Abstract
Hypertension constitutes a significant public health concern, characterized by a high incidence and mortality rate. Hypertensive kidney disease is a prevalent complication associated with hypertension and is the second leading cause of end-stage renal disease (ESRD). Renal fibrosis linked to hypertension has emerged as the third leading cause of disease in dialysis patients. Autophagy activity is crucial for maintaining homeostasis, vitality, and physiological function of kidney cells, while also protecting the kidneys from fibrosis. The deficiency of autophagy will increase the sensitivity of the kidney to the damage, leading to impaired renal function, accumulation of damaged mitochondria and more severe of renal fibrosis. However, enhancing autophagy by activating the PI3K/AKT, AMPK, and mTOR pathways, improves podocyte injury and renal pathological changes, and ameliorates renal function. Current clinical interventions aimed at halting or reversing renal fibrosis in hypertensive patients are notably limited in their efficacy. Here, we present Non-lethal Sonodynamic Therapy (NL-SDT), in which ultrasound is used to activate locally sonosensitizers, thereby stimulating the production of reactive oxygen species for the purpose of modulating cell function or fate, as a novel methodology to inhibit progression of hypertensive renal fibrosis.
To confirm whether NL-SDT can reduce hypertensive renal fibrosis and its mechanism. The mice model of hypertensive renal fibrosis was established by using osmotic minipumps (Alzet model 2004, Cupertino, CA) equipped with angiotensin-II (Ang II). The pumps were implanted in mice, ensuring constant infusion of Ang II at a dose of 1.0 µg/kg per minute for 4 weeks. The mice were exposed to 0.4 W/cm2 intensity ultrasonic radiation for 15 min at 4 h post injection of sinoporphyrin sodium (DVDMS) (4 mg/kg) into the caudal vein was repeated weekly for 4 treatments. The kidney from mice was stained with masson’s trichrome staining for collagen fiber expression, while alpha-smooth muscle actin (α-SMA) expression was determined via immunohistochemical staining. The protein levels of fibrosis parameters (α-SMA, collagen I, vimentin), pathway-related proteins (PI3K, AKT, mTORC1) and autophagy-related protein LC3B were determined using western blotting. Intracellular reactive oxygen species (ROS) levels were detected using DCFH-DA probe. Immunofluorescence was also used to observe the expression of α-SMA and E-cadherin in cells. Pathway-related protein inhibitors (the autophagy-related inhibitor 3-methyladenine (3-MA), chloroquine (CQ), ROS inhibitor N-acetyl-L-cysteine (NAC) were applied, and autophagosome changes were observed under transmission electron microscopy. Immunofluorescence was used to observe LC3 spot formation within cells.
We obtained the following results via animal and cellular research. In vivo, (1) The collagen area of renal tissue was increased significantly in Ang II group (50.6%). The positive expression of α-SMA was increased significantly (37.8%). (2) The collagen area decreased after NL-SDT treatment (34.8%). The expression of α-SMA was decreased too (48.9%). The expression of LC3B increased in NL-SDT group. (3) The effect of NL-SDT on reducing renal fibrosis can be changed by rapamycin and CQ. In vitro. (1) The expression of α-SMA, collagen I and vimentin were increased significantly in TGF-β1-induced NRK-52E cells. (2) The increase of autophagosomes was observed in TGF-β1-induced NRK-52E cells after NL-SDT. The levels of ROS were increased after NL-SDT (24.8%). The effect of NL-SDT on autophagy was reversed after administration of NAC. The expression of PI3K, P-AKT and P-mTORC1 was decreased in TGF-β1-induced NRK-52E cells after NL-SDT. NL-SDT inhibited the transition of epithelial cells into myofibroblasts by activating PI3K-AKT-mTORC1-autophagy pathway in TGF-β1-induced NRK-52E cells. (3) The administration of the pathway inhibitors showed a reciprocal effect on NL-SDT-inhibited epithelial-mesenchymal transition (EMT).
(1) NL-SDT reduced blood pressure temporarily in mice model of hypertensive renal fibrosis induced by Ang II. (2) NL-SDT alleviated renal fibrosis in mice model of hypertensive renal fibrosis induced by Ang II. (3) NL-SDT promoted autophagy by inhibiting PI3K-AKT-mTORC1 signaling pathway and alleviated renal fibrosis in mice model of hypertensive renal fibrosis induced by Ang II. NL-SDT is a non-invasive and efficacious regimen to inhibit renal fibrosis. It may be a new approach for clinical treatment of renal fibrosis, delaying or reducing the occurrence of ESRD.
Similar content being viewed by others
Introduction
During the past three decades, global hypertension prevalence has nearly doubled to be at 1.28billion1. It is a serious public health concern, characterized by a high mortality rate as well as a high prevalence2. Hypertension has a number of complications, among them kidney damage, which is becoming increasingly common around the world3. Currently, hypertensive nephropathy is the second leading cause of end-stage renal disease after diabetic nephropathy4,5,6. In clinical practice, there is currently no effective method to inhibit renal fibrosis. While ACEIs and ARBs have renal protective effects in patients with chronic kidney disease, a significant number still progress to end-stage renal disease. Prolonged use of ACEIs can lead to secondary increases in angiotensin II and aldosterone, also known as “ACE escape.“7. Discontinuation of ARB therapy can trigger a reflexive increase in renin and AngII concentrations through negative feedback within the renin-angiotensin system (RAS)8. Some traditional Chinese medicines (such as rhubarb), stem cells, purified or synthesized compounds (such as β-aminoisobutyric acid) show potential in treating renal fibrosis. However, the specific mechanisms and potential risks of these methods are still unclear and require further exploration. Therefore, understanding the pathogenesis of hypertensive nephropathy and identifying potential therapeutic targets have important scientific and clinical significance for the prevention and treatment of hypertensive nephropathy9.
The mechanism of hypertensive renal fibrosis is complex and currently unclear. Renal fibrosis is a histological hallmark observed during the progression to end-stage renal disease. Among the major characteristics of renal fibrosis are: (1) macrophages and dendritic cells infiltrate the tissue; (2) Myofibroblasts are stimulated and proliferated from various sources; (3) ECM components, with collagen, fibronectin and glycoproteins being produced and accumulated; (4) the microvascular rarefaction and the renal tubular atrophy10,11. Three phases can be identified: induction, inflammatory, and post-inflammatory. As the induction phase starts, mononuclear inflammatory cells invade the tubulointerstitial space. After infiltrating mononuclear cells, a phenotypically heterogeneous population of fibroblasts and tubular epithelial cells becomes activated fibroblasts and secretes abundant extracellular matrix. Tubular epithelial cells contribute to this process through EMT. The growing evidence has implicated EMT as a major pathway leading to the generation of matrix producing interstitial myofibroblasts in renal fibrosis12.
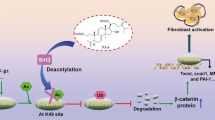
EMT describes phenotypical changes in which the membrane contacts between cells and the basement membranes are lost, and the cells acquire spindle shape and morphological characteristics similar to mesenchymal/myofibroblast cells. According to their functional consequences and biomarker context, EMT can be divided into three subtypes13. Mesenchymal cells are formed when type I EMT occurs, while fibroblasts form if type II EMT occurs. Type III EMT occurs when epithelial carcinoma cells become metastatic cancer cells in secondary epithelia. Secondary epithelial or endothelial cells undergo Type II EMT once they are differentiated into resident tissue or fibroblasts as a result of inflammation. This process may result in functional destruction of the respective tissue if the primary inflammatory insult is not removed14. Among the many mechanisms known to govern EMT, autophagy stands as one of the prime mechanisms15. Studies have shown that increasing the expression of Beclin-1 can improve the level of autophagy, inhibit the occurrence of EMT, and reduce renal fibrosis16,17. Importantly, SIRT1 as a cytokine that regulates and maintains cellular energy metabolism, enhances autophagy levels and reduces EMT, collagen accumulation and extracellular matrix deposition, thereby inhibiting kidney fibrosis18. In addition, lycopene promoted the generation of autophagosomes and then induces phagocytosis of injured mitochondria, ultimately maintaining tubular epithelial cell homeostasis19.
Autophagy is a highly conserved lysosomal protein degradation pathway carrying out degradation of damaged organelles, cytoplasmic components and even the invading pathogens20. As an important stress-responsive system, autophagy has been proved to be involved in the pathogenesis of various kidney diseases, including renal fibrosis11,21. It plays essential role in adult kidney resident cells and tightly involved in the progression of renal fibrosis. The activation of autophagy plays a protective effect on renal cells under stress condition22. Autophagic process is known to be involved in the regulation of EMT. Research has revealed that targeting epithelial autophagy by AdipoRon attenuates EMT process in the kidney and improves DOCA-salt hypertensive renal fibrosis23. However, the deficiency of autophagy will increase the sensitivity of the kidney to the damage, leading to more severe of renal fibrosis, accumulation of damaged mitochondria, impaired renal function and premature of kidney24.
Many studies have confirmed that a certain level of ROS can promote the expression of ATG genes and Beclin-1, thereby inducing autophagy25. When ROS levels rise, p85 preferentially binds to phosphatase and tensin homolog (PTEN), allowing the catalytic subunit 110β to bind with Rab5, ultimately enhancing autophagy as ROS levels increase. AKT is located downstream of PI3K, and AKT is involved in regulating ROS homeostasis, which is beneficial for cell growth and proliferation. AKT can inhibit autophagy by activating mTORC1. mTOR is a negative regulator of autophagy, also known as a gatekeeper molecule of autophagy26. Therefore, targeting the PI3K-AKT-mTOR pathway may be an appropriate treatment for hypertensive renal fibrosis, thereby promoting autophagic activity, improving renal pathological changes, and restoring renal function27.
NL-SDT can promote autophagy by generating low-concentration ROS. The basis for the numerous biological effects of the NL-SDT process, as well as the key to its therapeutic potential, lies in the reactive oxygen species (ROS) generated within cells28. Porphyrin derivatives make up the majority of the sonosensitizers utilized in SDT. The food and drug administration has approved sinoporphyrin sodium (DVDMS), a new sonosensitizer that was isolated from Photofrin, as a sensitizer. Stronger sonoactivity, reduced skin sensitivity, improved water solubility, and increased chemical purity are all attributes of DVDMS. As a result, DVDMS is given precedence in SDT applications29. Moreover, compared with tumor treatment, lower intensity of ultrasound is applied for atherosclerosis treatment30. We previously demonstrated that SDT inhibits fibrogenesis in rat cardiac fibroblasts induced by TGF-β131. Thus, in the present study, we used animal models and in vitro experiments to clarify whether NL-SDT rescued hypertensive renal and modulated autophagy, as well as address the underlying mechanisms.
Results
NL-SDT reduces renal fibrosis in mouse with angiotensin II-induced hypertensive nephropathy
We established the mice model of angiotensin II-induced hypertensive nephropathy. The blood pressure of the mice was measured using a noninvasive sphygmomanometer. The systolic blood pressure of the mice increased significantly at 1 week (before injecting Ang II: baseline 116 ± 4 mmHg vs. after injecting Ang II 1 week 163 ± 5 mmHg, ****P < 0.0001) and remained at high pressure for 4 weeks (4-week 196 ± 6 mmHg) (Figure S1C). As shown in Figure S1D-E, the first day after the third NL-SDT intervention and the fourth NL-SDT intervention showed a significant decrease in blood pressure, including systolic, diastolic, and mean arterial pressure, with the most notable change in systolic pressure. After the third NL-SDT intervention, the systolic blood pressure before treatment was 199 ± 15 mmHg compared to 158 ± 21 mmHg on the first day after treatment, **P < 0.01. After the fourth week of the NL-SDT intervention, the systolic blood pressure before treatment was 186 ± 13 mmHg compared to 149 ± 22 mmHg on the first day after treatment, ***P < 0.001. As mentioned above, NL-SDT can cause a temporary decrease in blood pressure. So, NL-SDT may have the potential to treat hypertension.
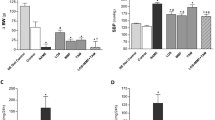
We further performed Masson’s trichrome staining and immunohistochemical staining of α-SMA to evaluate renal fibrosis at 28 days after infusion of Ang II. The levels of collagen I and α-SMA were significantly increased in the Ang II group (Fig. 1A-D). The proteins levels of collagen I and α-SMA were consistent with the pathological results (Fig. 1E-G). These effects were reversed after NL-SDT (Fig. 1A-G). Overall, these results suggest that NL-SDT can effectively mitigate renal fibrosis in mice with Angiotensin II-induced hypertensive nephropathy.
NL-SDT alleviates renal fibrosis in mice model of hypertensive renal fibrosis induced by Ang II.
(A, C) Representative images of Masson’s trichrome staining of kidney sections (Yellow arrow: collagen fiber; Black arrow: glomerulus; Green arrow: renal tubules) and quantification of the interstitial fibrosis area in each group. (B, D) Representative immunohistochemistry staining images of α-SMA-positive areas in kidney sections and quantification of α-SMA-positive expression in each group. Scale bar = 100 μm. (Ang II indicates Angiotensin-II); (E-G) Representative western blot images and quantitative measurements of the corresponding proteins in the kidneys of mice in each group. n = 6, and data were representative of three independent experiments. ANOVA followed by the Tukey’s multiple comparison test was applied. *P < 0.05, **P < 0.01.
NL-SDT activates autophagy in vitro
To determine how NL-SDT mitigate renal fibrosis in mice with Angiotensin II-induced hypertensive nephropathy. First, we exposed NRK-52E cells to either TGF-β1 or Ang II in a laboratory setting for identifying the most effective stimulus for cell model. We detected the levels of α-SMA and collagen I at different time points. A difference was observed during 72 h (Figure S2A-H). Interestingly, the addition of TGF-β1 resulted in a dose-dependent increase in α-SMA protein expression after 48 h (Figure S2A–B). We also observed a significant increase in collagen I protein levels after 72 h of TGF-β1 stimulation, as determined by western blot analysis. (Figure S2C-D). The effect of Ang II is significantly weaker than that of TGF-β1in vitro. Thus, we chose TGF-β1 as an inducer.
Next, to determine the best parameters for NL-SDT treatment, we assessed the intracellular accumulation of DVDMS using a multimode microplate reader and measured the mean fluorescence intensity. As shown in Figure. The cellular uptake of DVDMS increased rapidly within 1 h and reached a plateau at 5 h. After 6 h, the intracellular DVDMS value did not increase significantly. Therefore, in the following experiment, a DVDMS loading time of 5 h was selected (Figure S3A). DVDMS alone did not cause any toxic effects at concentrations below 0.4 µmol/L. Therefore, a drug concentration of 0.3 µmol/L was chosen for subsequent experiments (Figure S3B). As depicted in Figure S4A-D. There were no significant changes in the ratio of apoptotic or necrotic cells at ultrasonic intensities of 0.1 W/cm2 or 0.2 W/cm2. However, a noticeable change was observed at an ultrasonic intensity of 0.4 W/cm2 (Figure S4A-D). Furthermore, an ultrasonic intensity of 0.2 W/cm2 significantly promoted autophagy (Figure S3C-D). Hence, an ultrasonic intensity of 0.2 W/cm2 was utilized as the optimal NL-SDT-treatment parameter in vitro.
Emerging evidence suggests that autophagy plays a critical role in the development of renal fibrosis. We previously reported that low-intensity SDT mainly induced autophagy in cells. Therefore, we examined the expression of LC3. The results demonstrated that the LC3BII/LC3BI ratio was markedly elevated in the NL-SDT group (Fig. 5C-D). The administration of the lysosomal inhibitor CQ or 3-MA can attenuate these effects (Fig. 7I, J, M, N). Collectively, these results demonstrated that NL-SDT activates autophagy.
NL-SDT inhibits the PI3K-AKT–mTORC1 signaling pathway.
(A-D) Representative western blot images and quantitative measurements of proteins associated with PI3K-AKT–mTORC1 signaling in vivo. (E-H) Levels of proteins associated with PI3K-AKT–mTORC1–autophagy signaling in each group detected by western blot in vitro. For all experiments, n = 3 ~ 6, and experiments were performed independently in triplicate. ANOVA followed by the Tukey’s multiple comparison test was used for statistical analyses. *P < 0.05, **P < 0.01, ***P < 0.001.
NL-SDT inhibits the PI3K-AKT–mTORC1 signaling pathway
To investigate the molecular mechanism underlying NL-SDT induction of autophagy, we performed bioinformatics analysis of RNA sequencing data from the kidneys of mice treated with angiotensin II. The raw data are available in the GEO (GSE18430), (n = 3; Fig. 2). These results suggest that the PI3K-AKT cascade plays an essential role in renal fibrosis.
Effects of NL-SDT on intracellular ROS generation in TGF-β1-induced NRK-52E cells.
(A) Representative fluorescence photomicrographs of ROS generation of using the fluorescent probe DCFH-DA (Green. Scale bar: 200 μm. (B) Quantitative measurements of DCFH-DA fluorescence intensity by multimode microplate reader (n = 3), ANOVA followed by the Tukey’s multiple comparison test was used for statistical analyses. *P < 0.05. vs. control.
We then examined the protein expression of the PI3K-AKT–mTORC1 pathway in vivo. We found that the expression of PI3K, phosphorylated AKT and phosphorylated mTOR (Ser2448) increased markedly following Ang II treatment (Fig. 3A-D), and the expression of LC3BII decreased (Fig. 3C). However, the levels of PI3K, phosphorylated AKT and phosphorylated mTOR decreased after NL-SDT treatment, and the level of LC3II increased (Fig. 3A-D). Additionally, we examined PI3K-AKT-mTORC1 pathway activation in vitro and detected decreased levels of PI3K, decresed phosphorylation of AKT and decresed phosphorylation of mTORC1 following NL-SDT (Fig. 3E-H). These results indicate that NL-SDT inhibits the PI3K-AKT–mTORC1 pathway, contributing to the reduction of renal fibrosis.
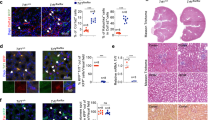
NL-SDT activates autophagy via the ROS-PI3K-AKT-mTORC1 signaling pathway in vitro.
(A, E) Electron micrograph showing the ultrastructural changes in autophagic vesicles (Red Arrow: Autophagosome Yellow Arrow: Myeloid Body). Scale bar: 2 μm. (B, F) Representative immunofluorescence images. Scale bar: 25 μm. (C, D) Western blot analysis of LC3B in different groups. (G-H) Levels of proteins associated with PI3K-AKT–mTORC1–autophagy signaling in NL-SDT-treated cells ± NAC, as detected by western blotting. n = 3 ~ 6, the differences among three or more groups were analyzed via ANOVA followed by the Tukey’s multiple comparison test. **P < 0.01, ****P < 0.0001.
NL-SDT activates autophagy via the PI3K-AKT–mTORC1 signaling pathway in vitro
To further confirm whether NL-SDT promotes autophagy through PI3K-AKT–mTORC1 pathway. We observed an increase in the number of autophagosomes in NL-SDT-treated cells through electron microscopy analysis (Fig. 5A, E). Immunofluorescence results demonstrated that NL-SDT induced clustering of the LC3B protein (Fig. 5B, F). The levels of ROS were significantly elevated in the NL-SDT group (Fig. 4). Pretreatment with NAC effectively suppressed the increase in the number of autophagosomes and the level of the LC3B protein (Fig. 5E-H). Furthermore, NAC successfully reversed the decreases in the levels of PI3K, phosphorylated AKT, and phosphorylated mTORC1 induced by NL-SDT (Fig. 5G). Additionally, the addition of the AKT inhibitor LY294002 and the mTORC1 inhibitor rapamycin resulted in increased levels of LC3BII in NL-SDT-treated cells (Fig. 7B, F). These findings indicate that the downregulation of mTORC1 through PI3K-AKT signaling is necessary for NL-SDT-mediated activation of autophagy.
NL-SDT blocks TGF-β1-induced NRK-52E cells transformation.
(A-C) Protein levels of α-SMA, vimentin and collagen I measured by western blot. (D) NRK-52E cells transformation measured in cells stained with an antibody to α-SMA (red), and DAPI (blue: labels nuclei) after treatment. (E) NRK-52E cells transformation measured in cells stained with an antibody to E-cadherin (green), and DAPI (blue: labels nuclei) after treatment. n = 6, and experiments were performed independently in triplicate. ANOVA followed by the Tukey’s multiple comparison test was used for statistical analyses. *P < 0.05 vs. control; **P < 0.01 vs. control; ***P < 0.001 vs. control.
NL-SDT inhibits renal fibrosis via PI3K-AKT–mTORC1–autophagy signaling.
(A-D) Cells were pretreated with LY294002 or rapamycin followed by NL-SDT. (I-P) Cells were pretreated with the autophagy inhibitors 3-MA or CQ followed by NL-SDT. (A–P) Relative protein levels of α-SMA, collagen I and LC3II/LC3I. n = 3 ~ 6. and data are shown as the means ± SD and representative of three independent experiments. ANOVA followed by the Tukey’s multiple comparison test was used for statistical analyses. *P < 0.05, **P < 0.01, ***P < 0.001.
NL-SDT decreases NRK-52E cell transformation the in vitro
Based on researches above, we have confirmed the molecular pathway of NL-SDT. To identify whether NL-SDT reduces renal fibrosis by inhibiting NRK-52E cells transformation on above pathway, we performed staining for α-SMA, collagen I, and E-cadherin in vitro. Cells treated with TGF-β1 exhibited a significant increase in α-SMA fluorescence intensity and a noticeable decrease in E-cadherin expression. However, NL-SDT reduced the transformation of NRK-52E cells (Fig. 6D-E). Additionally, we examined the expression levels of α-SMA, vimentin, and collagen I by western blotting to further confirm the inhibitory effect of NL-SDT on TGF-β1-induced NRK-52E cells transformation (Fig. 6A-C). In vitro, NL-SDT significantly suppressed the expression of α-SMA, vimentin, and collagen I, suggesting that NRK-52E cells transformation is inhibited by NL-SDT.
NL-SDT inhibits renal fibrosis via PI3K-AKT–mTORC1–autophagy signaling
To validate the involvement of the PI3K-AKT-mTORC1-autophagy pathway in the protective effects of NL-SDT on renal fibrosis. We administered a pathway-related protein molecule inhibitor, the mTORC1 inhibitor rapamycin, and the lysosomal inhibitor CQ in vivo. We conducted pathological analysis on consecutive slices of mouse kidney tissue. Renal collagen content was detected using Masson’s trichrome staining, and myofibroblasts were labeled with an α-SMA antibody. After statistical analysis, we found that the effect of NL-SDT treatment on renal fibrosis was promoted by CQ and rapamycin; however, no significant differences were observed between the groups (Figure S5A-F). These findings indicate that the PI3K-AKT-mTORC1-autophagy pathway may play a role in the modulatory effect of NL-SDT on renal fibrosis.
Next, we conducted in vitro cell experiments with LY294002, a highly selective AKT inhibitor and rapamycin. We found that LY294002 increased NL-SDT-mediated autophagy and further inhibited the expression of α-SMA and collagen I (Fig. 7A-D). However, the effect of NL-SDT on the expression of α-SMA and collagen I cannot be detected by an mTORC1 inhibitor. (Fig. 7E-H). Moreover, the administration of CQ or 3-MA attenuated the effects of NL-SDT on renal fibrosis, suggesting that NL-SDT-induced autophagy inhibition contributes to the alleviation of renal fibrosis (Fig. 7I-P). Overall, these results demonstrate that the ROS-PI3K-AKT-mTORC1-autophagy pathway is critically involved in the effects of NL-SDT on renal fibrosis.
Discussion
Hypertension constitutes a significant public health concern, characterized by a high incidence and mortality rate. It affects approximately 30% of the general population and 70% of the elderly demographic. A prevalent complication associated with hypertension is kidney damage. Renal fibrosis associated with hypertension is becoming the third leading cause of disease in dialysis patients, Hypertensive kidney disease is a prevalent complication associated with hypertension and is the second leading cause of end-stage renal disease (ESRD)32. Conventional clinical interventions aimed at halting or reversing renal fibrosis in hypertensive patients are notably limited in their efficacy. It is important to find effective methods for the treatment of clinical hypertensive renal fibrosis. Our study demonstrated the potential of NL-SDT as a noninvasive therapeutic tool for ameliorating hypertensive renal fibrosis. Activation of the PI3K-AKT-mTORC1-autophagy pathway appears to play a crucial role in the antifibrotic effects of NL-SDT (an overview is shown in Fig. 8).
Autophagy is necessary for renal cells to maintain homeostasis, vitality and physiological function; protect the kidney; and reduce fibrosis33. Induction of autophagy in renal tubular epithelial cells (RTECs) promotes mature TGF-β1 degradation and further inhibits the progression of renal fibrosis34. Autophagy deficiency in RTECs leads to the accumulation of deformed mitochondria and cytoplasmic inclusions, resulting in cellular hypertrophy and eventual degeneration35. NL-SDT is closely related to autophagy. The preliminary research of the research group found that NL-SDT promotes the conversion of M1 macrophages to M2 macrophages through the activation of the ROS-AMPKα-mTORC1 pathway-dependent autophagy. In addition, we found that NL-SDT can inhibit the fibrosis of rat cardiac fibroblasts induced by TGF-β1 by activating the AKT/GSK3β pathway, thereby exerting its anti-fibrotic effect. Therefore, we attempted to treat renal fibrosis by using NL-SDT in this study.
In this study, we investigated the changes in autophagy and EMT after NL-SDT treatment. We found that NL-SDT significantly suppressed the expression of α-SMA and collagen I. The relative protein levels of α-SMA and collagen I were increased after adding autophagy related inhibitors (3MA, CQ). the administration of CQ or 3-MA attenuated the effects of NL-SDT on renal fibrosis. And we examined the expression of LC3B. The results demonstrated that the LC3BII/LC3BI ratio was markedly elevated in the NL-SDT group. The administration of CQ or 3-MA can attenuate these effects. These results indicate that NL-SDT inhibited renal fibrosis by activating autophagy pathway. We observed an increase in autophagy and a decrease in fibrosis, which is consistent with previous studies21,34,36. These results suggest that autophagy in RTECs is beneficial for renal fibrosis.
NL-SDT promotes autophagy by generating low-level ROS. ROS is a byproduct generated during cellular metabolism which is necessary for the activation of the PI3K/AKT pathway37. AKT is located downstream of PI3K and involved in regulating ROS homeostasis, which is beneficial for cell growth and proliferation. AKT can inhibit autophagy by activating mTORC1. mTOR is a negative regulator of autophagy, also known as a gatekeeper molecule of autophagy38. In this study, the KEGG enrichment analysis results showed that the differentially expressed genes were significantly enriched in the PI3K-AKT pathway. Our results also revealed that NL-SDT activates the PI3K-AKT-mTORC1-autophagy pathway (Fig. 3). In this study, autophagy weakened after supplementing with NAC, which is consistent with the aforementioned research results. With NAC added, the inhibitory effect of NL-SDT on PI3K-AKT-mTORC1 pathway weakened. This suggests that NL-SDT can regulate PI3K-AKT-mTORC1 pathway through active oxygen. Further experimental design is needed to explore whether ROS exert their effects directly, indirectly through changes in signals such as Ca2+and ADP, or jointly initiate subsequent cascade reactions.
In this study, we found that the expression of α-SMA and collagen I was decreased after adding LY294002(AKT inhibitor). By observing the data, it can be found that compared with the NL-SDT group, the NL-SDT + rapamycin group did not achieve further definite therapeutic effects (Figure S5). We found a change in the results, but it was not statistically significant. The specific reason is still unclear, but increasing the sample size may be meaningful. In terms of experimental design, the addition of mTOR pathway related agonists may better elucidate the mechanism of mTOR in the renal fibrosis protective effect of NL-SDT. This is a shortcoming of this study and a part that needs to be improved in the future. These findings underscore the PI3K-AKT-mTORC1 cascade as a crucial regulator of renal cell transformation and a promising therapeutic target for NL-SDT to reduce renal fibrosis.
AKT regulation of autophagy is not solely dependent on mTOR. Under nutrient rich conditions, ULK1 binds to mTORC1, leading to ULK1 phosphorylation and inhibition of autophagy. Under low nutrient conditions, mTORC1 and ULK1 complexes separate and trigger autophagosome nucleation and elongation39. In summary, AKT regulates autophagy mediated metabolic processes through multiple signaling pathways. AKT plays dual roles in autophagy. On the one hand it can inhibit autophagy by activating mTOR; on the other hand, it can inhibit autophagy by inhibiting FoxO340. In this study, we tested the PI3K-AKT-mTORC1 pathway. It is not yet known whether it simultaneously inhibits FoxO3. And that, does mTORC1 also regulate renal fibrosis by influencing the AMPK pathway during the process of NL-SDT inhibiting the conversion of epithelial cells to myofibroblasts? Does the AMPK pathway interact with the AKT pathway? Are there other pathways that are also activated (e.g. metabolic pathways)? These questions remain unknown and require further research in the future.
The regulatory mechanisms of renal fibrosis are very complex. Multiple pathway involvement such as the MAPK pathway, ROCK pathway, and PPAR-γ pathway, among others. This suggests to us that renal fibrosis may be regulated by multiple pathways and mechanisms working together, rather than being controlled by a single regulatory mechanism. According to reports, AdipoRon (an adiponectin receptor agonist) can activate tubular epithelial cells. Blocking the activation of AMPK inhibits autophagy and can obstruct the inhibitory effect of AdipoRon on aldosterone-induced EMT. AdipoRon treatment can promote autophagy and improve renal fibrosis in DOCA-salt sensitive hypertensive mice23. In the protein detection of mouse kidney tissue, it was found that the NL-SDT group showed an increase in adiponectin expression, but the expression of TGF-β1 decreased (data not show). However, we did not investigate whether adiponectin alleviates renal fibrosis through certain signaling pathways. Further research is needed to confirm whether adiponectin alleviates renal fibrosis through certain signaling pathways.
In conclusion, this study demonstrates that NL-SDT inhibits EMT through the PI3K-AKT-mTORC1 pathway, thereby reducing the transformation of RTECs into mesenchymal cells. NL-SDT shows promise as a noninvasive therapeutic strategy for managing renal fibrosis in patients with chronic kidney disease. And there may be potential application prospects for renal fibrosis induced by other reasons and delaying or reducing the occurrence of ESRD. Further research is warranted to validate these findings and explore additional pathways involved in NL-SDT-induced autophagy.
Limitations
In this study, the animal model was established using only mice, whereas the effect of NL-SDT on renal fibrosis in other animal model of renal fibrosis (e.g. UUO, IRI, 5/6Nx) was not verified. This is a limitation of this study. In the future, the other model and a clinical trial would be needed to further confirm the efficacy and safety of NL-SDT. Blood pressure measurements were noninvasive, and future studies could benefit from more accurate measurements using telemetry transmitters. Additionally, we mainly focused on the PI3K-AKT-mTORC1-autophagy signaling pathway. Further research is needed to confirm whether other signaling pathways are involved (e.g. metabolic pathways). In the future, the patients receiving NL-SDT may need to avoid light for three days which will be safe, because DVDMS has photosensitivity.
Methods
Establishment of the mice model of hypertensive renal fibrosis
Male C57BL/6 mice (age: 8 weeks; weight: 25–30 g) from Harbin Medical University (Harbin, China) were maintained on a standard diet throughout the experiment. They were randomly separated into five treatment groups (n = 6/group; control (No intervention); Ang II; NL-SDT; NL-SDT + CQ; NL-SDT + rapamycin). For the Ang II group, Ang II (Sigma-Aldrich, St Louis, MO) was dissolved in distilled water, and the solution was administered to mice using osmotic minipumps (Alzet model 2004, Cupertino, CA). Pumps were subcutaneously implanted in mice, ensuring constant infusion of Ang II at a dose of 1.0 µg/kg per minute41,42. Ang II was administered to mice using osmotic minipumps for 4 weeks. Two investigators who were not informed of experimental groups performed noninvasive measurement of blood pressure at the tail using a sphygmomanometer (BP-98 A; Softron Co, Ltd, Tokyo, Japan). All animal protocols were in accordance with the regulations of the Ethics Committee of Harbin Medical University and approved by the ethical committee of Harbin Medical University. The ethical number: IACUC:2,020,117. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. All methods are reported in accordance with ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
NRK-52E cell culture
We obtained NRK-52E cells from the Department of Cardiology, Harbin Medical University, and cultured them in Dulbecco’s modified Eagle medium containing high-glucose (Gibco/Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum, (Gibco/ Life Technologies), then placed at 37 °C in a humidified atmosphere containing 5% CO2. Afterward, cells reached 70% confluent were used for the next experiments. In order to choose the appropriate inducer, the NRK-52E cells were divided into two groups: (1) TGF-β1group(0,5,10ng/ml), (2) Ang II group (0,100,1000nmol/L). The TGF-β1 group was treated with recombinant human TGF-β1 (Pepro Tech, Rocky Hill, NJ, USA) for 24,48,72 h, (Figure S2). The cells were cultured and treated in a 35 mm dish, and the volume of DMEM 2 ml in each dish.
The TGF-β1-induced cells were divided into 8 groups, control group(only induced by TGF-β1), DVDMS group, Ultrasound group; NL-SDT group (DVDMS + Ultrasound), NL-SDT + CQ(10µmol/L) group18, NL-SDT + 3-MA (10 mmol/L) group43, NL-SDT + NAC (5 mmol/L) group44. NL-SDT + LY294002 (50µmol/L) group45, NL-SDT + rapamycin(500nmol/L) group46. 3-MA is a PI3K selective inhibitor that blocks the formation of autophagosomes. LY294002 can suppress the PI3K/Akt signaling pathway, including inhibition of Akt phosphorylation. Rapamycin is a specific mTOR inhibitor that promotes autophagy. We have also validated the above drug concentrations at different concentrations (data not shown). After 24 h of treatment, the cells were harvested to determine the related parameter.
Application of specific inhibitors in vivo
The Lysosome inhibitor CQ (60 mg/kg) were dissolved in sterile PBS and administrated intraperitoneally to mice 2 h before ultrasonic radiation, according to previous studies47. The mTOR inhibitor rapamycin (2 mg/kg) was dissolved using a dilution of 0.25% Tween-80 and 0.2% carboxymethylcellulose46.
NL-SDT treatment protocol
NL-SDT treatment was performed as previously described48. Sonosensitizer for NL-SDT in this study was DVDMS. DVDMS is the property of Qinglong Hi-tech Co., Ltd. (Jiangxi, China) and was kindly provided by Professor Qicheng Fang from the Chinese Academy of Medical Sciences (Beijing, China). The ultrasound device was designed and assembled by Harbin Institute of Technology (Harbin, China) and had a diameter of 35 mm and resonance frequency of 1.0 MHz.
For the mice of hypertensive renal fibrosis, ultrasound gel was used to coat an ultrasound transducer, which was located on the skin above the kidneys on the back. Previous study showed that this dosage and exposure time for NL-SDT are safe and effective for myocardial fibrosis. Thus, we choose previous parameters in this study. The animals were anesthetized at 4 h after sinoporphyrin sodium (DVDMS 4 mg/kg) injection and received ultrasonic sonication (0.4 W/cm2, duty factor 30%) for 15 min48. The NL-SDT was repeated weekly throughout the study period. The mice were fed a standard diet after undergoing NL-SDT. The mice were shielded from light throughout the entire procedure and for one day following treatment. the cells were subjected to NL-SDT, following previously established methods44.
For cells, A 35 mm culture dish was submerged in a deoxygenated water bath located 30 cm from a customized ultrasonic transducer (diameter: 3.5 cm; resonance frequency: 1.0 MHz; duty factor:10%)44. The ultrasonic treatment lasted for 5 min, and the intensity was set to 0.2 W/cm2. Cells were incubated with 0.3µmol/L DVDMS in serum-free DMEM medium for 5 h before NL-SDT (Figure S3). The induced cells were pre-treated with the autophagy inhibitor 3-MA (10mmol/L) or CQ (10µmol/L) for 2 h according to previous studies27,43, and 5mM of NAC was added 1 h before NL-SDT31. Control (only induced by TGF-β1) and NL-SDT mice received sterile PBS.
Immunohistochemistry analysis and terminal deoxynucleotidyl transferase dUTP nickend labeling (TUNEL) staining
At the end of the experiments, the animals were deeply anesthetized and euthanized with an intraperitoneal injection of pentobarbital sodium (100 mg/kg). After heparinized saline perfusion, the mouse kidney was carefully removed. The kidney was cut into two parts along the center of the long axis, fixed with 4% paraformaldehyde, dehydrated, and serially cross-sectioned according to routine procedures. After deparaffinization, rehydration, and antigen retrieval, the sections were blocked and incubated with mouse monoclonal anti-α-SMA antibody (1: 200dilution, BIOSS, China) was subsequently incubated with goat anti-mouse/anti-rabbit secondary antibody and streptavidin-conjugated HRP. The slices were developed with 3, 3’-diaminobenzidine staining and counterstained with hematoxylin. Image J software was used to measure the percentage of the total positive area. DNA fragmentation in apoptotic cells was determined by an in situ cell death-detection kit according to manufacturer instructions.
Western blotting analysis
Kidney tissue or cells were lysed with pre-chilled RIPA buffer. Protease inhibitors and phosphatase inhibitors were added to prevent degradation. Following sonication, lysates were centrifuged at 13,500 × g for 10 min. Protein levels were assessed using the BCA assay. Equal amounts of protein samples were separated by SDS-PAGE and then transferred to PVDF membranes. Membranes were blocked using 5% milk for 1.5 h and incubated overnight at 4 °C with primary antibodies: anti-E- cadherin (1:1000 dilution, 20874-1-AP; Protein tech, China), vimentin (1:500 dilution, PTM-5376; PTM Bio, China), collagen I (1:500 dilution, BIOSS, China), anti-α-SMA antibody (1:500 dilution, BIOSS, China), PI3K (1:500 dilution, WL03380; Wan lei bio, China), anti-p-AKT(Ser473) (1:1000 dilution, 4058; Cell Signaling Technology USA), anti-AKT (1:1000 dilution, 4691; Cell Signaling Technology USA), anti-phospho-mTOR (Ser2448) (1:1000 dilution, 5536; Cell Signaling Technology USA), anti-mTOR (1:1000 dilution Cell Signaling Technology USA), anti-LC3B antibody (1:1000 dilution, ab192890; abcom UK); GAPDH (1:1000 dilution, AB clonal, China). Finally, color development was performed using western ECL solution in a gel imaging system, and the western blot bands were collected by Imaging System (LI-COR Biosciences, Lincoln, NE, USA). The intensity of each band was analyzed with Image J software (version 1.47v; National Institutes of Health) and normalized to the levels of GAPDH.
Immunofluorescence assay
For the immunofluorescence assay, NRK-52E cells were plated on chamber slides, fixed for 30 min with 4% formaldehyde, then cells were permeabilized with 0.1% Triton X-100 for 30 min and blocked with 3% BSA; Furtherly, cells were stained with primary antibody (anti-E-cadherin (1:100 dilution, 20874-1-AP; Protein tech, China; anti-α-SMA antibody (1:50 dilution, BIOSS, China) overnight. After washing in PBS, cells were incubated with secondary antibody (Molecular Probes, Invitrogen) for 1 h at room temperature. After washing with PBS, DAPI was added and the slides were incubated for 10 min at room temperature. Following two more washes in PBS, the slides were covered with immune-mount and a cover slip. Carl Zeiss Axio VertA1 microscope (Carl Zeiss Microimaging, Thornwood, NY, USA) was used to visualize cells and NIS Elements software was used to record images for further analysis.
Cell-viability assay
Differentiated cells (2 × 105 cells/mL) were seeded into 96-well plates, and various concentrations of DVDMS (0-0.8 µM) in serum-free DMEM medium were added and incubated for 48 h in the dark. After drug incubation, cells were rinsed, and 10 µL of CCK-8 solution was added to each well and incubated at 37 °C for an additional hour. Subsequently, the absorbance at 450 nm was measured using a microplate reader.
Statistical analysis
All experiments were preformed independently at least three times. The measurement data are presented as the mean ± standard deviation. The normality test was performed to determine whether the data were normally distributed. For normally distributed data, data were analyzed using a Student’s t test, one-way analysis of.
variance (ANOVA), followed by Tukey’s multiple comparison test or Dunnett’s multiple comparison test, or two-way ANOVA, followed by Sidak’s multiple comparison test, as appropriate. For data that were not normally distributed, a Kruskal–Wallis test, followed by Dunn’s multiple comparison test, was used. Statistical analysis was conducted using GraphPad Prism 6.0 from La Jolla, USA. A P value less than 0.05 was considered to indicate statistical significance. An expanded Methods section is available in the online-only Data Supplement.
Figures and figure legends.
Data availability
The datasets used and analysed during the current study are available from the Corresponding author on reasonable request.
References
Worldwide trends in. Hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants[J]. Lancet (London England). 398 (10304), 957–980 (2021).
Montaniel, K. R. & Harrison, D. G. Is Hypertens. Bone Marrow Disease?[J] Circulation, 134(18): 1369–1372. (2016).
Hill, N. R. et al. Hobbs. Global prevalence of chronic kidney disease - A systematic review and Meta-Analysis[J]. PloS One. 11 (7), e0158765 (2016).
Hypertension, T. K. A. Griffin. Hypertensive Kidney Injury and the Progression of Chronic Kidney Disease[J]. 2017, 70(4): 687–694. (1979).
Webster, A. C., Nagler, E. V. & Morton, R. L. Masson. Chronic kidney Disease[J]. Lancet (London England). 389 (10075), 1238–1252 (2017).
Tsuprykov, O. et al. The dipeptidyl peptidase inhibitor linagliptin and the angiotensin II receptor blocker telmisartan show renal benefit by different pathways in rats with 5/6 nephrectomy[J]. Kidney Int. 89 (5), 1049–1061 (2016).
Song, J. H., Cha, S. H., Hong, S. B. & Kim, D. H. Dual blockade of the renin-angiotensin system with angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in chronic kidney disease[J]. J. Hypertens. Supplement: Official J. Int. Soc. Hypertens. 24 (1), S101–S106 (2006).
Bolati, D., Shimizu, H., Higashiyama, Y., Nishijima, F. & Niwa, T. Indoxyl sulfate induces epithelial-to-mesenchymal transition in rat kidneys and human proximal tubular cells[J]. Am. J. Nephrol. 34 (4), 318–323 (2011).
Wang, M. Y. et al. OTUD1 promotes hypertensive kidney fibrosis and injury by deubiquitinating CDK9 in renal epithelial cells[J]. Acta Pharmacol. Sin. 45 (4), 765–776 (2024).
A. A. Eddy. Molecular basis of renal fibrosis[J]. Pediatric nephrology (Berlin, Germany), 15(3–4): 290–301. (2000).
Liu, Y. Cellular and molecular mechanisms of renal fibrosis[J]. Nat. Rev. Nephrol. 7 (12), 684–696 (2011).
Kalluri, R. & Neilson, E. G. Epithelial-mesenchymal transition and its implications for fibrosis[J]. J. Clin. Investig. 112 (12), 1776–1784 (2003).
Carew, R. M., Wang, B. & Kantharidis, P. The role of EMT in renal fibrosis[J]. Cell Tissue Res. 347 (1), 103–116 (2012).
Xue, C., Plieth, D., Venkov, C., Xu, C. & Neilson, E. G. The gatekeeper effect of epithelial-mesenchymal transition regulates the frequency of breast cancer metastasis[J]. Cancer Res. 63 (12), 3386–3394 (2003).
Nieto, M. A., Huang, R. Y. & Jackson, R. A. Thiery. EMT: 2016[J]. Cell 166 (1), 21–45 (2016).
Yang, X. et al. WNT1-inducible signaling protein-1 mediates TGF-β1-induced renal fibrosis in tubular epithelial cells and unilateral ureteral obstruction mouse models via autophagy[J]. J. Cell. Physiol. 235 (3), 2009–2022 (2020).
Bao, C. et al. Incremental load training improves renal fibrosis by regulating the TGF–β1/TAK1/MKK3/p38MAPK signaling pathway and inducing the activation of autophagy in aged mice[J]. Int. J. Mol. Med. 44 (5), 1677–1686 (2019).
Jin, D. et al. Jiedu Tongluo Baoshen formula enhances renal tubular epithelial cell autophagy to prevent renal fibrosis by activating SIRT1/LKB1/AMPK pathway[J]. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 160(114340. (2023).
Wang, Y. et al. Yu. LYC inhibits the AKT signaling pathway to activate autophagy and ameliorate TGFB-induced renal fibrosis[J]. Autophagy 20 (5), 1114–1133 (2024).
Mizushima, N., Levine, B. & Cuervo, A. M. Klionsky. Autophagy fights disease through cellular self-digestion[J]. Nature 451 (7182), 1069–1075 (2008).
Tang, C., Livingston, M. J., Liu, Z. & Dong, Z. Autophagy in kidney homeostasis and disease[J]. Nat. Rev. Nephrol. 16 (9), 489–508 (2020).
Zhang, Y. L., Zhang, J., Cui, L. Y. & Yang, S. Autophagy activation attenuates renal ischemia-reperfusion injury in rats[J]. Experimental biology and medicine. (Maywood N J). 240 (12), 1590–1598 (2015).
Li, Y., Song, B., Ruan, C., Xue, W. & Zhao, J. AdipoRon attenuates Hypertension-Induced epithelial-mesenchymal transition and renal fibrosis via promoting epithelial Autophagy[J]. J. Cardiovasc. Transl. Res. 14 (3), 538–545 (2021).
Liu, S. et al. Huber. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury[J]. Autophagy 8 (5), 826–837 (2012).
Liang, S., Wu, Y. S., Li, D. Y. & Tang, J. X. H F Liu Autophagy Ren. Fibrosis[J] Aging Disease, 13(3): 712–731. (2022).
Dai, R. et al. Autophagy in renal fibrosis: Protection or promotion?[J]. Frontiers in pharmacology, 13(963920. (2022).
Xuan, C., Xi, Y. M., Zhang, Y. D., Tao, C. H. & Zhang, L. Y. and W. F. Cao. Yiqi Jiedu Huayu Decoction Alleviates Renal Injury in Rats With Diabetic Nephropathy by Promoting Autophagy[J]. Frontiers in pharmacology, 12(624404. (2021).
Qian, X., Zheng, Y. & Chen, Y. Micro/Nanoparticle-Augmented Sonodynamic Therapy (SDT): Breaking the Depth Shallow of Photoactivation[J]. Advanced materials (Deerfield Beach, Fla.), 28(37): 8097–8129. (2016).
Wang, H. et al. Sinoporphyrin sodium, a novel sensitizer, triggers mitochondrial-dependent apoptosis in ECA-109 cells via production of reactive oxygen species[J]. Int. J. Nanomed. 9, 3077–3090 (2014).
Sun, X. et al. Rapid inhibition of atherosclerotic plaque progression by sonodynamic therapy[J]. Cardiovascular. Res. 115 (1), 190–203 (2019).
Guo, Y. et al. Sonodynamic Therapy inhibits Fibrogenesis in Rat Cardiac fibroblasts Induced by TGF-β1[J]. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology, 40(3–4): 579–588. (2016).
B. D. Humphreys. Mechanisms of Renal Fibrosis[J]. Annu. Rev. Physiol., 80(309–326. (2018).
De Rechter, S., Decuypere, J. P., Ivanova, E., van den Heuvel, L. P. & De Smedt, H. E. Levtchenko and D. Mekahli. Autophagy in renal diseases[J]. Pediatric nephrology (Berlin, Germany), 31(5): 737 – 52. (2016).
Ding, Y. et al. Autophagy regulates TGF-β expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction[J]. J. Am. Soc. Nephrology: JASN. 25 (12), 2835–2846 (2014).
Kimura, T. et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury[J]. J. Am. Soc. Nephrology: JASN. 22 (5), 902–913 (2011).
Li, H. et al. Atg5-mediated autophagy deficiency in proximal tubules promotes cell cycle G2/M arrest and renal fibrosis[J]. Autophagy 12 (9), 1472–1486 (2016).
Koundouros, N. & Poulogiannis, G. Phosphoinositide 3-Kinase/Akt Signaling and Redox Metabolism in Cancer[J]. Frontiers in oncology, 8(160. (2018).
Kma, L. & Baruah, T. J. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation[J]. Biotechnol. Appl. Chem. 69 (1), 248–264 (2022).
Alers, S., Löffler, A. S., Wesselborg, S. & Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks[J]. Mol. Cell. Biol. 32 (1), 2–11 (2012).
Zhao, J. et al. Goldberg. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells[J]. Cell Metabol. 6 (6), 472–483 (2007).
Aibara, Y. et al. Daily Low-intensity Pulsed Ultrasound Ameliorates Renal Fibrosis and Inflammation in Experimental Hypertensive and Diabetic Nephropathy[J]. Dallas, Tex.: 2020, 76(6): 1906–1914. (1979).
Ni, J. et al. Dual deficiency of angiotensin-converting enzyme-2 and Mas receptor enhances angiotensin II-induced hypertension and hypertensive nephropathy[J]. J. Cell. Mol. Med. 24 (22), 13093–13103 (2020).
Fan, L. et al. Experimental Study on Danggui Shaoyao San Improving Renal Fibrosis by Promoting Autophagy[J] (eCAM, 2022). 2022(6761453.
Yang, Y. et al. Non-lethal sonodynamic therapy facilitates the M1-to-M2 transition in advanced atherosclerotic plaques via activating the ROS-AMPK-mTORC1-autophagy pathway[J]. Redox biology, 32(101501. (2020).
Chen, L. et al. The PI3K-Akt-mTOR pathway mediates renal pericyte-myofibroblast transition by enhancing glycolysis through HKII[J]. J. Translational Med. 21 (1), 323 (2023).
Chen, G. et al. Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts[J]. PloS One. 7 (3), e33626 (2012).
Ding, W. X. et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice[J]. Gastroenterology 139 (5), 1740–1752 (2010).
Yao, J. et al. And Y. Tian. Sonodynamic Therapy Suppresses Neovascularization in Atherosclerotic Plaques via Macrophage Apoptosis-Induced Endothelial Cell Apoptosis[J]553–65 (JACC. Basic to translational science, 2020). 1.
Author information
Authors and Affiliations
Contributions
Tian ye and wang shu contributed to the conception of the study; Liu dandan, wang hui and li jialong performed the experiment; Liu dandan, wang hui, sheng siqi and li jialong contributed significantly to analysis and manuscript preparation; Liu dandanperformed the data analyses and wrote the manuscript; Tian ye helped perform the analysis with constructive discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, D., Wang, H., Li, J. et al. Non-lethal sonodynamic therapy mitigates hypertensive renal fibrosis through the PI3K/AKT/mTORC1-autophagy pathway. Sci Rep 15, 4534 (2025). https://doi.org/10.1038/s41598-025-86973-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86973-1