Abstract
The imbalance between estrogen and androgen may be an important mechanism of BPH, but the specific mechanism remains unclear. We used mixed sustained-release pellets made of testosterone and estradiol (T + E2) to stimulate the establishment of a BPH rat model. Compared to the prostate hyperplasia rat model using only androgens, the new prostate hyperplasia rat model can be observed to have better macroscopic and pathological characteristics of prostate hyperplasia. We used RNA-seq and bioinformatics to detect differentially expressed genes (DEGs) between the prostate tissue of the novel benign prostatic hyperplasia rat group and the control group, including 458 DEGs, of which 336 were upregulated and 122 were downregulated. Then, RT-qPCR confirmed the authenticity of sequencing results. The analysis results showed that Kif4a and Mki67 were the top core genes in the PPI network. Moreover, we found that these two genes have a positive correlation with each other in multiple cancer tissues, normal tissues, and cancer cells. The DEGs were mainly involved in mitotic nuclear division, nuclear chromosome segregation, and cytokine cell receptor interactions. DEGs were also regulated by 250 miRNAs. In conclusion, we built a novel T + E2-induced rat BPH model, and discovered potentially important genes, pathways, and miRNA-mRNA regulatory networks.
Similar content being viewed by others
Introduction
Benign prostatic hyperplasia (BPH) is a common disease in elderly men. BPH morbidity incidence can reach 50% in men over 50 years and exceed 80% in men over 70 years1. Prostatic hyperplasia usually occurs in the area around the proximal urethra of the prostate. The enlargement of the hyperplastic gland causes bladder outlet obstruction (BOO)2, which further leads to lower urinary tract symptoms (LUTS)3 and severely affects patient’s quality of life4. LUTS mainly includes urinary frequency, urgency, and nocturia5. As the disease progresses, more serious urinary complications develop, including urinary retention, bladder diverticula, hydronephrosis, bladder stones, and renal insufficiency6,7,8.
It is well known that androgens, including T and dihydrotestosterone (DHT), promote prostate hyperplasia by activating androgen receptors and are highly correlated with the onset and prognosis of prostate hyperplasia. T produced by the testes and adrenal glands is converted to DHT by type 2 5α-reductase in the prostate and other reproductive tissues7. DHT amplifies the role of T in the prostate, and based on this theory, 5α-reductase inhibitors (such as finasteride and dutasteride) have been proven to have therapeutic effects on prostate hyperplasia. However, it is interesting that in men with BPH, blood and tissue concentrations of DHT and T are not significantly higher than in normal controls9. A characteristic feature of BPH is the enlargement and alteration of the stromal compartment. As age increases, the generation of T and the conversion of DHT gradually decrease, and the estrogen/androgen ratio relatively increases. More and more evidence pointed out that the imbalance of estrogen and androgen levels will accelerate the progression of prostatic hyperplasia. Although prostate growth depends on androgens, a decrease in prostate androgen levels can lead to an imbalance in the molecular microenvironment and further promote prostate hyperplasia, for example, PKG1 may directly participate in the proliferation of prostate cells, while a decrease in androgen levels can increase the expression of PKG1, thereby promoting cell proliferation10,11. Estrogen may promote prostate hyperplasia through the proliferation of prostate stromal cells rather than prostate epithelial cells, which may be related to the regulation of inflammatory expression of prostate stromal cells by estrogen. However, the exact molecular role of estrogen in the occurrence of BPH is still unclear12. The animal model of BPH related to the imbalance of estrogen and androgen is helpful for the related research. However, traditional animal models of prostate hyperplasia induced by subcutaneous or intramuscular injection of androgen are prone to infection and greatly increase workload. There are limitations in establishing models on a larger scale. Building safer, more convenient, and high-quality models can promote the progress of related research. In addition, a single androgen cannot meet the current research needs. Moreover, there is a lack of large-scale studies about the changes in the molecular characteristics of hormonal imbalances in BPH. Recently, RNA sequencing has become a powerful technique for transcriptomes analysis due to its accurate quantification, high reproducibility, wide detection range and widespread use in transcriptome studies13. Thus, RNA-seq is an enabling technique for studying molecular mechanisms.
By a new method of hormone-induced, we successfully built T + E2-induced rat models and observed differences in general and pathological characteristics between T + E2 and other groups. Next, we used RNA-seq to examine the differential expression profiles of mRNAs between the T + E2 group and the Con group. Furthermore, we also constructed PPI networks of DEGs, performed GO and KEGG enrichment analyses, as well as GSVA analysis, and constructed miRNA-mRNA-related regulatory networks. Novel insights into the mechanisms of hormonal imbalances in BPH development are provided by this study.
Results
T + E2 treatment causes the characteristics of prostatic hyperplasia in rats combined with BOO
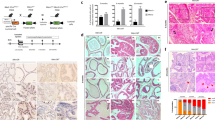
After 4 weeks of experimentation, all rats were alive and showed no complications. Food intake and water intake did not differ significantly, and urination did not show any obvious abnormalities between different groups. Most of the slow-release pellets had been absorbed when they were removed after 4 weeks (Fig. S1a). Compared to the Con group, the prostate and seminal vesicles in C group were reduced, while the prostate and seminal vesicles in T group and T + E2 group were increased (Fig. 1a). The prostate weight showed statistical differences among the four groups, with the T + E2 group having a greater prostate weight than the T group (Fig. 1b). Initially, there was no significant difference in body weight between different groups (Fig. S2a). In contrast, final body weight of the T group and T + E2 group was decreased compared to the Con group and C group after 4 weeks of hormone sustained-release pellets intervention (Fig. 1c). The weight added to the body of the T group and T + E2 group was decreased compared to the Con group and C group (P < 0.05, Fig. S2b).
There were differences in prostate weight ratios between each group, with the T + E2 group having the highest prostate weight ratio (Fig. 1d). Prostatic urethral length in the T group and T + E2 group increased compared to the Con group and C group, however, there was no statistically significant difference in prostate urethral length between the T and T + E2 groups (Fig. 1e). Testicular weight changes were not observed between different groups (Fig. S2c). Although bladder capacity was similar between these groups (Fig. S2d), bladder weight was higher in the T group and T + E2 group compared to the Con group and C group (Fig. S2e). Kidney volume did not change significantly between the four groups (Fig. S2f), but the kidney weight increased in the T group and T + E2 group compared to the Con group and C group (Fig. S2f). It was observed an increase in serum T in the T group and T + E2 group compared to the Con group and C group (Fig. S2h). The serum estradiol of T + E2 group was significantly higher than the other three groups (Fig. S2i). However, the highest E2/T ratio was observed in C group, which showed differences compared to other groups (Fig. S2j). T + E2 sustained-release pellets cause epithelial hyperplasia, mesenchymal cell hyperplasia, and immune cell infiltration in the prostate. In pathology, prostate gland is mainly divided in the prostatic duct system and interstitium14. Normal rats do not suffer from prostatic hyperplasia with age15, therefore, pathologically, there is no significant hyperplasia of glandular epithelium in the prostatic ducts of rats of Con group, and a small amount of interstitial tissue can be seen between prostate ducts (Fig. 2a). After castration, the epithelial cells of the prostate gradually shrink, and the interstitial tissue of the prostate increases relatively in C group (Fig. 2a). Pathology showed proliferation of prostate epithelial cells in T group, but there was no trend of proliferation in prostate stromal cells (Fig. 2c). The pathological results of the T + E2 group showed significant proliferation of prostate epithelial cells and stromal tissue, accompanied by a large amount of immune cell infiltration. Based on the shape and lobulation of immune cell nuclei, it was found that immune infiltrating cells were mainly lymphocytes (Fig. 2d).
DEGs analysis and verification via RT-qPCR
The transcriptome sequencing was used to determine mRNAs expression in prostate tissue. Overall, 458 mRNAs, including 336 upregulated and 122 downregulated mRNAs, were identified to be different in the test group compared to the Con group. Heatmap with hierarchical clustering (Fig. 3a) and volcano plots (Fig. 3b) shows differential gene expression between the T + E2 group and the Con group. PCA cluster analysis showed a strong gene expression grouping within groups and differences between groups (Fig. 3c). For verification, 8 mRNAs were analyzed using RT-qPCR. Figure 3d shows that all selected mRNA expressions matched sequencing results, and the differences between groups were significant.
GO and KEGG functional enrichment analysis of DEGs in BPH model
GO analysis indicated that the BP of DEGs was primarily enriched in nuclear division, cell division, chromosome segregation, mitotic nuclear division, and nuclear chromosome segregation (Fig. 4a). The CC was primarily enriched in spindle, chromosomal region, microtubule, chromosome, centromeric region and condensed chromosome (Fig. 4a). The MF was primarily enriched in tubulin binding, microtubule binding, glycosaminoglycan binding, immune receptor activity and cytokine receptor activity (Fig. 4a, Table S2). Moreover, KEGG pathway analysis was mostly enriched in Cytokine-cytokine receptor interaction and Cell cycle (Fig. 4b, Table S3).
PPI network and hub gene analysis
The PPI network was created by String with a total of 443 nodes and 3285 edges (Fig. 5a). After, Cytoscape’s plug-in, “Cytohubba,” was used to further examinate the PPI network. We calculated the degree of DEGs connectivity using all 12 algorithms. Finally, two hub genes (Kif4a and Mki67) shared by the top 100 genes of each algorithm were obtained (Fig. 5b, Table S4). PPI networks associated with hub genes were assessed with Cytoscape’s GeneMANIA plugin using co-expression, co-localization, and shared protein domains. The network contained 20 hub genes, including a number of kif genes (Fig. 5c).
In addition, it was found a significant positive correlation between Kif4a and Mki67 in all TCGA tumor samples (Fig. 5d). Similarly, in most normal tissues of GTEx, Kif4a and Mki67 showed a positive correlation (Fig. 5e). In most CCLE cancer cell lines except the prostate, urinary tract, and biliary tract, Kif4 positively correlated with Mki67 expression (Fig. 5f). It was further observed a significant correlation between Kif4a and Mki67 expression in prostate tumor and benign prostate tissue, but no significant positive correlation was found in prostate tumor cell lines (Fig. 5g–i).
GSVA analysis of DEGs in BPH model
Through GO enrichment function analysis by GSVA method, we found that after hormone induction, the BP of DEGs was enriched in Meiotic chromosome condensation. There was a regulation of t cell co-stimulation, positive regulation of chromosome condensation, positive regulation of blood vessel remodelling and a response to interleukin 15 (Table S5). The CC of DEGs was mostly enriched in Atg1 ulk1 kinase complex, Mcm complex, Alpha DBA polymerase primase complex, DNA replication preinitiation complex and Spindle midzone (Table S5). After assessing the MF terms, DEGs were significantly enriched. There were C-X3-C chemokine binding, neuregulin binding bound Interleukin 1 receptor activity, complement receptor activity, and Cxcr3 chemokine receptor binding (Table S5). According to the GSVA algorithm, after hormone induction, the differential genes in the test group were mainly enriched in the KEGG pathway associated cellular interaction, energy and metabolism, including cytokine cell receptor interactions, other glycan degradation, glycolipid biosynthesis colloid series, ribosomes and proteasomes (Fig. 6, Table S6).
Construction of miRNAs-DEmRNAs regulatory network and enrichment analysis of DEGs
Based on the targeting relationship between the predicted miRNAs and DEmRNAs, a miRNAs-DEmRNAs regulatory network was established. The network included 250 miRNAs and 238 mRNAs nodes, of which 171 mRNAs were up-regulated and 67 mRNAs were down regulated (Fig. S3a). The miRNA with the largest number of regulatory target genes was rno-mir-325-3p (regulating 36 target genes), and the DEmRNA regulated by the largest number of miRNAs was MMP16 (regulated by 75 miRNAs). There were 2447 interactions in the network. Some of these interactions were rno-mir-325-3p regulating targeted Fgf5, Foxp3, and Kif20b, and Mmp16 was regulated by miRNAs such as rno-mir-33-5p, rno-mir-192-5p and rno-mir-489-3p. GO and KEGG enrichment analysis of regulated DEGs in the network indicated that 101 GO terms and 4 KEGG pathways were significantly enriched (Fig. S3b, c, Table S7–8).
Discussion
The incidence of BPH increases with age, so an increase in BPH patients cannot be avoided as the population ages. In addition to placing a heavy financial burden on society, men with BPH suffer significant morbidity and poor quality of life16. Due to its complex etiopathogenesis, BPH is difficult to completely cure17. Some patients still had poor effects such as dysuria after BPH standard drug treatment, and in occasions, further surgical treatment is needed18. Therefore, new therapeutic targets are urgently needed. The prostate is a sex hormone dependent organ, and prostate hyperplasia depends on androgens. As age increases, androgen levels often gradually decrease. Relatively elevated estrogen may become an important factor in promoting the occurrence and development of prostate hyperplasia, which may be related to the potential mechanism by which estrogen affects the prostate. Hormonal imbalances are considered to be important risk factors for BPH progression19. In contrast, there are few large-scale, high-throughput transcriptomic studies that examine how hormonal imbalances cause BPH. Thus, understanding BPH gene expression profile is crucial to understand BPH molecular mechanisms in depth. In this study, rats were treated with T and E2 for 4 weeks to create a pathologically confirmed BPH rat model. Then, RNA-seq and comprehensive bioinformatics analysis were performed to evaluate the molecular characteristics of BPH related to hormonal imbalances.
Canines, mice, and rats are often used in BPH models. The advantage of canines is that they are more similar to humans physiologically, especially in terms of prostate structure and function. In particular, canines can develop BPH spontaneously, and T and E2 can also induce rapid hyperplasia of the canine prostate. However, canines are difficult to raise, and the costs are high, making it challenging to conduct large-scale research. The mice model is easy to genetically manipulate and reproduces quickly, but its small size complicates subsequent surgical operations on the mice model, resulting in fewer tissue samples being obtained. More importantly, it takes at least 3 months to induce a BPH mice models with steroid hormones, and the long induction time hinders the efficiency of the research20. The prostate tissue structure of rats is similar to that of mice, and they also reproduce quickly and cannot spontaneously develop BPH, but the time for steroids to induce BPH is significantly shorter than that of mice, which greatly improves modeling efficiency15. In addition, the amount of tissue obtained is also greater than that of mice, and they have a stronger ability to resist stress than mice. Rats are often used to establish BPH models and provide a reliable research vehicle for research. Among them, SD rats are one of the most commonly used rats because of their practicality and easy reproducibility. Other rat breeds are also used to establish BPH rat models. For example, the BPH models established in Wistar rats under the induction of estradiol and testosterone shows, in addition to increased wet weight, pathology suggests fibromuscular hyperplasia composed of fibroblasts, smooth muscle cells and collagen. However, the traditional method of inducing BPH in rats is time-consuming and often requires daily injection of androgens (with or without estrogen). SD rats were selected in this study mainly because of their advantages in practicality and repeatability, but the selection of other rat strains may provide special insights. Traditional methods not only increase working hours, but also cause the mice to experience additional pain stimuli once a day, which is not conducive to the welfare of animals. In addition, they are not suitable for large-scale production, and due to the need for multiple injections, they can easily lead to injection related complications, such as local inflammation and skin ulceration, which may affect the quality stability of prostate hyperplasia models and reduce the reliability of related research results. The general characteristics of the prostate rat model after adding E2 are more obvious than using T alone, more importantly, the addition of estrogen brings the internal hormone environment of the body closer to reality15, which is more conducive to preclinical research and real application. Previous studies had shown that estrogens in combination with androgens were more beneficial in promoting BPH21. Therefore, in this study, T with a small amount of E2 was used to create the BPH rat model. Only one minor operation was required to place the pellets under the skin of the rats. BPH rat model was obtained after 4 weeks, without the need of daily hormone injection, which reduced the risk of infection and shortened the treatment time. In addition, urethra elongation, which was an important factor causing BOO in human22, but this is a characteristic change of BPH that other rat animal models do not possess23,24,25.
RNA-seq has been widely used as a powerful tool for gene expression analysis26. The ability of RNA-seq to assess the expression level of the entire transcriptome makes a new valid method to effectively study gene expression, it can obtain comprehensive molecular expression information and facilitate the search for disease diagnostic markers and therapeutic targets27,28. For example, a recent RNA-seq study showed that the approach sheds light on VEGF-induced signaling in human retinal microvascular endothelial cells29. Another RNA-seq study revealed neural differentiation pathways and compound-induced developmental neurotoxicity in the human neural progenitor cell test (hNPT)30. We validated some sequencing results using RT-qPCR, for example, in the T + E2 group, Ccna2 was significantly elevated. As one of the proliferation markers, it plays an important role in regulating the eukaryotic cell division cycle and has potential functions in the pathogenesis of benign prostatic hyperplasia31,32. Cdk1 is a cell proliferation marker that mediates the early stages of microglial cell proliferation and may also play a role in the pathogenesis of hormone imbalance induced prostate hyperplasia33,34. IL-1b is a cytokine involved in inflammatory responses and various cellular activities, such as proliferation, differentiation, and apoptosis. Its pro-inflammatory response can trigger sustained neutrophil responses. This study found that T + E2 induction can induce tissue inflammation, which may be related to IL-1b-related signaling activation35,36. We found that the experimental group had high expression of cdc20 in prostate tissue, which is a cell cycle protein closely related to cell proliferation and cell cycle. Increased expression of cdc20 can regulate cell cycle and proliferation37. Mki67 is a marker of proliferation, and we found that the expression of Mki67 was significantly increased in the T + E2 group, indicating that the prostate tissue in the T + E2 group is in a state of active proliferation38. Ndc80 is associated with the regulation of cell cycle, mitosis, and proliferation, and may be involved in the mechanism of T + E2 induced BPH in this study39. Plk1 is involved in the process of mitosis and is closely related to cell proliferation40, and the proliferation mechanism associated with Plk1 may be related to T + E2 induced prostate hyperplasia and deserves further investigation. Racgap1 plays a role in promoting cell proliferation and inhibiting cell apoptosis in various cells, but there are currently no relevant literature reports on the relationship between Racgap1 and the pathogenesis of prostate hyperplasia, and further research is needed to determine whether it is involved in the mechanism of T + E2 induced prostate hyperplasia. In the list of differentially expressed genes, we also found that many genes related to steroid hormone signaling pathways were significantly elevated in the T + E2 group, such as Esrrb, Cited4, and Hsd17b7. The co-activation of these genes may promote pathological changes in the prostate through complex signaling network interactions. Therefore, these genes may play important roles in steroid hormone induced prostate hyperplasia and are worth further exploring their specific mechanisms and potential therapeutic targets in future research. In the future, we will further explore the proteomics of this model to analyze the changes and interactions of important proteins in this model, providing important evidence for understanding the pathogenesis of BPH. According to GO and KEGG analyses, DEGs were enriched in mitotic nuclear division, microtubule motor activity, cytokine − cytokine receptor interaction and cell cycle. As these biological processes and pathways are associated with cell proliferation41, cell cycle42 and immune infiltration43, they seem to play an important role in promoting prostate enlargement. Notably, the activation of cytokine and cytokine receptor may facilitate immune cells recruitment44, which might be an important factor contributing to the presence of the large number of immune cells which infiltrated in the interstitial rat prostate tissue. In addition, we found that in the top10 results of the GO functional enrichment analysis, there were some terms related to microtubule, such as microtubule, tubulin binding, microtubule binding and microtubule motor activity. Microtubule is a member of the family of filamentous assemblies, which plays an important role as part of the cytoskeleton and participates in vital processes, such as intracellular transport and chromosome segregation45. Furthermore, it is highly correlated with proliferation46, cell cycle47 and apoptosis48. GSVA was used to investigate the role of differential genes in BPH associated with androgen dysregulation progression. Genomic enrichment analysis using GSVA is a reliable method to estimate changes in sample pathway activity without supervision49. By enrichment analysis methods, we are more confident that many pathways associated with cell cycle, proliferation, and immune infiltration were enriched in this study. Enrichment analysis results of GSVA suggested that DEGs were also found in the GO terms related to microtubule, including microtubule plus end binding, microtubule motor activity and minus end directed microtubule motor activity. These results validate the accuracy of GO and KEGG analysis. We compared the enrichment analysis results of BPH rat differential genes in this study with the enrichment analysis results of RNA-seq of human BPH and normal prostate tissues, and found that they were all enriched in signaling pathways, such as Toll like receptor signaling pathway, Ras signaling pathway, MAPK signaling pathway50, At the same time, rats are also enriched in urinary tract tumor related pathways, such as Bladder cancer and Renal cell carcinoma. The rat model overlaps with the differentially enriched genes in the prostate of human BPH patients, indicating the effectiveness of the model. Co enrichment of similar pathways in humans and rats may contain potential therapeutic targets, which may be important entry points for developing new drugs and treatment methods. This can be validated and optimized in both human and rat models, and is also conducive to further clinical translation. In addition, we should also note that a large number of immune related pathways are involved in rat prostate hyperplasia in the rat model, such as T cell receptor signaling pathway, Primary immunodeficiency, Intestinal immune network for iga production, The reason for this difference may be the difference in immune systems between different species of rats and humans. At the same time, hormone changes in human BPH patients are gradual, while T + E2-induced hormone changes in rats are induced in a short period of time. More importantly, the mechanism by which sex hormones cause BPH has not been fully revealed. Hormone induced BPH rats may only be at a certain stage of human BPH disease progression, and BPH rats and their transcriptome data may not reveal the characteristics of all human BPH patients. Therefore, more research and verification are needed from basic scientific research to clinical application.
Kif4a and Mki67 were considered the top hub genes after creating the PPI network. Kif4a is part of the kinesin superfamily of proteins (KIFs) and holds a variety of biological functions, including microtubule poleward flux regulation, spindle elongation and chromosome formation51,52,53. Mki67 is a cell proliferation marker, which is known to enhance cell proliferation54. Kif4a and Mki67 were also found to be highly correlated with the network constructed by Genemania. Other Kif family members were found to be involved in this network. The main role of the KIF superfamily of proteins was to transport various substances to microtubule tracks55. Thus, the KIF family of proteins plays an important role in cell morphology and function. The two genes, Kif4a and Mki67, were found to be positively correlated with each other in normal tissues and tumor cells after searching on publicly available databases, such as TCGA, which further verifies the correlation between them. We hypothesised that the interplay of these two genes might affect BPH progression through intracellular transport function and cell proliferation and be a potential target for the treatment of BPH. Finally, the results of the PPI network analysis confirmed the reliability of the functional enrichment analysis results.
MiRNA is a short (22 nucleotide) non-coding RNA whose main function is to silence mRNA translation into proteins, thus participating in a wide range of physiological and pathological activities56. A total of 250 miRNAs in our predicted miRNA-mRNA network were involved in the DEGs regulation. Some of them had been already reported in BPH-related studies. L. Liu et al.57 found that mir-34c-5p is highly expressed in BPH prostate. In this study, mir-34c-5p was predicted to interact with down-regulated genes (grem2, RARB, slcl2a2 and per2), suggesting that mir-34c-5p may promote prostatic hyperplasia by downregulating the expression of these genes. Chen et al.21 found that mir-142-3p was low expressed in rats’ prostate with BPH, that mir-34c-5p could interact with up-regulated genes (itgb8 and Suco) and that reduced mir-34c-5p could attenuate the impact on these genes which accelerated the progression of prostate enlargement. In addition, these predicted results require further experimental validation to determine the exact mechanism of miRNA in prostate hyperplasia.
In the study, the shortcomings are identified as follows: First, the small sample size limited the results of this study. Second, we evaluated the overall weight changes of the prostate, but did not assess the changes in individual lobe weights, which cannot provide more detailed information for the study. Third, although RT-qPCR confirmed the reliability of sequencing results, the true diversity may be underestimated due to the small number of samples used for sequencing. In addition, the regulatory networks or mechanisms presented in this study are only based on bioinformatics analysis and lack experimental validation to verify the authenticity of these results. Therefore, more comprehensive analysis and further in-depth research are still needed through in vitro and in vivo experiments. Currently, our team is dedicated to investigating these potential mechanisms.
Conclusions
This study provides a new effective approach to create BPH rats associated with hormonal imbalances and identifies important genes, pathways, and miRNA-mRNA regulated network. The results may provide novel insights into the disease and suggest potential targets for clinical treatment.
Materials and methods
Animals and environmental conditions
In total, 35 healthy male 6–8 weeks old Sprague Dawley (SD) rats with an initial body weight of 200–230 g were purchased from Tengxin Biotechnology Co.Ltd (Chongqing, China). During their rearing, they were kept in a standard laboratory environment (12/12 hours of light/dark cycle, 22 ± 2 °C with 55 ± 5% relative humidity), and allowed to consume standard laboratory pellets and water ad libitum.
Prostatic hyperplasia induction and specimen collection
Rats were acclimatized for a week and randomized into 4 groups, including control group (Con group, n = 7), castration group (C group, n = 7), T-treated group (T group, n = 7), T and E2-treated group (T + E2 group, n = 8). All rats were anesthetized with 1% pentobarbital (30 mg/kg) intraperitoneally, the rats from Con group only underwent incision and suturing of the dorsal skin and scrotum, while the rats of other three groups underwent testicular resection surgery. The rats of C group do not receive any additional treatment except for incision and suturing of dorsal skin. We prepared sustained-release particles20 and subcutaneously implanted 4 particles (each containing 25 mg T) in group T rats, and 4 mixed slow-release particles (each containing 25 mg T and 2.5mg E2) subcutaneously in the T + E2 group rats for 4 weeks (Fig. S1b–d). T and E2 are derived from Sigma Chemical Co. (St. Louis, MO).
After 4 weeks of treatment, all rats were intraperitoneally injected with a lethal dose pentobarbital (180 mg/kg) anesthesia to be euthanized. Three rats were randomly selected from Con group and T + E2 group for RNA seq testing and validation using RT-qPCR, while the remaining rats underwent gross, blood, and histological testing. The tissues prepared for RNA-seq and RT-qPCR were freshly frozen in liquid nitrogen and placed in a refrigerator at -80 degrees Celsius. After settling for two hours at room temperature, the blood samples were centrifuged to obtain serum which was stored at -20 °C for spare. Prostate, urethra, bladder, kidney, and testis were measured and weighed with a precision caliper and weight scale. For histopathological evaluation, prostates were fixed with 10% neutral buffered formalin and embedded in paraffin.
Histopathological examination
Tissue was cut in sections of a thickness of 5 mm and paraffin embedded. Hematoxylin and eosin (HE) staining was performed according to a standard procedure for routine histopathology. Then, sections were dewaxed and rehydrated and observed using a light microscope (BX53F, Olympus, Tokyo, Japan). All pathological results were reviewed by at least two pathologists.
High-throughput RNA sequencing
To prepare RNA samples, total prostate tissue RNA was isolated (n = 3 for each group). Illumina TruSegTM RNA Sample Preparation Kit (Illumina Inc., San Diego, CA, USA) was used to prepare sequencing libraries. Libraries were sequenced on an Illumina HiSeg X-ten System (Illumina, San Diego, CA, USA) at Shanghai Biotechnology Corporation. Data from all RNA-seq are uploaded to the NCBI Gene Expression Omnibus (GEO) database (access number GSE217800). To review GEO accession GSE217800, go to https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE217800.
Differentially expressed gene analysis and hierarchical clustering
DESeq2 package in R software was used to identify DEGs. They were defined using the adjusted p value < 0.05 and |log2FC|>1 for significant differential expression. The aim of this study was to generate an overview of the mRNA expression profiles of the two groups and their differences. Screened DEGs were mapped in heatmap with a clustering and volcano map using the Complex Heatmap and ggplot2 packages. Additionally, we visualized sample-to-sample distances using PCA. Correlation and PCA analyses were performed in R software using the ggplot2 package.
Protein-protein interaction network (PPI) and correlation of identified gub genes
DEGs PPI networks were created using STRING (https://string-db.org/), which were used to retrieve interacting genes for evaluation of protein-PPIs. DEGs PPI network was created using default parameter values. Cytohubba plug-in of Cytoscape v. 3.9.1 was used to identify the top hub genes from 100 hub genes with each algorithm. Next, genes intersection was analyzed in another network using GeneMANIA of Cytoscape. Additionally, high throughput expression data were downloaded from TCGA (Dataset ID: tcga_RSEM_gene_tpm), and GTEx (Dataset ID: gtex_RSEM_gene_tpm) from the UCSC Xena database58 (https://xena.ucsc.edu/) and CCLE (Dataset ID: CCLE 2019 Files) from the CCLE database59 (https://sites.broadinstitute.org/ccle) to verify the correlation between the top hub genes. In different cancers, different cell lines, normal tissues, and the top hub genes were correlated and visualized by the ggplot2 package in R.
GO functional and KEGG pathway analyses
Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway were used to assess the functional and biological implications of DEGs using the R package cluster profiler. The significance P value cutoff was set at 0.05. Biological process (BP), cellular component (CC), and molecular function (MF) are the three categories of GO terms analyzed during the enrichment analysis. Furthermore, the GOCircle plotting was created using the GOplot R package.
Gene set variation analysis (GSVA)
As a nonparametric and unsupervised method, GSVA evaluates the enrichment of KEGG gene sets60. There are two files that GSVA requires: a gene set and a gene expression matrix. R GSVA package was used to further evaluate hub genes’ signaling pathways. Then, GSVA package was used to integrate the gene set and the gene expression matrix. KEGG pathway matrices were obtained. Significant KEGG pathways were identified using the Limma package. As a reference to GSVA, C2 and C5 gene sets (KEGG pathways and GO entry for Rattus norvegicus) from MSigDB by msigdbr package were used. GSVA terms were visualized using the clusterProfiler package. Statistical significance was determined when P < 0.05.
miRNA-mRNA regulatory network construction
It is all known that a miRNA can regulate dozens of transcripts of mRNA, and a target gene’s mRNA can be simultaneously regulated by multiple miRNAs. MiRNAs regulate target mRNA by repressing translational activity or degrading it. MiRNA to mRNA binding plays a crucial role in regulating mRNA levels and protein expression. Then TargetScan (http://www.targetscan.org/) was used to search for the potential miRNA-mRNA binding region. Finally, miRNA–DEmRNAs interaction network was created and visualized by Cytoscape.
Real-time quantitative polymerase chain reaction (RT-qPCR)
In this study, total RNA was extracted from prostate tissue using TRIzol Reagent (Invitrogen, Thermo Fisher Scientific, Inc., MA, USA). The concentration of RNA was determined by measuring absorbance at 260/280 nm. The first-strand cDNA was synthesized using a first-strand cDNA synthesis kit (Takara, Japan). GAPDH was used as internal control for RT-qPCR. RT-qPCR was performed using SYBR Green Mix (Takara, Japan) and BIO-RAD CFX ConnectTM Optics Module (Bio-Rad, USA). The relative gene expression values were calculated using the 2−△△CT method. Each experiment was repeated three times. All primers were synthesized by Sangon Biotech Co. Ltd. (Shanghai). Table S1 shows the primers used for RT-qPCR.
Statistical analysis
The samples were taken randomly using Excel Rand function (Microsoft Corp, Redmond, WA). R software (version 4.1.3) and GraphPad Prism 9.3.0 were used for the statistical analyses and visualization. Data were compared using unpaired two-tailed Student’s t-test or Wilcox test between two groups. One-way analysis of variance (One-way ANOVA) was used for multiple group comparisons. Spearman correlation was used to assess correlations. A p-value < 0.05 was considered statistically significant.
Gross observations in different rat groups. (a) Gross view of prostate, Bladder, urethra, and seminal vesicles of different groups. (b) Prostate weight. (c) Final body weight. (d) Prostatic index: “Prostate weight” / “Final body weight” x 100%. (e) Prostatic urethral length. B, bladder; AP, anterior prostate; VP/LP, ventral prostate/ lateral prostate; U, urethra; SV, seminal vesicle; DP, dorsal prostate; ****, p < 0.0001; ns, not significant.
Rat prostate pathological examination. (a) Prostate tissue of the Con group stained with HE (Scale bar: 100 μm). (b) Prostate tissues in the C group stained with HE (Scale bar = 100 μm). (c) Prostate tissue of the T group stained with HE (Scale bar: 100 μm). (d) Prostate tissue of the T + E2 group stained with HE (Scale bar: 100 μm).
DEGs identification and validation based on gene expression profiles. (a) Heatmap diagram of DEGs in control and test tissues are shown. (b) Volcano plot showing DEGs. (c) PCA plots showing all DEGs clustering the samples. (d) RT-qPCR used to validate eight differentially expressed genes.*, p < 0.05; **, p < 0.01.
A PPI network of DEGs was constructed and hub genes were analyzed. (a) PPI network and top hub genes (Kif4a and Mki67) are highlighted in yellow. (b) Flower plot showing the results of 12 algorithms to identify hub genes. (c) Overview of the GeneMANIA network: the black circle represents a GeneMANIA input and the gray circle represents a GeneMANIA hub. (d) Relationship between the hub genes expression level in different cancer types. (e) Using different normal tissues to compare the Kif4a and Mki67. (f) Different cell lines analyzed for variations in hub gene expression levels. (g) In TCGA, PRAD hub genes (Kif4a and Mki67) showed a positive correlation with their expression values. (h) Relationship between hub genes expression in the normal tissues. (i) Hub genes expression in cells and their relationship.
Data availability
Data from all RNA-seq are uploaded to the NCBI Gene Expression Omnibus (GEO) database (access number GSE217800). To review GEO accession GSE217800, go to https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE217800.
References
Yu, Z. J. et al. Efficacy and side effects of drugs commonly used for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia. Front. Pharmacol. 11, 658. https://doi.org/10.3389/fphar.2020.00658 (2020).
He, W. et al. Changes in the expression and function of the PDE5 pathway in the obstructed urinary bladder. J. Cell. Mol. Med. 24, 13181–13195. https://doi.org/10.1111/jcmm.15926 (2020).
Russo, G. I. et al. The Investigative Role of statins in ameliorating lower urinary tract symptoms (LUTS): a systematic review. J. Clin. Med. 10, 859. https://doi.org/10.3390/jcm10030416 (2021).
Jin, B. R. et al. 6'-Sialyllactose ameliorates in vivo and in vitro benign prostatic hyperplasia by regulating the E2F1/pRb-AR pathway. Nutrients 2019, 11. https://doi.org/10.3390/nu11092203 (2019).
Porst, H. et al. Effects of once-daily tadalafil on erectile function in men with erectile dysfunction and signs and symptoms of benign prostatic hyperplasia. Eur. Urol. 56, 727–735. https://doi.org/10.1016/j.eururo.2009.04.033 (2009).
Oelke, M. et al. Age and bladder outlet obstruction are independently associated with detrusor overactivity in patients with benign prostatic hyperplasia. Eur. Urol. 54, 419–426. https://doi.org/10.1016/j.eururo.2008.02.017 (2008).
Kim, E. H., Larson, J. A. & Andriole, G. L. Management of benign prostatic hyperplasia. Annu. Rev. Med. 67, 137–151. https://doi.org/10.1146/annurev-med-063014-123902 (2016).
Tavani, A. et al. Intake of selected micronutrients and the risk of surgically treated benign prostatic hyperplasia: a case-control study from Italy. Eur. Urol. 50, 549–554. https://doi.org/10.1016/j.eururo.2005.11.027 (2006).
Kim, N. H. et al. The effects of aronia melanocarpa extract on testosterone-induced benign prostatic hyperplasia in rats, and quantitative analysis of major constituents depending on extract conditions. Nutrients 12, 859. https://doi.org/10.3390/nu12061575 (2020).
Aversa, A., Duca, Y., Condorelli, R. A., Calogero, A. E. & La Vignera, S. Androgen deficiency and phosphodiesterase type 5 expression changes in aging male: therapeutic implications. Front. Endocrinol. 10, 225. https://doi.org/10.3389/fendo.2019.00225 (2019).
Driscoll, I. & Resnick, S. M. Testosterone and cognition in normal aging and Alzheimer’s disease: an update. Curr. Alzheimer Res. 4, 33–45. https://doi.org/10.2174/156720507779939878 (2007).
Chen, B. et al. Estrogen regulates the proliferation and inflammatory expression of primary stromal cell in benign prostatic hyperplasia. Translational Androl. Urol. 9, 322–331. https://doi.org/10.21037/tau.2020.02.08 (2020).
Roberson, E. D. O. et al. Alterations of the primary cilia gene SPAG17 and SOX9 locus noncoding RNAs identified by RNA-Sequencing analysis in patients with systemic sclerosis. Arthritis Rheumatol. 75, 108–119. https://doi.org/10.1002/art.42281 (2023).
Zins, K. et al. Inhibition of stromal PlGF suppresses the growth of prostate cancer xenografts. Int. J. Mol. Sci. 14, 17958–17971. https://doi.org/10.3390/ijms140917958 (2013).
Zhang, J. et al. Animal models of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 24, 49–57. https://doi.org/10.1038/s41391-020-00277-1 (2021).
Xu, X. F. et al. α1-Blockers and 5α-Reductase inhibitors are the most recommended drugs in treating Benign Prostatic Hyperplasia: an evidence-based evaluation of clinical practice guidelines. Front. Pharmacol. 11, 311. https://doi.org/10.3389/fphar.2020.00311 (2020).
Sheng, J. et al. M2 macrophage-mediated interleukin-4 signalling induces myofibroblast phenotype during the progression of benign prostatic hyperplasia. Cell. Death Dis. 9, 755. https://doi.org/10.1038/s41419-018-0744-1 (2018).
Yang, B. Y. et al. 5-ARI induces autophagy of prostate epithelial cells through suppressing IGF-1 expression in prostate fibroblasts. Cell. Prolif. 52, e12590. https://doi.org/10.1111/cpr.12590 (2019).
Huang, D. Y. et al. Oral exposure of low-dose bisphenol A promotes proliferation of dorsolateral prostate and induces epithelial-mesenchymal transition in aged rats. Sci. Rep. 8, 490. https://doi.org/10.1038/s41598-017-18869-8 (2018).
Tang, X. et al. Comparative RNA-sequencing analysis of the prostate in a mouse model of benign prostatic hyperplasia with bladder outlet obstruction. Mol. Cell. Biochem. 478, 2721–2737. https://doi.org/10.1007/s11010-023-04695-2 (2023).
Chen, X. et al. Regulation of microRNAs by rape bee pollen on benign prostate hyperplasia in rats. Andrologia 52, e13386. https://doi.org/10.1111/and.13386 (2020).
Agrawal, V., Khullar, R. & Jha, A. K. Assessment of posterior urethra in benign prostatic hyperplasia and after its surgery. Urol. Ann. 12, 63–68. https://doi.org/10.4103/ua.Ua_118_18 (2020).
Li, L. Y. et al. Alterations of gut microbiota diversity, composition and metabonomics in testosterone-induced benign prostatic hyperplasia rats. Mil Med. Res. 9, 12. https://doi.org/10.1186/s40779-022-00373-4 (2022).
Jin, B. R., Lim, C. Y., Kim, H. J., Lee, M. & An, H. J. Antioxidant mitoquinone suppresses benign prostatic hyperplasia by regulating the AR-NLRP3 pathway. Redox Biol. 65, 102816. https://doi.org/10.1016/j.redox.2023.102816 (2023).
Kim, M., Tran, P., Yin, J., Song, J. & Kim, H. Cinnamomum cassia and Rosa laevigata mixture improves benign prostatic hyperplasia in rats by regulating androgen receptor signaling and apoptosis. Nutrients 15 https://doi.org/10.3390/nu15040818 (2023).
Li, D. et al. Baiting out a full length sequence from unmapped RNA-seq data. BMC Genom. 22, 857. https://doi.org/10.1186/s12864-021-08146-4 (2021).
Verdes, A. et al. Expression patterns, and distribution of novel ribbon worm predatory and defensive toxins. Mol. Biol. Evol. 39, 589. https://doi.org/10.1093/molbev/msac096 (2022).
Rivellese, F. et al. Rituximab versus tocilizumab in rheumatoid arthritis: synovial biopsy-based biomarker analysis of the phase 4 R4RA randomized trial. Nat. Med. 28, 1256–1268. https://doi.org/10.1038/s41591-022-01789-0 (2022).
Ramshekar, A., Bretz, C. A. & Hartnett, M. E. RNA-Seq provides insights into VEGF-Induced Signaling in Human Retinal Microvascular endothelial cells: implications in retinopathy of Prematurity. Int. J. Mol. Sci. 23, 896. https://doi.org/10.3390/ijms23137354 (2022).
de Leeuw, V. C. et al. Neuronal differentiation pathways and compound-induced developmental neurotoxicity in the human neural progenitor cell test (hNPT) revealed by RNA-seq. Chemosphere 304, 135298. https://doi.org/10.1016/j.chemosphere.2022.135298 (2022).
Yang, J. et al. Integrated genomic and transcriptomic analysis reveals unique characteristics of hepatic metastases and pro-metastatic role of complement C1q in pancreatic ductal adenocarcinoma. Genome Biol. 22, 4. https://doi.org/10.1186/s13059-020-02222-w (2021).
Liu, F., Dong, J., Zhang, P., Zhou, D. & Zhang, Q. Transcriptome sequencing reveals key genes in three early phases of osteogenic, adipogenic, and Chondrogenic differentiation of bone marrow mesenchymal stem cells in rats. Front. Mol. Biosci. 8, 782054. https://doi.org/10.3389/fmolb.2021.782054 (2021).
Tan, W.. et al. Distinct phases of adult microglia proliferation: a myc-mediated early phase and a Tnfaip3-mediated late phase. Cell. Discov 8, 34. https://doi.org/10.1038/s41421-022-00377-3 (2022).
Bouguéon, M. et al. A rule-based multiscale model of hepatic stellate cell plasticity: critical role of the inactivation loop in fibrosis progression. PLoS Comput. Biol. 20, e1011858. https://doi.org/10.1371/journal.pcbi.1011858 (2024).
Chu, F. et al. The genetic and immunologic landscape underlying the risk of malignant progression in laryngeal dysplasia. Cancers 15, 8963. https://doi.org/10.3390/cancers15041117 (2023).
Guerrero, D. et al. Evaluation of cutaneous immune response in a controlled human in vivo model of mosquito bites. Nat. Commun. 13, 7036. https://doi.org/10.1038/s41467-022-34534-9 (2022).
Guo, L. et al. PER2 integrates circadian disruption and pituitary tumorigenesis. Theranostics 13, 2657–2672. https://doi.org/10.7150/thno.82995 (2023).
Aguilar, C. et al. Helicobacter pylori shows tropism to gastric differentiated pit cells dependent on urea chemotaxis. Nat. Commun. 13, 5878. https://doi.org/10.1038/s41467-022-33165-4 (2022).
Zhang, Y. J., Sun, Y. Z., Gao, X. H. & Qi, R. Q. Integrated bioinformatic analysis of differentially expressed genes and signaling pathways in plaque psoriasis. Mol. Med. Rep. 20, 225–235. https://doi.org/10.3892/mmr.2019.10241 (2019).
de Cárcer, G. et al. Plk1 overexpression induces chromosomal instability and suppresses tumor development. Nat. Commun. 9, 3012. https://doi.org/10.1038/s41467-018-05429-5 (2018).
Bhushan, B. et al. TCPOBOP-Induced hepatomegaly and hepatocyte proliferation are attenuated by combined disruption of MET and EGFR Signaling. Hepatol. (Baltimore Md). 69, 1702–1718. https://doi.org/10.1002/hep.30109 (2019).
Haworth, S. et al. Low-frequency variation in TP53 has large effects on head circumference and intracranial volume. Nat. Commun. 10, 357. https://doi.org/10.1038/s41467-018-07863-x (2019).
Malhotra, D. et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat. Immunol. 13, 499–510. https://doi.org/10.1038/ni.2262 (2012).
Li, P. et al. Messenger RNA sequencing and pathway analysis provide novel insights into the susceptibility to Salmonella enteritidis infection in chickens. Front. Genet. 9, 256. https://doi.org/10.3389/fgene.2018.00256 (2018).
Brändén, G. et al. Coherent diffractive imaging of microtubules using an X-ray laser. Nat. Commun. 10, 2589. https://doi.org/10.1038/s41467-019-10448-x (2019).
Feng, Q. et al. ID09, A newly-designed tubulin inhibitor, regulating the proliferation, migration, EMT process and apoptosis of oral squamous cell carcinoma. Int. J. Biol. Sci. 18, 473–490. https://doi.org/10.7150/ijbs.65824 (2022).
Kazmi, N. et al. Rho GTPase gene expression and breast cancer risk: a mendelian randomization analysis. Sci. Rep. 12, 1463. https://doi.org/10.1038/s41598-022-05549-5 (2022).
Yan, Y. et al. Sulforaphane downregulated fatty acid synthase and inhibited microtubule-mediated mitophagy leading to apoptosis. Cell. Death Dis. 12, 917. https://doi.org/10.1038/s41419-021-04198-2 (2021).
Zhou, Z. et al. Hematopoietic gene expression regulation through m(6)a methylation predicts prognosis in stage III colorectal cancer. Front. Oncol. 10, 572708. https://doi.org/10.3389/fonc.2020.572708 (2020).
Xiao, H. et al. Identification and functional activity of matrix-remodeling associated 5 (MXRA5) in benign hyperplastic prostate. Aging 12, 8605–8621. https://doi.org/10.18632/aging.103175 (2020).
Steblyanko, Y. et al. Microtubule poleward flux in human cells is driven by the coordinated action of four kinesins. EMBO J. 39, e105432. https://doi.org/10.15252/embj.2020105432 (2020).
Vukušić, K., Ponjavić, I., Buđa, R., Risteski, P. & Tolić, I. M. Microtubule-sliding modules based on kinesins EG5 and PRC1-dependent KIF4A drive human spindle elongation. Dev. Cell. 56, 1253–1267e1210. https://doi.org/10.1016/j.devcel.2021.04.005 (2021).
Paulson, J. R., Hudson, D. F., Cisneros-Soberanis, F. & Earnshaw, W. C. Mitotic chromosomes. Semin. Cell Dev. Biol. 117, 7–29. https://doi.org/10.1016/j.semcdb.2021.03.014 (2021).
Kumar, D. et al. T-follicular helper cell expansion and chronic T-cell activation are characteristic immune anomalies in Evans syndrome. Blood 139, 369–383. https://doi.org/10.1182/blood.2021012924 (2022).
Sati, L., Seval-Celik, Y., Unek, G., Korgun, E. T. & Demir, R. The presence of kinesin superfamily motor proteins KIFC1 and KIF17 in normal and pathological human placenta. Placenta 30, 848–854. https://doi.org/10.1016/j.placenta.2009.07.005 (2009).
Lan, T., Shiyu, H., Shen, Z., Yan, B. & Chen, J. New insights into the interplay between miRNAs and autophagy in the aging of intervertebral discs. Ageing Res. Rev. 65, 101227. https://doi.org/10.1016/j.arr.2020.101227 (2021).
Liu, L. et al. miRNA Regulation network analysis in qianliening capsule treatment of benign prostatic hyperplasia. Evid. Based Complement. Altern. Medicine: eCAM 2015, 365484. https://doi.org/10.1155/2015/365484 (2015).
Goldman, M. J. et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 38, 675–678. https://doi.org/10.1038/s41587-020-0546-8 (2020).
Nusinow, D. P. et al. Quantitative proteomics of the cancer cell line encyclopedia. Cell 180, 387–402e316. https://doi.org/10.1016/j.cell.2019.12.023 (2020).
Quan, Y., Zhang, X. & Ping, H. Construction of a risk prediction model using m6A RNA methylation regulators in prostate cancer: comprehensive bioinformatic analysis and histological validation. Cancer Cell. Int. 22, 33. https://doi.org/10.1186/s12935-021-02438-1 (2022).
Funding
This work was supported by the National Natural Science Foundation of China (No. 82260157).
Author information
Authors and Affiliations
Contributions
XT, and GL conceived and designed the study. XT, ZL, and HZ performed the experiment. XT, ZL, and YT contributed significantly to the analysis and manuscript preparation. XT, and JR performed the data analyses and wrote the manuscript. XT, ZL, and HZ structurally revised the manuscript. XT, JH, and ZS helped perform the analysis with constructive discussions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, XH., Liu, ZY., Ren, JW. et al. Comprehensive RNA-seq analysis of benign prostatic hyperplasia (BPH) in rats exposed to testosterone and estradiol. Sci Rep 15, 2750 (2025). https://doi.org/10.1038/s41598-025-87205-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87205-2