Abstract
In most patients with type 1 xanthinuria caused by mutations in the xanthine dehydrogenase gene (XDH), no clinical complications, except for urinary stones, are observed. In contrast, all Xdh(− / −) mice die due to renal failure before reaching adulthood at 8 weeks of age. Hypoxanthine or xanthine levels become excessive and thus toxic in Xdh(− / −) mice because enhancing the activity of hypoxanthine phosphoribosyl transferase (HPRT), which is an enzyme that uses hypoxanthine as a substrate, slightly increases the life span of these mice. In this study, we targeted the mouse intestinal sodium-dependent nucleobase transporter (SNBT) gene (Slc23a4), which is a pseudogene in humans. Hprt(high)Xdh(− / −)Slc23a4(− / −) mice had a longer life span and reached adulthood. The urinary xanthine excretion of these mice was 20-fold greater than that of patients with type 1 xanthinuria. The urinary hypoxanthine/xanthine ratio of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice was lower than that of patients with type 1 xanthinuria. Hprt(high)Xdh(− / −)Slc23a4(− / −) mice exhibited renal impairment, accompanied by high plasma creatinine levels and anemia. Moreover, female Hprt(high)Xdh(− / −)Slc23a4(− / −) mice produced offspring that did not survive. In conclusion, for the first time, we established that Xdh(− / −) mice survive to adulthood.
Similar content being viewed by others
Introduction
The final step of purine metabolism in humans, namely, the breakdown of hypoxanthine to xanthine and xanthine to uric acid, is carried out intracellularly by xanthine dehydrogenase (XDH); this is a dehydrogenation reaction that uses a coenzyme NAD+. In type 1 xanthinuria, which is caused by a loss of XDH activity due to mutations in the xanthine dehydrogenase (XDH) gene, uric acid production in the body is lost, and the oxypurines xanthine and hypoxanthine are excreted as purine end products, resulting in the clinical symptom of urinary stones. However, similar to patients with renal hypouricemia, no clinical complications are observed in many patients with xanthinuria1. On the other hand, it has been reported that all Xdh(− / −) mice, which serve as a model of type 1 xanthinuria, die before reaching adulthood at 8 weeks of age2,3,4. Therefore, it has not been possible to use adult Xdh(− / −) mice to study the biological function of XDH by comparing mice with or without XDH expression.
Even among the most closely related mammals, namely, humans and mice, there are large species-specific differences in purine metabolism. In mammals other than apes, uric acid is further degraded, and allantoin is excreted as the final product of purine metabolism. However, apes, including humans, lack uricase (Uox), which converts uric acid to allantoin, so uric acid is excreted as the final product of purine metabolism5. Uox(− / −) mice, which are considered a model of normal human physiology, die within 2 weeks after birth6, similar to Xdh(− / −) mice, which die soon after birth. On the other hand, patients deficient in hypoxanthine phosphoribosyltransferase (HPRT), which converts hypoxanthine into inosine monophosphate (IMP) and reuses it as purine salvage pathway, develop Lesch-Nyhan disease and exhibit neurological symptoms, including recurrent self-injurious behavior7; however, Hprt(− / −) mice are completely asymptomatic8. Thus, it has been impossible to use mice to study human purine metabolism because of the large differences between species.
We hypothesized that the reason why Xdh(− / −) mice die soon after birth is that the levels of hypoxanthine or xanthine, which should be degraded by XDH, become excessive and toxic. Since HPRT has low activity in laboratory mice, we generated Hprt(high) mice by introducing a high-activity Hprt allele derived from field mice9, and then, we generated Hprt(high)Xdh(− / −) mice. Individual mice that survived until 24 weeks of age were obtained. This survival indicated that a reduction in intracellular hypoxanthine levels is necessary for increasing the lifespan of Xdh(− / −) mice10.
There are species-specific differences between humans and mice not only in purine metabolism but also in purine kinetics. Because the Na+-dependent intestinal purine base transporter SNBT1 (Slc23a4), which is a pseudogene in humans, is expressed in mice11, the intestinal absorption of hypoxanthine and xanthine may be greater in mice than in humans, and the abundance of these substances in the body may become excessive. In this study, we targeted Slc23a4 with the aim of reducing hypoxanthine and xanthine absorption in the intestinal tract and extending the lifespan of Hprt(high)Xdh(− / −) mice. The Hprt(high)Xdh(− / −)Slc23a4(− / −) mice that were generated in this study exhibited successfully extended lifespans compared to Hprt(low)Xdh(− / −)Slc23a4(+ / +) mice.
Results
Survival of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice
The median survival time of the 108 Hprt(high)Xdh(− / −)Slc23a4(− / −) mice was 191.8 days. However, the median survival times of the 6 Hprt(high)Xdh(− / −)Slc23a4(+ / −) and 7 Hprt(high)Xdh(− / −)Slc23a4(+ / +)mice were 64.05 and 83.3 days, respectively (Fig. 1A). There was no significant difference in median survival between Hprt(high)Xdh(− / −)Slc23a4(+ / +) mice and Hprt(high)Xdh(− / −)Slc23a4(+ / −) mice. On the other hand, the median survival of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice was significantly longer than that of both Hprt(high)Xdh(− / −)Slc23a4(+ / −) mice and Hprt(high)Xdh(− / −)Slc23a4(+ / +) mice.
(A) Kaplan–Meier curves depicting the survival of Hprt(high)Xdh(− / −)Slc23a4 mice. Hprt(high)Xdh(− / −)Slc23a4(− / −) mice (n = 108, solid line); Hprt(high)Xdh(− / −)Slc23a4(+ /−) mice (n = 6, dashed line); Hprt(high)Xdh(− / −)Slc23a4(+ / +) (n = 7, dotted line). (B) Body weights of Hprt(high)Xdh(− / −)Slc23a4(− / −) male mice (n = 6, black squares) compared with Hprt(high)Xdh(− / −)Slc23a4(− / −) female mice (n = 8, white circles).
Body weight increased with age in the six male and nine female Hprt(high)Xdh(− / −)Slc23a4(− / −) mice that were used for blood and urine collection (Fig. 1B). The mating of male and female Hprt(high)Xdh(− / −)Slc23a4(− / −) mice produced offspring several times. However, all the offspring died within a few days after birth. In contrast, the mating of male Hprt(high)Xdh(− / −)Slc23a4(− / −) mice with female Hprt(high)Xdh(+ /-)Slc23a4(− / −) mice produced offspring that grew without problems. These results suggested that female Xdh(− / −) mice could not raise offspring, which may have been caused by insufficient lactation.
Urinary oxypurine excretion in Hprt(high)Xdh(− / −)Slc23a4(− / −) mice and the effect of a low-purine diet
The urinary hypoxanthine/creatinine ratio of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice that were fed a normal diet was 0.4335 ± 0.0864 (mol/mol Cr), whereas the ratio was significantly reduced to 14.2% to 0.0615 ± 0.0346 (mol/mol Cr) in the mice that were fed a low-purine purified diet (Fig. 2A). The urinary hypoxanthine/creatinine ratio of Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice that were fed a normal diet was 0.0488 ± 0.0088 (mol/mol Cr), whereas it was 0.0290 ± 0.0041 (mol/mol Cr) in the mice that were fed a low-purine purified diet, indicating that there were no significant differences between the effects of the two diets. Compared with Hprt(high)Xdh(− / −)Slc23a4(− / −) mice, the ratio was significantly reduced by the normal diet, but no significant difference was observed in mice that were fed the low-purine purified diet (Fig. 2A).
Profiles of purine metabolism in Hprt(high)Xdh(− / −)Slc23a4(− / −) and Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice that were fed a normal diet or a low-purine purified diet. (A) urinary hypoxanthine/creatinine ratio, (B) urinary xanthine/creatinine ratio, (C) total urinary purine/creatinine ratio. The data represent the means ± SDs. *p < 0.05, **p < 0.01 vs. the Xdh(− / −) MRA group; #p < 0.05, ##p < 0.01 vs. the Xdh(− / −) AIN group; ††p < 0.01 vs. the Xdh(+ / −) MRA group. P values were calculated via one-way ANOVA. Normal diet; MRA. Low-purine purified diet; AIN.
The urinary xanthine/creatinine ratio of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice that were fed a normal diet was 3.761 ± 0.299 (mol/mol Cr), whereas the ratio was significantly reduced by 40.4% to 1.520 ± 0.260 (mol/mol Cr) in mice that were fed the low-purine purified diet (Fig. 2B). The urinary xanthine/creatinine ratio of Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice that were fed a normal diet was 0.015 ± 0.008, whereas the ratio was 0.017 ± 0.007 in Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice that were fed a low-purine purified diet , indicating that there were no significant differences between the effects of the two diets. But the urinary xanthine/creatinine ratio of Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice was significantly lower than that in Hprt(high)Xdh(− / −)Slc23a4(− / −) mice when the mice were fed both normal and low-purine diets (Fig. 2B). In addition, the urinary hypoxanthine/xanthine ratio of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice that were fed a normal diet was 0.115 (mol/mol Cr), whereas it was 0.040 (mol/mol Cr) in Hprt(high)Xdh(− / −)Slc23a4(− / −) mice fed a low-purine purified diet (Fig. 2A, B).
The ratio of total urinary purine metabolites, including allantoin and uric acid, to creatinine in Hprt(high)Xdh(− / −) Slc23a4(− / −) mice was 4.248 ± 0.3686 (mol/mol Cr) when the mice were fed a normal diet and 1.626 ± 0.2574 (mol/mol Cr) when the mice were fed a low-purine diet, which was a significant decrease of 61.7% (Fig. 2C). The ratio of Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice was 6.478 ± 0.5065 (mol/mol Cr) when the mice were fed a normal diet and 3.977 ± 0.2700 (mol/mol Cr) when the mice were fed a low-purine diet, which was a significant decrease of 61.4%. Compared with that in Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice, the total urinary purine/creatinine ratio in Hprt(high)Xdh(− / −)Slc23a4(− / −) mice was significantly lower when the mice were fed both normal and low-purine diets (Fig. 2C).
Plasma oxypurine concentrations in Hprt(high)Xdh(− / −)Slc23a4(− / −) mice
The plasma hypoxanthine concentration in Hprt(high)Xdh(− / −)Slc23a4(− / −) mice was 0.4764 ± 0.0569 (mg/dL) when the mice were fed a normal diet and 0.3286 ± 0.0549 (mg/dL) when the mice were fed a low-purine purified diet, which was not significantly different (Fig. 3A). On the other hand, the plasma xanthine concentration was 1.855 ± 0.1595 (mg/dL) in the normal diet group and was significantly reduced to 0.9942 ± 0.07633 (mg/dL) in the low-purine purified diet group (Fig. 3B). Neither the plasma hypoxanthine concentration nor the plasma xanthine concentration in Hprt(high)Xdh(+ /−)Slc23a4(− / −) mice was detectable (data not shown).
Profiles of purine metabolism in Hprt(high)Xdh(− / −)Slc23a4(− / −) mice that were fed a normal diet or a low-purine purified diet. (A) plasma hypoxanthine concentration, (B) plasma xanthine concentration, (C) hypoxanthine clearance (CLHX)/creatinine clearance (CLCr) ratio, (D) xanthine clearance (CLXA)/CLCr ratio. The data represent the means ± SDs. **p < 0.01 vs. the Xdh(− / −) MRA group. P values were calculated via Student’s t test. Normal diet; MRA. Low-purine purified diet; AIN.
The hypoxanthine clearance (CLHX)/creatinine clearance (CLCr) ratio of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice that were fed a normal diet was 0.4595 ± 0.0798. Since it was less than 1.0, it was concluded that hypoxanthine was reabsorbed in the kidney (Fig. 3C). When the mice were fed a low-purine purified diet, this ratio significantly decreased to 0.0609 ± 0.0259, and 94% of the hypoxanthine in the glomerular filtrate was reabsorbed in the kidney (Fig. 3C). On the other hand, the xanthine clearance (CLXA)/CLCr ratio of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice that were fed a normal diet was 1.163 ± 0.090, suggesting that xanthine may be slightly secreted by the kidney (Fig. 3D). When the mice were fed the low-purine purified diet, this ratio was significantly reduced to 0.7912 ± 0.0599, and approximately 20% of the xanthine in the glomerular filtrate was reabsorbed in the kidney because of the decrease in purine intake (Fig. 3D).
Renal dysfunction in Hprt(high)Xdh(− / −)Slc23a4(− / −) mice
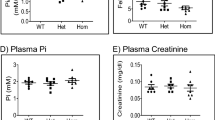
The plasma creatinine concentration in Hprt(high)Xdh(− / −)Slc23a4(− / −) mice was 0.4395 ± 0.0440 (mg/dL) when the mice were fed a normal diet and 0.4619 ± 0.0707 (mg/dL) when the mice were fed a low-purine diet, which were not significantly different (Fig. 4A). The plasma creatinine concentrations of Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice were 0.06875 ± 0.01848 (mg/dL) when the mice were fed a normal diet and 0.05775 ± 0.008577 (mg/dL) when the mice were fed a low-purine diet, which were not significantly different (Fig. 4A). Therefore, the plasma creatinine concentrations of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice were significantly increased by 6.39-fold when the mice were fed a normal diet and by 8.00-fold when the mice were fed a low-purine purified diet compared with those of Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice. Therefore, renal dysfunction was observed in Hprt(high)Xdh(− / −)Slc23a4(− / −) mice.
Profiles of blood from Hprt(high)Xdh(− / −)Slc23a4(− / −) and Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice that were fed a normal diet or a low-purine purified diet. (A) plasma creatinine concentration. (B) hematocrit values. The data represent the means ± SDs. *p < 0.05, **p < 0.01 vs. the Xdh(− / −) MRA group; ##p < 0.01 vs. the Xdh(− / −) AIN group. P values were calculated via one-way ANOVA. Normal diet; MRA. Low-purine purified diet; AIN.
The hematocrit value of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice that were fed a normal diet was 35.00 ± 1.16%, whereas that of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice that were fed a low-purine purified diet was 34.30 ± 1.52%, and no significant difference between the groups was observed (Fig. 4B). Similarly, the hematocrit values of Hprt(high)Xdh(+ /-)Slc23a4(− / −) mice that were fed normal diet were 59.50 ± 0.96% and 56.00 ± 1.87%, respectively, with no significant difference between the groups (Fig. 4B). Therefore, the hematocrit values of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice were significantly lower (0.588-fold when the mice were fed a normal diet and 0.613-fold when the mice were fed a low-purine purified diet) than those of Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice. Thus, the high creatinine level and anemia of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice were not improved by the short-term attenuation of the renal oxypurine levels due to consumption of a low-purine diet.
Discussion
Xanthinuria and Xdh(− / −) mice
All type 1 xanthinuria model mice (Xdh(− / −) mice) die by 8 weeks of age (56 days after birth)2,3,4. It has also been reported that type 2 xanthinuria model mice die by 8 weeks of age (average life span of 28.4 days)12. We reported that Hprt(high)Xdh(− / −) mice have an extended life span of up to 24 weeks of age, and we suggested that increased intracellular hypoxanthine levels may contribute to the death of Xdh(− / −) mice10. In this study, the median survival of Hprt(high)Xdh(− / −)Slc23a4(+ / +) mice was 83.3 days, which is longer than that of Hprt(low)Xdh(− / −)Slc23a4(+ / +) mice, which are reported to die within 56 days of birth. Therefore, Xdh(− / −) mice, which express high levels of Hprt similar to humans and field mice, are considered have longer lifespans than mice with low HPRT activity, such as laboratory mice. The transporter gene Slc23a4, which absorbs hypoxanthine and xanthine from the intestinal tract, is pseudogenized in humans. Therefore, we targeted Slc23a4 to account for species-specific differences between mice and humans. Although the median survival of Hprt(high)Xdh(− / −)Slc23a4(+ / −) mice was not longer than that of Hprt(high)Xdh(− / −)Slc23a4(+ / +) mice, the median survival of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice was significantly longer (191.8 days), making it possible, for the first time, to use adult Xdh(− / −) mice for breeding and research purposes.
A previous report on Hprt(low)Xdh(− / −)Slc23a4(+ / +) mice, whose kidney have exhibited interstitial fibrosis through aberrant lipid and purine accumulation in renal tubules, revealed that the plasma hypoxanthine concentration was approximately 20 µg/mL (2 mg/dL) and that the plasma xanthine concentration was approximately 30 µg/mL (3 mg/dL) at 4 weeks of age13. In Hprt(high)Xdh(− / −)Slc23a4(− / −) mice, the plasma hypoxanthine concentration was approximately 0.5 mg/dL, which is a quarter of that in Hprt(low)Xdh(− / −)Slc23a4(+ / +) mice. The plasma xanthine concentration was approximately 1.9 mg/dL, which is two-thirds that of Hprt(low)Xdh(− / −)Slc23a4(+ / +) mice. In addition, both the urinary xanthine/creatinine ratio and hypoxanthine/creatinine ratio of 4-week-old Hprt(low)Xdh(− / −)Slc23a4(+ / +) mice have been reported to be approximately 4013, whereas the urinary xanthine/creatinine ratio of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice is approximately 3.8, which is 1/10 that of Hprt(low)Xdh(− / −)Slc23a4(+ / +) mice. Moreover, the urinary hypoxanthine/creatinine ratio is approximately 0.4, which is 1/100 that of Hprt(low)Xdh(− / −)Slc23a4(+ / +) mice. Although the difference in age may have an effect, targeting Slc23a4 may decrease the absorption of hypoxanthine and xanthine in the intestine, contributing to the increased lifespan.
The activity of serum xanthine oxidase (XO), the oxidase form of XDH gene product, is high in mice but almost nonexistent in humans14, and the expression of Xdh in the mouse liver is 100-fold greater than that of human XDH15; thus, the XDH activity of Xdh(+ / −) mice is much greater than that of wild-type humans. The total purine excretion in the urine of Hprt(high)Xdh(+ / +)Slc23a4(+ / +) mice that was previously reported16 is almost equal to that of Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice, as shown in Fig. 2C. If we assume that the common ancestor of mice and humans had high XDH activity similar to that of modern mice and that XDH activity decreased during the evolution of humans, the Slc23a4 mutation is considered neutral because no difference in traits due to Slc23a4 mutation appears when XDH activity is high. Furthermore, the effect of the Slc23a4 mutation on total urinary purine excretion was apparent only in homozygous Xdh mutant mice after XDH activity was reduced. These findings suggest that when XDH activity decreased during human evolution, only the homozygous Slc23a4 mutant had a renoprotective effect, leading to selection and the development of a pseudogene.
Although female Xdh(− / −) mice gave birth to pups, the pups died a few days after birth, suggesting that female Xdh(− / −) mice may have lost the ability to secrete milk. Although lactation is reportedly affected in Xdh(+ / −) mice2, the Xdh(− / −) mouse strain that was used in this study could be maintained with Xdh(+ / −) mice3, so it is believed that lactation is not affected in the Xdh(+ / −) mice we used. By generating Xdh(− / −) mice of childbearing age, it was revealed for the first time that homozygous Xdh mutations affect lactation. Although agalactia has recently been reported in a patient with xanthinuria17, a study of the milk of a female patient with xanthinuria reported that no XO activity was detected in this patient’s milk18. Therefore, it is unlikely that all female patients with xanthinuria exhibit agalactia. Moreover, mammary gland-specific Xdh-knockout mice exhibit delayed lactation but no problems19; thus, multiple factors other than Xdh deficiency may be related to agalactia in female Xdh(− / −) mice.
Oxypurine kinetics
As shown in Table 1, which listed oxypurine kinetics of patients with type 1 xanthinuria whose XDH gene mutation was confirmed by DNA sequencing, the urinary xanthine/creatinine ratio of patients with type 1 xanthinuria was 0.188 ± 0.021 (mol/mol Cr), and the urinary xanthine/creatinine ratio of Hprt(High)Xdh(− / −)Slc23a4(− / −) mice that were fed a normal diet and those that were fed a low-purine diet was 20.0-fold and 8.09-fold greater than that of patients with type 1 xanthinuria, respectively. Compared with patients with type 1 xanthinuria, Hprt(High)Xdh(− / −)Slc23a4(− / −) mice excrete excessive levels of xanthine in their urine. The urinary hypoxanthine/creatinine ratio of Hprt(High)Xdh(− / −)Slc23a4(− / −) mice was 0.061 ± 0.010 (mol/mol Cr) when the mice were fed a normal diet, which was 7.11-fold greater than that of patients with type 1 xanthinuria, but when the mice were fed a low-purine diet, it was reduced to almost the same level as that of patients with type 1 xanthinuria. Because the low-purine diet reduced urinary xanthine and hypoxanthine excretion, the purine metabolites that are excreted in excess in the urine as xanthine in Xdh(− / −) mice and as allantoin in Xdh(+ / −) mice are thought to be the purines that are absorbed in the digestive tract. Compared with that in mice, intestinal purine base absorption that is mediated by SNBT1/Slc23a4 is lost in humans, and the activity of XDH, which converts absorbed purines to uric acid in intestinal epithelial cells, might be attenuated in humans. Thus, the reduced purine absorption in the digestive tract may decrease urinary oxypurine excretion.
As shown in Table 1, the plasma xanthine concentration of patients with type 1 xanthiuria was 0.371 ± 0.090 (mg/dl), which was 5.00 and 2.68 times lower than that of Hprt(High)Xdh(− / −)Slc23a4(− / −) mice that were fed a normal diet and those that were a low-purine diet, respectively. The plasma hypoxanthine concentration of patients with type 1 xanthinuria was 0.141 ± 0.035 (mg/dl), which was 3.38 and 2.33 times lower than that of Hprt(High)Xdh(− / −)Slc23a4(− / −) mice that were fed a normal diet and those that were fed a low-purine diet, respectively. It is thought that the mechanism for hypoxanthine reabsorption in the kidneys was active and that the hypoxanthine plasma concentration was maintained even when purine intake was reduced. The renal tubular reabsorption rate of xanthine in patients with type 1 xanthinuria has been reported to be 3% to 35%, and that of hypoxanthine is 93% to 79%20. The renal tubular reabsorption rates of xanthine and hypoxanthine in Hprt(High)Xdh(− / −)Slc23a4(− / −) mice were similar to those in patients with type 1 xanthinuria, suggesting that the hypoxanthine reabsorption mechanism in these mice may be similar to that in humans.
The urinary hypoxanthine/xanthine ratio of patients with type 1 xanthinuria, except two patients with renal impairment, was 0.378 ± 0.049, which was higher than that of Hprt(High)Xdh(− / −)Slc23a4(− / −) mice, suggesting that remaining species-specific differences may contribute to the difference in the urinary hypoxanthine/xanthine ratios between humans and mice. The decrease in the urinary hypoxanthine/xanthine ratio indicates the oxidation or dehydrogenation of hypoxanthine to xanthine, but whether there are species-specific differences in this reaction pathway remains to be investigated. Moreover, the urinary hypoxanthine/xanthine ratios of patients with type 1 xanthinuria with renal impairment are low at 0.047 and 0.01117,21, and these ratios are similar to those of Hprt(High)Xdh(− / −)Slc23a4(− / −) mice. Therefore, we cannot exclude the possibility that the low urinary hypoxanthine/xanthine ratio in Hprt(High)Xdh(− / −)Slc23a4(− / −) mice might occur due to renal dysfunction rather than to remaining species-specific differences.
Renal dysfunction associated with increased urinary excretion of oxypurine
All Hprt(High)Xdh(− / −)Slc23a4(− / −) mice exhibited chronic kidney damage accompanied by anemia. Although some patients with type 1 xanthinuria develop urinary stones and urinary stone-associated acute kidney injury, many patients are completely asymptomatic. Compared with patients with type 1 xanthinuria, Hprt(High)Xdh(− / −)Slc23a4(− / −) mice presented 7.11-fold increased urinary hypoxanthine excretion and 20.0-fold increased urinary xanthine excretion. Furthermore, the urinary hypoxanthine/xanthine ratio was decreased in the same way as that in patients with type 1 xanthinuria with renal dysfunction, suggesting that increased urinary xanthine excretion may be related to the cause of renal dysfunction.
Hypoxanthine and uric acid are reabsorbed at the renal tubules at more than 90% of the glomerular filtrate, but xanthine is excreted in the urine without significant reabsorption. Similar to patients with renal hypouricemia, in whom uric acid reaches the collecting duct without being reabsorbed by the renal tubules, it is assumed that urinary uric acid excretion increases due to purine breakdown during exercise in patients with renal hypouricemia, and it is known that these patients develop acute kidney injury after exercise22,23. Therefore, it is possible that increased urinary excretion of xanthine, which is a purine metabolite that is not reabsorbed by the renal tubules, might be related to decreased renal function, similar to that in renal hypouricemia patients.
Allopurinol reportedly cannot suppress the progression of renal injury in patients with chronic kidney disease24. Xdh(− / −) mice, which exhibit increased urinary xanthine excretion, exhibit renal injury, so it is possible that inhibition of XDH activity by allopurinol increases xanthine production in patients with chronic kidney disease and that the renal injury caused by increased urinary excretion of xanthine might offset the renoprotective effect by suppressing uric acid production.
Adenine nephropathy is a known mouse model of kidney damage that is caused by purine metabolites25. When the mice are fed a 0.20% adenine diet (200 mg adenine/100 g diet), the amount of adenine, which is normally salvaged to adenine monophosphate (AMP) by adenine phosphoribosyltransferase (APRT), becomes excessive. Excess adenine is converted to 2,8-dihydroxyadenine by XDH and excreted in the urine, which causes kidney damage. However, normal human purine intake is approximately 10–20 mg/100 g diet, and 2,8-dihydroxyadenine is not excreted in urine except in patients with APRT deficiency26,27. In contrast, xanthine, which is deaminated at the 6-position of 2,8-dihydroxyadenine, is excreted in urine even in healthy individuals. It is possible that the xanthine load per single nephron increases with decreasing nephron number, causing progressive deterioration of renal function. Therefore, Hprt(High)Xdh(− / −)Slc23a4(− / −) mice may be a better model of human chronic kidney disease.
In this study, targeting Slc23a4 attenuated renal damage in Xdh(− / −) mice and increased their life span. Since Slc23a4 is expressed in the intestine and its product is a transporter that functions in the absorption of purine bases, it is thought that purines that are absorbed in the intestine may play a role in causing renal damage. Uric acid did not contribute to the increased life span of Hprt(High)Xdh(− / −)Slc23a4(− / −) mice, suggesting a mechanism different from the initial hypothesis that the loss of uric acid resulting from XDH deficiency is the cause of renal damage in Xdh(− / −) mice. In addition, in this study, the mice were fed a low-purine diet for one week after being fed a normal diet, but no improvement was observed in the plasma creatinine concentration or hematocrit values. These results suggest that this model mouse has irreversible renal damage rather than a functional decrease in the GFR. In the future, it will be necessary to investigate whether Hprt(High)Xdh(− / −)Slc23a4(− / −) mice do not develop renal damage if they are fed a low-purine diet from birth.
Conclusion
We succeeded in extending the life span of Xdh(− / −) mice and obtaining adult mice by the induction of high HPRT activity and pseudogenization of Slc23a4, as observed in humans. However, there are still differences between Xdh(− / −) mice and patients with type 1 xanthinuria in terms of excessive urinary excretion of oxypurines, renal dysfunction with anemia in all cases, and failure of offspring to live, presumably due to lactation failure in female Xdh(− / −) mice. It is necessary to establish a mouse model of type 1 xanthinuria that is more similar to patients with type 1 xanthinuria by accounting for the species-specific differences between Xdh(− / −) mice and humans not only in purine metabolism but also in purine kinetics.
Materials and methods
Establishment of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice
Knockout-first allele: Slc23a4-targeted mice carrying the promoter-driven selection cassette were by importing cryopreserved sperm (049682-UCD-SPERM, C57BL/6N-Slc23a4tm1a(KOMP)Wtsi/Mmucd) from MMRRC UC Davis; and recovering litters from the cryopreserved sperm at the RIKEN BioResource Center. Mating the recovered litters with cre mice23 resulted in offspring with the lac z sequence that lost exon 4 of Slc23a4 and the neo cassette. Mating the recovered litters first with flp mice24 resulted in offspring that lost the lac z sequence and neo cassette. Then, mating the offspring from flp mice with cre mice resulted in Slc23a4(+ / −) mice without the lac z sequence. By crossing these Slc23a4(+ / −) mice with Hprt(high)Xdh(+ /−) mice on the B6 background, Hprt(high)Xdh(− / −)Slc23a4(− / −) mice and Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice were generated. In addition, Hprt(high)Xdh(− / −)Slc23a4(+ / +) and Hprt(high)Xdh(− / −)Slc23a4(+ / −) mice were obtained during the establishment process.
All animal experiments and the experimental protocols were approved as Teikyo Animal Ethics #21-007 by the animal ethics committee of Teikyo University. All methods were performed in accordance with the relevant guidelines and regulations. Anesthesia in this study was 3.5% isoflurane only at the ear punching for genotyping. Euthanasia was conducted with 100% CO2 and deep freezing. All methods are reported in accordance with ARRIVE guidelines for the reporting of animal experiments.
Genotyping
The Hprt genotype was determined as previously reported9. The genotypes of Xdh and Slc23a4 were determined via PCR via the use of 10 μL of the TaKaRa PCR Carryover Prevention Kit (Takara Bio Inc. Shiga, Japan) and DreamTaq™ Hot Start Green DNA Polymerase (Thermo Fisher Sci. USA) together with 0.5 μL of DNA extracted from an ear punch via Phire Tissue Direct PCR Master Mix (Thermo Fisher Sci. USA).
The PCR conditions for the Xdh gene were as follows: (25 °C for 10 min) - (95 °C for 2 min) - (95 °C for 10 s - 56 °C for 10 s - 72 °C for 40 s) × 40 cycles - (72 °C for 1 min) - 10 °C. The sense primer was 5ʹ-gcaccgtgatgatctccaagta-3ʹ, and the wild-type antisense primer was 5ʹ -ttctga ggcggaaagaaccag-3ʹ to produce a 205-bp product of the wild-type amplicon. The sense primer was used with the targeted antisense primer 5ʹ -aaaattcctgggtgagaggaca-3ʹ to produce a 445-bp product of the targeted amplicon.
The PCR conditions for the Slc23a4 gene with the lac z sequence were as follows: (25 °C for 10 min) - (95 °C for 2 min) - (95 °C for 10 s - 55 °C for 10 s - 72 °C for 45 s) × 40 cycles - (72 °C for 1 min.) - 10 °C. The sense primer was 5ʹ-agctagacacacccacaacgaaa-3ʹ and the wild-type antisense primer was 5ʹ-agtttgagacggggtgtgt-3ʹ to produce a 175-bp product of the wild-type amplicon. The sense primer was used with the targeted antisense primer 5ʹ-ataaagtgaccctcccaacagc-3ʹ to produce a 316-bp product of the targeted amplicon. The PCR conditions for the Slc23a4 gene without the lac z sequence were the same as those described above, and the sense primer was used with another targeted antisense primer 5ʹ-ggcttcacttcctctggagtt-3ʹ to produce a 437-bp targeted amplicon.
Measurement of blood and urinary oxypurine levels
Mice of various species were bred using MR-A1 feed (Nihon Nosan) as a normal diet and AIN93M (Oriental Yeast Co., Ltd., Japan) as a low-purine purified diet for one week. The mice were placed in a plastic container and weighed, and 30 μL of spotted urine was collected. The mice were placed in a rodent restrainer, and the tail vein was punctured with a 27G blood collection needle to collect 20 μL of blood via a heparinized microcapillary (Drummond). After being sealed, the blood was centrifuged at 6000×g for 3 min to measure the hematocrit values, and plasma samples were obtained. The urine and plasma samples were stored at − 20 °C until measurement.
The quantitative analysis of the measured substances was carried out via Chromaster (Hitachi) high-performance liquid chromatography (HPLC) with a Unison US-C18 250 × 2.0 mm column (Imtakt US026). The mobile phase was 10 mM ammonium formate 0.200 mL/min at 25 °C, and the absorbance was measured at 234 nm. For the urine samples, 2 μL of spotted urine was diluted with 100 μL of the mobile phase. For the plasma samples, 2 μL of plasma was diluted with 40 μL of 80% acetonitrile solution, deproteinized with a centrifugal filter, evaporated to dryness with a centrifugal concentrator, and redissolved in 10 μL of the mobile phase.
The supplemental figure shows typical chromatograms of standards (Suppl. Fig. A), urine samples of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice (Suppl. Fig. B) and Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice (Suppl. Fig. C), and plasma samples of Hprt(high)Xdh(− / −)Slc23a4(− / −) mice (Suppl. Fig. D) and Hprt(high)Xdh(+ / −)Slc23a4(− / −) mice (Suppl. Fig. E). The standards that were used were 10 mg/L allantoin, 1 mg/L sodium urate, creatinine, hypoxanthine, and sodium xanthine.
Statistical analysis
Statistical comparisons between groups and treatments were performed via one-way analysis of variance (ANOVA). Student’s t tests were used to compare two groups. A p value of < 0.05 was considered to indicate statistical significance. The data are presented as the means ± SDs.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Simmonds, H. A., Reiter, S. & Nishino, T. Hereditary xanthinuria. In The Metabolic and Molecular Bases of Inherited Diseases (eds Scriver, C. R. et al.) (McGraw-Hill Inc., 1995).
Vorbach, C., Scriven, A. & Capecchi, M. R. The housekeeping gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: gene sharing in the lactating mammary gland. Genes Dev. 16, 3223–3235 (2002).
Ohtsubo, T., Rovira, I. I., Starost, M. F., Liu, C. & Finkel, T. Xanthine oxidoreductase is an endogenous regulator of cyclooxygenase-2. Circ. Res. 95, 1118–1124 (2004).
Piret, S. E. et al. A mouse model of early-onset renal failure due to a xanthine dehydrogenase nonsense mutation. PLoS One 7, e45217 (2012).
Kratzer, J. T. et al. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc. Natl. Acad. Sci. U. S. A. 111, 3763–3768 (2014).
Wu, X. et al. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 91, 742–746 (1994).
Jinnah HA. HPRT1 Disorders. In: Adam MP et al. (eds) GeneReviews(R). (1993).
Finger, S., Heavens, R. P., Sirinathsinghji, D. J., Kuehn, M. R. & Dunnett, S. B. Behavioral and neurochemical evaluation of a transgenic mouse model of Lesch-Nyhan syndrome. J. Neurol. Sci. 86, 203–213 (1988).
Watanabe, T. et al. The mechanism of false in vitro elevation of uric acid level in mouse blood. Biol. Pharm. Bull. 39, 1081–1084 (2016).
Hosoyamada, M., Tomioka, N. H., Ohtsubo, T. & Ichida, K. Xanthine oxidoreductase knockout mice with high HPRT activity were not rescued by NAD(+) replenishment. Nucleosides Nucleotides Nucleic Acids 39, 1465–1473 (2020).
Yamamoto, S. et al. Identification and functional characterization of the first nucleobase transporter in mammals: implication in the species difference in the intestinal absorption mechanism of nucleobases and their analogs between higher primates and other mammals. J. Biol. Chem. 285, 6522–6531 (2010).
Sedda, D. et al. Deletion of mocos induces xanthinuria with obstructive nephropathy and major metabolic disorders in mice. Kidney 360 2, 1793–1806 (2021).
Ohtsubo, T. et al. Xanthine oxidoreductase depletion induces renal interstitial fibrosis through aberrant lipid and purine accumulation in renal tubules. Hypertension 54, 868–876 (2009).
Al-Khalidi, U. A. & Chaglassian, T. H. The species distribution of xanthine oxidase. Biochem. J. 97, 318–320 (1965).
Xu, P., LaVallee, P. & Hoidal, J. R. Repressed expression of the human xanthine oxidoreductase gene. E-box and TATA-like elements restrict ground state transcriptional activity. J. Biol. Chem. 275, 5918–5926 (2000).
Hosoya, T. et al. Effect of animal diets on urate kinetics in uricase knockout mice. Gout Uric Nucleic Acids 47, 133–140 (2023).
Goncalves, P. L. et al. Kidney failure secondary to hereditary xanthinuria due to a homozygous deletion of the XDH gene, in the absence of overt kidney stone disease. Nephron 148, 578–583 (2024).
Gibbs, D., Allsop, J. & Watts, R. The absence of xanthine oxidase (E. C 1. 2. 3. 2) from a xanthiuric patient’s milk. J. Mol. Med. Int. J. Uniflying Clin. Med. Mol. Biol. 1, 167–170 (1976).
Monks, J. et al. Xanthine oxidoreductase mediates membrane docking of milk-fat droplets but is not essential for apocrine lipid secretion. J. Physiol. 594, 5899–5921 (2016).
Arikyants, N. et al. Xanthinuria type I: a rare cause of urolithiasis. Pediatr. Nephrol. 22, 310–314 (2007).
Ichida, K. et al. Identification of two mutations in human xanthine dehydrogenase gene responsible for classical type I xanthinuria. J. Clin. Investig. 99, 2391–2397 (1997).
Hosoyamada, M. Hypothetical mechanism of exercise-induced acute kidney injury associated with renal hypouricemia. Biomedicines 9, 1847 (2021).
Hosoya, T. et al. Xanthine oxidoreductase inhibitors suppress the onset of exercise-induced AKI in high HPRT activity Urat1-Uox double knockout mice. J. Am. Soc. Nephrol. 33, 326–341 (2022).
Badve, S. V. et al. Effects of allopurinol on the progression of chronic kidney disease. N. Engl. J. Med. 382, 2504–2513 (2020).
Yang, Q., Su, S., Luo, N. & Cao, G. Adenine-induced animal model of chronic kidney disease: current applications and future perspectives. Ren. Fail. 46, 2336128 (2024).
Cartier, P., Hamet, M. & Hamburger, J. A new metabolic disease: the complete deficit of adenine phosphoribosyltransferase and lithiasis of 2,8-dihydroxyadenine. C. R. Acad. Hebd. Seances Acad. Sci. D 279, 883–886 (1974).
Van Acker, K. J., Simmonds, H. A. & Cameron, J. S. Complete deficiency of adenine phosphoribosyltransferase: report of a family. Adv. Exp. Med. Biol. 76A, 295–305 (1977).
Gok, F., Ichida, K. & Topaloglu, R. Mutational analysis of the xanthine dehydrogenase gene in a Turkish family with autosomal recessive classical xanthinuria. Nephrol. Dial. Transplant. 18, 2278–2283 (2003).
Jurecka, A., Stiburkova, B., Krijt, J., Gradowska, W. & Tylki-Szymanska, A. Xanthine dehydrogenase deficiency with novel sequence variations presenting as rheumatoid arthritis in a 78-year-old patient. J. Inherit. Metab. Dis. 33(Suppl 3), S21-24 (2010).
Stiburkova, B. et al. Novel mutations in xanthine dehydrogenase/oxidase cause severe hypouricemia: biochemical and molecular genetic analysis in two Czech families with xanthinuria type I. Clin. Chim. Acta 413, 93–99 (2012).
Eggermann, T., Spengler, S., Denecke, B., Zerres, K. & Mache, C. J. Multi-exon deletion in the XDH gene as a cause of classical xanthinuria. Clin. Nephrol. 79, 78–80 (2013).
Mraz, M. et al. Modern diagnostic approach to hereditary xanthinuria. Urolithiasis 43, 61–67 (2015).
Iguchi, A. et al. A case of xanthinuria type I with a novel mutation in xanthine dehydrogenase. CEN Case Rep. 5, 158–162 (2016).
Xu, T. et al. A novel mutation in xanthine dehydrogenase in a case with xanthinuria in Hunan province of China. Clin. Chim. Acta 504, 168–171 (2020).
Peretz, H. et al. Classical xanthinuria in nine Israeli families and two isolated cases from Germany: Molecular biochemical and population genetics aspects. Biomedicines 9, 788 (2021).
Maes, B. & Dedeurwaerdere, F. Hypouricaemia in a patient with hereditary xanthinuria type I. Lancet 403, 1493 (2024).
Acknowledgements
This study was partly supported by Grants-in Aid for Scientific Research from Japan Society for the Promotion of Science (to M. H., Grant Number: No. 26460347, No. 17K08605 and No. 22K08320)
Author information
Authors and Affiliations
Contributions
K. T. contributed draft of the work; T. W., N. Y., T. O., S. S. and K. I. contributed substantively revised it; and M.H. contributed all of this study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Terada, K., Watanabe, T., Yasuno, N. et al. Pseudogenization of the Slc23a4 gene is necessary for the survival of Xdh-deficient mice. Sci Rep 15, 3250 (2025). https://doi.org/10.1038/s41598-025-87751-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87751-9