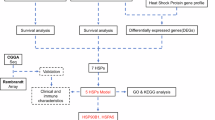
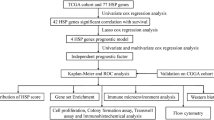
Abstract
Sphingosine kinase 1 (SPHK1) is a member of the SPHK family, enzymes essential for the phosphorylation of sphingosine to sphingosine-1-phosphate (S1P). Previous studies have revealed important roles of SPHK1 in inflammatory, anti-apoptotic, immune processes, and cancer. Although the predictive significance and possible roles of SPHK1 in gliomas have recently been examined, the precise molecular mechanisms remain unclear. We comprehensively examined SPHK1 and investigated its correlation with glioma survival time using different datasets. The correlation between SPHK1 and various cancer pathways was analyzed using the Kyoto encyclopedia of genes and genomes (KEGG) analysis. The SPHK1 influence on glioma migration was examined using transwell and wound healing experiments. M2 macrophage infiltration experiments investigated SPHK1’s role in the glioma immune microenvironment. We identified SPHK1 downstream pathways and further elucidated their regulatory relationship. Survival analysis illustrated that patients with high-SPHK1 expression, particularly glioblastoma and IDH-wildtype, tended to have a shorter survival time. The Cox regression model (COX) results demonstrated that SPHK1 was an independent prognostic factor affecting the survival of patients with glioma. Functional experiments illustrated that SPHK1 suppression led to a reduction in the migration capacity of glioma cells. Enrichment analysis and Western blotting revealed that SPHK1 functions as a JAK2/STAT3 pathway controller. The SPHK1 overexpression-induced migration was suppressed by the JAK2/STAT3 pathway suppressor (AG490). We found that SPHK1 promotes M2 macrophage infiltration. Further study indicated that SPHK1 could serve as a prognostic indicator of glioma and promote cell migration, providing new insights for glioma therapy.
Similar content being viewed by others
Introduction
Glioma affects human central nervous system health and is thought to develop from neural progenitors or stem cells1. Glioma treatment typically includes maximal tumor resection along with radiotherapy and temozolomide chemotherapy2. According to the World Health Organization (WHO) 2021 classification, adult-type diffuse gliomas are divided into three entities: Astrocytoma IDH-mutant, oligodendroglioma IDH-mutant and 1p/19q co-deleted, and glioblastoma (GBM) IDH-wildtype3. Gliomas grading includes grades 2–4, with increasing levels of malignancy; grade 4 gliomas can include various types, such as glioblastomas (GBM) and IDH-mutated astrocytomas, which can range from grade 2 to grade 4. Typically, patients with GBM have a worse prognosis, with a median overall survival of 14.6 months4. Additionally, grade 2 gliomas had a relatively good prognosis, with a median survival of 7.9 years, while grade 3 gliomas were in the middle of the range, with a median survival of 33.5 months5. However, achieving complete surgical removal is difficult because of the tumor’s aggressive nature and its ability to penetrate the adjacent brain tissue. The blood-brain barrier also limits systemic chemotherapy effectiveness. The glioma pathogenesis remains controversial, and effective treatment faces enormous challenges6.
Gliomas produce an immunosuppressive microenvironment that hinders the immune response7. Gliomas contain large numbers of tumor-associated macrophages (TAMs), and immunosuppressive TAMs are a barrier to emerging immunotherapies8. The tumor-immune cell infrastructure, a hallmark of high-grade gliomas, primarily comprises TAMs, mainly M2 macrophages9. Shi et al.10 reported that M2 macrophage numbers were higher in gliomas than in the normal brain, linking them to glioma tumor development. Studies suggest that M2 macrophages promote tumor cell proliferation and migration by secreting tumor-promoting factors and influence tumor progression by regulating the immune response. For instance, M2 macrophages have been found to promote angiogenesis through Vascular endothelial growth factor A (VEGFA) secretion, thereby supporting glioma growth11. In addition, PTEN-deficient glioma cells secrete high levels of galectin-9, which further promotes tumor progression by activating the TIM-3 receptor in M2 macrophages11. Another study revealed that M2 macrophages enhanced glioma cell invasion and migration by releasing interleukin (IL)-10 and TGF-β112. It has also been demonstrated that M2 macrophage polarization is closely related to the interaction of tumor stem cells, and this interaction may promote the malignant progression of tumors by regulating immune status in the tumor microenvironment13.
Sphingosine kinases (SPHK), including SPHK1 and SPHK2, are essential enzymes that phosphorylate sphingosine to sphingosine 1-phosphate (S1P)14. This protein and its product, S1P, play vital roles in the tumor necrosis factor-alpha signaling and NF-κB activation pathways, which are essential in inflammatory, anti-apoptotic, and immune processes. It was reported that SPHK1 contributes to cell lung cancer development by promoting STAT315. SPHK1 catalyzes the phosphorylation of sphingosine to generate S1P. It has been suggested that S1P affects glioma M2 macrophage infiltration, pointing to the S1P-S1PR axis as an attractive target for glioma therapy16. Hou et al.18 demonstrated that SPHK1 facilitates the development of salivary adenoid cystic carcinoma mediated by PI3K/Akt signaling17. Cell cycle blockade in cell lung cancer indirectly targets the SPHK1/PBX1 axis. Additionally, SPHK1 has been reported in gliomas. The glioma cell anti-apoptosis is promoted by SPHK1 regulation of the Akt/FOXO3a/Bim pathway19. Pavlakis et al.20 found that two isoforms of paired box 6 (PAX6) downregulated lipid kinase SPHK1, resulting in decreased glioma survival, diminished transfer, and increased cell death. However, these studies did not fully explore the exact mechanism through which SPHK1 plays a role in glioma development. Our study described SPHK1 expression in gliomas and the underlying mechanisms in detail. We investigated whether SPHK1 influenced the glioma immune microenvironment.
Materials and methods
Survival analysis
Nine glioma cohorts were selected to generate Kaplan–Meier curves to assess the correlation between SPHK1 and glioma prognosis. Expression and survival data for these cohorts were obtained using the Gliovis platform21. The sample size of the glioma cohort was as follows: CGGA with 1013 cases, TCGA with 667 cases, Gravendeel with 276 cases, Rembrandt with 444 cases, Kamoun with 180 cases, LeeY with 191 cases, Phillips with 100 cases, and Freije with 85 cases. We performed a Cox analysis to assess whether SPHK1 can be used as an independent prognostic marker for gliomas. Receiver Operating Characteristic Curve (ROC) curves were plotted to determine SPHK1 prognosis value for 1-, 3-, and 5-year survival.
Immune infiltration analysis
Enrichment scores for the 24 immune cells were analyzed using the R package “GSVA.” The genetic set of these immune cells was obtained from previous studies22,23. Chen and Mellman et al. proposed the cancer-immunity cycle, which is a cell-killing procedure by the immune system and is divided into seven steps24,25. The organism can die cells by the cancer-immunity cycle, and immunotherapy can amplify or activate this mechanism to enhance the immune system’s role. Based on the TCGA database, we analyzed the relationship between SPHK1 and the cancer-immunity cycle.
Enrichment analysis
Using the “limma” package, differentially expressed genes (DEGs) were determined for SPHK1 in glioma26. To analyze the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) of DEGs27,28, the package “clusterProfiler” in R was employed29. We conducted GO, KEGG, and GSEA analyses to explore the molecular mechanisms related to SPHK1 functional localization and its role in glioma.
Immunohistochemistry
Tissue sections were obtained from the Department of Pathology, comprised Grades I, II, III, and IV (five samples each level). The experimental steps for immunohistochemistry were consistent with previous studies30. We used the SPHK1 antibody from Boster Biological (Wuhan, China) at a dilution of 1:70.
Cell culture
Glioma cell lines (U251, T98G, U87, and A172), THP-1, and HA cells were obtained from the Procell Life Science. The glioma cell lines and human astrocytes were maintained in DMEM. THP-1 cells were cultured in 1640 medium. Han Biotech (Shanghai, China) designed the siRNA and overexpression plasmid. After 48–72 h of transfection, the successfully transfected cells were collected for further examination.
RT-qPCR and Western blotting
The RT-qPCR and Western blotting steps followed established protocols from prior research1. The RT-qPCR analysis was performed using the SYBR qPCR Mix kit (Vazyme, Nanjing, China), and antibodies for Western blotting were sourced from Abcam.
M2 macrophage infiltration assay
With 100 ng/ml PMA (Beyotime; Shanghai, China) for 24 h. M2 macrophages were then polarized with 20 ng/ml IL-4 (Nearshore protein; Suzhou; China) and 20 ng/ml IL-13 (Nearshore protein; Suzhou; (China) in the presence of PMA for 48 h. Cell morphology was observed microscopically, and M0 and M2 macrophage-associated markers were detected to determine the success of induction. For the M2 macrophage infiltration assay, the upper chamber was inoculated with 300 µL of serum-free 1640 medium, and 5.0 × 104 M2 macrophages were placed into it. The lower chamber was inoculated with an equal number of U251 and T98G cells, and 700 µL of serum-containing DMEM medium was inoculated. After 24 h, the upper chamber was fixed and stained, and the number of migrated cells was observed under a microscope.
Migration assay
Glioma cells and 300 µL serum-free medium were inoculated into the upper chamber of a plate. The lower chamber was supplemented with 700 µL of serum-containing medium. After 24 h, cells were fixed with 4% paraformaldehyde and stained with crystal violet. After rinsing off the excess crystal violet with phosphate-buffered saline, the cells were allowed to dry and photographed.
Wound healing assay
Glioma cells were placed in 6-well dishes, and a pipette tip was used to produce an elongated well by scratching. Cell cultures were incubated in a serum-free medium, and plots were obtained after 0 and 24 h. Width changes were measured using ImageJ software to assess the cells’ migratory capacity.
Xenograft experiment
Male BALB/c nude mice (four weeks) were selected for the experiments, all nude mice were purchased from Huafu Kang (Beijing, China). Mice were injected with tumor cells (5 × 106 per mouse) into the subcutaneous tissue. After 28 days, mice were anesthetized with Pentobarbital sodium by intraperitoneal injection, subcutaneous xenografts from mice were obtained for further investigation. Nude mice were euthanized by overdose of Pentobarbital sodium. The xenograft volume was calculated using V = (W2 × L)/2.
Statistical analysis
The experiment was performed thrice, and the results are presented as mean and standard deviation. Statistics were performed using R (version 4.2.1) and Prism 8 (GraphPad Inc., USA) software. Student’s t-test and one-way analysis of variance were utilized to assess the significance between groups. Statistical significance was determined at p < 0.05.
Ethical statements
All experiments were approved by the Ethics Committee of the Second Hospital of Hebei Medical University and strictly followed National Institutes of Health guidelines for animal care and use. The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Results
Abnormal expression of SPHK1 was observed in glioma and various other cancer types
In the Genotype-Tissue Expression (GTEx) database, SPHK1 was found in different normal human tissues, including the brain and mammary glands (Supplementary Figure S1A). The pan-cancer research was conducted using the UCSCXenaShiny tool at https://hiplot-academic.com/31. Our investigation revealed that SPHK1 was higher in most of the 33 types of tumor tissues than in normal tissues (Supplementary Figure S1B). cBioPortal analysis revealed that the missense in SPHK1 was mainly concentrated on K27E (Supplementary Figure S1C). TIMER analysis revealed that the three tumors with the highest SPHK1 mutation rates were UCEC (15/531), SKCM (11/468), and STAD (8/439) (Supplementary Figure S1D). Various tumors exhibited SPHK1 alterations, including glioma amplification and deep deletion in ovarian cancer (Supplementary Figure S1E).
Survival and clinical correlation analysis
Survival studies indicated that elevated SPHK1 expression was affected in TCGA (hazard ratio [HR]: 2.61, 95% confidence interval [CI]: 1.98–3.42), CGGA (HR: 1.59, 95% CI: 1.28–1.97), Gravendeel (HR: 1.43, 95% CI: 1.11–1.85), Rembrandt (HR: 1. 34, and 95% CI: 1.07–1.67), LeeY (HR: 1.51, 95% CI: 1.12–2.04), Freije (HR: 1.24, 95% CI: 0.74–2.07), Murat (HR: 1.97, and 95% CI: 1.19–3.26), Phillips (HR: 2.18, 95% CI: 1.30–3.67) and Kamoun (HR: 2.41, 95% CI: 1.26–4.62), and had a shorter median overall survival compared to those with low expression (Fig. 1A–I). Meta-analysis of these results demonstrated a mean HR of 1.63 and 95% CI of 1.47–1.81 (Fig. 1J). Additionally, according to the WHO’s updated glioma classification in 2021, patients with high-SPHK1 expression have reduced survival in all three categories, especially for GBM, IDH-wildtype (Supplementary Figure S2). Subsequently, we investigated the relationship between SPHK1 and gliomas’ clinical features. We found that SPHK1 expression was elevated in the higher WHO classification, GBM IDH wild-type, 1p/19q non-codeleted, and IDH wild-type groups (Fig. 2A–C). Univariate and multifactorial prognostic analyses revealed that SPHK1 served as an independent prognostic factor for patients with glioma (Fig. 3A–F). A meta-analysis of the results reinforces this (Supplementary Figure S3).
Overall survival curves of SPHK1 in different databases. (A–I) Aberrant high expression of SPHK1 leads to a worse prognosis. (J) A meta-analysis of these databases yielded mean HR values for SPHK1. The experiment was performed thrice, and the results are presented as mean and standard deviation. Statistical significance was determined at p < 0.05.
SPHK1 dysregulation created an immunosuppressive microenvironment
Our results demonstrated that SPHK1 expression level was directly correlated with the number of immunosuppressive cells, including M2 macrophages, Th2 cells, and neutrophils (Fig. 4A–E). Anti-tumor immunity is performed in an array of steps called the “cancer-immunity cycle,” as described in previous studies1. The findings illustrated that SPHK1 was directly associated with scores at steps 1 and 4 in the cancer-immunization cycle and negatively correlated with scores in the remaining steps (Fig. 4F–G). We analyzed SPHK1 and 23 immune response types to determine whether gliomas exhibit a heat immunophenotype. The relevant genes were derived from previous studies32. The results illustrated that the immune phenotype tended to be “hot” with elevated SPHK1 expression (Fig. 4F). These findings demonstrate that SPHK1 may modulate the cancer-immunity cycle and immune infiltration, leading to an immune-suppressive microenvironment.
Relationship between SPHK1 and the immune microenvironment of gliomas. (A) Correlation of SPHK1 with 24 immune cells. (B) SPHK1 expression is associated with multiple immune cell types. (C) Relationship between SPHK1 and macrophages. (D) SPHK1 and neutrophils. (E) SPHK1 and Th2 cells. (F) Relationship between SPHK1 and immunity in innate, adaptive, and cancer-immunity cycle. (G) Enrichment scores of the cancer-immunity cycle and SPHK1 expression levels. Statistical significance was determined at p < 0.05.
Genes enrichment analysis
We investigated the potential mechanisms of SPHK1, analyzing DEGs between patients with low and high SPHK1 expression levels in the TCGA. A total of 4,437 DEGs were acquired, which contained 453 downregulated genes and 3,984 upregulated genes (Fig. 5A). GSEA results indicated an upregulation of the JAK-STAT pathway and cell cycle checkpoints in patients with elevated SPHK1 expression. The GO and KEGG analysis revealed that these DEGs were abundantly involved in the humoral immune response, immunological synapse, receptor-ligand activity, and JAK-STAT pathway (Fig. 5B–G).
SPHK1 augments the migration ability of glioma cells
Immunohistochemical analysis indicated that SPHK1 in glioma tissues increased with increasing WHO grades (Fig. 6A). Compared to HA cells, the levels of SPHK1 expression were elevated in U251, T98G, A172, and U87 cell lines (Fig. 6B). Because of the significant overexpression of SPHK1 in U251 and T98G cells, we conducted cellular experiments using them. Figure 6C demonstrates a reduction in SPHK1 expression following siRNA transfection, whereas an increase was observed after plasmid transfection in U251 and T98G cells. Transwell and wound healing assays demonstrated that SPHK1 upregulation increased glioma cell migration, whereas its downregulation reduced this capability (Fig. 7A–B). We chose the inhibitor of SPHK1, ABC294640 purchased from Selleck Chemicals. glioma cells in the ABC294640 group showed reduced expression levels of SPHK1 and decreased migration compared to the control group (Supplementary Figure S4).
Figure 8A illustrates the cellular morphological changes during THP-1 cells induction into M2 macrophages using classical induction methods. RT-qPCR results exhibited a corresponding elevation in M2 macrophage markers (Fig. 8B). Figure 8C demonstrates a simple procedure for the M2 macrophage infiltration experiment. The extent of M2 macrophage infiltration decreased with SPHK1 knockdown and increased with SPHK1 overexpression in glioma cells (Fig. 8D).
Determination of SPHK1 expression levels and knockdown versus overexpression. (A) Immunohistochemistry reveals SPHK1 expression levels in glioma tissues of different WHO grades. (B) Expression levels of SPHK1 in HA versus different glioma cell lines. (C) Knockdown and overexpression of SPHK1 in U251 and T98G cell lines. The experiment was performed thrice, and the results are presented as mean and standard deviation. Statistical significance was determined at p < 0.05.
Expression level of SPHK1 affects the migration ability of glioma cells. (A) Transwell assay reveals SPHK1 affects glioma cell migration. (B) Wound healing assay reveals SPHK1 affects glioma cell migration. The experiment was performed thrice, and the results are presented as mean and standard deviation. Statistical significance was determined at p < 0.05.
SPHK1 enhances the M2 macrophage infiltration capabilities of glioma cells
SPHK1 affects M2 macrophage infiltration in the glioma microenvironment. (A) Morphologic changes in the induction of THP-1 cells to M2 macrophages. (B) RT-qPCR validation of markers for M2 macrophages. (C) Schematic representation of the M2 macrophage infiltration assay. (D) SPHK1 expression level affects the number of M2 macrophage infiltrations. (E) JAK2/STAT3 pathway is inhibited upon SPHK1 knockdown and activated upon SPHK1 overexpression. The experiment was performed thrice, and the results are presented as mean and standard deviation. Statistical significance was determined at p < 0.05.
SPHK1 may influence glioma progression through the JAK2/STAT3 axis
We used Western blotting to verify the database results, revealing a positive relationship between SPHK1 and p-JAK2/JAK2 and p-STAT3/STAT3 (Fig. 8E). The migratory changes induced by SPHK1 overexpression in U251 and T98G cell lines were successfully reversed by AG490, the JAK2/STAT3 inhibitor, which was selected for rescue experiments (Fig. 9A–B). Western blotting analysis also confirmed that AG490 could counteract the elevation of p-JAK2/JAK2 and p-STAT3/STAT3 due to SPHK1 overexpression (Fig. 9C). This process suggests that SPHK1 may play a biological role through the JAK2/STAT3 pathway.
SPHK1 facilitates glioma progress by the JAK2/STAT3 pathway. (A–B) The JAK2/STAT3 pathway inhibitor AG490 reverses the overactivation of glioma cell migration ability due to SPHK1 overexpression. (C) AG490 reversed the abnormal elevation of p-JAK2/JAK2 and p-STAT3/STAT3 induced by SPHK1 overexpression. The experiment was performed thrice, and the results are presented as mean and standard deviation. Statistical significance was determined at p < 0.05.
SPHK1 affects glioma progression in vivo through the JAK2/STAT3 pathway
Tumorigenic experiments in nude mice confirmed that, in an in vivo setting, the SPHK1 expression level can influence glioma progression, and AG490 can reverse the progression of this hyperactivation (Fig. 10A–B). The volume and weight were measured after tumor removal. With SPHK1 overexpression, the tumors were larger and heavier in mass, and AG490 reversed this phenomenon (Fig. 11C). Western blotting analysis with subcutaneous tumors confirmed that AG490 could reverse the overactivation of the JAK2/STAT3 pathway by SPHK1 overexpression (Fig. 10D). RT-qPCR results demonstrated that high SPHK1 expression in the in vivo environment elevated M2 macrophage-associated markers and TGF-β1 cytokines, but AG490 reversed this phenomenon (Fig. 10E).
In vivo experiment. (A–B) Results in nude mice after anesthesia for tumor formation and after tumor stripping. (C) Volume and weight were measured after tumor removal. (D) Western blotting experiments in vivo illustrated that AG490 reversed the SPHK1 overexpression-induced elevation of p-JAK2/JAK2 and p-STAT3/STAT3. (E) CD206 and TGF-β1 expression level were detected after extracting RNA from tumor tissues. The experiment was performed thrice, and the results are presented as mean and standard deviation. Statistical significance was determined at p < 0.05.
Discussion
Research has indicated that TAMs significantly contribute to glioma progression, angiogenesis, immune escape, and treatment resistance33. TAMs are the predominant immune cells infiltrating the TME of gliomas and initiate either an immunostimulatory (M1) or immunosuppressive (M2) reaction by their state of polarization34. Recently, several investigators have discovered the role of TAMs in gliomas. Li et al. demonstrated that Ferritin light chain (FTL) fosters a pro-tumorigenic microenvironment by enhancing the M2 polarity of TAMs. They further exhibited that TAMs overexpressing FTL dramatically contribute to glioma cell growth, invasion, and metastasis33. Increasing evidence suggests that TAMs facilitate glioma progression and lead to an adverse prognosis by inducing an M2-like manifestation35.
M2 macrophages are generally considered tumor-promoting factors that support tumor cell growth and survival by secreting various cytokines and growth factors36,37. In the glioma microenvironment, M2 macrophage polarization is regulated by signaling molecules released by tumor cells, thereby enhancing tumor growth and metastasis38,39. TAMs support tumor progression by promoting angiogenesis and suppressing immune responses. M2 macrophages can secrete factors that promote angiogenesis, such as Vascular endothelial growth factor (VEGF), which not only promote the blood supply to the tumor but also enhance tumor cell aggressiveness40. Meanwhile, M2 macrophages further impair the host anti-tumor immune response by inhibiting cytotoxic T-cell activity. Additionally, the interaction between M2 macrophages and tumor-associated fibroblasts (CAFs) is considered an important mechanism that promotes glioma progression. The M2 macrophage polarization induced by CAFs secretes CXCL12, which enhances tumor cell proliferation and metastatic capacity41. Treatment strategies targeting TAMs are emerging as a new approach for glioma treatment. Tumor growth and metastasis are effectively inhibited by reprogramming M2 to M1 macrophages or directly targeting TAMs42.
Our study focused on the SPHK1 effect on TAMs in gliomas, which has not been performed previously. Prior studies have identified abnormal SPHK1 expression in various tumors, such as colon, lung, and prostate cancers. Colorectal cancer progression and metastasis were promoted by SPHK1 via modulating TRAF6-induced ULK1-mediated autophagy. The SPHK1-TRAF6-ULK1 pathway is critical for colorectal cancer progression43. Liu et al.44 illustrated that PF-543, a potent anti-hepatocellular carcinoma drug, targets the SPHK1/S1P/PFKFB3 axis to inhibit the development of hepatocellular carcinoma by disrupting glycolytic energy supply that drives tumor angiogenesis. It has also been suggested that SPHK1-MTA3 sustains the immunosuppressive state in the TME and encourages tumor immune escape by regulating PD-L1 positively in black melanoma cells, reducing the ratio of TILs, and independently inhibiting tumor-specific CTL activation45. Although SPHK1 has been reported in gliomas, studies have been limited to its effect on drug resistance in glioma cells and its role as a signaling pathway molecule in gliomas. In a public database analysis of SPHK1, we found positive correlations with macrophages, neutrophils, Th2, CD8T cells, and others, particularly macrophages and neutrophils. Cellular experiments were performed to validate the analysis based on these databases. Small chambers of the transwell plates were used to mimic the glioma microenvironment. Tumor cells and M2 macrophages were placed in the lower chamber, and the number of cells crossing the upper chamber membrane was observed under the same conditions. Our study demonstrated that abnormally high SPHK1 expression could affect TAMs in the glioma TME by influencing the infiltration of M2 macrophages, thereby creating an inhibitory immune microenvironment that promotes glioma progression. Additionally, we systematically explored the effect of abnormally high SPHK1 expression on the glioma phenotype using in vivo and in vitro experiments, revealing its potential influence on glioma through the JAK2/STAT3 pathway.
Many studies have focused on the JAK2/STAT3 pathway, a common pathway in tumor pathogenesis. Various fundamental biological processes such as differentiation, progression, apoptosis, and inflammation are regulated by STAT protein46. Phosphorylated STAT3 forms dimers and travels to the nucleus to mobilize genes influencing tumor cell behavior. The JAK2/STAT3 pathway has a significant role in numerous cancers, particularly gliomas. He et al. suggested that KIAA0040 overexpression was associated with the malignant behavior of gliomas through the JAK2/STAT3 pathway47. It has been reported that IFITM3 expressed in a tumor subpopulation (GSC) of GBM regulates the JAK/STAT3 pathway, stimulates bFGF production, and promotes tumor angiogenesis48. A plant extract, nitrotidine chloride, suppresses epithelial mesenchymal transition (EMT) in gliomas and the nature of glioma stem-type cells through the JAK2/STAT3 pathway49. The tumorigenic characteristics of glioma cells expressing the novel convergent protein KLC1-ROS1 fusion are influenced by JAK-STAT pathway suppression50. These studies exhibited that JAK2/STAT3 plays a pivotal role in gliomas progression, EMT of glioma cells, and oxygen metabolism. However, previous studies have not explored the connection between SPHK1 and its mechanism of action, and we found that SPHK1 may affect glioma metastatic ability through the JAK2/STAT3 pathway.
We combined public databases with in vivo and in vitro experiments to elucidate SPHK1’s effects on gliomas. We explored that SPHK1 is a prognostic marker and its abnormally high expression led to the short prognosis of patients with gliomas. Dysregulation of SPHK1 expression promotes the formation of an inhibitory tumor-immune microenvironment in glioma cells and enhances M2 macrophage infiltration. Additionally, SPHK1 promoted the glioma cell migration through the JAK2/STAT3 pathway. Our study has some shortcomings; for instance, the sample size of the clinical tissue specimens was small. In the future, this will be improved by performing multicenter experiments.
Conclusion
Our findings suggest that SPHK1 is a prognostic marker, and its high expression affects the prognosis of patients with glioma. We identified that SPHK1 promotes the infiltration of M2 macrophages, and the JAK2/STAT3 axis may be a downstream pathway of SPHK1, allowing it to influence glioma cell metastasis.
Data availability
The data used to support the findings of this study are included in the article.
References
Song, Z. et al. Arylsulfatase D is a prognostic biomarker that promotes glioma cells progression through JAK2/STAT3 pathway and M2 macrophage infiltration. Front. Oncol. 13, 1228426 (2023).
Behnan, J., Finocchiaro, G. & Hanna, G. The landscape of the mesenchymal signature in brain tumours. Brain J. Neurol. 142 (4), 847–866 (2019).
Kalluri, A. L., Lee, J. H., Lucas, C. G., Rincon-Torroella, J. & Bettegowda, C. Implications of molecular classifications in glioma surgery. J. Neuro-oncology (2024).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352 (10), 987–996 (2005).
Ozturk-Isik, E. et al. Spatial characteristics of newly diagnosed grade 3 glioma assessed by magnetic resonance metabolic and diffusion tensor imaging. Transl. Oncol. 5 (1), 10–18 (2012).
Buerki, R. A., Chheda, Z. S. & Okada, H. Immunotherapy of primary brain tumors: facts and hopes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 24 (21), 5198–5205 (2018).
Gielen, P. R. et al. Elevated levels of polymorphonuclear myeloid-derived suppressor cells in patients with glioblastoma highly express S100A8/9 and arginase and suppress T cell function. Neuro-oncology 18 (9), 1253–1264 (2016).
Müller, S. et al. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 18 (1), 234 (2017).
Rahman, M. et al. Adult immuno-oncology: using past failures to inform the future. Neuro-oncology 22 (9), 1249–1261 (2020).
Shi, Y. et al. miR-106b-5p inhibits IRF1/IFN-β signaling to promote M2 macrophage polarization of Glioblastoma. OncoTargets Therapy. 13, 7479–7492 (2020).
Ni, X. et al. Interrogating glioma-M2 macrophage interactions identifies Gal-9/Tim-3 as a viable target against PTEN-null glioblastoma. Sci. Adv. 8 (27), eabl5165 (2022).
Li, M. et al. Tumor-derived exosomes deliver the tumor suppressor mir-3591-3p to induce M2 macrophage polarization and promote glioma progression. Oncogene 41 (41), 4618–4632 (2022).
Pan, T. et al. Glioma-stem-cell-derived exosomes remodeled glioma-associated macrophage via NEAT1/miR-125a/STAT3 pathway. Cancers 16(14) (2024).
Jin, L. et al. Targeting SphK1/2 by SKI-178 inhibits prostate cancer cell growth. Cell Death Dis. 14 (8), 537 (2023).
Ma, Y. et al. SphK1 promotes development of non–small cell lung cancer through activation of STAT3. Int. J. Mol. Med. 47 (1), 374–386 (2021).
Arseni, L. et al. Sphingosine-1-Phosphate recruits macrophages and Microglia and induces a pro-tumorigenic phenotype that favors glioma progression. Cancers 15(2) (2023).
Hou, C. X. et al. Sphk1 promotes salivary adenoid cystic carcinoma progression via PI3K/Akt signaling. Pathol. Res. Pract. 227, 153620 (2021).
Lin, Z. et al. Targeting SPHK1/PBX1 axis induced cell cycle arrest in non-small cell lung cancer. Int. J. Mol. Sci. 23(21) (2022).
Guan, H. et al. Sphingosine kinase 1 regulates the Akt/FOXO3a/Bim pathway and contributes to apoptosis resistance in glioma cells. PloS One. 6 (5), e19946 (2011).
Pavlakis, E., Tonchev, A. B., Kaprelyan, A., Enchev, Y. & Stoykova, A. Interaction between transcription factors PAX6/PAX6-5a and specific members of miR-183-96-182 cluster, may contribute to glioma progression in glioblastoma cell lines. Oncol. Rep. 37 (3), 1579–1592 (2017).
Bowman, R. L., Wang, Q., Carro, A., Verhaak, R. G. & Squatrito, M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro-oncology 19 (1), 139–141 (2017).
Hänzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 14, 7 (2013).
Jia, Q. et al. Local mutational diversity drives intratumoral immune heterogeneity in non-small cell lung cancer. Nat. Commun. 9 (1), 5361 (2018).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39 (1), 1–10 (2013).
Xu, L. et al. TIP: a web server for resolving tumor immunophenotype profiling. Cancer Res. 78 (23), 6575–6580 (2018).
Ritchie, M. E. et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43 (7), e47 (2015).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44 (D1), D457–462 (2016).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28 (1), 27–30 (2000).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics J. Integr. Biology. 16 (5), 284–287 (2012).
Song, Z. et al. STEAP3 is a prognostic biomarker that promotes glioma progression by regulating immune microenvironment and PI3K-AKT pathway. Cancer Biomark. A. 38 (4), 505–522 (2023).
Wang, S. et al. UCSCXenaShiny: an R/CRAN package for interactive analysis of UCSC Xena data. Bioinf. (Oxford England). 38 (2), 527–529 (2022).
García-Mulero, S. et al. Lung metastases share common immune features regardless of primary tumor origin. J. Immunother. Cancer 8(1) (2020).
Li, H. et al. Ferritin light chain promotes the reprogramming of glioma immune microenvironment and facilitates glioma progression. Theranostics 13 (11), 3794–3813 (2023).
Xu, C. et al. Origin, activation, and targeted therapy of glioma-associated macrophages. Front. Immunol. 13, 974996 (2022).
Pinton, L. et al. The immune suppressive microenvironment of human gliomas depends on the accumulation of bone marrow-derived macrophages in the center of the lesion. J. Immunother. Cancer. 7 (1), 58 (2019).
Ruffell, B., Affara, N. I. & Coussens, L. M. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 33 (3), 119–126 (2012).
Nusblat, L. M., Carroll, M. J. & Roth, C. M. Crosstalk between M2 macrophages and glioma stem cells. Cell. Oncol. (Dordrecht Netherlands). 40 (5), 471–482 (2017).
Zarif, J. C., Taichman, R. S. & Pienta, K. J. TAM macrophages promote growth and metastasis within the cancer ecosystem. Oncoimmunology 3 (7), e941734 (2014).
Georgieva, P. B. et al. Long-lived tumor-associated macrophages in glioma. Neuro-oncology Adv. 2 (1), vdaa127 (2020).
Toledo, B. et al. Deciphering the performance of macrophages in tumour microenvironment: a call for precision immunotherapy. J. Hematol. Oncol. 17 (1), 44 (2024).
Jiang, H., Ge, H., Shi, Y., Yuan, F. & Yue, H. CAFs secrete CXCL12 to accelerate the progression and cisplatin resistance of colorectal cancer through promoting M2 polarization of macrophages. Med. Oncol. (Northwood Lond. Engl.). 40 (3), 90 (2023).
Li, C. et al. Tumor-associated macrophages: potential therapeutic strategies and future prospects in cancer. J. Immunother. Cancer 9(1) (2021).
Chen, D. et al. SPHK1 potentiates colorectal cancer progression and metastasis via regulating autophagy mediated by TRAF6-induced ULK1 ubiquitination. Cancer Gene Ther. 31 (3), 410–419 (2024).
Liu, X. T. et al. Targeting the SphK1/S1P/PFKFB3 axis suppresses hepatocellular carcinoma progression by disrupting glycolytic energy supply that drives tumor angiogenesis. J. Translational Med. 22 (1), 43 (2024).
Lau, P. et al. Sphingosine kinase 1 promotes tumor immune evasion by regulating the MTA3-PD-L1 axis. Cell Mol. Immunol. 19 (10), 1153–1167 (2022).
Chun, K. S., Jang, J. H. & Kim, D. H. Perspectives regarding the intersections between STAT3 and oxidative metabolism in Cancer. Cells 9(10) (2020).
He, J. et al. KIAA0040 enhances glioma growth by controlling the JAK2/STAT3 signalling pathway. J. Cell. Mol. Med. 28 (8), e18332 (2024).
Xiong, Z. et al. IFITM3 promotes glioblastoma stem cell-mediated angiogenesis via regulating JAK/STAT3/bFGF signaling pathway. Cell Death Dis. 15 (1), 45 (2024).
Jia, M. et al. Nitidine chloride suppresses epithelial-mesenchymal transition and stem cell-like properties in glioblastoma by regulating JAK2/STAT3 signaling. Cancer Med. 10 (9), 3113–3128 (2021).
Fujii, T. et al. KLC1-ROS1 Fusion exerts Oncogenic Properties of Glioma Cells via specific activation of JAK-STAT pathway. Cancers ; 16(1). (2023).
Acknowledgements
Hebei Provincial Natural Fund Grant Program (H2021206037), Hebei Provincial Government Grant Program for Excellent Talents in Clinical Medicine (303-16-20-06), Hebei Provincial Medical Science Research Project Program (20230031), Central Guided Local Science and Technology Development Funding Program (236Z7752G).
Author information
Authors and Affiliations
Contributions
Zihan Song conducted experiments, analyzed, and interpreted data, and wrote the original draft. Zijun Zhao, Xuehua Liu, and Yiran Song were involved in animal experiments. Siyu Zhu, Ziyang Jia, Boyu Sun and Yijie Li were involved in cell experiments. Zairan Wang, Qianxu Jin and Shiyang Zhang collected the samples and data. Zihan Song created tables, graphs, and figures. Liqiang Liu and Zongmao Zhao conceived the idea for the project, revised the paper, and received funding for the project. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, Z., Zhao, Z., Liu, X. et al. Sphingosine kinase 1 promotes M2 macrophage infiltration and enhances glioma cell migration via the JAK2/STAT3 pathway. Sci Rep 15, 4152 (2025). https://doi.org/10.1038/s41598-025-88328-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88328-2