Abstract
Esophageal cancer is one of the most prevalent malignancies. This study aimed to examine the impact of factors such as immunotherapy, altitude, radiotherapy target volume, and radiotherapy dose on the prognosis of patients with locally advanced and advanced esophageal cancer who are receiving definitive radiotherapy and living in high-altitude regions. We retrospectively collected data from all patients with locally advanced and advanced esophageal cancer who completed definitive radiotherapy at Yunnan Cancer Hospital between January 2017 and January 2023. A total of 274 patients were included, with a median follow-up time of 24.5 months. The median overall survival (OS) and progression-free survival (PFS) were 15.0 months and 11.0 months, respectively. Adjuvant therapy (including chemotherapy, immunotherapy, and antiangiogenic targeted therapy, P = 0.004) and gross target volume (GTV, P = 0.015) were independent predictors of overall survival, whereas body mass index (BMI, P = 0.037) was an independent predictor of progression-free survival. Patients with a smaller planning target volume (PTV), clinical target volume (CTV), GTV, and gross tumor volume of metastatic regional lymph nodes (GTVnd), as well as those with a smaller New target volume, had a better prognosis. Treatment efficacy affects patient prognosis, with those showing early therapeutic effectiveness having a better prognosis than those for whom the treatment is ineffective. Patients who experienced disease progression within three months after the end of radiotherapy had a poorer prognosis. The altitude and radiotherapy dose had no significant impact on the prognosis of esophageal cancer patients. The location of the lesion, GTV, and simultaneous integrated boost (SIB) radiotherapy were factors influencing the occurrence of esophageal fistulas.
Similar content being viewed by others
Introduction
Esophageal cancer (EC) is one of the most common malignant tumors in China. In Yunnan Province, esophageal cancer ranks 10th in incidence but 6th in mortality rate1. The prognosis of EC patients in Yunnan Province is worse than expected, which may be related to factors such as a high-altitude hypoxic environment and poor lifestyle habits. Yunnan Province has an average altitude of 2,000 m, with the highest altitude reaching 6,740 m, classifying it as a high-altitude area. Studies have shown that the plateau hypoxic environment is associated with hypoxia-inducible factor (HIF)2. Hypoxia activates the HIF signaling pathway3, which in turn affects vascular endothelial growth factor (VEGF) and stimulates angiogenesis4, thereby improving the tumor hypoxic environment of tumors and promoting tumor growth and metastasis. Research has shown that altitude is directly proportional to the risk of cancer and death2.
In Yunnan, most esophageal cancer patients are diagnosed only after they present with significant dysphagia, often at a locally advanced or advanced stage. Definitive chemoradiotherapy (dCRT) is the mainstay treatment, yet the prognosis remains suboptimal. The median survival for patients undergoing dCRT is only 10.3 to 15.2 months5,6,7, and the 3-year overall survival (OS) rate ranges from 12.4–39.3%5. Immunotherapy works by modulating the body’s immune system to exert antitumor effects and has shown promising outcomes in the treatment of esophageal cancer9,10. However, hypoxia can reduce the efficacy of immunotherapy, as there is an established link between hypoxia and an immunosuppressive tumor microenvironment11. Hypoxia has been shown to suppress CD4 + effector T cells while enhancing the immunosuppressive activity of regulatory T cells12.
Radiotherapy can alter the body’s immune microenvironment, enhancing the antitumor effects of immunotherapy. This mechanism may be related to radiation-induced tumor cell death, the release of new tumor antigens, and the upregulation of PD-L1 expression13. The combination of radiotherapy with anti-PD-1 antibody therapy promotes the migration of dendritic cells (DCs) and macrophages closer to tumor cells14, which could be a potential mechanism for enhancing the antitumor immune response. Additionally, immunotherapy has been shown to reduce HIF-1 levels15. Patients treated with both radiotherapy and immunotherapy have shown better prognoses than those receiving concurrent chemoradiotherapy16. Therefore, combining immunotherapy with radiotherapy may offer more benefits to esophageal cancer patients in high-altitude regions.
Radiation of regional lymph nodes remains controversial, particularly regarding elective nodal irradiation (ENI) versus involved-field irradiation (IFI). IFI refers to irradiating only the lymph nodes that are confirmed to have metastasis, whereas ENI involves irradiating not only the metastatic lymph nodes but also high-risk lymph nodes that have not yet shown signs of metastasis. Compared with EN I, IFI results in a smaller irradiation field. Studies have reported no significant differences in median progression-free survival (PFS) ( 20.3 months vs. 21.4 months ) or OS ( 32.5 months vs. 34.9 months ) between the ENI and IFI groups17. The current trend in regional lymph node irradiation is to focus on the involved field. The size of the irradiation field influences the treatment design and completion rate, which may subsequently affect the efficacy and prognosis. Research indicates that patients with smaller gross target volumes (GTV) may gain more significant benefits from higher radiotherapy doses for local lesions (34.7 months vs. 30.3 months)18. The clinical target volume (CTV) and planning target volume (PTV)19 can also impact patient prognosis by affecting the completion rate of concurrent chemotherapy. The volume of radiotherapy targets has critical predictive value for treatment outcomes and prognosis, serving as a reference for formulating individualized treatment plans.
In the era of immunotherapy, several challenges remain in developing treatment plans for esophageal cancer patients in high-altitude regions. Factors such as immunotherapy, altitude, radiotherapy target volume, radiotherapy dose, and induction chemotherapy all influence patient prognosis. Our team has conducted systematic research on these issues to offer more options for clinical decision-making.
Materials and methods
The clinical data of all esophageal cancer patients who received radiotherapy at Yunnan Cancer Hospital from January 2017 to January 2023 were retrospectively analyzed. The inclusion criteria were: a pathological diagnosis of esophageal malignancy, AJCC version 8 stage III-IV, and radiotherapy with a total dose of ≥ 50 Gy, administered in 25–35 fractions with a single dose of 1.8–2.0 Gy. The exclusion criteria included incomplete radiotherapy, nonesophageal irradiation sites, preoperative or postoperative radiotherapy, the presence of other malignancies, incomplete medical records, and loss to follow-up.
Specifically, for delineation of radiotherapy target areas, the gross target volume (GTV) includes the primary esophageal tumor as clinically evaluated before treatment. The gross tumor volume of metastatic regional lymph nodes (GTVnd) encompasses lymph nodes with confirmed metastasis or those where metastasis cannot be ruled out. The clinical target volume (CTV) is defined as a 3 cm expansion in the cranial and caudal directions from the GTV, and a 0.5–1.0 cm three-dimensional expansion around the GTVnd. The lymphatic drainage area can include either elective nodal irradiation or involved-field irradiation, depending on the specific delineation performed by the physician. The planning target volume (PTV) is created by expanding the CTV by 0.5–1.0 cm in all directions.
The volumes of the PTV, CTV, GTV, and GTVnd can be calculated from the Monaco and Pinnacle radiotherapy planning systems, with units measured in cm³. The New volume (New) is defined as the CTV minus the GTV and GTVnd, representing the expanded range of the CTV. Simultaneous integrated boost (SIB) refers to the simultaneous administration of different radiation doses to the GTV and PTV during radiotherapy, with the GTV receiving a higher dose than the PTV. The radiotherapy techniques used include intensity modulated radiation therapy (IMRT) and three-dimensional conformal radiotherapy (3DCRT), with IMRT being the predominant technique, accounting for more than 99% of the usage.
The chemotherapy regimen is formulated with reference to the Clinical Practice Guidelines for Malignant Tumors published annually by the National Comprehensive Cancer Network (NCCN) and the Chinese Society of Clinical Oncology (CSCO). The final drug regimen and dosage are determined by the clinician on the basis of the patient’s specific tolerance. Induction chemotherapy refers to chemotherapy administered before radiotherapy, whereas concurrent chemotherapy is defined as chemotherapy given from the first day of radiotherapy until the final day of radiotherapy. Adjuvant therapy includes all treatments received after the completion of radiotherapy, such as chemotherapy, immunotherapy, antiangiogenic targeted therapy, and other treatments, but excludes treatments given after recurrence or disease progression.
The patients included in this analysis received chemotherapy regimens such as TP (paclitaxel + cisplatin/carboplatin/nedaplatin/lobaplatin), PF(paclitaxel + fluorouracil), DP(docetaxel + cisplatin/nedaplatin/lobaplatin), EP(etoposide + cisplatin/nedaplatin/lobaplatin), NP(vinorelbine + nedaplatin), XELOX(capecitabine + oxaliplatin), FOLFOX(fluorouracil + leucovorin + oxaliplatin), and capecitabine, with TP and PF being the most commonly used regimens. The chemotherapy drugs administered included paclitaxel, cisplatin, carboplatin, nedaplatin, lobaplatin, docetaxel, etoposide, vinorelbine, fluorouracil, S-1, capecitabine, and oxaliplatin. Induction chemotherapy ranged from 1 to 6 cycles, concurrent chemotherapy consisted of 1 to 2 cycles, and adjuvant chemotherapy ranged from 1 to 6 cycles. The immunotherapy agents included camrelizumab, tislelizumab, pembrolizumab, toripalimab, durvalumab, and sintilimab. The number of treatment cycles ranged from 1 to 11 cycles. The antiangiogenic targeted agents used included anlotinib, nivolumab, and bevacizumab.
Overall survival (OS) was defined as the time from the completion of radiotherapy to death from any cause or the last follow-up. Progression-free survival (PFS) was defined as the time from the completion of radiotherapy to tumor progression or death from any cause. Short-term efficacy was assessed via an esophageal barium swallow combined with a CT examination20,21. The evaluation outcomes were classified into complete response (CR), partial response (PR), and nonresponse or disease progression (NR). CR and PR were defined as effective responses, whereas NR was defined as ineffective. The efficacy rate was calculated as (CR + PR) / total population.
X-Tile software was used to determine the cutoff value for the target volume. Data statistics and analysis were conducted via SPSS 26.0 software. The Kaplan‒Meier method was used to plot the survival curves for each group, and the log-rank test was used to compare the median survival and survival rates between groups. Logistic regression analysis was performed to examine the influencing factors of esophageal fistula. The Cox proportional hazards regression model was applied for univariate and multivariate prognostic correlation analysis.
Results
Overall population
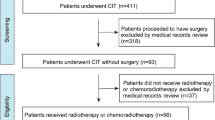
A total of 274 patients were included in the final analysis on the basis of the inclusion and exclusion criteria (Fig. 1). The mean age was 60.7 years (± 9.9), with a median body mass index (BMI) of 20.2 (range, 13.8 ~ 31.6). The median altitude of the patients’ residences was 1750 m (IQR:1420–1891 m). Most of the patients were male (266 patients, 97.1%) and were diagnosed with squamous cell carcinoma (263 patients, 96%). The median length of the lesion was 5.6 cm ( range, 0.6~14 cm), which was predominantly located in the mid-thoracic segment (126 patients, 46%), with only 13 patients (4.7%) presenting with lesions in the cervical segment. All patients, except one, had an ECOG performance status score of 0–1. The distribution of clinical stages was as follows: 121 patients (44.2%) were in stage III, 95 patients (34.7%) were in stage IVA, and 58 patients (21.2%) were in stage IVB. A total of 263 patients (96%) received chemotherapy, 239 patients (87.2%) received induction chemotherapy, and 167 patients (60.9%) received concurrent chemotherapy. Additionally, 101 patients (36.9%) received immunotherapy. The detailed baseline characteristics are shown in Table 1.
The median follow-up period for all patients was 24.5 months, and the median overall survival (OS) and progression-free survival (PFS) were 15.0 and 11.0 months, respectively. The OS and PFS rates at 1, 2, and 3 years were 57%, 34.3%, and 27%, respectively, and 46.1%, 19%, and 12.5%, respectively. Clinical baseline characteristics and treatment factors were included in the Cox univariate and multivariate analyses. Univariate Cox regression analysis demonstrated that BMI (P = 0.018), lesion location (P = 0.036), adjuvant therapy (P < 0.001), immunotherapy (P = 0.011), and GTV (P = 0.026) were significant factors influencing OS. The analysis also revealed that immunotherapy (P = 0.012) and adjuvant therapy (P = 0.004) significantly affected PFS (Table 3). In the multivariate analysis, adjuvant therapy (P = 0.004, HR = 1.871) and GTV (P = 0.015, HR = 1.005) were identified as independent predictors of OS (Table 2). Additionally, the analysis indicated that BMI (P = 0.037, HR = 0.946) was an independent predictor of PFS.
Immunotherapy
Patients were divided into two groups based on whether they received immunotherapy: the immunotherapy group (n = 101, 36.9%) and the non-immunotherapy group (n = 173, 63.1%). Compared to the nonimmunotherapy group, the immunotherapy group had significantly higher proportions of concurrent chemotherapy (P = 0.015), antiangiogenic targeted therapy (P = 0.014), adjuvant therapy (P < 0.001), standard radiotherapy dose (P = 0.002), and metastasis (P = 0.010). No significant differences were observed in the other baseline characteristics. The median OS and PFS were 22.0 and 12.0 months, respectively, for the immunotherapy group, and 14.0 and 10.0 months for the nonimmunotherapy group, respectively, with statistically significant differences (P = 0.010 for both OS and PFS).
The immunotherapy group was further subdivided into a preradiotherapy group (n = 47, 46.5%) and a postradiotherapy group (n = 54, 53.5%) on the basis of the timing of immunotherapy administration. The postradiotherapy group included patients who received immunotherapy during or after radiotherapy. No significant differences in baseline characteristics were observed between the two groups. Compared to the preradiotherapy group, the postradiotherapy group demonstrated superior OS (25.7 months vs. 14.0 months, P = 0.039) and PFS (16.0 months vs. 11.0 months, P = 0.079). The 1-year OS and PFS rates were 79.5% and 64.7%, respectively, for the postradiotherapy group, and 69.1% and 44.9% for the preradiotherapy group, respectively. The survival curves are presented in Fig. 2.
Univariate analysis revealed that age (P = 0.024, HR = 0.486) and the sequence of immunotherapy and radiotherapy (P = 0.044, HR = 0.509) were significant factors influencing OS, whereas age (P = 0.021, HR = 0.494) was a significant factor influencing PFS. Multivariate analysis indicated that age (P = 0.004, HR = 0.328) and the sequence of immunotherapy and radiotherapy (P = 0.007, HR = 0.308) were independent predictors of OS in patients receiving immunotherapy. Furthermore, age (P = 0.032, HR = 0.487), GTV (P = 0.028, HR = 2.062), and the sequence of immunotherapy and radiotherapy (P = 0.017, HR = 0.428) were independent predictors of PFS in these patients.
Kaplan-Meier survival analysis related to immunotherapy. (A) Overall survival (OS) curves for patients who received immunotherapy versus those who did not. (B) Progression-free survival (PFS) curves for patients who received immunotherapy versus those who did not. (C) OS curves for patients who received immunotherapy before versus after radiotherapy. (D) PFS curves for patients who received immunotherapy before versus after radiotherapy.
Volume of radiotherapy target
Owing to variations in physician delineation practices and missing data, the collected volumes for PTV, CTV, GTV, GTV-nd, and New were 228, 192, 214, 149, and 191 cases, respectively. The median volumes were as follows: PTV, 334.24 cm2 (IQR: 230.76–485.46 cm2); CTV, 187.25 cm2 (IQR: 114.21–286.16 cm2); GTV, 36.95 cm2 (IQR: 22.80–64.32 cm2); GTVnd, 10.98 cm2 (IQR: 4.26–25.86 cm2); and New, 137.13 cm2 (IQR: 72.18–212.12 cm2). Using X-Tile software, the cutoff values for PTV, CTV, GTV, GTV-nd, and New were determined to be 300 cm2, 178 cm2, 35 cm2, 10 cm2, and 117 cm2, respectively. The analysis revealed that patients with PTV < 300 cm2, CTV < 178 cm2, GTV < 35 cm2, GTVnd < 10 cm2, and New < 117 cm2 had longer median survival times. Smaller target volumes were associated with better patient prognoses (Table 4).
Radiotherapy dose and altitude
According to the Guidelines for the Diagnosis and Treatment of Esophageal Cancer (2022 Edition)22, the recommended dose for definitive radiotherapy is 60 Gy, although it can be reduced to 50–54 Gy for definitive concurrent chemoradiotherapy. In this study, a cutoff of 54 Gy was used, with doses > 54 Gy classified as the high-dose group, and doses ≤ 54 Gy as the standard-dose group. The high-dose group included 152 patients with a mean dose of 61.0 Gy (range: 55.8–70.0 Gy), whereas the standard-dose group included 122 patients with a mean dose of 50.7 Gy (range: 50.0–54.0 Gy). There was no significant difference in OS (14.0 months vs. 15.0 months, P = 0.662) or PFS (10.4 months vs. 11.4 months, P = 0.932) between the two groups (Fig. 3A-B). To rule out the effect of immunotherapy on outcomes, subgroup analyses were performed on patients who did and did not receive immunotherapy. There were no statistically significant differences in OS (P = 0.899, P = 0.186) or PFS (P = 0.760, P = 0.160) between the groups. The median altitude of patients’ residences was 1750 m (IQR: 1420–1891 m). Patients were divided into a high-altitude group (> 1500 m, 195 patients, 71.2%) and a low-altitude group (≤ 1500 m, 79 patients, 28.8%). No statistically significant differences in OS or PFS were observed between these two groups (Supplemental Table 1).
Induction chemotherapy
Induction chemotherapy was defined as chemotherapy administered prior to radiotherapy, with 239 patients receiving induction chemotherapy. On the basis of whether they received concurrent chemotherapy after induction chemotherapy, 147 patients (61.5%) were classified into the concurrent chemoradiotherapy group, whereas 92 patients (38.5%) were in the radiotherapy-only group. The median OS and PFS for the entire induction chemotherapy population were 15 and 11 months, respectively. There was no statistically significant difference in median OS (15.0 months vs. 14.0 months, P = 0.773) or PFS (12.0 months vs. 11.0 months, P = 0.656) between the concurrent chemoradiotherapy and radiotherapy-only groups.
Multivariate analysis revealed that age (P = 0.028), adjuvant therapy (P = 0.020), and short-term efficacy (P = 0.043) were independent predictors of OS, whereas adjuvant therapy (P = 0.023) and short-term efficacy (P = 0.018) were independent predictors of PFS. Esophageal fistula occurred in 22 patients (9.2%) who received induction chemotherapy. The incidence of esophageal fistula increased with increasing target volume, including PTV (7.8% vs. 6.8%, P = 0.016), CTV (7.5% vs. 5%, P = 0.021), GTV (9.5% vs. 3.4%, P = 0.240), and New (7.1% vs. 5.4%, P = 0.144).
Esophageal fistula
In the overall population, 24 patients (8.8%) developed esophageal fistula, and the median survival time for patients with esophageal fistula was significantly shorter than that for those without fistula (8.4 months vs. 15.0 months, P = 0.003). Logistic regression analysis was performed, incorporating relevant factors. The results indicated that lesion location (OR = 0.382, 95% CI: 0.171–0.854, P = 0.019), GTV (OR = 1.016, 95% CI: 1.003–1.030, P = 0.014), and SIB (OR = 17.309, 95% CI: 1.725–175.653, P = 0.015) were statistically significant factors for predicting esophageal fistula. The lower the lesion location is, the lower the risk of esophageal fistula. For every 1 cm³ increase in the GTV, the risk of esophageal fistula increased by 0.01-fold. The risk of esophageal fistula in patients receiving SIB was 16.309 times greater.
Short-term efficacy and progression
A combination of CT and barium meal was used to evaluate the short-term outcomes of 91 patients. Among them, 1 patient achieved complete remission, 53 patients achieved partial remission, and 37 patients experienced no remission or disease progression, resulting in an overall response rate of 59.3%. Patients who responded positively to short-term efficacy had significantly better OS and PFS than did those who did not (median OS: 22.0 months vs. 11.0 months, P = 0.004; median PFS: 14.5 months vs. 8.4 months, P = 0.001).
Patients were categorized into a local progression group (41 patients, 59.4%) and a distant metastasis group (28 patients, 40.6%). Local recurrence included the recurrence of primary esophageal lesions and regional lymph nodes. There was no statistically significant difference in median OS (16.0 months vs. 12.7 months, P = 0.567) or PFS (13.0 months vs. 11.4 months, P = 0.298) between the local recurrence group and the distant metastasis group (Fig. 3C).
Furthermore, the prognosis of patients whose disease progressed within three months of the end of radiotherapy was significantly worse than that of patients whose disease progressed after three months of radiotherapy (median OS: 11.0 months vs. 16.0 months, P = 0.005). The timing of disease progression was identified as a prognostic factor (Fig. 3D).
Discussion
Immunotherapy is a known prognostic factor in patients with esophageal cancer23. In our study, patients who received immunotherapy had better median OS (22 months vs. 12 months, P = 0.010) and PFS (14 months vs. 10 months, P = 0.010) than those who did not. However, the multivariate analysis did not reveal significant differences. More data and research are needed to validate the effectiveness of immunotherapy. The sequence of radiotherapy and immunotherapy can influence patient prognosis. A patient study24 reported that patients treated with sintilimab within three months after radiotherapy achieved favorable median OS and PFS, indicating that three months may be a critical time point. In our study, the postradiotherapy immunotherapy group exhibited superior OS (25.7 months vs. 14.0 months, P = 0.039) and PFS (16.0 months vs. 11.0 months, P = 0.079) than did the preradiotherapy group. Nonetheless, no significant statistical differences were observed in the multivariate analysis. Radiotherapy may improve the immune microenvironment, potentially enhancing the antitumor efficacy of immunotherapy; however, further extensive clinical studies are needed to confirm this.
The size of the irradiation field impacts patient prognosis. An expanded irradiation area may increase the occurrence of adverse events, which could negatively affect survival. The size of the irradiation field can be measured by the target volume. A larger planning target volume (PTV) has been associated with an increased incidence of grade 4 lymphopenia25, which is correlated with poorer outcomes. A PTV greater than 521.2 cm³ has been identified as an independent risk factor for lymphopenia26. In this study, patients with larger PTV (P = 0.016) and CTV (P = 0.021) had higher incidences of esophageal fistula. Smaller target volumes were linked to better prognoses, which is consistent with previous findings. particularly in the population receiving immunotherapy. In the immunotherapy cohort, patients with a smaller New volume had a longer median OS (33.0 months vs. 14.0 months, P = 0.134, HR = 1.680). Moreover, survival outcomes in the postradiotherapy group were better than those in the preradiotherapy group (P = 0.039), potentially because there was a greater proportion of patients with New volumes < 117 cm³ in the postradiotherapy group than in the preradiotherapy group (53.2% vs. 21.6%, P = 0.004). These findings suggest that reducing the CTV could minimize radiotherapy-induced immune system damage and improve patient prognosis. The impact of the radiotherapy target size on prognosis is not absolute. It is essential to consider the radiotherapy dose, the effect of the irradiation range on the body’s immune environment, and the overall intensity of the treatment regimen.
The dose of radiotherapy also affects patient prognosis. Both the INT0123 study27 and the ARTDECO study28 revealed that higher doses of radiotherapy did not improve local control or survival, thus recommending 50.4 Gy is recommended as the standard radiation dose. Our findings align with these studies, as increasing the radiotherapy dose did not significantly increase patient survival. In a phase 1b study29 in which where camrelizumab was administered concurrently with chemoradiotherapy (60 Gy), 65% of patients achieved an objective response after receiving 40 Gy of irradiation. When combined with immunotherapy and chemotherapy, several challenges remain, such as determining the optimal radiotherapy dose and formulating individualized radiotherapy plans.
The hypoxic environment reduces oxidative DNA damage, and in a mouse study, median tumor-free survival increased by approximately 50%30. On the other hand, hypoxia, via the HIF-1α/A2 adenosine pathway, can mediate immunosuppression31. Hypoxia and HIF are also key factors contributing to treatment resistance32. In this study, there was no significant difference in the outcomes of patients living at different altitudes, and the residence altitude did not affect short-term efficacy. However, in terms of long-term survival, the 3-year OS rate of patients living at high altitudes was lower than that of patients living at low altitudes(25% vs. 32.7%), This may be due to the relatively short survival time of EC patients, so the effect of altitude on survival may not be immediately apparent.
Furthermore, among the 91 patients whose short-term efficacy data were collected in this study, only one patient achieved CR (1.1%), which is much lower than that reported in previous studies (12.9–24%)7. Possible reasons for this include overly strict evaluation criteria. When assessed solely by esophageal barium radiography, the CR rate was 14.8%. Another possible factor is the influence of the high-altitude hypoxic environment. The hypoxic environment here refers to the altitude of the hospital during treatment rather than the altitude of residence, and further experiments are needed to confirm this hypothesis.
A phase II clinical trial34 compared the effects of definitive chemoradiotherapy (CRT) following induction chemotherapy with those of CRT alone. The overall response rate was similar between the two groups (74.5% vs. 61.8%, P = 0.152). In this study, induction chemotherapy did not significantly increase survival (median OS: 15 months vs. 16 months, P = 0.890), which is consistent with previous findings. Induction therapy before radiotherapy can be combined with immunotherapy, with pembrolizumab combined with chemotherapy achieving an objective response rate as high as 87.2%35. In another ongoing multicenter, prospective clinical study36, patients receiving immunotherapy combined with chemotherapy underwent induction therapy; those who responded significantly to the treatment proceeded with definitive chemoradiotherapy, while those with less favorable responses were treated surgically.
This study analyzed prognostic factors related to esophageal cancer patients in the plateau region of Yunnan Province. However, several limitations remain. Since the research was conducted at a single medical institution, the sample size was relatively small, and we were unable to control for all potential confounding factors, which may have affected the statistical significance of the results and the inference of causal relationships. Additionally, as this is a retrospective study, missing data or inconsistencies in documentation may impact the reliability of the findings.
Conclusion
Adjuvant therapy and gross tumor volume (GTV) were identified as independent predictors of overall survival (OS), with patients with smaller radiotherapy target volumes having better prognoses. Neither the altitude of residence nor the radiotherapy dose had a significant effect on prognosis. In the era of immunotherapy, further clinical trials are needed to explore individualized treatment plans for patients, considering factors such as radiotherapy target volume, radiotherapy dose, immunotherapy, high-altitude hypoxic environments, and chemotherapy.
Data availability
The data are available from the corresponding author on reasonable request.
References
Zheng, R. S. et al. Cancer statistics in China, 2016. Chin. J. Oncol. 45 (3), 212–219 (2023).
Garrido, D. I. & Garrido, S. M. Cancer risk associated with living at high altitude in Ecuadorian population from 2005 to 2014. Clujul Med. 91, 188–196 (2018).
Dzhalilova, D. S. & Makarova, O. V. HIF-dependent mechanisms of relationship between hypoxia tolerance and tumor development. Biochemistry (Mosc). 86, 1163–1180 (2021).
Dodd, K. M. & Tee, A. R. STAT3 and mTOR: Co-operating to drive HIF and angiogenesis. Oncoscience 2, 913–914 (2015).
Pai, C. P., Chien, L. I., Huang, C. S., Hsu, H. S. & Hsu, P. K. Treatment outcomes and risk factors for incomplete treatment after definitive chemoradiotherapy for non-resectable or metastatic esophageal cancer. Cancers (Basel). 15, 5421 (2023).
van Ruler, M. P. et al. Clinical outcomes of definitive chemoradiotherapy using carboplatin and paclitaxel in esophageal cancer. Dis. Esophagus. 30, 1–9 (2017).
Tsuchiya, N. et al. Chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Langenbecks Arch. Surg. 407, 1911–1921 (2022).
Zhang, P. et al. Clinical efficacy and failure pattern in patients with cervical esophageal cancer treated with definitive chemoradiotherapy. Radiother. Oncol. 116, 257–261 (2015).
Kato, K. et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 20, 1506–1517 (2019).
Kojima, T. et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J. Clin. Oncol. 38, 4138–4148 (2020).
Guan, R. Bioinformatics Study of Hypoxia and Immunosuppressive Microenvironment in Colorectal cancer (Shantou University, 2022).
Chen, P. Identification of Hypoxia-Associated Signature in Colon Cancer to Assess Tumor Immune Microenvironment and Predict Prognosis Based on 14 Hypoxia-Associated Genes (Hebei Medical University, 2023).
Dovedi, S. J. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 74, 5458–5468 (2014).
Ma, X. et al. Spatial distribution and predictive significance of dendritic cells and macrophages in esophageal cancer treated with combined chemoradiotherapy and PD-1 blockade. Front. Immunol. 12, 786429 (2022).
Liu, X. Effect of immunotherapy combined with targeted therapy on serum HIF-1α, VEGF, AFP and PHD2 levels in patients with advanced liver cancer. Med. Innov. China. 19 (34), 1–4 (2022).
Wei, H. et al. Comparison of dynamic changes in the peripheral CD8 + T cells function and differentiation in ESCC patients treated with radiotherapy combined with anti-PD-1 antibody or concurrent chemoradiotherapy. Front. Immunol. 13, 1060695 (2022).
Lyu, J. et al. Comparison between the effects of elective nodal irradiation and involved-field irradiation on long-term survival in thoracic esophageal squamous cell carcinoma patients: A prospective, multicenter, randomized, controlled study in China. Cancer Med. 9, 7460–7468 (2020).
Wang, X. M. et al. [Long-term efficacy and safety of simultaneous integrated boost radiotherapy in non-operative esophageal squamous cell carcinoma: A multicenter retrospective data analysis (3JECROG R-05)]. Zhonghua Zhong Liu Za Zhi. 43, 889–896 (2021).
Wang, S. et al. Factors affecting the completion of concurrent chemotherapy and impact of non-completion on survival in locally advanced esophageal squamous cell carcinoma. Esophagus 19, 717–725 (2022).
Ren, X. J. A Study of Barium Swallow Combined with CT to Evaluate the short-term Efficacy of Radiotherapy for Esophageal cancer (Hebei Medical University, 2012).
Han, C. Discussion on the new criteria for the evaluation of short-term efficacy after radiotherapy for esophageal cancer. In Radiation Oncology Committee of Chinese Anti-Cancer Association, Sino-American Radiation Oncology Association (SANTRO).Academic Conference of the Academic Conference of the Chinese Anti-Cancer Association (SANTRO) 2012 Jinan International Radiation Oncology Forum (2012).
Chinese Society of Clinical Oncology Radiotherapy Committee, Chinese Medical Association Radiotherapy Oncology Branch, & Chinese Anti-Cancer Association Radiation Oncology Branch. Guidelines for radiotherapy of esophageal cancer in China (2022 edition). Int. J. Oncol. 49, 641–657 (2022).
Luo, H. C. et al. Real-world treatment patterns and survival for locally advanced esophageal squamous cell carcinoma. Medicine 102 (34), e34647 (2023).
Xu, S., Xu, X. & Zhu, H. Pre-immunotherapy radiotherapy enhanced the efficacy of multi-line sintilimab in unresectable advanced esophageal squamous cell carcinoma. Front. Immunol. 14, 960339 (2023).
Ni, W. et al. Severe radiation-induced lymphopenia during postoperative radiotherapy or chemoradiotherapy has poor prognosis in patients with stage IIB-III after radical esophagectomy: a post hoc analysis of a randomized controlled trial. Front. Oncol. 12, 936684 (2022).
Zhao, Q. et al. Prognostic value of absolute lymphocyte count in patients with advanced esophageal cancer treated with immunotherapy: A retrospective analysis. Ann. Transl. Med. 10 (13), 744 (2022).
Minsky, B. D. et al. INT 0123 (Radiation Therapy Oncology Group 94 – 05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J. Clin. Oncol. 20, 1167–1174 (2002).
Hulshof, M. C. C. M. et al. Randomized study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer (ARTDECO study). J. Clin. Oncol. 39, 2816–2824 (2021).
Zhang, W. et al. Addition of camrelizumab to docetaxel, cisplatin, and radiation therapy in patients with locally advanced esophageal squamous cell carcinoma: A phase 1b study. Oncoimmunology 10, 1971418 (2021).
Sung, H. J. et al. Ambient oxygen promotes tumorigenesis. PLoS One. 6, e19785 (2011).
Hatfield, S. M. & Sitkovsky, M. V. Antihypoxic oxygenation agents with respiratory hyperoxia to improve cancer immunotherapy. J. Clin. Invest. 130, 5629–5637 (2020).
Schöning, J. P., Monteiro, M. & Gu, W. Drug resistance and cancer stem cells: The shared but distinct roles of hypoxia-inducible factors HIF1α and HIF2α. Clin. Exp. Pharmacol. Physiol. 44 (2), 153–161 (2017).
Liu, Y. et al. Comparison of concurrent chemoradiotherapy with radiotherapy alone for locally advanced esophageal squamous cell cancer in elderly patients: A randomized, multicenter, phase II clinical trial. Int. J. Cancer. 151, 607–615 (2022).
Liu, S. et al. Induction chemotherapy followed by definitive chemoradiotherapy versus chemoradiotherapy alone in esophageal squamous cell carcinoma: A randomized phase II trial. Nat. Commun. 12, 4014 (2021).
Lin, W. et al. Pembrolizumab combined with paclitaxel and platinum as induction therapy for locally advanced esophageal squamous cell carcinoma: A retrospective, single-center, three-arm study. J. Gastrointest. Oncol. 13 (6), 2758–2768 (2022).
Li, C. et al. Stratified treatment of localized cervical esophageal squamous cell carcinoma induced by neoadjuvant immunotherapy plus chemotherapy (SCENIC). J. Thorac. Dis. 14 (9), 3277–3284 (2022).
Acknowledgements
We thank our colleagues from the Yunnan Cancer Hospital for their valuable suggestions and discussions.
Funding
Young talents of Yunnan Xingdian Talents Support Program (XDYC-QNRC-2023-0189).
Author information
Authors and Affiliations
Contributions
L.W., Y.X. and J.Z. contributed to the conception and design of the study; H.B. and D.Z. collected the data; F.H., J.Z. and F.L. analyzed and interpreted the data; J.Z. and L.W. wrote sections of the manuscript. All the authors contributed to manuscript revision, and read, and approved the submitted version. Consent for publication was obtained from all the authors and participants in this article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
This study was approved by the Ethics Committee of Yunnan Cancer Hospital (approval number: KYLX2024-029). All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects and/or their legal guardian(s).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, J., Bai, H., Zhao, D. et al. Factors affecting prognosis in patients with locally advanced and advanced esophageal cancer receiving definitive radiotherapy in plateau regions. Sci Rep 15, 11017 (2025). https://doi.org/10.1038/s41598-025-86655-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86655-y
Keywords
This article is cited by
-
Real-world study of first-line immunotherapy combined with chemoradiotherapy in esophageal squamous cell carcinoma
Scientific Reports (2025)
-
68Ga-FAPI and 18F-FDG PET/CT for predicting pathologic response and progression-free survival in locally advanced esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy
European Journal of Nuclear Medicine and Molecular Imaging (2025)