-85826-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85826-1